Abstract
The aim of this study is to examine the predictive value of ultrasound diagnostics for the assessment of traumatic lesions of the posterior ligament complex (PLC) in burst fractures of the thoracolumbar spine. This was a prospective validating cohort study. Judgment about instability and treatment of burst fractures depends on the condition of the PLC. There have been some studies describing underdiagnosis of PLC injuries due to classification problems in ligamentary distraction type fractures. The gold standard for assessing these lesions is magnetic resonance imaging (MRI). Even then, there are often limits in contemporary operational availability and technical limitations of MRI. Ultrasound was described being an alternative. In a prospective study, 54 levels of 18 patients with acute burst fractures of the thoracic and lumbar spine have been examined by ultrasound and additional MRI scans preoperatively. The condition (intact vs. ruptured) of supraspinous ligament (SSL) and the interspinous ligament has been assessed for the ligaments separately. Hematoma below the SSL has also been evaluated as an indirect sign of an injured PLC. In all the patients the primary performed operative treatment was a posterior spinal instrumentation. Postoperatively the blinded results of the ultrasound procedures have been matched against intraoperative and MRI findings. Assessments of all target structures have been contributed to the calculation of the sensitivity and specificity of ultrasound. A total of 18 patients, 14 males and 4 females, with acute burst fractures have been qualified for inclusion in the study. The patients’ mean age was 43.4 years. Comparing intraoperative findings with preoperatively performed investigations, ultrasound archived a sensitivity of 0.99 and a specificity of 0.75 (P < 0.05) to detect traumatic lesions to the PLC. As hypothesized the obtained predictive value using ultrasound correlates closely with intraoperative findings. Anyway MRI still seems to be the superior diagnostic method for examining the PLC. However, ultrasound can be considered to be an adequate alternative method in cases with contraindications for MRI such as ferromagnetic side effects, claustrophobia, availability or emergency diagnostics in multiple injuries.
Keywords: Posterior ligament complex, Burst fracture, Ultrasound, Instability, Predictive value
Introduction
The posterior ligament complex (PLC) includes the supraspinous ligament (SSL), interspinous ligament (ISL), ligamentum flavum (LF) and the facet joint capsules [31]. The importance of these posterior structures for spinal stability has been emphasized for more than 20 years in literature [11, 15]. Injuries to the PLC structures still influence the way of its treatment. Even so the most common classification systems—the AO comprehensive fracture classification system according to Magerl and the Denis classification, e.g., pay attention to injuries of the dorsal column [6, 7, 13, 19–21]. Further description of fracture morphology in this study is classified according to the AO system [19]. In this classification system impaction-, split- and burst fractures are subsumed under category A. Type B injuries include distraction, flexion–distraction and hyperextension–shear injuries including an injured PLC. Type C injuries include all types with a rotational injury mechanism.
How to treat best and to achieve an effective prognosis is still the subject of controversy and recently new treatment scores have been proposed to identify unstable fractures with high reliability [32].
In recent years, improvements of imaging techniques have been developed. Injuries to the disco-ligamentous structures as well are able to be diagnosed and often lead to a more invasive treatment, especially if the fracture morphology initially seems not to be that serious [2, 3, 24].
The correct evaluation of both—the bony and ligament components of the injury—therefore seems to be critical to achieve a good outcome [5].
The standard diagnostics used to assess the extent of osseous lesions are conventional X-rays and computed tomography (CT) with the option of sagittal, axial and coronal reconstruction [22]. However, these diagnostic methods only provide indirect signs of ligament lesions and spinal instability, such as kyphosis angle, misalignment and widening of the interspinous space. Instability may be present even in the absence of these signs [5, 14, 26]. Furthermore, the inter-rater reliability in assessing the stability of facet joint for example by CT or magnetic resonance imaging (MRI) had just a fair to moderate agreement. However, MRI that is accepted to be the standard method in investigating most of soft tissue injuries also is a highly sensitive method for examining the PLC [17, 22]. On the other hand, contraindications, availability and high costs often limit the use of this method in normal clinical settings. In an emergency situation and the initial treatment of multiple blunt trauma patients MRI often occurs not to be suitable because of narrow space, magnetic area as well as organizational problems and time loss. These circumstances may explain that MRI is rarely performed as a matter of routine in every case of an acute burst fracture in absence of neurological deficits [22].
The present study aimed to investigate the sensitivity and specificity of ultrasound in the assessment of the PLC. We hypothesized that ultrasound is a sensitive method for detecting ligament injuries of the PLC in thoracolumbar burst fractures. For this purpose, results of sonography have been compared to the intraoperative and MRI findings.
Patients and methods
This prospective study includes 54 levels of 18 patients with acute burst fractures of the thoracic and lumbar spine without neurological deficit (Types A2.3, A3.1, A3.3 and B1.2 according to the Magerl classification [19], all patients ASIA Impairment Scale E).
The local review board approved the study. All patients have been informed of participation in a clinical study and written consent was received.
Fractures older than 2 weeks after trauma, conservative treated fractures and pathologic compression fractures due to tumor or osteoporosis have not been included in this study. Furthermore, focusing on the problem to differentiate A- and B-fracture types, patients with radiological signs for C-fractures as rotational components like sagittal or axial misalignment of segments, luxation or fracture of the facet joints and lamina fractures were also excluded.
In addition to clinical and standard diagnostic examinations using conventional X-rays and CT of the injured region, ultrasound scans of the PLC have been performed. In addition, if possible and no contraindications were given, MRI was preoperatively performed.
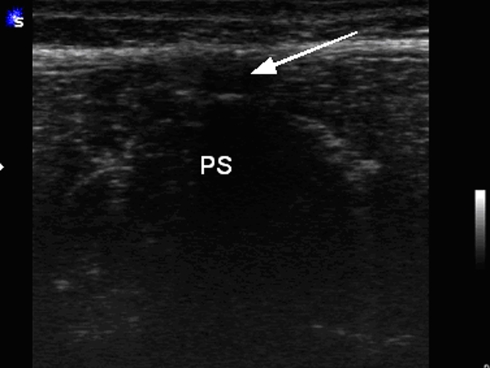
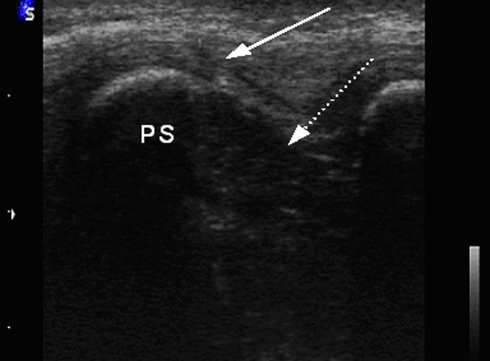
Ultrasound has been carried out in prone or lateral position using a 7.5-MHz linear probe (Siemens Inc. Sonoline) in B mode performing transverse and sagittal planes (Figs. 1, 2, 3, 4).
Fig. 1.
Positioning of hand probe in transverse
Fig. 2.
Positioning of hand probe in sagittal plane
Fig. 3.
Positioning of hand probe in transverse with corresponding ultrasound image (Fig. 1). PS processus spinalis. Arrow indicates SSL
Fig. 4.
Positioning of hand probe in sagittal plane with corresponding ultrasound image (Fig. 2). PS processus spinalis. Dotted arrow indicates ISL, Arrow indicates SSL
According to Moon et al. [23] following ultrasound criteria for detecting PLC injuries were used:
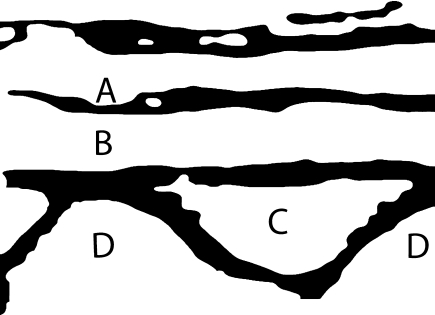
Disruption of the first continuous echogenic layer as a sign for a lesion of the subcutaneous fat and fascial structures (Fig. 5—A).
Disruption of the continuous hypoechoic line between spinous processes as a sign for SSL and ISL lesions (Fig. 5—B = SSL, C = ISL).
Identification of spinous process as echogenic demarcation with posterior acoustic shadow (Fig. 5—D).
Appearance of hypoechoic cysts as a indirect sign for hematoma and disruption.
Fig. 5.
Schematic drawing of normally corresponding ultrasound structures (A subcutaneous layer with facial structures, B SSL, C ISL, D spinous process)
Corresponding ultrasonic structures are shown in Fig. 6.
Fig. 6.
Corresponding ultrasound image to Fig. 5
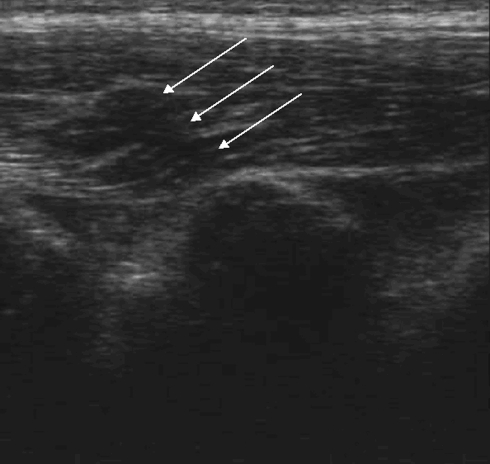
Disruptions of the SSL and ISL have been noted as direct signs of lesions of the PLC (Fig. 7). Hematoma, an indirect sign of a PLC lesion has also been recorded (Fig. 8). These lesions have been considered separately. Hematoma of the SSL needed to be included to pay attention to PLC stretching without disruption.
Fig. 7.
Ultrasound findings with a rupture of the SSL (arrows)
Fig. 8.
Hematoma (arrows) without lesion of ISL/SSL
The clinical examination likewise investigated local tenderness to pressure and palpable interspinous gaps. One spine surgeon has performed all ultrasound examinations, who was trained before with healthy volunteers and some patients with spinal fractures, in order to anticipate the well-known learning curve of the ultrasound technique.
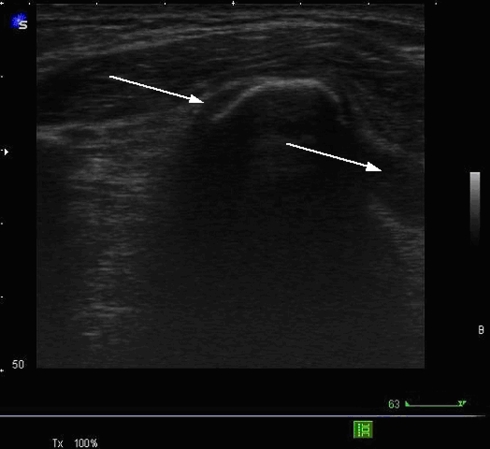
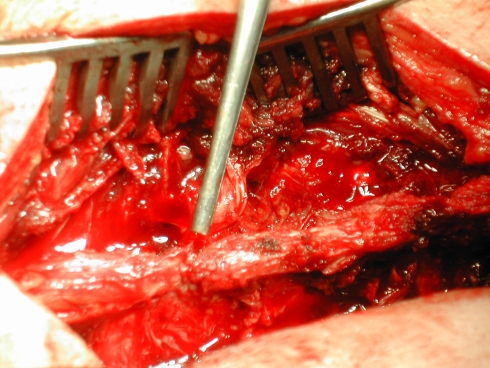
Results of all performed examinations have not been revealed before the operative treatment. The surgeon, performing the operation, has been blinded, instructed to classify the injury and note the condition of the PLC (Fig. 9, corresponding finding to Fig. 7).
Fig. 9.
Corresponding intraoperative finding with rupture of the supraspinous ligament (SSL)
All examination results have then been compared postoperatively.
Specificity and sensitivity have been calculated and the Fischer-Test was used to assess the P value.
Results
A total of 18 patients, 14 males and 4 females, with acute burst fractures were qualified for inclusion in the study. The patients’ mean age was 43.4 years (range 16–70 years).
With ultrasound the injured level (n = 18) and the adjacent lower and upper segments have been evaluated (n = 36). Overall the PLC of 54 levels has been investigated by ultrasound. In 12 patients (67%) MRI was performed additionally. MRI evidenced a specificity and sensitivity of 100%. However, as only 67% of the patients were examined by both methods, ultrasound and MRI, a direct comparison is not feasible.
The cause of injury was a fall from a great height in 12 (48%) and a road traffic accident (pedestrian, motorcycle or car) in 10 (40%) of the patients.
In 13 patients a Type A fracture according to Magerl et al. [19] without injury to the PCL and in 5 patients a Magerl Type B fracture combined with a ruptured PCL have been prospectively diagnosed (Table 1).
Table 1.
Preoperative diagnostic, results of preoperative ultrasound and intraoperative control
| No. | Gender, age (year) | Tested levels | Level of injury | Type of fracture according to Magerl | Clinical symptoms | Ultrasound SSL | ISL | Hem | Preoperative MRI estimation for PLC | Operative findings for PLC | Estimation of ultrasound |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M, 40 | L1-4 | L 3 | A2.3 | Local pressure pain | No | No | No | Intact | Intact | Correct |
| 2 | M, 51 | Th 11-L2 | L 1 | A2.3 | Back pain | No | No | No | Intact | Intact | Correct |
| 3 | M, 16 | Th 3-Th6 | TH 5 | B1.2 | Back pain | Ruptured | Ruptured | No | Ruptured | Ruptured | Correct |
| 4 | M, 53 | Th10-L1 | TH 12 | B1.2 | Local pressure pain | Ruptured | Ruptured | No | MRI not performed | Ruptured | Correct |
| 5 | M, 21 | Th12-L3 | L 2 | A3.1 | Back pain | No | No | No | Intact | Intact | Correct |
| 6 | F, 52 | Th11-L2 | L 1 | A3.3 | Local pressure pain | No | No | No | MRI not performed | Intact | Correct |
| 7 | M, 70 | Th10-L2 | TH 12 | A3.1 | No pain | Ruptured | No | No | Intact | Intact | False positive |
| 8 | F, 50 | Th11-L2 | L 1 | A3.3 | Local pressure pain | No | No | Hem | MRI not performed | Intact | False positive |
| 9 | M, 25 | Th6-Th9 | TH 8 | A3.3 | Back pain | No | No | Hem | Intact | Intact | False positive |
| 10 | M, 54 | Th11-L2 | L 1 | A3.2 | Back pain | No | No | No | MRI not performed | Intact | Correct |
| 11 | M, 39 | Th3-Th6 | TH 5 | B1.2 | Local Pressure pain | Ruptured | Ruptured | Hem | Ruptured | Ruptured | Correct |
| 12 | M, 70 | Th9-Th12 | TH 11 | B1.2 | Gap | Ruptured | Ruptured | Hem | Ruptured | Ruptured | Correct |
| 13 | M, 19 | Th11-L2 | L 1 | A3.3 | Back pain | No | No | No | MRI not performed | Intact | Correct |
| 14 | M, 20 | Th11-L2 | L 1 | A3.1 | Local pressure pain | No | No | No | Intact | Intact | Correct |
| 15 | F, 61 | Th10-L1 | TH 12 | A2.3 | Local pressure pain | No | No | No | Intact | Intact | Correct |
| 16 | F, 61 | Th12-L3 | L 2 | A3.1 | Local pressure pain | No | No | No | Intact | Intact | Correct |
| 17 | M, 19 | Th10-L1 | TH 12 | B1.2 | Local pressure pain | Ruptured | Ruptured | No | Ruptured | Ruptured | Correct |
| 18 | M, 60 | L2-L5 | L 4 | A2.3 | Local pressure pain | No | No | No | MRI not performed | Intact | Correct |
SSL supraspinous ligament, ISL interspinous ligament, Hem hematoma
The clinical investigations could not differ between A- and B-fractures. By performing investigations through ultrasound attention have been paid to the different anatomical components of the PCL. Specificity and sensitivity of the techniques have been calculated to each of these structures and even to indirect findings like hematoma (Table 2).
Table 2.
Results of 54 evaluated levels by ultrasound
| SSL | ISL | Hematoma | PLC | |
|---|---|---|---|---|
| Sensitivity | 0.990** | 0.916** | 0.619a | 0.990* |
| Specificity | 0.892** | 0.996** | 0.800a | 0.750* |
| Positive predictive value | 0.785 | 0.990 | 0.714 | 0.611 |
| Negative predictive value | 0.996 | 0.964 | 0.727 | 0.995 |
SSL supraspinous ligament, ISL interspinous ligament, Hem hematoma
* P < 0.05 calculated with Fischer-Test
** P < 0.001 calculated with Fischer-Test
aNo significance
Evaluation of the supraspinous ligament
Over all cases five injuries of the SSL have been seen. All these five injuries have been detected by ultrasound. This corresponds to a sensitivity of 0.99. One SSL injury has been overrated, giving a specificity of 0.89. The positive predictive value was 0.785, the negative was 0.998. The P value was <0.00070.
Evaluation of the interspinous ligament
In five lesions to the ISL¸ all have been identified in ultrasound. This corresponds to a sensitivity of 0.916 and a specificity of 0.996. The positive predictive value was 0.99, the negative was 0.964. The P value was 0.00012.
Evaluation of hematoma
Hematoma of the SSL has been noted as an indirect sign of injury. In this case, ultrasound has been found to provide a sensitivity of 0.625 and a specificity of 0.80. There was no significance (P > 0.05).
The individual evaluations of the three shown structures resulted in overall sensitivity values of 0.99, and specificity values of 0.75 for ultrasound. The positive predictive value was 0.61, the negative was 0.995. The P value was <0.05 (0.00654).
All results (PLC/SSL/ISL/hematoma) are concluded in Table 2.
Discussion
Integrity of the ligamentous structures strongly influences the stability of the spinal column [1]. However, the judgment about instability is still a field of controversy [11, 17, 33].
In biomechanical loading studies, Gillespie and Dickey [8] have recently shown that the PLC as a whole plays a primary role in dictating the flexion resistance of the thoracolumbar spine. The SSL and ISL are mentioned to be the main load-bearing elements. Even then these are often the first ligamentous structures been torn in a flexion injury [25].
Evidence of a ruptured PLC seems thus to be a good indicator of the severity and grade of stability of the injury itself. In literature different classification systems according to Denis, McAfee, McCormack, Magerl, Vaccaro and Whiteside can be found [6, 13, 19, 21, 32]. But a few of them are used in daily clinical routine. An ideal classification for thoracolumbar fractures still remains to be difficult [7, 16, 24, 33].
Especially in Europe Magerl’s classification [19] has gained acceptance in everyday clinical use not at least because its attention to the ligamentous component of the injury and the mechanics of the trauma. On the other hand, the Denis classification [6] has been widely accepted in North America. Both the Denis and the AO system for the classification of spine fractures only provide moderate reliability and repeatability [11].
Anyway a classification system used in daily clinical work still needs to be kept simple.
New classification scores paying intention to the integrity of the dorsal ligament complex [11, 31–33] have been developed. For example, by using the Thoracolumbar Injury Classification and Severity Score [7, 32] the integrity of the PLC, injury morphometries, injury morphology and neurological impairment should lead to the correct treatment decision.
In conclusion for most of the common classification systems ligamentous instability is an important issue. CT and X-ray signs for B-type fractures have been reported to be a diastasis of the spinal processes over 7 mm, segmental angulation over 15°, anterior loss of height of the vertebra over 50%, distraction of the posterior elements (facet joints, dorsal aspect of vertebra) [29]. Nevertheless up to 30–41.9% of all injuries to the PCL are reported to be overseen initially by only performing X-ray and CT scans [18, 29]. So the danger of delayed adequate treatment has to be considered even in mild fracture patterns. The reliability of diagnostics seems to be an important factor. Especially if conservative treatment is to be considered, adequate diagnosis especially to the PLC because of the lack of intraoperative control is mandatory.
Which diagnostic options are achievable?
In routine diagnostics like X-ray and CT scans only indirect signs of ligamentous injuries could be seen often, but still remains to be uncertain signs, even being investigated by experienced spine surgeons and radiologists [17, 18].
Up to now standardized evaluation of the posterior ligaments using MRI preoperatively [4, 5, 17, 24] or intraoperative exploration is the common standard.
The advantage of MRI remains that anterior ligaments, the LF and the disc will be evaluated at the same time. Anyway, according to current literature, this appears to have a subordinate role as predictors of instability by comparison with the PLC [8].
Magnetic resonance imaging is often impossible to perform because of contraindications (claustrophobia, presence of implants for example pacemakers or steel hip prosthesis’s, circulatory and respiratory instability) and infrastructural obstacles. Furthermore, in most clinics MRI is performed for assessing neurological injuries. In the clinical practice it is not often indicated for injuries of the ligament complex. For ventilated polytraumatized patients the diagnostic options involving complex equipment are particularly limited because of the stress they incur during transport [10, 12].
Magnetic resonance imaging is assumed to have specificity and sensitivity levels of up to 100% for the thoracolumbar spine [27, 30]. Haba et al. [9] examined 35 patients with burst fractures using MRI and reported sensitivity levels of 90.5% for the SSL and 94.3% for the ISL. Lee et al. [17] reported sensitivity levels of 92.9% for the SSL and 100% for the ISL, with specificity levels of 80% (SSL) and 75% (ISL).
Nevertheless ultrasound which is widely accepted to provide an excellent possibility to investigate soft tissue was also shown to provide the possibility of diagnostics to spinal cord from anterior, intraoperative [28] and the posterior ligamentous spinal complex [23].
In 2002 Moon et al. [23] described the use of the ultrasound method as an alternative to MRI. Reliable statements regarding sensitivity and specificity especially are missing.
This prospective study allows to evaluate the specificity and sensitivity of ultrasound for the assessment of injuries to the PLC and later intraoperative evaluation of the PLC. The results show that, with a specificity of 75% and a sensitivity of 91% for the evaluation of the PLC, ultrasound has a predictive reliability. The sensitivity for the assessment of hematoma was considerably low. Nevertheless it was included in the calculation of sensitivity and specificity for the PLC in order to take into account of transitional states such as elongation of the PLC.
With a gently inclined learning curve it is straightforward to use, cost-effective, and furthermore permits reliable differentiation between type A and type B injuries of the thoracolumbar spine even in mild fracture types where normally no MRI would be performed.
In the thoracolumbar spine limitations of sonography have not been experienced as described by Moon et al. [23]. Even overlapping spinal processes have not interfered with diagnosis. Tilting the ultrasound probe has made it possible to look into the interspinous space in every case.
Identifying the exact height of the injury has been difficult using ultrasound. A comparison with conventional imaging techniques might be useful.
Concluding the shown findings, ultrasound can be postulated to be an additive diagnostic method. In cases MRI is not possible or not practical to be performed it might be seen as an alternative diagnostic tool. It may reduce the complexity of the diagnostic procedure especially in trauma patients and could influence the treatment.
Magnetic resonance imaging remains to be the gold standard not only for assessing the PLC in spinal fractures but also to detect injuries to the anterior ligaments, the LF, the posterior longitudinal ligament and swelling or injury of the spinal cord itself.
Anyway in cases of unavailability or contraindications of MRI ultrasound has shown to be a good alternative to investigate the posterior ligamentous complex to judge stability of spinal injuries. Ultrasound has been seen as an additive valid diagnostic tool, assessing the dorsal ligament complex, necessary to use a reliable classification system.
References
- 1.Adams MA. Mechanical testing of the spine. An appraisal of methodology, results, and conclusions. Spine. 1995;20:2151–2156. doi: 10.1097/00007632-199510000-00015. [DOI] [PubMed] [Google Scholar]
- 2.An HS, Vaccaro A, Cotler JM, Lin S. Low lumbar burst fractures. Comparison among body cast, Harrington rod, Luque rod, and Steffee plate. Spine. 1991;16:S440–S444. doi: 10.1097/00007632-199110001-00019. [DOI] [PubMed] [Google Scholar]
- 3.Been HD, Bouma GJ. Comparison of two types of surgery for thoraco-lumbar burst fractures: combined anterior and posterior stabilisation vs. posterior instrumentation only. Acta Neurochir (Wien) 1999;141:349–357. doi: 10.1007/s007010050310. [DOI] [PubMed] [Google Scholar]
- 4.Benzel EC, Hart BL, Ball PA, Baldwin NG, Orrison WW, Espinosa MC. Magnetic resonance imaging for the evaluation of patients with occult cervical spine injury. J Neurosurg. 1996;85:824–829. doi: 10.3171/jns.1996.85.5.0824. [DOI] [PubMed] [Google Scholar]
- 5.Brightman RP, Miller CA, Rea GL, Chakeres DW, Hunt WE. Magnetic resonance imaging of trauma to the thoracic and lumbar spine. The importance of the posterior longitudinal ligament. Spine. 1992;17:541–550. doi: 10.1097/00007632-199205000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8:817–831. doi: 10.1097/00007632-198311000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Fassett DR. Classification systems for acute thoracolumbar trauma. Thoracolumbar spine. Curr Opin Orthop. 2007;18:253. doi: 10.1097/BCO.0b013e32810498d0. [DOI] [Google Scholar]
- 8.Gillespie KA, Dickey JP. Biomechanical role of lumbar spine ligaments in flexion and extension: determination using a parallel linkage robot and a porcine model. Spine. 2004;29:1208–1216. doi: 10.1097/00007632-200406010-00010. [DOI] [PubMed] [Google Scholar]
- 9.Haba H, Taneichi H, Kotani Y, Terae S, Abe S, Yoshikawa H, Abumi K, Minami A, Kaneda K. Diagnostic accuracy of magnetic resonance imaging for detecting posterior ligamentous complex injury associated with thoracic and lumbar fractures. J Neurosurg. 2003;99:20–26. doi: 10.3171/spi.2003.99.1.0020. [DOI] [PubMed] [Google Scholar]
- 10.Harris MB, Sethi RK. The initial assessment and management of the multiple-trauma patient with an associated spine injury. Spine. 2006;31:S9–S15. doi: 10.1097/01.brs.0000217924.56853.0d. [DOI] [PubMed] [Google Scholar]
- 11.Harrop JS, Vaccaro AR, Hurlbert RJ, Wilsey JT, Baron EM, Shaffrey CI, Fisher CG, Dvorak MF, Oner FC, Wood KB, Anand N, Anderson DG, Lim MR, Lee JY, Bono CM, Arnold PM, Rampersaud YR, Fehlings MG (2006) Intrarater and interrater reliability and validity in the assessment of the mechanism of injury and integrity of the posterior ligamentous complex: a novel injury severity scoring system for thoracolumbar injuries. Invited submission from the joint section meeting on disorders of the spine and peripheral nerves, March 2005. J Neurosurg Spine 4:118–122. doi:10.3171/spi.2006.4.2.118 [DOI] [PubMed]
- 12.Ho HS. Safety of metallic implants in magnetic resonance imaging. J Magn Reson Imaging. 2001;14:472–477. doi: 10.1002/jmri.1209. [DOI] [PubMed] [Google Scholar]
- 13.Holdsworth F. Fractures, dislocations, and fracture-dislocations of the spine. J Bone Joint Surg Am. 1970;52:1534–1551. [PubMed] [Google Scholar]
- 14.Holmes JF, Mirvis SE, Panacek EA, Hoffman JR, Mower WR, Velmahos GC. Variability in computed tomography and magnetic resonance imaging in patients with cervical spine injuries. J Trauma. 2002;53:524–529. doi: 10.1097/00005373-200209000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs RR, Casey MP. Surgical management of thoracolumbar spinal injuries. General principles and controversial considerations. Clin Orthop Relat Res. 1984;189:22–35. [PubMed] [Google Scholar]
- 16.Kriek JJ, Govender S. AO-classification of thoracic and lumbar fractures—reproducibility utilizing radiographs and clinical information. Eur Spine J. 2006;15:1239–1246. doi: 10.1007/s00586-005-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HM, Kim HS, Kim DJ, Suk KS, Park JO, Kim NH. Reliability of magnetic resonance imaging in detecting posterior ligament complex injury in thoracolumbar spinal fractures. Spine. 2000;25:2079–2084. doi: 10.1097/00007632-200008150-00012. [DOI] [PubMed] [Google Scholar]
- 18.Leferink VJ, Veldhuis EF, Zimmerman KW, ten Vergert EM, ten Duis HJ. Classificational problems in ligamentary distraction type vertebral fractures: 30% of all B-type fractures are initially unrecognised. Eur Spine J. 2002;11:246–250. doi: 10.1007/s00586-001-0366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magerl F, Aebi M, Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184–201. doi: 10.1007/BF02221591. [DOI] [PubMed] [Google Scholar]
- 20.McAfee PC, Yuan HA, Fredrickson BE, Lubicky JP. The value of computed tomography in thoracolumbar fractures. An analysis of one hundred consecutive cases and a new classification. J Bone Joint Surg Am. 1983;65:461–473. [PubMed] [Google Scholar]
- 21.McCormack T, Karaikovic E, Gaines RW. The load sharing classification of spine fractures. Spine. 1994;19:1741–1744. doi: 10.1097/00007632-199408000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Mhuircheartaigh NN, Kerr JM, Murray JG. MR imaging of traumatic spinal injuries. Semin Musculoskelet Radiol. 2006;10:293–307. doi: 10.1055/s-2007-972000. [DOI] [PubMed] [Google Scholar]
- 23.Moon SH, Park MS, Suk KS, Suh JS, Lee SH, Kim NH, Lee HM. Feasibility of ultrasound examination in posterior ligament complex injury of thoracolumbar spine fracture. Spine. 2002;27:2154–2158. doi: 10.1097/00007632-200210010-00015. [DOI] [PubMed] [Google Scholar]
- 24.Oner FC, Ramos LM, Simmermacher RK, Kingma PT, Diekerhof CH, Dhert WJ, Verbout AJ. Classification of thoracic and lumbar spine fractures: problems of reproducibility. A study of 53 patients using CT and MRI. Eur Spine J. 2002;11:235–245. doi: 10.1007/s00586-001-0364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oner FC, Gils AP, Faber JA, Dhert WJ, Verbout AJ. Some complications of common treatment schemes of thoracolumbar spine fractures can be predicted with magnetic resonance imaging: prospective study of 53 patients with 71 fractures. Spine. 2002;27:629–636. doi: 10.1097/00007632-200203150-00012. [DOI] [PubMed] [Google Scholar]
- 26.Petersilge CA, Emery SE. Thoracolumbar burst fracture: evaluating stability. Semin Ultrasound CT MR. 1996;17:105–113. doi: 10.1016/S0887-2171(96)90010-4. [DOI] [PubMed] [Google Scholar]
- 27.Petersilge CA, Pathria MN, Emery SE, Masaryk TJ. Thoracolumbar burst fractures: evaluation with MR imaging. Radiology. 1995;194:49–54. doi: 10.1148/radiology.194.1.7997581. [DOI] [PubMed] [Google Scholar]
- 28.Raynor RB. Intraoperative ultrasound for immediate evaluation of anterior cervical decompression and discectomy. Spine. 1997;22:389–395. doi: 10.1097/00007632-199702150-00006. [DOI] [PubMed] [Google Scholar]
- 29.Schnake KJ, Scotti F, Haas NP, Kandziora F. Type-B-distraction injuries of the thoracolumbar spine—misinterpretations of the integrity of the posterior ligament complex using radiologic diagnostics. Unfallchirurg. 2008;111:977–984. doi: 10.1007/s00113-008-1503-z. [DOI] [PubMed] [Google Scholar]
- 30.Terk MR, Hume-Neal M, Fraipont M, Ahmadi J, Colletti PM. Injury of the posterior ligament complex in patients with acute spinal trauma: evaluation by MR imaging. AJR Am J Roentgenol. 1997;168:1481–1486. doi: 10.2214/ajr.168.6.9168711. [DOI] [PubMed] [Google Scholar]
- 31.Vaccaro AR, Lehman RA, Jr, Hurlbert RJ, Anderson PA, Harris M, Hedlund R, Harrop J, Dvorak M, Wood K, Fehlings MG, Fisher C, Zeiller SC, Anderson DG, Bono CM, Stock GH, Brown AK, Kuklo T, Oner FC. A new classification of thoracolumbar injuries: the importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine. 2005;30:2325–2333. doi: 10.1097/01.brs.0000182986.43345.cb. [DOI] [PubMed] [Google Scholar]
- 32.Vaccaro AR, Zeiller SC, Hulbert RJ, Anderson PA, Harris M, Hedlund R, Harrop J, Dvorak M, Wood K, Fehlings MG, Fisher C, Lehman RA, Jr, Anderson DG, Bono CM, Kuklo T, Oner FC. The thoracolumbar injury severity score: a proposed treatment algorithm. J Spinal Disord Tech. 2005;18:209–215. [PubMed] [Google Scholar]
- 33.Whang PG, Vaccaro AR, Poelstra KA, Patel AA, Anderson DG, Albert TJ, Hilibrand AS, Harrop JS, Sharan AD, Ratliff JK, Hurlbert RJ, Anderson P, Aarabi B, Sekhon LH, Gahr R, Carrino JA. The influence of fracture mechanism and morphology on the reliability and validity of two novel thoracolumbar injury classification systems. Spine. 2007;32:791–795. doi: 10.1097/01.brs.0000258882.96011.47. [DOI] [PubMed] [Google Scholar]