Abstract
Nuclear factor-kappa B (NF-κB) is a gene transcriptional regulator of inflammatory cytokines. We investigated the transduction efficiency of NF-κB decoy to dorsal root ganglion (DRG), as well as the decrease in nerve injury, mechanical allodynia, and thermal hyperalgesia in a rat lumbar disc herniation model. Forty rats were used in this study. NF-κB decoy–fluorescein isothiocyanate (FITC) was injected intrathecally at the L5 level in five rats, and its transduction efficiency into DRG measured. In another 30 rats, mechanical pressure was placed on the DRG at the L5 level and nucleus pulposus harvested from the rat coccygeal disc was transplanted on the DRG. Rats were classified into three groups of ten animals each: a herniation + decoy group, a herniation + oligo group, and a herniation only group. For behavioral testing, mechanical allodynia and thermal hyperalgesia were evaluated. In 15 of the herniation rats, their left L5 DRGs were resected, and the expression of activating transcription factor 3 (ATF-3) and calcitonin gene-related peptide (CGRP) was evaluated immunohistochemically compared to five controls. The total transduction efficiency of NF-κB decoy–FITC in DRG neurons was 10.8% in vivo. The expression of CGRP and ATF-3 was significantly lower in the herniation + decoy group than in the other herniation groups. Mechanical allodynia and thermal hyperalgesia were significantly suppressed in the herniation + decoy group. NF-κB decoy was transduced into DRGs in vivo. NF-κB decoy may be useful as a target for clarifying the mechanism of sciatica caused by lumbar disc herniation.
Keywords: Nuclear factor-kappa B, Decoy, Nerve injury, Pain, Lumbar disc herniation
Introduction
Radicular pain, a common symptom of lumbar disc herniation, is induced by mechanical compression and inflammation [28, 29]. Prostaglandins (PGs) and cytokines generated at the site of inflammation are responsible for producing the associated pain [26]. Recently, cytokines such as interleukins and tumor necrosis factor alpha (TNF-alpha), and cyclooxygenase-2 (COX-2) have been strongly linked to the effects of nucleus pulposus on nerve roots [12, 27, 28]. It was recently reported that infliximab, a TNF-alpha inhibitor, was effective in treating sciatic pain caused by lumbar disc herniation and that patients experienced no significant side effects [16]. However, in another report, infliximab was compared to a placebo by the same Finnish group that carried out the first randomized controlled trial and the results were disappointing [17].
Recently, it was reported that the transcription factor nuclear factor-kappa B (NF-κB) plays a crucial role in regulating proinflammatory cytokine gene expression and the transfer of nociceptive information [2, 3]. NF-κB is activated in dorsal root ganglia (DRG) after partial sciatic nerve injury and is crucial for hyperalgesia [4, 20]. Thus, NF-κB is regarded as one of the most important targets for therapeutic intervention against inflammatory diseases such as rheumatoid arthritis, asthma, or inflammatory bowel disease [1, 8, 33, 35, 40].
Recent experiments to inhibit proinflammatory cytokine gene expression have found that NF-κB decoy oligodeoxynucleotides effectively block the activation of promoters of proinflammatory cytokine genes. NF-κB decoys have been shown to suppress cytokine expression in DRG, reduce thermal hyperalgesia after spinal nerve ligation, and reduce mechanical hyperalgesia and thermal hyperalgesia in a peripheral inflammatory pain model [19, 31]. In a previous report, we showed that an NF-κB decoy was conveyed and transduced into DRG in both an in vivo and in vitro model [11]. Additionally, NF-κB decoys reduced mechanical allodynia and thermal hyperalgesia in a rat foot inflammatory pain model [11].
In this report, we describe the transduction efficiency of NF-κB decoys into DRG neurons. In addition, we examine changes in nerve injury and inflammatory neurotransmitters using activating transcription factor 3 (ATF-3), a marker of nerve injury, and calcitonin gene-related peptide (CGRP), a marker of inflammatory neuropeptides, and look at the behavioral effects of intrathecal injection of the NF-κB decoy in reducing pain sensation in a rat model of lumbar disc herniation.
Materials and methods
All protocols involving animal procedures were approved by the Ethics Committees of our institution following the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (1996 revision). A total of 40 rats were used in the current study.
In vivo transduction efficiency of nuclear factor-kappa B decoy oligodeoxynucleotides-FITC
Five 6-week-old male Sprague–Dawley rats (200–250 g) were anesthetized by an intraperitoneal (i.p.) injection of sodium pentobarbital (40 mg/kg) and laminectomies were performed at the L5 level. A 20-μl solution of NF-κB decoy–FITC (10 mg/ml, AnGes MG, Inc., Osaka, Japan) was intrathecally injected using a 30-gauge needle. After injection of the solution, the muscle and skin were sutured. Seven days after surgery, the rats were transcardially perfused with 0.9% saline, followed by 500 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The left L5 dorsal root ganglia were resected and immersed in the fixative solution overnight at 4°C, after which they were transferred to a 20% sucrose solution in phosphate buffered saline for 24 h. Next, the DRG were cut into 10-μm sections on a cryostat and samples were mounted on poly-l-lysine-coated slides. Ten sections were randomly selected, and the percentage of FITC-positive neurons was analyzed. The sections were examined using a fluorescent microscope (Nikon, Tokyo, Japan).
Rat lumbar disc herniation model
Thirty rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.) and laminectomies were performed in which the left L5 nerve roots and associated dorsal root ganglia were exposed. Next, a 23-gauge needle was inserted at a central point in the DRG to place mechanical pressure on the DRG. Rats were then injected at the base of the tail with 1 ml of 1% lidocaine for local anesthesia and the tails were ligated with a 2-0 silk surgical suture to avoid continuous bleeding during the experiment. The tails were then amputated and autologous nucleus pulposus was harvested and applied to the left L5 nerve roots just proximal to the DRG [37]. Nucleus pulposus was harvested from between the second and third coccygeal vertebra of each tail and the amount harvested was approximately equal for all the animals. Rats were classified into three groups of ten rats each. The herniation + decoy group received 20 μl of NF-κB decoy (10 mg/ml, AnGes MG), which was injected intrathecally during surgery. The herniation + oligo group had 20 μl of scrambled oligonucleotide injected and the herniation only group had no additional treatment.
Evaluation of mechanical allodynia and thermal hyperalgesia
Fifteen rats were evaluated for mechanical allodynia and thermal hyperalgesia. The evaluation was performed prior to surgery (day 0) and on days 1, 2, 3, 7, 10, 14, and 28 after surgery. There were no animal dropouts on any day. Mechanical thresholds were measured using von Frey filaments with logarithmically incremental rigidity of 0.41, 0.70, 1.20, 2.00, 3.63, 5.50, 8.50, and 15.1 g (Stoelting, Wood Dale, IL), to calculate the 50% probability thresholds for mechanical paw withdrawal, as previously described [5]. Beginning with the 2.00-g probe, filaments were applied to the left plantar surface of the hind paw, corresponding to the L5 dermatome, for 6–8 s in a stepwise ascending or descending order following negative or positive withdrawal responses until six consecutive responses were noted [38]. Fifty percent withdrawal thresholds were calculated according to the method of Dixson [6]. In the absence of foot withdrawal in response to the application of a 15.1-g von Frey filament, 15.1 g was assigned as the mechanical threshold.
Thermal nociceptive thresholds in the rat hind paws were evaluated using a Hargreaves device (Ugo Basile, Varese, Italy). Rats were deposited into individual transparent acrylic boxes in a 17 × 22-cm plexiglass testing chamber with the floor maintained at 28°C. A heat stimulus (150 mcal/s/cm2) was delivered using a 0.5-cm-diameter radiant heat source positioned under the plantar surface of the paw to be tested. The heat source was placed alternately under each hind paw, after randomly selecting the side of the first paw stimulation to avoid anticipation by the animal. We used a cutoff time of 20 s after it was ascertained that no tissue damage was produced within this time period. Three trials were performed at each time point, and the latencies of paw withdrawal for all trials were averaged.
ATF-3 and CGRP immunohistochemical detection in lumbar disc herniation
Fifteen rats in the herniation groups and five control rats were evaluated for ATF-3 and CGRP expression. At 10 days after surgery, rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.) and perfused transcardially with 500 ml 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The left L5 DRG were resected and cut into 10-μm sections on a cryostat and samples were mounted on poly-l-lysine-coated slides. Sections were incubated in a blocking solution containing 0.3% Triton X-100 and 5% skim milk in 0.01 M phosphate buffered saline for 60 min at room temperature. Sections were processed for immunohistochemical detection of ATF-3 and CGRP by incubation with a rabbit anti-ATF3 polyclonal antibody (1:800; Santa Cruz Biotechnology Inc., Santa Cruz, CA) or CGRP (1:1,000; Chemicon, Temecula, CA) for 20 h at 4°C. Sections were then incubated with the fluorescein conjugated goat anti-rabbit antibody Alexa 488 (1:400; Molecular Probes Inc., Eugene, OR). The sections were examined using a fluorescent microscope (Nikon, Tokyo, Japan) with a FITC filter. We counted 10 sections each of DRG immunoreactive cells at 400× magnification using a counting grid. The number of ATF-3 and CGRP immunoreactive cells per 0.0225 mm2 was counted in ipsilateral DRG sections in each animal. The ratio of ATF-3 and CGRP immunoreactive neurons to the total number of neurons in each section was then calculated.
Statistical analysis
The paw withdrawal latency of the hind paws was compared using a one-way analysis of variance (ANOVA) for repeat measurements. For multiple comparisons, we used Dunnett’s test. Comparison of the number of ATF-3 and CGRP immunoreactive neurons among the groups was made using one-way ANOVA with Dunnett’s test.
Results
In vivo transduction efficiency of NF-κB decoy oligodeoxynucleotides-FITC
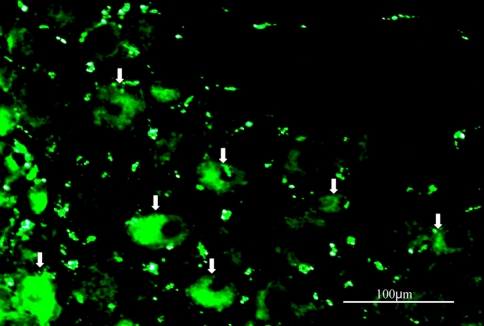
FITC labeled NF-κB decoy was transduced into DRG neurons. The ratio of FITC labeled neurons per total number of DRG neurons was 10.8 ± 1.2% (mean ± SEM). Figure 1 shows decoy-FITC-positive DRG neurons.
Fig. 1.
Photomicrograph showing decoy FITC-positive DRG neurons (arrows)
Mechanical allodynia and thermal hyperalgesia caused by disc herniation
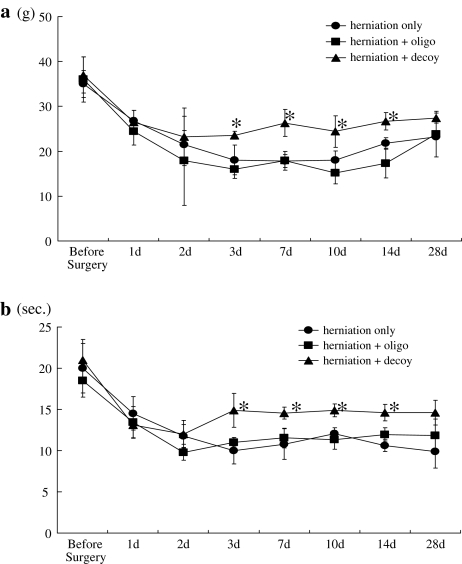
Compared with the basal line, animals in the disc herniation groups experienced significant mechanical allodynia and thermal hyperalgesia that continued through day 28 (P < 0.05). Mechanical allodynia and thermal hyperalgesia were significantly suppressed in the herniation + decoy group compared with the herniation only and herniation + oligo groups from days 3–14 and 3–14, respectively (P < 0.05) (Fig. 2a, b).
Fig. 2.
Animals in the disc herniation groups experienced significant mechanical allodynia (a) and thermal hyperalgesia (b) that continued through day 28, compared with the basal line (P < 0.05). Mechanical allodynia and thermal hyperalgesia were significantly suppressed in the herniation + decoy group compared with the herniation only or herniation + oligo groups from 3 to 14 days, and from 3 to 14 days, respectively (*P < 0.05)
ATF-3 and CGRP expression in DRG neurons
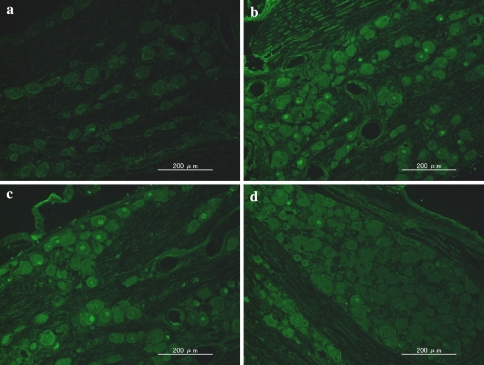
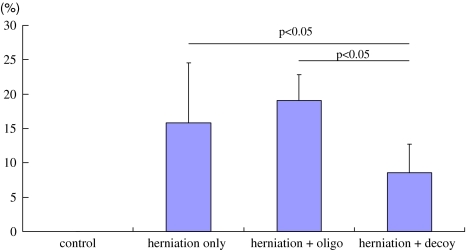
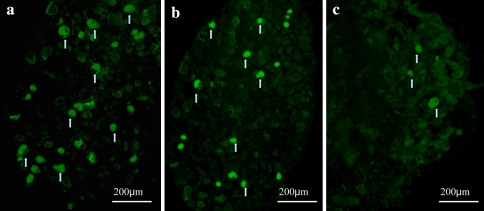
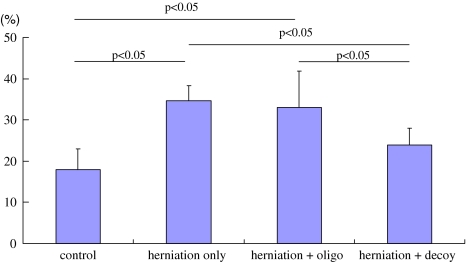
ATF-3 immunoreactivity was not observed in DRG neurons in the control animals, but was observed in the DRG neurons in the three herniation groups. The ratio of ATF-3 immunoreactive neurons to the total number of DRG neurons in the herniation only (16 ± 9%), and herniation + oligo groups (19 ± 5%) was significantly higher than in the herniation + decoy group (9 ± 4%) (P < 0.05) (Figs. 3, 4). On the other hand, CGRP immunoreactivity was observed in DRG neurons in the control group as well as the three herniation groups. The ratio of CGRP immunoreactive neurons to the total number of DRG neurons in the control group was significantly less than those in the herniation only and herniation + oligo groups (P < 0.05). Many CGRP positive DRG neurons were observed in the herniation only and herniation + oligo groups; however, the number of CGRP immunoreactive DRG neurons in the herniation + decoy group decreased after application of NF-κB decoy (P < 0.05) (Figs. 5, 6). The ratio of CGRP positive neurons to total DRG neurons in each group was as follows: control, 18 ± 5%; herniation only group, 35 ± 2%; herniation + oligo group, 33 ± 9%; herniation + decoy group, 23 ± 5%.
Fig. 3.
Photomicrographs show ATF-3 expression in the control group (a), herniation only group (b), herniation ± oligo group (c), and herniation ± NF-κB decoy group (d). The ratio of ATF-3 immunoreactive neurons in the herniation + NF-κB group was significantly less than those in the herniation only and herniation + oligo groups (P < 0.05)
Fig. 4.
The graph shows the ratio of ATF-3 immunoreactive cells to the total number of DRG neurons in the control, herniation only, herniation + oligo, and herniation + decoy groups. The ratios of ATF-3 positive DRG neurons in the herniation only and herniation + oligo groups were significantly higher than that in the herniation + decoy group (P < 0.05)
Fig. 5.
CGRP positive DRG neurons were observed in the herniation only (a) and herniation + oligo groups (b); however, the number of CGRP positive DRG neurons in the herniation + decoy group (c) decreased after application with NF-κB decoy. Arrows indicate CGRP positive DRG neurons
Fig. 6.
The graph shows the ratio of CGRP positive DRG neurons to the total number of DRG neurons in the control, herniation only, herniation + oligo, and herniation + decoy groups. CGRP expression significantly increased in herniation only and herniation + oligo groups compared with control group (P < 0.05). The ratio of CGRP positive DRG neurons in the herniation + decoy group was significantly less than those in the herniation only and herniation + oligo groups (P < 0.05)
Discussion
In this study, we demonstrated that lumbar disc herniation produces mechanical allodynia and thermal hyperalgesia, and increases ATF-3 and CGRP in DRG in rats. NF-κB decoy was transduced into DRG neurons in vivo leading to a decrease of mechanical allodynia and thermal hyperalgesia and decreased ATF-3 and CGRP expression.
Previous studies in animal models involving lumbar disc herniation have reported the occurrence of thermal hyperalgesia or mechanical allodynia [13, 14]. Application of nucleus pulposus onto nerve roots has been shown to induce edema of the nerve roots and dorsal root ganglia, and to alter the conduction velocity [41]. Proinflammatory cytokines, including IL-1, IL-6, and TNF-alpha, as well as COX-2, are known mediators of the peripheral inflammatory response caused by nucleus pulposus [10, 28, 30, 36]. These molecules are also synthesized and released during various types of nerve injury [26].
Recent studies have indicated that NF-κB decoy oligodeoxynucleotides are effective in suppressing inflammatory cytokine expression through inhibition of proinflammatory cytokine gene expression [9, 15, 18, 22, 32]. Previous reports have found that perineural injection of NF-κB decoy just distal to the dorsal root ganglion suppressed cytokine expression in the dorsal root ganglion and also reduced thermal hyperalgesia after spinal nerve ligation. Furthermore, NF-κB decoy injected intrathecally reduced mechanical alloying and thermal hyperalgesia after complete Freund’s adjuvant injection into rat hind paws [19, 31]. We have also reported that NF-κB decoy could be introduced into DRG neurons effectively in an in vitro and in vivo model, and that NF-κB decoy suppressed mechanical and thermal allodynia in a rat inflammatory foot pain model [11]. We did not examine proinflammatory cytokines in DRG neurons; however, considering previous reports and the results of the current study, NF-κB decoy may act to decrease the level of several cytokines, resulting in a decrease in pain behavior in a lumbar disc herniation model.
On the other hand, it has been reported that several cytokines that induce nerve injury are associated with ATF-3 expression. TNF-alpha plays a crucial role in peripheral nerve damage and recent studies have revealed that TNF-alpha activation in nucleus pulposus-induced apoptosis at the surface of the DRG in a lumbar disc herniation model [23]. TNF application to DRG has also been shown to induce histological changes in a dose-dependent manner in rats [24]. Selective inhibition of TNF-alpha prevents nucleus pulposus-induced histologic changes in DRG [25]. Interleukin has been shown to be produced in Schwann cells following sciatic nerve injury, and is also induced in resident Schwann cells in the Wallerian degeneration of sciatic nerves [34]. After peripheral nerve injury, CGRP in the small DRG neurons decreases; however, CGRP, which is not generally produced in medium and large DRG neurons under physiological conditions, starts to be expressed in these neurons [21]. This expression of CGRP in large DRG neurons induces mechanical allodynia [21]. Increased expression of CGRP in DRG has been reported during the development of inflammation in the rat [7]. In a study of human lumbar disc herniation, preoperative plasma levels of CGRP were significantly correlated with the extent of sciatica as determined by visual analogue scale [39]. In addition, plasma levels of CGRP significantly decreased after lumbar discectomy, which was in line with the disappearance of pain symptoms [39]. Thus, NF-κB decoy may suppress nerve damage and inflammation associated with decreased ATF-3 and CGRP expression.
Conclusion
In conclusion, NF-κB decoy was transduced into DRG neurons in vivo. When NFκ-B decoy was injected intrathecally, it suppressed nerve injury and influenced the production of peptides involved in pain perception. NFκ-B decoy may be useful as a target to clarify the mechanism of sciatica.
References
- 1.Aupperle K, Bennett B, Han Z, Boyle D, Manning A, Firestein GS. NF-kappa B regulation by I kappa B kinase-2 in rheumatoid arthritis synoviocytes. J Immunol. 2001;166:2705–2711. doi: 10.4049/jimmunol.166.4.2705. [DOI] [PubMed] [Google Scholar]
- 2.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/S0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 3.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 4.Chan CF, Sun WZ, Lin JK, Lin-Shiau SY. Activation of transcription factors of nuclear factor kappa B, activator protein-1 and octamer factors in hyperalgesia. Eur J Pharmacol. 2000;402:61–68. doi: 10.1016/S0014-2999(00)00431-3. [DOI] [PubMed] [Google Scholar]
- 5.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 6.Dixson WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 7.Donaldson LF, Harmar AJ, McQueen DS, Seckl JR. Increased expression of preprotachykinin, calcitonin gene-related peptide, but not vasoactive intestinal peptide messenger RNA in dorsal root ganglia during the development of adjuvant monoarthritis in the rat. Brain Res Mol Brain Res. 1992;16:143–149. doi: 10.1016/0169-328X(92)90204-O. [DOI] [PubMed] [Google Scholar]
- 8.Han Z, Boyle DL, Manning AM, Firestein GS. AP-1 and NF-kappaB regulation in rheumatoid arthritis and murine collagen-induced arthritis. Autoimmunity. 1998;28:197–208. doi: 10.3109/08916939808995367. [DOI] [PubMed] [Google Scholar]
- 9.Hölschermann H, Dürfeld F, Maus U, Bierhaus A, Heidinger K, Lohmeyer J, Nawroth PP, Tillmanns H, Haberbosch W. Cyclosporine a inhibits tissue factor expression in monocytes/macrophages. Blood. 1996;88:3837–3845. [PubMed] [Google Scholar]
- 10.Igarashi T, Kikuchi S, Shubayev V, Myers RR. 2000 Volvo Award winner in basic science studies: exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine. 2000;25:2975–2980. doi: 10.1097/00007632-200012010-00003. [DOI] [PubMed] [Google Scholar]
- 11.Inoue G, Ochiai N, Ohtori S, Nakagawa K, Gemba T, Doya H, Ito T, Koshi T, Moriya H, Takahashi K. Injection of nuclear factor-kappa B decoy into the sciatic nerve suppresses mechanical allodynia and thermal hyperalgesia in a rat inflammatory pain model. Spine. 2006;31:2904–2908. doi: 10.1097/01.brs.0000248424.46652.67. [DOI] [PubMed] [Google Scholar]
- 12.Kawakami M, Matsumoto T, Kuribayashi K, Tamaki T. mRNA expression of interleukins, phospholipase A2, and nitric oxide synthase in the nerve root and dorsal root ganglion induced by autologous nucleus pulposus in the rat. J Orthop Res. 1999;17:941–946. doi: 10.1002/jor.1100170620. [DOI] [PubMed] [Google Scholar]
- 13.Kawakami M, Tamaki T, Hayashi N, Hashizume H, Matsumoto T, Minamide A, Kihira T. Mechanical compression of the lumbar nerve root alters pain-related behaviors induced by the nucleus pulposus in the rat. J Orthop Res. 2000;18:257–264. doi: 10.1002/jor.1100180214. [DOI] [PubMed] [Google Scholar]
- 14.Kawakami M, Tamaki T, Weinstein JN, Hashizume H, Nishi H, Meller ST. Pathomechanism of pain-related behavior produced by allografts of intervertebral disc in the rat. Spine. 1996;21:2101–2107. doi: 10.1097/00007632-199609150-00009. [DOI] [PubMed] [Google Scholar]
- 15.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 16.Korhonen T, Karppinen J, Malmivaara A, Autio R, Niinimäki J, Paimela L, Kyllönen E, Lindgren KA, Tervonen O, Seitsalo S, Hurri H. Efficacy of infliximab for disc herniation-induced sciatica: one-year follow-up. Spine. 2004;29:2115–2119. doi: 10.1097/01.brs.0000141179.58778.6c. [DOI] [PubMed] [Google Scholar]
- 17.Korhonen T, Karppinen J, Paimela L, Malmivaara A, Lindgren KA, Bowman C, Hammond A, Kirkham B, Järvinen S, Niinimäki J, Veeger N, Haapea M, Torkki M, Tervonen O, Seitsalo S, Hurri H. The treatment of disc herniation-induced sciatica with infliximab: results of a randomized, controlled, 3-month follow-up study. Spine. 2005;30:2724–2728. doi: 10.1097/01.brs.0000190815.13764.64. [DOI] [PubMed] [Google Scholar]
- 18.Kunz D, Walker G, Eberhardt W, Nitsch D, Pfeilschifter J. Interleukin 1 beta-induced expression of nitric oxide synthase in rat renal mesangial cells is suppressed by cyclosporin A. Biochem Biophys Res Commun. 1995;216:438–446. doi: 10.1006/bbrc.1995.2642. [DOI] [PubMed] [Google Scholar]
- 19.Lee KM, Kang BS, Lee HL, Son SJ, Hwang SH, Kim DS, Park JS, Cho HJ. Spinal NF-κB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur J Neurosci. 2004;19:3375–3381. doi: 10.1111/j.0953-816X.2004.03441.x. [DOI] [PubMed] [Google Scholar]
- 20.Ma W, Bisby MA. Increased activation of nuclear factor kappa B in rat lumbar dorsal root ganglion neurons following partial sciatic nerve injuries. Brain Res. 1998;797:243–254. doi: 10.1016/S0006-8993(98)00380-1. [DOI] [PubMed] [Google Scholar]
- 21.Miki K, Fukuoka T, Tokunaga A, Noguchi K. Calcitonin gene-related peptide increase in the rat spinal dorsal horn and dorsal column nucleus following peripheral nerve injury: upregulation in a subpopulation of primary afferent sensory neurons. Neuroscience. 1998;82:1243–1252. doi: 10.1016/S0306-4522(97)00258-3. [DOI] [PubMed] [Google Scholar]
- 22.Morishita R, Sugimoto T, Aoki M, Kida I, Tomita N, Moriguchi A, Maeda K, Sawa Y, Kaneda Y, Higaki J, Ogihara T. In vivo transfection of cis element “decoy” against nuclear factor-kappaB binding site prevents myocardial infarction. Nat Med. 1997;8:894–899. doi: 10.1038/nm0897-894. [DOI] [PubMed] [Google Scholar]
- 23.Murata Y, Nannmark U, Rydevik B, Takahashi K, Olmarker K. Nucleus pulposus-induced apoptosis in dorsal root ganglion following experimental disc herniation in rats. Spine. 2006;31:382–390. doi: 10.1097/01.brs.0000199618.85326.17. [DOI] [PubMed] [Google Scholar]
- 24.Murata Y, Onda A, Rydevik B, Takahashi I, Takahashi K, Olmarker K. Changes in pain behavior and histologic changes caused by application of tumor necrosis factor-alpha to the dorsal root ganglion in rats. Spine. 2006;31:530–535. doi: 10.1097/01.brs.0000201260.10082.23. [DOI] [PubMed] [Google Scholar]
- 25.Murata Y, Onda A, Rydevik B, Takahashi K, Olmarker K. Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced histologic changes in the dorsal root ganglion. Spine. 2004;29:2477–2484. doi: 10.1097/01.brs.0000144406.17512.ea. [DOI] [PubMed] [Google Scholar]
- 26.Myers RR, Wagner R, Sorkin LS (1999) Hyperalgesic actions of cytokines on peripheral nerves. In: Watkins LR, Maier SF (eds) Cytokines and pain. Birkhauser, Basel, pp 133–157
- 27.Ohtori S, Takahashi K, Aoki Y, Doya H, Ozawa T, Saito T, Moriya H. Spinal neural cyclooxygenase-2 mediates pain caused in a rat model of lumbar disk herniation. J Pain. 2004;5:385–391. doi: 10.1016/j.jpain.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Olmarker K, Larsson K. Tumor necrosis factor alpha and nucleus-pulposus-induced nerve root injury. Spine. 1998;23:2538–2544. doi: 10.1097/00007632-199812010-00008. [DOI] [PubMed] [Google Scholar]
- 29.Olmarker K, Rydevik B, Nordborg C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine. 1993;18:1425–1432. doi: 10.1097/00007632-199318110-00005. [DOI] [PubMed] [Google Scholar]
- 30.Rand N, Reichert F, Floman Y, Rotshenker S. Murine NP-derived cells secrete interleukins-1-b, -6, and -10, granulocyte-macrophage colony-stimulating factor in cell culture. Spine. 1997;22:2598–2602. doi: 10.1097/00007632-199711150-00002. [DOI] [PubMed] [Google Scholar]
- 31.Sakaue G, Shimaoka M, Fukuoka T, Hiroi T, Inoue T, Hashimoto N, Sakaguchi T, Sawa Y, Morishita R, Kiyono H, Noguchi K, Mashimo T. NF-kappa B decoy suppresses cytokine expression and thermal hyperalgesia in a rat neuropathic pain model. Neuroreport. 2001;12:2079–2084. doi: 10.1097/00001756-200107200-00008. [DOI] [PubMed] [Google Scholar]
- 32.Sakurai H, Shigemori N, Hisada Y, Ishizuka T, Kawashima K, Sugita T. Suppression of NF-kappa B and AP-1 activation by glucocorticoids in experimental glomerulonephritis in rats: molecular mechanisms of anti-nephritic action. Biochim Biophys Acta. 1997;1362:252–262. doi: 10.1016/s0925-4439(97)00068-9. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477–484. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shamash S, Reichert F, Rotshenker S. The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J Neurosci. 2002;22:3052–3060. doi: 10.1523/JNEUROSCI.22-08-03052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stacey MA, Sun G, Vassalli G, Marini M, Bellini A, Mattoli S. The allergen Der p1 induces NF-kappaB activation through interference with IkappaB alpha function in asthmatic bronchial epithelial cells. Biochem Biophys Res Commun. 1997;236:522–526. doi: 10.1006/bbrc.1997.6997. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi H, Suguro T, Okazima Y, Motegi M, Okada Y, Kakiuchi T. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine. 1996;21:218–224. doi: 10.1097/00007632-199601150-00011. [DOI] [PubMed] [Google Scholar]
- 37.Takebayashi T, Cavanaugh JM, Cuneyt Ozaktay A, Kallakuri S, Chen C. Effect of nucleus pulposus on the neural activity of dorsal root ganglion. Spine. 2001;26:940–945. doi: 10.1097/00007632-200104150-00018. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi Y, Chiba T, Kurokawa M, Aoki Y. Dermatomes and the central organization of dermatomes and body surface regions in the spinal cord dorsal horn in rats. J Comp Neurol. 2003;462:29–41. doi: 10.1002/cne.10669. [DOI] [PubMed] [Google Scholar]
- 39.Takeuchi H, Kawaguchi S, Ohwada O, Kobayashi H, Hayakawa M, Takebayashi T, Torigoe T, Sato N, Yamashita T. Plasma neuropeptides in patients undergoing lumbar discectomy. Spine. 2007;32:79–84. doi: 10.1097/01.brs.0000252204.88750.cf. [DOI] [PubMed] [Google Scholar]
- 40.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yabuki S, Onda A, Kikuchi S, Myers RR. Prevention of compartment syndrome in dorsal root ganglia caused by exposure to nucleus pulposus. Spine. 2001;26:870–875. doi: 10.1097/00007632-200104150-00008. [DOI] [PubMed] [Google Scholar]