Abstract
The objective of this study was to analyse the presentation, aetiology, conservative management, and outcome of non-tuberculous pyogenic spinal infection in adults. We performed a retrospective review of 56 patients (35 women and 21 men) of pyogenic spinal infection presenting over a 7-year period (1999–2006) to the Department of Spinal Surgery of Hesperia Hospital. The medical records, radiologic imaging, bacteriology results, treatment, and complications of all patients were reviewed. The mean age at presentation was 47.8 years (age range 35–72 years), the mean follow-up duration was 12.5 months. The most common site of infection was lumbar spine (n: 48), followed by the thoracic spine (n: 8). Most patients were symptomatic for between 4 and 10 weeks before presenting to hospital. The frequently isolated pathogen was Staphylococcus aureus in 31 of 56 cases (57.6%). Percutaneous biopsies were diagnostic in 57% of patients; the open biopsy was indicated if closed biopsy failed and when the infection was not accessible by percutaneous technique. The patients were managed by conservative measures alone, including antibiotic therapy and spinal bracing. The mean period of antibiotic therapy was 8.5 weeks (range 6–9 weeks), followed by oral antibiotics for 6 weeks. All patients had a supportive spinal brace for mean 8 weeks (range 6–10 weeks). The duration of the administration of oral antibiotics was dependent on clinical and laboratory evidence (white cell count, erythrocyte sedimentation rate, C-reactive protein) that the infection was resolved. The follow-up MR gadolinium scans were essential to monitor the response to medical treatment. The diagnosis of pyogenic spinal infection should be considered in any patient presenting with severe localised unremitting back and neck pain, especially when accompanied with systemic features, such as fever and weight loss and in the presence of elevated inflammatory markers. The conservative management of infection with antibiotic therapy and spinal bracing was very successful.
Introduction
Cases of pyogenic spondylodiscitis are relatively rare events that concern 2–7% of cases of osteomyelitis [50]. Incidence varies from 1 per 100,000/year to 1 per 250,000/year [1, 11]. Ninety-five percent of infections involve the vertebrae, while 5% of infections involve the posterior elements of the spine [52].
The infection prefers the male sex with a male/female ratio of 3:1. The age affected has a bimodal distribution: a first peak during childhood (discitis) and a second peak during the fifth–sixth decade.
An increase in the incidence of vertebral infections has recently been noted, due to the prolonging of average age, malnutrition, immunodeficiency, diabetes mellitus, drug abuse, the spreading of the use of endovascular and genitourinary devices, HIV, septicaemia, and the chronic use of steroids [2, 13, 19].
Owing to the low frequency and initial aspecific nature of signs and symptoms, diagnosis is often delayed; literature agrees in referring a diagnostic delay of 2–6 months from the onset of clinical symptomatology [4, 10, 51].
The aim of the retrospective study was to highlight the relation between vertebral infections and the persistent algic refractory symptomatology to anti-inflammatory and painkilling drugs, to underline the importance of the coexistence of risk factors and comorbidity, to highlight the correlation between early diagnosis and successful results obtained with suitable conservative treatment [29, 33, 37, 54].
Materials and methods
Our study will contain the retrospective analysis of case studies of patients suffering from non-specific spondylodiscitis treated at the Vertebrae Surgery Centre at Hesperia Hospital in Modena during the period of 7 years.
Seventy patients were treated from July 1999 to June 2006, and 56 patients responded to follow-up. Two cases of infection in paediatric patients were excluded from the study.
Treatment of the infections was carried out by orthopaedic surgeons working with internal disease and infectious disease specialists.
The following were retrospectively assessed for all patients, such as personal data, predisposing factors, comorbidity, aetiology, and duration of symptoms.
The patients were subjected to clinical, radiological, laboratory, and microbiological tests in order to diagnose non-specific vertebral infection. During the therapeutic process, a multidisciplinary approach was essential in which clinical evaluation, imaging, and inflammation indicators were vital for analysing the efficacy of antibiotic treatment.
Patients affected by tubercular vertebral infections were excluded.
We analysed demographic data (gender, age), risk factors, comorbidity, clinical symptomatology, peripheral neurological deficit, the vertebral level involved (which anatomic segment, single or multiple), blood count and inflammation indicators (haemoglobin, leukocyte formula, PLTS, ESR, CRP, fibrinogen), haematochemical tests (hepatic enzymes, kidney functions, sideraemia and transferrin, glycaemia), microbiological results (blood culture, guided percutaneous or open CT biopsy), radiological imaging (X-rays in two positions, MRI with and without contrast, scintigraphy with marked leukocytes Indium 111), therapeutic treatment (immobilisation, use of antibiotics, surgery), the results.
Results
Demographic
The analysed population comprises 56 patients: 35 males and 21 females; at the time when the treatment was begun the mean age was 51.9 years with a range from 32 to 72 years.
The duration of average follow-up at the end of the treatment was between 12 and 56 months with a mean value of 28 months.
Clinical evaluation
The patients were brought to our notice about 8 weeks (range between 4 and 12 weeks) from the onset of algic symptomatology.
All the patients (56) had backache and rigidity of the dorsal-lumbar rachis, especially during the flexion and extension actions; pain did not respond to therapy. Twenty-seven patients were found to suffer from anorexia, asthenia, and loss of weight.
An increase in the pain symptoms was observed when digital pressure was applied to the vertebral area involved in 14 cases, the pain also irradiated to the homolateral periumbilical area. Eighteen patients suffered radicular irradiation in the sciatica or crural fascia area; positive liquoral signs were found in ten patients. Peripheral radicular deficit of the lower limbs was found in two patients only. Body temperature >38°C in 14 patients.
Kyphosis was present only in one case. No cases or paraparesis and incontinence of the sphincter were diagnosed.
Comorbidity and risk factors
Twenty-nine patients were found to have single positivity or positivity associated with comorbidity and risk factors, such as high blood pressure (n: 20), diabetes (n: 12), previous infection loci (n: 5), prolonged use of steroids (n: 4), chronic kidney failure (n: 3), serious traumas (n: 3), liver failure (n: 2), tumours and chemotherapy (n: 2), rheumatoid arthritis (n: 2), and use of drugs (n: 1).
Haematochemical tests and inflammation indicators
Leukocytosis WBC > 4–11,102/l was found in 26 patients, anaemia Hb < 11.5 g/dl in 12 patients, alteration of ESR (20–54 mm/h n: 40; 55–100 mm/h n: 10; >100 mm/h n: 6), CRP (>5 mg/l n: 20) and fibrinogen (>400 mg/dl n: 17). In 14 patients, we found a reduction of transferrin and ferritin. Glycaemia was >115 mg/dl in 10 patients and >140 mg/dl in 12 patients. Kidney function (creatinine <0.60 n: 4) and liver function (AST, ALT, g-GT n: 2) were found to be altered in six patients.
Microbiology
A CT-guided percutaneous trans-peduncle biopsy was carried out on all patients (n: 56); an open biopsy was carried out in 10 patients. Twenty-four patients underwent blood culture tests.
Positivity due to bacterial growth was found during culture tests in 57.14% (n: 32); 53.6% (n: 30) in the CT-guided or open biopsy, and 41.6% (n: 10) during blood culture, respectively. Blood culture was diagnosed in two patients.
The isolated microorganisms are Staphylococcus aureus 65% (n: 21), Staphylococcus aureus MRSA 9.3% (n: 3), Staphylococcus epidermidis 6.25% (n: 2), E. coli 6.25% (n: 2), Pseudomonas aeruginosa 6.25% (n: 2), Haemophilus influenzae 3.12% (n: 1), Enterococcus 3.12% (n: 1).
Radiological imaging
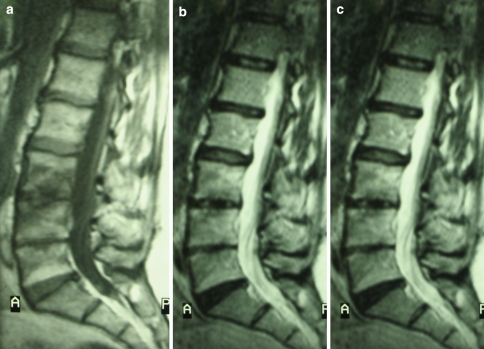
The patients also underwent radiological evaluations (n: 56), MRI with and without means of contrast (n: 56), axial computerised tomography (n: 20), Indium 111 marked leukocytes scintigraphy (n: 20), and CT-PET (n: 18). During the radiological checks, bone alterations typical of the infections, such as the anterolateral degeneration of the soma and reduction of the inter-vertebral space were found in 36 patients. During CT scans, characteristic alterations were found in 16 patients; MRI testing showed typical alterations of the pyogenic vertebral infection in all patients: hypo-intensity in the T1 weighing, 96.4% (n: 54); hyper-intensity in T1 weighing, 100% (n: 56); the body’s hypercaptation of the means of contrast; 94.6% (n: 52). The resonance evaluation was carried out according to the protocol, via the windows in T1, T2, STIR, T1 with means of contrast, showing a high specificity and sensitivity at diagnosis (Fig. 1a–c) at the intermediate follow-up and at healed stage (Fig. 2a–c). The use of gadolinium was found to be essential at the time of diagnosis. CT-PET with Leu-Indium 111 was found to be positive in all the patients, who underwent the test (n: 18).
Fig. 1.
MRI testing showed typical alterations of the pyogenic vertebral infection in patients: hypo-intensity in the T1 weighing (a); hyper-intensity in T2 weighing (b); the body’s hyper-captation of the means of contrast (c)
Fig. 2.
MRI testing showed the healing: iso-intensity in the T1 weighing (a); iso-intensity in T2 weighing (b); no enhancement (c)
Anatomic distribution
There were a total of 62 vertebrae levels involved; single level n: 50/62, dual level n: 6/62.
The anatomic segments were n: 3/62 cervical, n: 13/62 dorsal, n: 12 dorsal-lumbar passage, n: 27/62 lumbar, and n: 5/62 lumbar–sacral. The most affected anatomic segment is the lumbar area, in 43.5% of levels involved; the dorsal segment in 21% (n: 13/62).
Treatment
Mean hospitalisation period was 28 days, with a range from 9 to 71 days. Mean hospitalisation duration in the group of patients treated without surgery was 27 days (range from 9 to 51 days); mean hospitalisation in the group of patients, who underwent surgery was 37 days (range from 14 to 45 days).
Medical treatment comprised the unloading of the spine: bust in plaster for 8 weeks (range 6–10 weeks); at the end of this period a reinforced canvas corset was worn, to be kept for at least 4 weeks, with a following gradual removal.
Antibiotic therapy was specific (in the event of positivity found in biopsy and/or blood culture) or broad spectrum (in the cases in which the pathogenic agent was not isolated). Antibiotics were administered in association with each other; initially parenterally, for a period of 6–9 weeks (mean 8.5 weeks) and then a single antibiotic given orally for an average period of 6 weeks, until laboratory and radiological imaging values returned to normal.
The antibiotic associations most frequently used were levofloxacin + rifampicin, teicoplanin + rifampicin, vancomycin + rifampicin.
Patients were assessed based on the completion of antibiotic therapy (mean period at 14 weeks) and underwent clinical evaluation, haematochemical, and instrumental tests (X-ray of rachis in two positions and MRI with and without means of contrast) and the possible removal of plaster corset or replacement with reinforced canvas corset. Check ups were then carried out at 6 months, 1 year, and at the minimum follow-up of 24 months.
The patients who did not respond to the conservative treatment that they underwent, with active septic centres of infection with abscesses, low improvement trend, presence of deteriorating neurological deficit, instability or progressive kyphosis were then subjected to surgery.
Clinical results
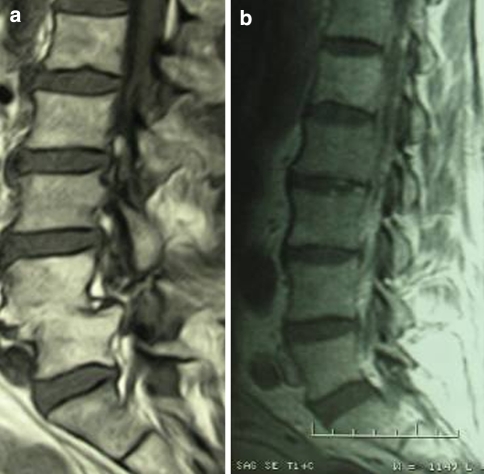
Forty-eight (85.7%) patients were cured through conservative treatment; healing occurred by the process of fusion, degeneration or restitutio ad integrum (Fig. 3a, b). No invalidating peripheral neurological deficit remained; five patients had sthenic and aesthetic alterations in the lower limbs. All patients are currently independently mobile and no cases of reactivation of the infectious process have occurred.
Fig. 3.
MRI testing showed the healing: fusion (a); restitution ad integrum (b)
Eight patients (14.3%) underwent surgery; the operation carried out was decompression (n: 2), abscess drainage (n: 3), surgical cleaning and stabilisation (n: 2), decompression, and intersomatic fusion (n: 1).
One patient with acute paraplegia due to T8–T9 medullary compression showed a significant neurological recovery after decompression and stabilisation surgery. He is currently able to walk with the aid of a walking frame (Frankel C).
Two patients with spondylodiscitis in the lumbar region developed cauda equine syndrome. Following decompression surgery, the patients recovered completely from the neurological symptomatology.
Twelve patients (21%) recovered with residual pain; two patients (3.6%) recovered with kyphosis.
Discussion
Cases of pyogenic spondylodiscitis are events that concern 2–7% of cases of osteomyelitis [1, 11, 50].
It is an event whose incidence is increasing, complicated in young subjects by abscesses. Epidemiological studies have shown an increase in incidence, from five cases per 1 million inhabitants [25] at the end of the 1980s, to values of between 0.2 and 2 cases per 100,000 per year in the most recent studies [7, 36, 56].
An increase in incidence in males has been proven, with the values of 1.5–3:1 [42, 49, 51]. The results we obtained confirm this trend with a male:female ratio of 1.4:1.
Patients’ age has a bimodal distribution, with peaks in paediatric age and in the fifth and sixth decade of life, with a preference for the adults; indeed, mean age values found in literature are 64 years [14, 30, 44, 47]. Our study mainly looks at vertebral infections in adults, with a mean age of 51.9 years, partly confirming the epidemiological trend.
Diagnosis of the pyogenic vertebral infection is made by clinical, radiological, and microbiological evaluation. The delay in diagnosis is 2–6 months, with average values of 10 weeks, and may determine significant bone damage and the onset of neurological damage [4, 9, 10]. In our experience, patients came under observation about 4–12 weeks (mean time 8 weeks) after the beginning of pain symptomatology; this data were found to be vital for rapid diagnosis, but above all for the successful result of conservative treatment. Early diagnosis allows lower progression of the infectious process and a better response to therapy.
Clinical symptoms are aspecific, and often treacherous; the patients do not always have a fever, as confirmed in our case study, which is positive in 25% of the cases. Rachis rigidity is found in all patients; it is often associated with anorexia, lethargy, weight loss, and vomiting [12, 15, 19, 23, 46].
A clinical sign is in fact missing in the vertebral infections that can be considered to be distinctively pathognomonic for diagnosis and for differential diagnosis; to the point that Digby and Kersley defined the infection as “not sufficiently common to be easily recognised and not sufficiently rare to constitute medical curiosity” [18].
To obtain a definite diagnosis, it is necessary to isolate a pathogen via blood culture or biopsy. In a patient in an acute phase of the infection, biopsy is necessary; it can be carried out using a fluoroscopy-guided or CT-guided technique [5, 8, 24]. A percutaneous biopsy has a accuracy rate of 70–100% [22, 28, 31]; Brugieres estimated a 63% accuracy rate in the thoracic site and 67% in the lumbar site [8]. In the event of failure using the percutaneous technique, if the infection locus cannot be reached percutaneously or if significant bone damage is found, an open biopsy must be performed. This is diagnostic in 80% of patients [39]. In our studies, we discovered positivity due to bacterial growth in 57.2% of cases, using the percutaneous or open technique. The data obtained would appear to agree with literature.
Radicular compressions with radiculopathy, neurological deficit in the peripheral ends, or deteriorating paraplegia are the clinical signs of invasion of the purulent material into the epidural space. Untreated vertebral infections may progress to secondary instability that may develop towards kyphosis deformity with paraplegia or tetraplegia. Only 14.3% of the population examined by us underwent surgery due to segment instability and/or the onset of peripheral neurological deficit. The results assessed in literature show percentages, patients who underwent surgery, between 27 and 29% [11, 48].
X-rays must be carried out on all patients with a suspected vertebral infection. This allows us to assess the degree to which the bone has been affected, analysing the deformities caused by the infection on a sagittal or coronal plane.
X-rays have a sensitivity and specificity rate of 82 and 57%, respectively. In 75% of cases, the first alterations are visible on the segment involved after 2–3 weeks from the onset of the infectious process [41]. Considering the large percentage of patients suffering from spondylarthrosis, the degenerative situation of the vertebrae is not easy to distinguish from the initial phase of infection. In our case studies, all the patients underwent X-rays which decreed an initial suspicion of diagnosis that was worthy of further in-depth investigation.
Magnetic resonance represents the gold-standard in the imaging of pyogenic spinal infections, especially in the initial phase when traditional radiology is negative and nuclear scintigraphy is not adequately specific.
With 96% sensitivity, 94% specificity and 92% accuracy, MRI shows pathological alterations with improved anatomic detail [17, 21, 22, 26, 40, 53]. Alterations of the signal are the consequence of medullary, bone destruction, fibrosis, sclerosis, and adipose degeneration. The use of a means of contrast such as gadolinium allows us to view the signal alteration at the infection focus.
Generally speaking, the region most commonly affected at the early stage in adults is spongeous bone under the somatic limits; the infection spreads rapidly to the disc and the adjacent vertebra. The view during MRI depends on the extension of the infection and on the organism’s response. The patients who came under our observation had infections that had reached at least the disc and two adjacent vertebrae [16, 31].
All the patients in our study underwent MRI; the use of gadolinium permitted improved sensitivity and specificity in diagnosis. MRI was used in the follow-up period to monitor the healing of the infectious process at the same time as normalisation of signals; it also allowed, indirectly, an evaluation of the efficacy of the antibiotic therapy being used.
Further confirmation of the ongoing infection was found via scintigraphy that offer a high rate of sensitivity (87–98%) and specificity (91–100%) [38, 58]. In our experience, this diagnostic method was used in 20 cases, the cases where it was necessary to make a differential diagnosis and where the MRI images needed in-depth diagnostic study.
Most patients, n: 48 (85.7%) recovered through conservative treatment, comprising a plaster corset and antibiotic therapy. The data we obtained appear to be in line with the data in literature; O’Daly presented positive recovery results in 72% [34]; Bulter instead, had positive results in 73% [11]. The excellent results we obtained are due, in our opinion, to the early stage of diagnosis and the prompt commencement of treatment; the almost total absence of abscesses in the population treated must also not be underestimated.
There is currently no single consensus on the duration and type of treatment. The optimal duration of parenteral antibiotic therapy and the continuation of oral therapy have still not been defined. Esteemed authors describe parenteral treatments of 6–8 weeks up to a maximum of 3 months [51, 55]; others from 6 to 18 weeks; others again describe a 14-week antibiotic treatment, at the end of which it is necessary to assess the evolution of the infection using MRI [36, 43, 57].
Wearing the corset for a period of 6–10 weeks is instead confirmed by most authors.
Conservative treatment in non-specific spondylodiscitis is to be considered a failure when the symptoms persist or worsen, if the inflammatory indicators do not decrease or if imaging does not show a tendency towards signal normalisation after a month of therapy [36].
Surgical treatment must be addressed with precise indications, such as the onset of peripheral neurological deficit, cauda equine syndrome, vertebral instability, progressive vertebral deformity, and paravertebral abscesses. The aim of surgery the was to avoid neurological damage, block the development of kyphosis, and allow patient mobilisation at the earliest possible moment [35, 45].
Thirty percent of patients (n: 17) recovered with not fully satisfactory results: 12 patients recovered with residual pain, five patients had neurological deficit residue that was not, however, invalidating for mobility. Seventy percent of patients achieved total regression of pain with the recovery from the infectious process. Persistence of pain after recovery from infection has a negative effect on the patient’s psychological and functional state. The unsatisfactory results contained in our study agree with the results reported in literature [6, 15, 27, 32].
Mortality linked to vertebral infection is decreasing; the most recent data published indicated a value of 2%, mostly in immunodepressed patients [20]. Our cases studies did not show any cases of mortality.
Conclusion
The study shows the efficacy of non-surgical treatment in 87.6% of patients; only a minimum number needed surgery (12.4%).
Early diagnosis has an effect on the success of conservative treatment; this must be carried out scrupulously with close patient monitoring. The unloading of the spine is essential; antibiotic therapy must be targeted, with pharmacological association and for long periods.
Haematochemical tests and inflammation indicators must be repeated to assess the evolution of the infection, initially on a weekly basis, then monthly, until values return to normal.
NMR offers good sensitivity, specificity, and accuracy in diag nosis and in monitoring the evolution of the vertebral infection; it must always be carried out with and without means of contrast.
The resolving of the infection does not always correspond with the resolving symptoms of pain.
Conflict of interest statement
None of the authors has any potential conflict of interest.
Contributor Information
N. Bettini, Phone: +39-0348-8468428, FAX: +39-0322-50293, Email: nbettini@centroscoliosi.it
M. Girardo, Phone: +39-0333-8456660, FAX: +39-0322-50293, Email: massimogirardo@libero.it
References
- 1.Acosta FL, Jr, Chin CT, Quiñones-Hinojosa A, Ames CP, Weinstein PR, Chou D. Diagnosis and management of adult pyogenic osteomyelitis of the cervical spine. Neurosurg Focus. 2004;17:E2. doi: 10.3171/foc.2004.17.6.2. [DOI] [PubMed] [Google Scholar]
- 2.Acosta FL, Galvez LF, Ames C. Recent advances: infections of the spine. Curr Infect Dis Rep. 2006;8:390–393. doi: 10.1007/s11908-006-0050-4. [DOI] [PubMed] [Google Scholar]
- 3.An HS, Seldomridge JA (2006) Spinal infections: diagnostic tests and imaging studies [DOI] [PubMed]
- 4.Arnold PM, Baek PN, Bernardi RJ, Luck EA, Larson SJ. Surgical management of nontuberculous thoracic and lumbar vertebral osteomyelitis: report of 33 cases. Surg Neurol. 1997;47:551–561. doi: 10.1016/S0090-3019(96)00242-X. [DOI] [PubMed] [Google Scholar]
- 5.Babu NV, Titus VT, Chittaranjan S, Abraham G, Prem H, Korula RJ. Computed tomographically guided biopsy of the spine. Spine. 1994;19:2436–2442. doi: 10.1097/00007632-199411000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Belzunegui J, Del Val N, Intxausti JJ, Dios JR, Queiro R, González C, Rodríguez-Valverde V, Figueroa M. Vertebral osteomyelitis in northern Spain. Report of 62 cases. Clin Exp Rheumatol. 1999;17:447–452. [PubMed] [Google Scholar]
- 7.Blake C, Codd MB, O’Meara YM. The Short form 36 (SF-36) health survey: normative data for the Irish population. Ir J Med Sci. 2000;169:195–200. doi: 10.1007/BF03167695. [DOI] [PubMed] [Google Scholar]
- 8.Brugieres P, Revel MP, Dumas JL. Ct-guided vertebral biopsy: a report of 89 cases. J Neuroradiol. 1991;18:351–359. [PubMed] [Google Scholar]
- 9.Buranapanitkit B, Lim A, Geater A. Misdiagnosis in vertebral osteomyelitis: problems and factors. J Med Assoc Thai. 2001;84:1743–1750. [PubMed] [Google Scholar]
- 10.Buranapanitkit B, Lim A, Kiriratnikom T. Clinical manifestation of tuberculous and pyogenic spine infection. J Med Assoc Thai. 2001;84:1522–1526. [PubMed] [Google Scholar]
- 11.Butler JS, Shelly MJ, Timlin M, Powderly WG, O’Byrne JM. Nontuberculous pyogenic spinal infection in adults: a 12-year experience from a tertiary referral center. Spine. 2006;31:2695–2700. doi: 10.1097/01.brs.0000244662.78725.37. [DOI] [PubMed] [Google Scholar]
- 12.Cahill DW, Love LC, Rechtine GR. Pyogenic osteomyelitis of the spine in the elderly. J Neurosurg. 1991;74:878–886. doi: 10.3171/jns.1991.74.6.0878. [DOI] [PubMed] [Google Scholar]
- 13.Calderone RR, Larsen JM. Overview and classification of spinal infections. Orthop Clin North Am. 1996;27:1–8. [PubMed] [Google Scholar]
- 14.Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1997;79:874–880. doi: 10.1302/0301-620X.79B5.8078. [DOI] [PubMed] [Google Scholar]
- 15.Chelsom J, Solberg CO. Vertebral osteomyelitis at a Norwegian university hospital 1987–1997: clinical features, laboratory findings and outcome. Scand J Infect Dis. 1998;30:147–151. doi: 10.1080/003655498750003537. [DOI] [PubMed] [Google Scholar]
- 16.Dagirmanjian A, Schils J, McHenry M, Modic MT. MR imaging of vertebral osteomyelitis revisited. AJR Am J Roentgenol. 1996;67:1539–1543. doi: 10.2214/ajr.167.6.8956593. [DOI] [PubMed] [Google Scholar]
- 17.Dagirmanjian A, Schils J, McHenry M. MR imaging of spinal infections. Magn Reson Imaging Clin N Am. 1999;7:525–538. [PubMed] [Google Scholar]
- 18.Digby JM, Kersley JB. Pyogenic non-tuberculous spinal infection: an analysis of thirty cases. J Bone Joint Surg Br. 1979;61(1):47–55. doi: 10.1302/0301-620X.61B1.370121. [DOI] [PubMed] [Google Scholar]
- 19.Gasbarrini AL, Bertoldi E, Mazzetti M, Fini L, Terzi S, Gonella F, Mirabile L, Barbanti Bròdano G, Furno A, Gasbarrini A, Boriani S. Clinical features, diagnostic and therapeutic approaches to haematogenous vertebral osteomyelitis. Eur Rev Med Pharmacol Sci. 2005;9:53–66. [PubMed] [Google Scholar]
- 20.Govender S. Spinal infections. J Bone Joint Surg Br. 2005;87:1454–1458. doi: 10.1302/0301-620X.87B11.16294. [DOI] [PubMed] [Google Scholar]
- 21.Grollmus J, Perkins RK, Rusel W. Erythrocyte sedimentation rate as a possible 16. Indicator of early disc space infection. Neurochirurgia (Stuttg) 1974;17:30–35. doi: 10.1055/s-0028-1090460. [DOI] [PubMed] [Google Scholar]
- 22.Kallio MJ, Unkila-Kallio L, Aalto K, Peltola H. Serum C-reactive protein, erythrocyte sedimentation rate and white blood cell count in septic arthritis in children. Pediatr Infect Dis. 1997;16:411–413. doi: 10.1097/00006454-199704000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Kapeller P, Fazekas F, Krametter D, Koch M, Roob G, Schmidt R, Offenbacher H. Pyogenic infectious spondylitis: clinical, laboratory and MRI features. Eur Neurol. 1997;38:94–98. doi: 10.1159/000113167. [DOI] [PubMed] [Google Scholar]
- 24.Kornblum MB, Wesolowski DP, Fischgrund JS, Herkowitz HN. Computed tomography-guided biopsy of the spine: a review of 103 patients. Spine. 1998;23:81–85. doi: 10.1097/00007632-199801010-00018. [DOI] [PubMed] [Google Scholar]
- 25.Krogsgaard MR, Wagn P, Bengtsson J. Epidemiology of acute vertebral osteomyelitis in Denmark: 137 cases in Denmark 1978–1982, compared to cases reported to the National Patient Register 1991–1993. Acta Orthop Scand. 1998;69:513–517. doi: 10.3109/17453679808997789. [DOI] [PubMed] [Google Scholar]
- 26.Ledermann HP, Schweitzer ME, Morrison WB, Carrino JA. MR imaging findings in spinal infections: rules or myths? Radiology. 2003;228:506–514. doi: 10.1148/radiol.2282020752. [DOI] [PubMed] [Google Scholar]
- 27.Lerner RK, Esterhai JL, Jr, Polomono RC, Cheatle MC, Heppenstall RB, Brighton CT. Psychosocial, functional, and quality of life assessment of patients with posttraumatic fracture nonunion, chronic refractory osteomyelitis, and lower extremity amputation. Arch Phys Med Rehabil. 1991;72:122–126. [PubMed] [Google Scholar]
- 28.Mallya RK, Beer FC, Berry H. Correlation of clinical parameters of disease activity in rheumatoid arthritis with serum concentration of C-reactive protein and erythrocyte sedimentation rate. J Rheumatol. 1982;9:224–228. [PubMed] [Google Scholar]
- 29.Mampalam TJ, Rosegay H, Andrews BT. Nonoperative treatment of spinal epidural infections. J Neurosurg. 1989;71:208–210. doi: 10.3171/jns.1989.71.2.0208. [DOI] [PubMed] [Google Scholar]
- 30.Matsui H, Hirano N, Sakaguchi Y. Vertebral osteomyelitis: an analysis of 38 surgically treated cases. Eur Spine J. 1998;7:50–54. doi: 10.1007/s005860050027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Modic MT, Feiglin DH, Piraino DW, Boumphrey F, Weinstein MA, Duchesneau PM, Rehm S. Vertebral osteomyelitis: assessment using MR. Radiology. 1985;157:157–166. doi: 10.1148/radiology.157.1.3875878. [DOI] [PubMed] [Google Scholar]
- 32.Nolla JM, Ariza J, Gómez-Vaquero C, Fiter J, Bermejo J, Valverde J, Escofet DR, Gudiol F. Spontaneous pyogenic vertebral osteomyelitis in nondrug users. Semin Arthritis Rheum. 2002;31:271–278. doi: 10.1053/sarh.2002.29492. [DOI] [PubMed] [Google Scholar]
- 33.Nussbaum ES, Rigamonti D, Standiford H. Spinal epidural abscess: a report of 40 cases and review. Surg Neurol. 1992;38:225–231. doi: 10.1016/0090-3019(92)90173-K. [DOI] [PubMed] [Google Scholar]
- 34.O’Daly BJ, Morris SF, O’Rourke SK. Long-term functional outcome in pyogenic spinal infection. Spine. 2008;33:E246–E253. doi: 10.1097/BRS.0b013e31816b8872. [DOI] [PubMed] [Google Scholar]
- 35.Ogden AT, Kaiser MG. Single-stage debridement and instrumentation for pyogenic spinal infections. Neurosurg Focus. 2004;17:E5. doi: 10.3171/foc.2004.17.6.5. [DOI] [PubMed] [Google Scholar]
- 36.Quinones-Hinojosa A, Jun P, Jacobs R, Rosenberg WS, Weinstein PR. General principles in the medical and surgical management of spinal infections: a multidisciplinary approach. Neurosurg Focus. 2004;17:e1. doi: 10.3171/foc.2004.17.6.1. [DOI] [PubMed] [Google Scholar]
- 37.Redekop GJ, Del Maestro RF. Diagnosis and management of spinal epidural abscess. Can J Neurol Sci. 1992;19:180–187. [PubMed] [Google Scholar]
- 38.Rodiek SO. Diagnostic methods in spinal infections. Radiology. 2001;41:976–986. doi: 10.1007/s001170170034. [DOI] [PubMed] [Google Scholar]
- 39.Ross PM, Fleming JL. Vertebral body osteomyelitis: spectrum and natural history. A retrospective analysis of 37 cases. Clin Orthop Relat Res. 1976;118:190–198. [PubMed] [Google Scholar]
- 40.Rothman SL. The diagnosis of infections of the spine by modern imaging techniques. Orthop Clin North Am. 1996;27:111–123. [PubMed] [Google Scholar]
- 41.Sapico FL, Montgomerie JZ. Pyogenic vertebral osteomyelitis: report of nine cases and review of the literature. Rev Infect Dis. 1979;1:754–776. doi: 10.1093/clinids/1.5.754. [DOI] [PubMed] [Google Scholar]
- 42.Sapico FL, Montgomerie JZ. Vertebral osteomyelitis. Infect Dis Clin North Am. 1990;4:539–550. [PubMed] [Google Scholar]
- 43.Sapico FL. Microbiology and antimicrobial therapy of spinal infections. Orthop Clin North Am. 1996;27:9–13. [PubMed] [Google Scholar]
- 44.Schimmer RC, Jeanneret C, Nunley PD, Jeanneret B. Osteomyelitis of the cervical spine: a potentially dramatic disease. J Spinal Disord Tech. 2002;15:110–117. doi: 10.1097/00024720-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Siddiq F, Chowfin A, Tight R, Sahmoun AE, Smego RA., Jr Medical vs surgical management of spinal epidural abscess. Arch Intern Med. 2004;164:2409–2412. doi: 10.1001/archinte.164.22.2409. [DOI] [PubMed] [Google Scholar]
- 46.Slucky AV, Eismont FJ. Spinal infections. In: Bridwell KH, De-Wald RL, editors. The textbook of spinal surgery. Philadelphia: Lippincott-Raven; 1997. pp. 2141–2183. [Google Scholar]
- 47.Garcia Solis, del Pozo J, Soto MV, Solera J. Vertebral osteomyelitis: long-term disability and prognostic factors. J Infect. 2007;54:129–134. doi: 10.1016/j.jinf.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 48.Still JM, Abranson R, Law EJ. Development of an epidural abscess following staphylococcal septicaemia in an acutely burned patient: case report. J Trauma. 1995;38:158–159. doi: 10.1097/00005373-199506000-00027. [DOI] [PubMed] [Google Scholar]
- 49.Tali ET. Spinal infections. Eur J Radiol. 2004;50:120–133. doi: 10.1016/j.ejrad.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 50.Thelander U, Larsson S. Quantitation of C-reactive protein levels and erythrocyte sedimentation rate after spinal surgery. Spine. 1992;17:400–404. doi: 10.1097/00007632-199204000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Tsiodras S, Falagas ME. Clinical assessment and medical treatment of spine infections. Clin Orthop Relat Res. 2006;444:38–50. doi: 10.1097/01.blo.0000203454.82264.cd. [DOI] [PubMed] [Google Scholar]
- 52.Tyrell PNM, Cassar-Pollucino VN, McCall IW. Spinal infection. Eur Radiol. 1999;9:1066–1077. doi: 10.1007/s003300050793. [DOI] [PubMed] [Google Scholar]
- 53.Varma R, Lander R, Assaf A. Imaging of pyogenic infectious spondylodiscitis. Radiol Clin North Am. 2001;39:203–213. doi: 10.1016/S0033-8389(05)70273-6. [DOI] [PubMed] [Google Scholar]
- 54.Verner EF, Musher DM. Spinal epidural abscess. Med Clin North Am. 1995;69:375–384. doi: 10.1016/s0025-7125(16)31049-5. [DOI] [PubMed] [Google Scholar]
- 55.Vinas FC, King PK, Diaz FG. Spinal aspergillus osteomyelitis. Clin Infect Dis. 1999;28:1223–1229. doi: 10.1086/514774. [DOI] [PubMed] [Google Scholar]
- 56.Ware JE. SF-36 health survey manual and interpretation guide. Boston: Nimrod Press; 1993. [Google Scholar]
- 57.Weinberg J, Silber JS. Infections of the spine: what the orthopaedist needs to know. Am J Orthop. 2004;33:13–17. doi: 10.1007/s00132-003-0575-2. [DOI] [PubMed] [Google Scholar]
- 58.Whalen JL, Brown ML, McLeod R, Fitzgerald RH., Jr Limitations of indium leukocyte imaging for the diagnosis of spine infections. Spine. 1991;16:193–197. [PubMed] [Google Scholar]