Abstract
The objective of the study was to evaluate the breathing pattern in patients with chronic non-specific low back pain (LBP) and in healthy subjects, both at rest and during motor control tests. Ten healthy subjects and ten patients with chronic LBP participated at this case–control study. The breathing pattern was evaluated at rest (standing and supine position during both relaxed breathing and deep breathing) and while performing clinical motor control tests, i.e. bent knee fall out and active straight leg raise. A blinded observer analyzed the breathing pattern of the participants using visual inspection and manual palpation. Costo-diaphragmatic breathing was considered as optimal breathing pattern. Subjects filled in visual analog scales for the assessment of pain intensity during the tests. At rest, no significant differences were found between the breathing pattern of patients and healthy subjects (P > 0.05). In contrast, significantly more altered breathing patterns were observed in chronic LBP-patients during motor control tests (P = 0.01). Changes in breathing pattern during motor control tests were not related to pain severity (P > 0.01), but were related to motor control dysfunction (P = 0.01).
Keywords: Motor control impairment, Non-specific low back pain, Chronic, Breathing pattern
Introduction
The relationship between spinal stability and low back pain (LBP) has been the focus of research over the last decades. It has been shown that m. transversus abdominis and m. multifidus are consistently activated in an anticipatory manner to prepare the trunk against the reactive moments from movements [21]. Recent evidence also suggests the diaphragm may contribute to spinal stability [18]. In an experimental situation in healthy subjects, stimulation of the diaphragm by activation of the phrenic nerve resulted in an increase in intra-abdominal pressure with subsequently enhanced spinal stiffness, without overt activity of abdominal and back muscles [18]. Contraction of the diaphragm thus modulates intra-abdominal pressure and contributes to trunk stability, in addition to maintaining of ventilation [18, 19]. In healthy subjects, the diaphragm is able to perform this dual task (trunk stability and respiration) when trunk stability is challenged [22].
In the presence of pain or after a painful experience, the strategies used by the central nervous system to control trunk muscles (i.e. motor control) may be altered [16]. A delayed contraction of m. transversus abdominis [21] and inhibition of m. multifidus [14] have been observed in LBP-patients. These changes are associated with impaired spinal stability and are considered motor control deficits. Research has focussed on these motor control deficits as a potential mechanism for ongoing symptoms in low back and pelvic pain [2]. For example, impaired kinematics of diaphragm and pelvic floor were observed during a motor control test in patients with sacro-iliac joint pain [36]. More than half of the patients displayed complete breath hold during this test. Normalization of breathing patterns and diaphragmatic kinematics were found in the patients after following an individualized motor learning intervention [35]. In healthy individuals, increased diaphragmatic activity has been found during incorrect performance of a motor control test [1].
Researchers and clinicians therefore suggested that breathing patterns should be evaluated during motor control tests, as breath holding and increased diaphragmatic activity can be considered as compensatory strategies to enhance lumbopelvic stability [1, 5]. The guidelines for stability retraining advise sustained normal breathing during exercises [37]. Clinically, physiotherapists are aware that altered breathing patterns may be present in a proportion of individuals with chronic LBP during stability retraining. However, no data is presently available regarding the proportion of patients with altered breathing patterns during motor control exercises.
In conclusion, there is some evidence suggesting a relation between respiration and low back pain. A non-optimal coordination of postural and respiratory functions of trunk muscles was proposed as an explanation for this relationship. Postural and motor control impairments have been reported extensively in chronic LBP-patients during laboratory testing [20, 33], but information about the influence of LBP on respiratory patterns during clinical tests assessing motor control of the lumbopelvic region is lacking.
Study aims
The purpose of this study was therefore to evaluate the breathing pattern in chronic LBP-patients and in healthy subjects, in both standing and supine position, and under three different types of conditions: spontaneous breathing, deep breathing and during motor control tests. Three clinical tests were employed to examine the influence of motor control on the lumbopelvic region during hip movements. We hypothesized that healthy subjects would be able to perform motor control exercises without changing their breathing pattern, while a change in breathing pattern would be observed in the patients during these performances. Furthermore, we hypothesized that patients would have more difficulties than healthy subjects to dissociate hip and lumbar movements during the clinical motor control tests.
Methods
Research design
In a case–control study, breathing patterns of chronic LBP-patients and healthy subjects were evaluated, both at rest and during performances in which trunk stability muscles are challenged. Prior to participation, all subjects received verbal and written information addressing the nature of the study. Participants were asked to read an information leaflet explaining the purpose of the research, and to seek additional explanations if necessary. In case of agreement, they were asked to sign a document stating their informed consent. To guarantee blinding of the examiner, the subjects were recruited by a nurse, by their physiotherapists or by the first author, all of whom were not involved in the clinical examination part of the study. Participants were told the clinical examiner (LV) had to be blinded about their medical condition during the complete clinical evaluation. Demographic information was recorded by the time of testing and the patients were asked to fill in several questionnaires. The Human Research Ethics Committee of the University Hospital of Antwerp approved the study protocol and the information leaflet.
Subject recruitment
A sample of ten chronic LBP-patients (6 women and 4 men) was recruited from a private physiotherapy practice and hospital outpatient physical therapy division. Inclusion criteria were chronic pain LBP (>3 months), which was of sufficient intensity to limit function, for which they had sought medical treatment, and that was of insidious onset, rather than the result of gross trauma. Further inclusion criteria for LBP-patients were an age range of 18–65 years, a diagnosis of non-specific LBP made by a physician and Dutch as the native language. Patients with specific underlying pathology as cause of LBP or with primary respiratory diseases were excluded [41]. A standardized checklist was used for the recruitment of the patients. In order to ascertain that no subjects with specific LBP entered the study, the study investigator re-evaluated the in- and exclusion criteria after performance and scoring of the clinical tests.
Ten healthy people (4 women and 6 men) were recruited among researchers’ acquaintances. Inclusion criteria were an age range of 18–65 years, a good health, no history of LBP or respiratory diseases and Dutch as the native language. Characteristics of all subjects are presented in Table 1.
Table 1.
Characteristics of healthy subjects and patients
| Healthy n = 10 |
Patients n = 10 |
P value | |
|---|---|---|---|
| Age (years) | 38.5 (7.0) | 44.3 (7.3) | 0.20 |
| Length (cm) | 171.4 (9.3) | 167.8 (9.2) | 0.32 |
| Weight (kg) | 73.4 (16.5) | 67.3 (8.2) | 0.20 |
| VAS (mm) | 0 | 24.4 (30.6) | 0.01 |
| Duration symptoms (years) | 0 | 13 (8) | <0.01 |
| PVAQ | 39.5 (13.3) | ||
| PCS | 18.3 (14.8) |
Instrumentation
The pressure biofeedback unit (PBU) consists of an inflatable inelastic bag connected to a pressure gauge and inflation device, and was developed to monitor lumbopelvic movement by recording pressure changes during assessment [38]. Excessive pressure changes indicate movement of the lumbopelvic region, which may suggest uncontrolled movement) during the test performance [47].
The visual analog scale (VAS, 100 mm) was used for the assessment of the severity of lumbar pain. The VAS pain score is believed to be reliable, valid, and sensitive to change [24, 32]. The psychometric properties of the Dutch versions of the pain catastrophizing scale (PCS) [45] and the pain, vigilance and awareness questionnaire (PVAQ) [39, 40] have been described elsewhere.
Procedure
The breathing pattern was evaluated at rest and during two motor control tests of the lumbopelvic region: the active straight leg raising (ASLR) and the bent knee fall out (BKFO). Furthermore, motor control patterns of the lumbopelvic region were evaluated during the knee lift abdominal test (KLAT) and the BKFO with the use of a pressure biofeedback unit [42]. These clinical tests were employed to examine the influence of motor control on the lumbopelvic region during hip movements. The order of the tests was randomly assigned to avoid order effects. After each test, participants were asked to fill in a VAS score and a Borg score for perceived effort, which was not handed out to the clinical examiner prior to the end of the test, to guarantee complete blinding of the examiner during testing. In three 2-h training sessions prior to data collection, the examiner was trained in performing the tests. Two experts with both clinical and research experience in the field of manual therapy (NR, JN) and respiratory physiotherapy (JN) leaded these sessions.
The thoraco-abdominal motion during breathing was assessed in rest, both in standing and in supine position, as described previously [31, 41]. Movements of the thorax and the abdomen during respiration were inspected and palpated during spontaneous quiet breathing and during deep breathing. A normal breathing pattern (costo-diaphragmatic breathing) was defined as a displacement of the ribcage in cranial, lateral outward and ventral direction and an outward movement of the abdomen during inspiration and the reverse pattern during expiration [6, 31]. All other breathing patterns were defined as asynchronous breathing patterns.
Motor control of the lumbopelvic region was analyzed by evaluating the subjects’ ability to control movement of lumbopelvic region, while performing simple movements in the hips [28]. The participants were instructed to maintain neutral spine position (i.e. preventing uncontrolled spinal movement) during lower extremity movement (knee flexion, hip flexion or hip abduction/rotation). KLAT (see Fig. 1) was based on the abdominal exercises described by Sahrmann [43] and on the isometric stability test (level 1) performed by Wohlfahrt et al. [44]. The subjects were positioned in crook lying. They were asked to lift one foot off the table and to raise the leg to 90° of hip flexion with knee flexion, keeping the lumbar spine stable. KLAT was scored with a PBU, which was placed horizontally under the spine of the participant, with the lower edge at the level of the posterior superior iliac spines. The pressure in the PBU was inflated to 40 mmHg (baseline pressure) [38]. A pre-testing trial was organized to familiarize the subjects with the PBU and the clinical test. Prior to the test session, the subjects performed two inspirations and expirations. Small increase and decrease in pressure (less than 2 mmHg) were observed during inspiration and expiration in rest. The pressure was then readjusted to 40 mmHg.
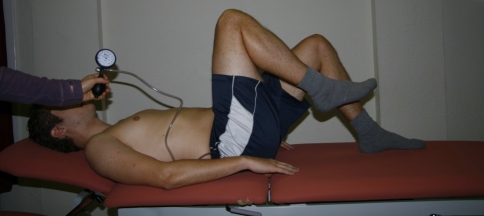
Fig. 1.
Knee lift abdominal test (KLAT). The subject is instructed to lift one foot off the table to 90° of hip flexion with knee flexion, keeping the lumbar spine stable. The pressure biofeedback is positioned under the lumbar spine. The pressure is read on the manometer
For the BKFO (see Fig. 2), the subject was positioned supine in partial crook lying position, as described by Comerford and Mottram [5]. The participant then slowly lowered the bent leg to approximately 45° of abduction/lateral rotation, while keeping the foot supported beside the straight leg, and then returned to the starting position. Abdominal muscles should then activate to stabilize the trunk in coordination with the adductors, which eccentrically lower the leg to abduction/lateral rotation [5]. The BKFO was also scored with a PBU, which was positioned vertically under the lumbar spine, with the lower edge 2 cm caudal of the posterior superior iliac spines, on the heterolateral side of the bent knee. A folded towel was placed near the PBU, so as to keep both sides of the lumbar spine at the same height. The pressure in the PBU was inflated to 40 mmHg (baseline pressure) [38], and the same protocol as mentioned above was used for the scoring method of BKFO. High inter-observer reliability (ICC > 0.85) has been found for the use of the PBU during KLAT and moderate to high inter-observer reliability (ICC varying between 0.68 and 0.87) has been found for the BKFO [42].
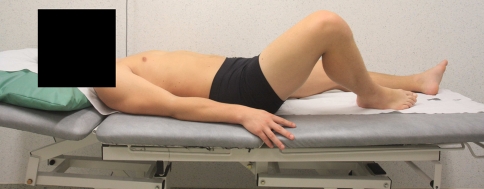
Fig. 2.
Bent knee fall out (BKFO). The subject is instructed to lower out the bent leg to approximately 45° of abduction/lateral rotation, while keeping the foot supported beside the straight leg, and then to return to the starting position
The breathing pattern was evaluated during the performance of two motor control tests, ASLR and BKFO. The ASLR (see Fig. 3) was performed as described by Mens et al. [30] and Roussel et al. [41] to assess the ability of the pelvic girdle to transfer loads between the lumbopelvic region and the legs. In brief, the subject was instructed to raise a straight leg 20 cm above the table starting from a supine position. This position was hold during 10 s. The ASLR was not evaluated with a PBU, but the patients’ Borg score for perceived effort was used to score the test [41]. To avoid influence on the breathing pattern, the subjects were not told that the breathing pattern was assessed while performing motor control tests. Therefore, the thoraco-abdominal motion (spontaneous breathing pattern) during the motor control test was only assessed with visual inspection and not with palpation.
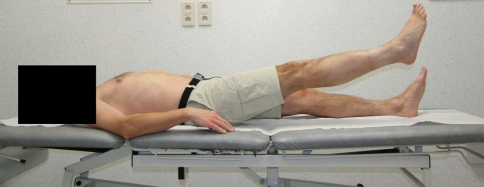
Fig. 3.
Active straight leg raise (ASLR). The subject is instructed to lift one extended leg 20 cm above the table and to hold this for 10 s, keeping the lumbar spine stable
Statistical analysis
All data were analyzed using SPSS 12.0© for Windows (SPSS Inc. Headquarters, 233 s. Wacker Drive, 11th floor, Chicago, IL 60606, USA) and SigmaStat. (Systat Software, Inc. 1735, Technology Drive, Ste 430, San Jose, CA 95110, USA) Appropriate descriptive statistics were used. A 1-sample Kolmogorov–Smirnov goodness-of-fit test was used to examine whether the variables were normally distributed. We expected that less than 10% of the healthy subjects (based on clinical observations) and 50–60% of the patients (based on a previous uncontrolled research [41]) would display an asynchronous breathing pattern during motor control tests. A power analysis therefore determined that 20 subjects were necessary to establish statistical significance at a power of 0.80. A Mann Whitney U test (for ASLR, BKFO and KLAT) and a Fisher exact test (for evaluation of the breathing pattern) were used to compare patients with healthy subjects. Spearman correlation coefficients were computed to analyze correlations between clinical tests and self-reported measurements. The significance level was set at 0.05, except for the correlation analysis, where the significance level was set at 0.01 to help protect against potential type I errors.
Results
At rest, no significant differences were found between the breathing pattern of patients and healthy subjects in supine position (P > 0.05). In standing, no differences were found during quiet breathing, but there was a significant difference during a deep breath (P < 0.05). Significantly more altered breathing patterns were observed in chronic LBP-patients during performance of the motor control testing (P < 0.05). All patients were able to carry out the clinical motor control tests. Five and six out of ten patients presented with an altered breathing pattern during the ASLR and BKFO, respectively, while none of the healthy subjects changed their breathing pattern during ASLR or BKFO (see Table 2). Changes in breathing pattern during motor control tests were not related to the severity of the pain during test performance (P > 0.05).
Table 2.
Breathing pattern (BP) during the active straight leg raise (ASLR) and bent knee fall out (BKFO) in healthy subjects and patients with low back pain
| Abnormal BP in healthy (%) n = 10 |
Abnormal BP in patients (%) n = 10 |
P value | |
|---|---|---|---|
| Standing, SBP | 1 (10) | 3 (30) | 0.29 |
| Standing, DBP | 1 (10) | 6 (60) | 0.03 |
| Supine, SBP | 0 | 0 | |
| Supine, DBP | 1 (10) | 2 (20) | 0.5 |
| ASLR, left | 1 (10) | 5 (50) | 0.05 |
| ASLR, right | 0 | 5 (50) | 0.01 |
| BKFO, left | 0 | 6 (60) | <0.01 |
| BKFO, right | 0 | 7 (70) | <0.01 |
SBP spontaneous quit breathing pattern, DBP deep breathing pattern
Patients demonstrated increased pressure deviation from baseline value when compared to the healthy subjects (see Table 3). The results of KLAT did not differ significantly between patients and healthy participants (P > 0.05). However, significant differences between patients with LBP and healthy subjects were found for the BKFO on the right side (P < 0.05) and a trend toward significance was found for the left side.
Table 3.
Mean (X) and standard deviations (SD) are given for the pressure results, evaluated with the pressure biofeedback unit, of bent knee fall out (BKFO) and knee lift abdominal test (KLAT) in healthy subjects and patients with low back pain
| Data healthy X (SD) mmHg n = 10 |
Data patients X (SD) mmHg n = 10 |
P value | |
|---|---|---|---|
| BKFO, left | 36.2 (3.71) | 34.1 (2.13) | 0.07 |
| BKFO, right | 36.4 (2.27) | 34.2 (1.99) | 0.03 |
| KLAT, left | 44.2 (5.45) | 46.2 (5.70) | >0.05 |
| KLAT, right | 43.2 (4.54) | 45.7 (7.09) | >0.05 |
No significant correlations were found either between the VAS score and the test results (r varying between −0.05 and 0.64, P > 0.05), or between the questionnaires and the test results (r varying between −0.70 and 0.25, P > 0.05). Significant correlations were found between the evaluation of the breathing pattern during the BKFO and an increase in pressure during this test (r > 0.50, P = 0.01). At least 25% (r2) of the variance in pressure increase can thus be explained by a change in breathing pattern.
Discussion
The results of this study suggest that chronic LBP-patients exhibit altered breathing patterns during performances in which the trunk stability muscles are challenged and that changes in breathing pattern during motor control tests are not related to pain severity.
The diaphragm is the principal muscle for inspiration [7], but evidence suggests it may also contribute to trunk stiffness and postural control [17]. Also m. transversus abdominis is involved in both postural/motor control [21] and respiration [8]. The coordination of both postural/motor control and respiration seems nevertheless to be complicated, particularly when either postural/motor control or respiratory demand is increased [19]. Therefore, the present study was undertaken to analyze the breathing pattern in relation to motor control in chronic LBP-patients.
The subjects’ breathing pattern was evaluated with palpation and visual inspection in the present study. Palpation and visual inspection of abdominal and upper chest wall motion have been suggested for the evaluation of diaphragmatic excursion [3], as prior research confirmed that the observed rib cage and abdominal motion actually reflects intercostal and diaphragm contribution during breathing [11]. Costo-diaphragmatic breathing was defined as normal breathing pattern [6, 31], as it allows maximal lung expansion and therefore maximal gas exchange. Paradoxical breathing pattern, upper costal breathing, mixed breathing pattern and breath holding were all considered as asynchronous breathing motions [29], as these asynchronous breathing patterns are likely to prevent effective alveolar ventilation [3].
All subjects displayed a costo-diaphragmatic breathing pattern in supine position during spontaneous quiet breathing. Also in standing, no significant differences were found between breathing patterns of patients and healthy subjects during spontaneous quiet breathing. In contrast, significantly more altered breathing patterns were present in chronic LBP-patients during deep breathing in standing but not in supine position. The results of these clinical observations are in line with the results of the studies performed in laboratory settings. Respiratory movements represent a greater disturbance to posture and body balance in LBP-patients compared to healthy subjects [12, 13]. Some patients in the present study may have favored the postural function of diaphragm and transversus abdominis, thereby disadvantaging the respiration, with altered breathing patterns as result.
To verify this hypothesis, motor control tests were performed in the present study. More than half of the patients displayed an asynchronous breathing pattern during the motor control tests, i.e. BKFO and ASLR, while they all had a synchronous breathing pattern in rest when lying supine. These results confirm our previous uncontrolled research findings [41], i.e. that approximately 50–60% of the patients with chronic LBP change their breathing pattern in situations in which trunk stability is challenged. It is therefore plausible that several patients utilize their diaphragm as a kind of regulating mechanism, to influence trunk stability and more specifically segmental stability of the lumbar spine, which interfere with their breathing pattern. A change in breathing pattern was indeed related with a significant increase in pressure during the motor control tests, while only small pressure changes were observed during the respiratory cycle in rest. As no electromyography of the diaphragm was used in the present study, further research is required to verify this hypothesis.
While evidence is available for the relationship between postural and respiratory control [12, 13, 17, 19, 26], only few studies analyzed the relationship between respiration and motor control. A PBU was used in the present study to score the motor control tests. Calibration studies demonstrated that pressure recordings resulted from lumbopelvic movement and positional changes and that they were independent of the individual body weight [25]. The PBU is sensitive to small movements associated with deep muscle recruitment within 2 mmHg of pressure change [10]. A high level of agreement has been found between the results of the prone abdominal drawing-in test, recorded with the PBU and converted to categories and a delayed contraction of transversus abdominis [15]. Furthermore, a blinded observer was able to detect the presence or absence of LBP with the use of the prone abdominal drawing-in test, recorded with the PBU [4]. Although it is unnatural to keep the spine rigid during movements, previous research demonstrated that healthy subjects with good trunk stabilization are able to maintain neutral spine position while moving the legs [25]. Jull et al. [25] developed a method to measure active control of the trunk by loading in the sagittal plane (unilateral leg load). These unilateral leg movements were considered to be low load. In this study, subjects who automatically pre-activated the transversus abdominis and internal oblique controlled the lumbopelvic position during the application of low load, with low pressure changes as result. Jull et al. [25] therefore suggested that ‘excessive’ pressure changes during low loaded exercise reflect an inability to maintain isometric contraction of the abdominal muscles, resulting in uncontrolled movement of the lumbar spine. They did, however, not define these ‘excessive’ pressure changes.
Healthy subjects are able to lift their leg without disturbing intra-abdominal and intra-thoracal pressure fluctuations associated with respiration, and demonstrate only a slight increase in intra-abdominal pressure associated with the ASLR [2]. In contrast, increased diaphragmatic activity was found in healthy subjects during incorrect performance of a motor control test, evaluated with the PBU [1]. Higher pressure rates during motor control tests, similar to the tests used in the present study, were related to increased activity of both diaphragm and rectus abdominis, confirmed by electromyography, without concomitant increase in activity of the internal oblique/transversus abdominis. Conversely, correct test performances consisted of low pressure changes and a significant increase in activity of the internal oblique/transversus abdominis without dominance of rectus abdominis or diaphragm. We did not use electromyography, but an increase in pressure during the BKFO was related with a change in breathing pattern in the present study.
In patients with sacro-iliac joint pain, impaired kinematics of diaphragm and pelvic floor were also observed during a motor control test in patients [36]. More than half of the patients displayed complete breath hold during ASLR performance. Normalization of breathing patterns and diaphragmatic kinematics were found in the patients after following an individualized motor learning intervention [35].
To date, however, no other studies regarding the relationship between altered breathing patterns and motor control exercises in patients with LBP have been published. Respiration has a continuous fluctuating effect on intradiskal pressure [44]. These respiration-induced intradiskal pressure changes may play a role in the nutrition and consequently in the mechanical behavior of the intervertebral disk [27]. A correct breathing pattern could therefore be very important in LBP-patients, as many of them show degenerated or impaired disks.
In the present study, patients displayed more pressure changes than healthy subjects during the performance of the motor control tests. These results of impaired motor control in LBP-patients are in accordance with literature results of both clinical and fundamental studies [4, 15, 21, 34, 46]. Differences in temporal patterns of activation between healthy subjects and LBP-patients have been found during KLAT, indicating a lack of synergistic co-activation between the examined muscle sites in the patients [23]. If the deep intrinsic (local) spinal muscles fail to provide optimal control of segmental motion, several compensatory mechanisms may occur. First, uncontrolled movement of the lumbar spine and/or pelvis may occur. Second, global muscles such as external obliques or rectus abdominis may be recruited to provide global control of the lumbo-pelvic region. Also the diaphragm may be used to enhance spinal stability, thereby disadvantaging respiration (decreased diaphragmatic excursion or breath holding). Finally, there is evidence that also the pelvic floor may be dysfunctional in patients with LBP [9]. It seems plausible that the pelvic floor may be used as well for improving the spinal stability. The fact that different mechanisms may be used by the patients to enhance the lumbo-pelvic stability, as compensation for dysfunctional local muscles, could explain the rather small mean differences in pressure found in the present study, as not all these compensations lead to the increased pressure during motor control tests. The differences were significant for the BKFO on the right side and nearly significant on the left side. Patients with chronic LBP do have impaired motor control of the lumbo-pelvic region, but it may manifest in several ways. In our study, only 25% of the variance in pressure is for example explained by a change in breathing pattern. Differentiating subgroups in the heterogeneous population of patients with non-specific LBP in terms of direction of uncontrolled movement and of compensatory strategy chosen by the patient, is therefore crucial.
Methodological considerations
The results of this study should be interpreted in the light of several methodological issues. First, no reliability assessment was made by the investigator for the assessment of the breathing pattern. The inter-observer reliability of the assessment of breathing patterns is generally fair to moderate [41]. Therefore, all subjects were examined by the same observer, and efforts were made to train the examiner in the evaluation of the breathing pattern. The examiner was completely blinded to the medical condition of the subject in order to reduce potential bias. However, the intra-observer reliability of this evaluation method remains to be examined. Second, only manual palpation and/or visual inspection were used to assess the breathing patterns, which is a rather subjective evaluation method. Further research with the use of electromyography is required, to analyze the relationship between diaphragmal activity and motor control tests. Finally, prospective studies are needed, to determine the cause and effect relationship of these observations.
Conclusion
The results of this study suggest that more than half of the patients with chronic non-specific low back pain exhibit altered breathing patterns during performances in which the trunk stability muscles are challenged, but that these altered breathing patterns are not related to pain intensity during test performance. Clinicians should therefore evaluate the breathing pattern in patients with low back pain, as a subgroup of patients with low back pain may recruit the diaphragm to enhance lumbopelvic stability. Further research regarding the role of the diaphragm in the occurrence and/or recurrence of low back pain, and possible therapeutically implications, is required.
Acknowledgments
This study was financially supported by a PhD grant (‘Motor control of the lumbopelvic region in dancers and patients with low back pain’, G806) supplied by the Artesis University College of Antwerp. The authors would like to thank all the participants, physicians and physical therapists for kindly cooperating in this study. Special thanks to Cathy de Vel and Geraldine Clarke for editing the manuscript.
Conflict of interest statement No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization in which the authors are associated.
References
- 1.Allison GT, Kendle K, Roll S, et al. The role of the diaphragm during abdominal hollowing exercises. Aust J Physiother. 1998;44:95–102. doi: 10.1016/s0004-9514(14)60369-x. [DOI] [PubMed] [Google Scholar]
- 2.Beales D, O’Sullivan PB, Briffa NK. Motor control patterns during an active straight leg raise in pain-free subjects. Spine. 2009;34(1):E1–E8. doi: 10.1097/BRS.0b013e318188b9dd. [DOI] [PubMed] [Google Scholar]
- 3.Cahalin LP, Braga M, Matsuo Y, et al. Efficacy of diaphragmatic breathing in persons with chronic obstructive pulmonary disease: a review of the literature. J Cardiopulm Rehabil. 2002;22:7–21. doi: 10.1097/00008483-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Cairns M, Harrison K, Wright C. Pressure Biofeedback: a useful tool in the quantification of abdominal muscular dysfunction. Physiotherapy. 2000;86:127–138. doi: 10.1016/S0031-9406(05)61155-8. [DOI] [Google Scholar]
- 5.Comerford MJ, Mottram SL. Functional stability re-training: principles and strategies for managing mechanical dysfunction. Man Ther. 2001;6:3–14. doi: 10.1054/math.2000.0389. [DOI] [PubMed] [Google Scholar]
- 6.Groote A, Wantier M, Cheron G, et al. Chest wall motion during tidal breathing. J Appl Physiol. 1997;83:1531–1537. doi: 10.1152/jappl.1997.83.5.1531. [DOI] [PubMed] [Google Scholar]
- 7.Troyer A. Actions of the respiratory muscles or how the chest wall moves in upright man. Bull Eur Physiopathol Respir. 1984;20:409–413. [PubMed] [Google Scholar]
- 8.Troyer A, Estenne M, Ninane V, et al. Transversus abdominis muscle function in humans. J Appl Physiol. 1990;68:1010–1016. doi: 10.1152/jappl.1990.68.3.1010. [DOI] [PubMed] [Google Scholar]
- 9.Eliasson K, Elfving B, Nordgren B et al (2008) Urinary incontinence in women with low back pain. Man Ther 13:206–212 [DOI] [PubMed]
- 10.Falla DL, Campbell CD, Fagan AE, et al. Relationship between cranio-cervical flexion range of motion and pressure change during the cranio-cervical flexion test. Man Ther. 2003;8:92–96. doi: 10.1016/S1356-689X(03)00008-0. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert R, Auchincloss JH, Jr, Peppi D. Relationship of rib cage and abdomen motion to diaphragm function during quiet breathing. Chest. 1981;80:607–612. doi: 10.1378/chest.80.5.607. [DOI] [PubMed] [Google Scholar]
- 12.Grimstone SK, Hodges PW. Impaired postural compensation for respiration in people with recurrent low back pain. Exp Brain Res. 2003;151:218–224. doi: 10.1007/s00221-003-1433-5. [DOI] [PubMed] [Google Scholar]
- 13.Hamaoui A, Mc Do, Poupard L, et al. Does respiration perturb body balance more in chronic low back pain subjects than in healthy subjects? Clin Biomech (Bristol, Avon) 2002;17:548–550. doi: 10.1016/S0268-0033(02)00042-6. [DOI] [PubMed] [Google Scholar]
- 14.Hides JA, Richardson CA, Jull GA. Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine. 1996;21:2763–2769. doi: 10.1097/00007632-199612010-00011. [DOI] [PubMed] [Google Scholar]
- 15.Hodges P, Richardson C, Jull G. Evaluation of the relationship between laboratory and clinical tests of transversus abdominis function. Physiother Res Int. 1996;1:30–40. doi: 10.1002/pri.45. [DOI] [PubMed] [Google Scholar]
- 16.Hodges PW. The role of the motor system in spinal pain: implications for rehabilitation of the athlete following lower back pain. J Sci Med Sport. 2000;3:243–253. doi: 10.1016/S1440-2440(00)80033-X. [DOI] [PubMed] [Google Scholar]
- 17.Hodges PW, Butler JE, McKenzie DK, et al. Contraction of the human diaphragm during rapid postural adjustments. J Physiol. 1997;505(pt 2):539–548. doi: 10.1111/j.1469-7793.1997.539bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodges PW, Eriksson AE, Shirley D, et al. Intra-abdominal pressure increases stiffness of the lumbar spine. J Biomech. 2005;38:1873–1880. doi: 10.1016/j.jbiomech.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Hodges PW, Gurfinkel VS, Brumagne S, et al. Coexistence of stability and mobility in postural control: evidence from postural compensation for respiration. Exp Brain Res. 2002;144:293–302. doi: 10.1007/s00221-002-1040-x. [DOI] [PubMed] [Google Scholar]
- 20.Hodges PW, Moseley GL. Pain and motor control of the lumbopelvic region: effect and possible mechanisms. J Electromyogr Kinesiol. 2003;13:361–370. doi: 10.1016/S1050-6411(03)00042-7. [DOI] [PubMed] [Google Scholar]
- 21.Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain. A motor control evaluation of transversus abdominis. Spine. 1996;21:2640–2650. doi: 10.1097/00007632-199611150-00014. [DOI] [PubMed] [Google Scholar]
- 22.Hodges PW, Gandevia S. Activation of the human diaphragm during a repetitive postural task. J Physiol. 2000;522(part 1):165–175. doi: 10.1111/j.1469-7793.2000.t01-1-00165.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubley-Kozey CL, Vezina MJ. Differentiating temporal electromyographic waveforms between those with chronic low back pain and healthy controls. Clin Biomech (Bristol, Avon) 2002;17:621–629. doi: 10.1016/S0268-0033(02)00103-1. [DOI] [PubMed] [Google Scholar]
- 24.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27:117–126. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 25.Jull G, Richardson CA, Toppenberg R, et al. Towards a measurement of active muscle control for lumbar stabilisation. Aust J Physiother. 1993;39:187–193. doi: 10.1016/S0004-9514(14)60481-5. [DOI] [PubMed] [Google Scholar]
- 26.Kantor E, Poupard L, Le Bozec S, et al. Does body stability depend on postural chain mobility or stability area? Neurosci Lett. 2001;308:128–132. doi: 10.1016/S0304-3940(01)01986-3. [DOI] [PubMed] [Google Scholar]
- 27.Keller TS, Holm SH, Hansson TH, et al. 1990 Volvo Award in experimental studies. The dependence of intervertebral disc mechanical properties on physiologic conditions. Spine. 1990;15:751–761. doi: 10.1097/00007632-199008010-00004. [DOI] [PubMed] [Google Scholar]
- 28.Luomajoki H, Kool J, Bruin E, et al. Movement control tests of the low back; evaluation of the difference between patients with low back pain and healthy controls. BMC Musculoskelet Disord. 2008;9:170. doi: 10.1186/1471-2474-9-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maitre B, Similowski T, Derenne JP. Physical examination of the adult patient with respiratory diseases: inspection and palpation. Eur Respir J. 1995;8:1584–1593. [PubMed] [Google Scholar]
- 30.Mens JM, Vleeming A, Snijders CJ, et al. Reliability and validity of the active straight leg raise test in posterior pelvic pain since pregnancy. Spine. 2001;26:1167–1171. doi: 10.1097/00007632-200105150-00015. [DOI] [PubMed] [Google Scholar]
- 31.Nijs J, Adriaens J, Schuermans D, et al. Breathing retraining in patients with chronic fatigue syndrome: a pilot study. Physiother Theory Pract. 2008;24:83–94. doi: 10.1080/09593980701429406. [DOI] [PubMed] [Google Scholar]
- 32.Ogon M, Krismer M, Söllner W, et al. Chronic low back pain measurement with visual analogue scales in different settings. Pain. 1996;64:425–428. doi: 10.1016/0304-3959(95)00208-1. [DOI] [PubMed] [Google Scholar]
- 33.O’Sullivan P. Diagnosis and classification of chronic low back pain disorders: maladaptive movement and motor control impairments as underlying mechanism. Man Ther. 2005;10:242–255. doi: 10.1016/j.math.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 34.O’Sullivan P, Twomey L, Allison G, et al. Altered patterns of abdominal muscle activation in patients with chronic low back pain. Aust J Physiother. 1997;43:91–98. doi: 10.1016/s0004-9514(14)60403-7. [DOI] [PubMed] [Google Scholar]
- 35.O’Sullivan PB, Beales DJ. Changes in pelvic floor and diaphragm kinematics and respiratory patterns in subjects with sacroiliac joint pain following a motor learning intervention: a case series. Man Ther. 2007;12:209–218. doi: 10.1016/j.math.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 36.O’Sullivan PB, Beales DJ, Beetham JA, et al. Altered motor control strategies in subjects with sacroiliac joint pain during the active straight-leg-raise test. Spine. 2002;27:E1–E8. doi: 10.1097/00007632-200201010-00015. [DOI] [PubMed] [Google Scholar]
- 37.Richardson C, Hodges P, Hides J. Therapeutic exercise for lumbopelvic stabilization: a motor control approach for the treatment and prevention of low back pain. Edinburgh: Churchill Livingstone; 2004. [Google Scholar]
- 38.Richardson CA, Jull GA, Toppenberg R, et al. Techniques for active stabilisation for spinal protection: a pilot study. Aust J Physiother. 1992;38:105–112. doi: 10.1016/S0004-9514(14)60555-9. [DOI] [PubMed] [Google Scholar]
- 39.Roelofs J, Peters ML, McCracken L, et al. The pain vigilance and awareness questionnaire (PVAQ): further psychometric evaluation in fibromyalgia and other chronic pain syndromes. Pain. 2003;101:299–306. doi: 10.1016/S0304-3959(02)00338-X. [DOI] [PubMed] [Google Scholar]
- 40.Roelofs J, Peters ML, Muris P, et al. Dutch version of the pain vigilance and awareness questionnaire: validity and reliability in a pain-free population. Behav Res Ther. 2002;40:1081–1090. doi: 10.1016/S0005-7967(02)00008-6. [DOI] [PubMed] [Google Scholar]
- 41.Roussel NA, Nijs J, Truijen S, et al. Low back pain: clinimetric properties of the Trendelenburg test, active straight leg raise test, and breathing pattern during active straight leg raising. J Manipulative Physiol Ther. 2007;30:270–278. doi: 10.1016/j.jmpt.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Roussel NA, Nijs J, Mottram S, Van Moorsel A, Truijen S, Stassijns G (2009) Altered lumbopelvic movement control but not generalised joint hypermobility is associated with increased injury in dancers: a prospective study. Man Ther. doi:10.1016/j.math.2008 [DOI] [PubMed]
- 43.Sahrmann SA. Diagnosis and treatment of movement impairment syndromes. St Louis: Mosby; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sato K, Kikuchi S, Yonezawa T. In vivo intradiscal pressure measurement in healthy individuals and in patients with ongoing back problems. Spine. 1999;24:2468–2474. doi: 10.1097/00007632-199912010-00008. [DOI] [PubMed] [Google Scholar]
- 45.Severeijns R, Hout M, Vlaeyen J, et al. Pain catastrophizing and general health status in a large Dutch community sample. Pain. 2002;99:367–376. doi: 10.1016/S0304-3959(02)00219-1. [DOI] [PubMed] [Google Scholar]
- 46.Silfies SP, Squillante D, Maurer P, et al. Trunk muscle recruitment patterns in specific chronic low back pain populations. Clin Biomech (Bristol, Avon) 2005;20:465–473. doi: 10.1016/j.clinbiomech.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Wohlfahrt D, Jull G, Richardson C. The relationship between dynamic and static function of abdominal muscles. Aust J Physiother. 1993;39:9–13. doi: 10.1016/S0004-9514(14)60464-5. [DOI] [PubMed] [Google Scholar]