Abstract
The projectional nature of radiogram limits its amount of information about the instrumented spine. MRI and CT imaging can be more helpful, using cross-sectional view. However, the presence of metal-related artifacts at both conventional CT and MRI imaging can obscure relevant anatomy and disease. We reviewed the literature about overcoming artifacts from metallic orthopaedic implants at high-field strength MRI imaging and multi-detector CT. The evolution of multichannel CT has made available new techniques that can help minimizing the severe beam-hardening artifacts. The presence of artifacts at CT from metal hardware is related to image reconstruction algorithm (filter), tube current (in mA), X-ray kilovolt peak, pitch, hardware composition, geometry (shape), and location. MRI imaging has been used safely in patients with orthopaedic metallic implants because most of these implants do not have ferromagnetic properties and have been fixed into position. However, on MRI imaging metallic implants may produce geometric distortion, the so-called susceptibility artifact. In conclusion, although 140 kV and high milliamperage second exposures are recommended for imaging patients with hardware, caution should always be exercised, particularly in children, young adults, and patients undergoing multiple examinations. MRI artifacts can be minimized by positioning optimally and correctly the examined anatomy part with metallic implants in the magnet and by choosing fast spin-echo sequences, and in some cases also STIR sequences, with an anterior to posterior frequency-encoding direction and the smallest voxel size.
Keywords: CT, MRI, Artifacts
Introduction
The projectional nature of radiogram limits its amount of information about the instrumented spine, whereas cross-sectional view with MRI and CT imaging can be more helpful.
Thanks to the continuos development of new imaging equipment, the clinical use of high-field strength magnetic resonance (MRI) imaging and multidetector computed tomography (CT) is increasing in the evaluation of instrumented spine. However, in patients who have metallic orthopaedic implants, artifacts due to misregistration at MRI and to beam hardening at CT often have prevented the accurate evaluation of region of interest near the implants [1]. Moreover, the use of high magnetic field strength at MRI produces more obtrusive artifacts than does the use of lower field strength. In contrast, multidetector CT performed on a scanner with more than four channels does not produce more pronounced artifacts than CT performed on a scanner with fewer channels.
Materials and methods
We tried to assess the new state of the art of the evaluation of instrumented spine with high-field strength MRI and multidetector CT, by reviewing the literature on the argument, from 1985 till date.
Results and discussion
CT
Metallic hardware causes severe beam hardening and dramatically attenuates the X-ray beam, degrading image quality to the extent that the resultant image is either incomplete or is a faulty projection of the data with consequent reconstruction artifacts [2–4]. The generation of artifacts at CT from metal hardware is related to the X-ray kilovolt peak, tube current (in mA) pitch, hardware composition, geometry (shape), location, and the image reconstruction parameters [5].
Imaging parameters
X-ray kilovolt peak
A higher X-ray kilovolt peak can increase the ability of the X-ray beam to penetrate metal [6].
X-ray tube current (mA)
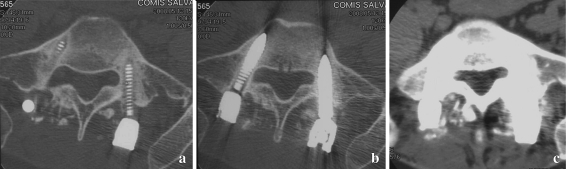
A higher tube current setting may increase the ability of the X-ray beam to penetrate metal. These settings are possible with multichannel CT because of its lower pitch settings (Fig. 1a, b).
Fig. 1.
a 140 kV peak, smooth filter, bone window (see the mobilization). b 120 kV peak, smooth filter, bone window. c 120 kV peak, hard filter, soft tissue window
Pitch
With multichannel CT, a lower pitch setting allows the collection of redundant data, thereby increasing the likelihood that adequate projection data will be collected. In addition, lower pitch settings reduce splay artifacts (radially alternating bands of higher- and lower-attenuation projection across the image) which are inherent in multichannel CT. Given that scanner setup and technical factors remain unchanged, splay artifacts are reduced as the number of detector rows increases.
Metal composition
The artifacts generated by orthopaedic hardware are related to the composition of the hardware. Materials with lower X-ray beam attenuation coefficients (density) produce fewer artifacts (e.g., plastic < titanium < vitallium < stainless steel < cobalt–chrome) [4, 7–10].
Geometric factors
The amount of artifacts generated at CT is also related to the cross-sectional area of the hardware. Artifacts generally predominate in the direction of the maximal thickness of the hardware; in fact, the degree of X-ray beam attenuation is proportional to the thickness of the hardware. If it is possible, the affected body part should be positioned so that the X-ray beam passes the metal at its smallest cross-sectional area, although the repositioning of body parts is generally not possible for spine imaging. X-ray beam attenuation is also greatest in the regions of greatest patient girth and bone mass. Hence, X-ray beam attenuation in the cervical spine is less than that in the thoracic spine, which in turn is less than that in the lumbar and sacral spine.
Image reconstruction parameters
Filter (Kernel)
Selection of an appropriate reconstruction filter may play a critical role in the appearance of a metal-related artifact. The use of a standard or smooth reconstruction filter is preferred, particularly in the presence of dense metallic hardware and in patients with a large body habitus.
Multiplanar reconstruction
Reformatted images can be thicker than those initially acquired by averaging pixel values within slices. On these thicker reformats, metal-induced artifacts are reduced and signal-to-noise ratio is enhanced.
Volumetric reconstructions
Surface 3D or volumetric rendered images are occasionally useful in providing an additional view on the relationship of hardware to the bones or by providing a global view of the regional anatomy. They can display metal hardware fracture than is easily overlooked on multiplanar reconstruction (MPR). Volumetric rendering techniques provide semitransparent views of bones that tend to reduce metal artifacts. They clearly show location of metal hardware and their relationship to the adjacent bone.
Window levels
The use of wide windows to review or to film images (3,000–4,000 HU window width, 800 window level) facilitates visualization of structures adjacent to metal hardware and reduce the effects of metal artifacts. The use of extended-scale CT and dedicated workstations also will decrease the appearance of metal artifacts by allowing for large window widths.
Clinical indications for MDCT of the patient with metal hardware
MDCT is an effective imaging tool for the evaluation of orthopaedic hardware. The detection rate of hardware complication (hardware malplacement or fracture, persistent non-union or perihardware fracture, septic or mechanical loosening) is higher with MDCT than with radiography, both in the spine and in the appendicular skeleton [11].
In the early postoperative period after joint fusion, MDCT can be used to demonstrate the exact position of the material and its anatomic relationship with respect to adjacent structures such as cortical bone, nerve, dural sac and vessels [12, 13]. In the late postoperative period, cortical, and trabecular bone continuity reflects successful joint fusion. Failed fusion with persistent mobility can be shown with bone resorption around material and the lack of bone continuity. Migration or fracture of metal or graft can also be displayed.
MR
Artifacts on MRI images obtained in patients with metallic implants are produced by the large differences between the magnetic properties of human tissues and those of the implanted metals [14]. The artifacts are more marked when the differences in magnetic susceptibilities between the metallic object and the surrounding matter are substantial, creating local magnetic field inhomogeneities, altering the phase and frequency of local spins. Thus, the spins are subsequently mapped to an erroneous location within the image. The results are distortion of the shape of the metallic object along the axes of frequency encoding and section selection, and loss of signal within the metallic object. A rim of high signal intensity appears around the metallic object as a result of the mismapping of a disproportionate number of spins to that location [15, 16].
Factors that influence the production of metal-related artifacts at MRI imaging include the composition, size, and orientation of the metallic object with regard to the direction of the external magnetic field; the type of pulse sequences applied; and the sequence parameters, including magnetic field strength, voxel size (determined by field of view, image matrix, and section thickness), and echo train length. To demonstrate these factors, we performed MRI imaging in a phantom and a patient. Reduction of artifacts can be made paying attention to patient positioning, choosing adequate imaging parameters and selecting different pulse sequences.
Composition, size, and orientation
Titanium implants are non-ferromagnetic and produce much less severe artifacts than do ferromagnetic implants made of stainless steel [4, 16–19]. In addition, the artifact size is affected by the implant size with larger implants producing more obtrusive artifacts [18, 19].
When the direction of the main magnetic field (z axis of the scanner) is aligned parallel with the longitudinal axis of the hardware device, there is a significant relative reduction in artifact [4, 16, 18–21]. The limitations in patient positioning resulting from restrictive MRI scanner bore diameters can be overcome in magnets with an “open” configuration.
Pulse sequence selection
MRI degradation caused by metal hardware is primarily the result of a series of MRI artifacts produced by the ferromagnetic properties of the metallic device [22]). These artifacts include intravoxel dephasing, diffusion-related signal loss, slice thickness variation, misregistration artifacts, and inhomogeneous or paradoxical tissue-selective signal suppression with spectral (frequency-selective) fat-saturation techniques. The type and severity of metal-related artifacts are linked to the pulse sequence and operator selection of individual sequence parameters. Some MRI sequences are more susceptible to artifacts than others.
Gradient recalled echo (GRE) sequences are extremely sensitive to the presence of metal (Fig. 2). Intravoxel dephasing is the predominant cause of signal loss on GRE imaging [23], resulting in a dark or black area (signal loss) around the metal on the processed images. Shortening the MRI parameter echo time (TE) and decreasing voxel size can be used to reduce the degree of intravoxel dephasing seen on GRE acquisition [4, 16, 24].
Fig. 2.
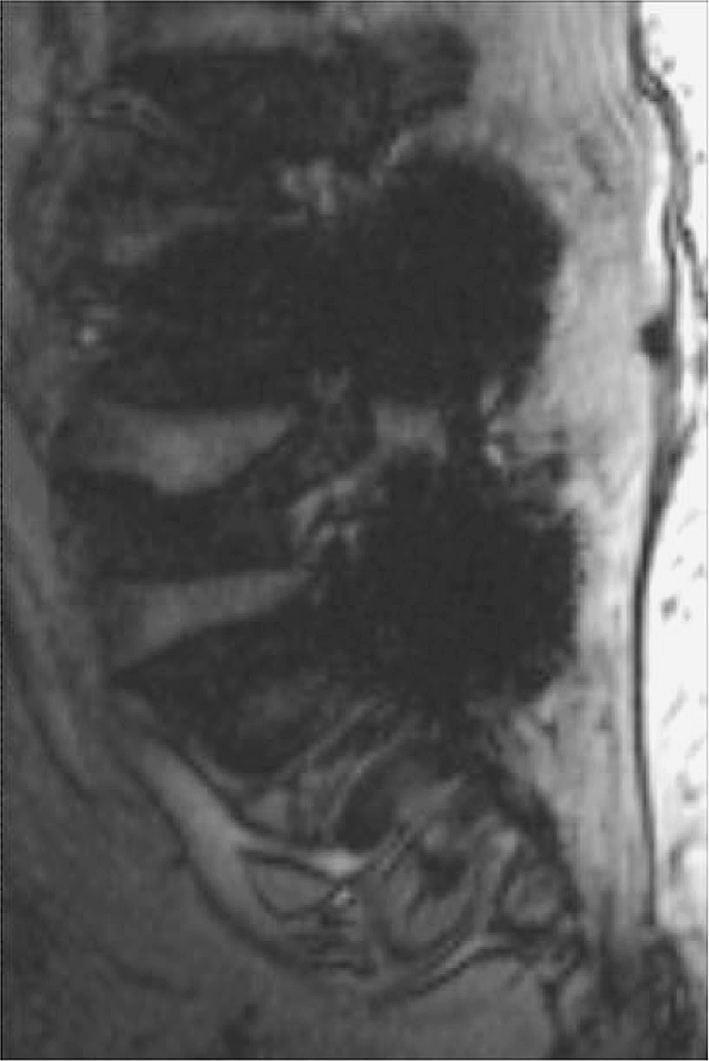
Sagittal gradient-echo sequence
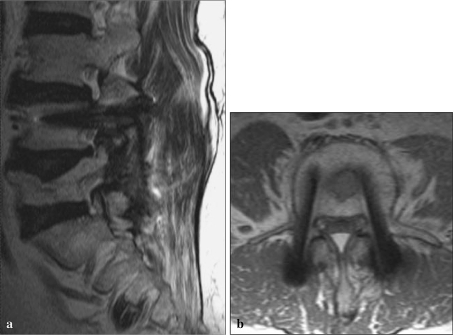
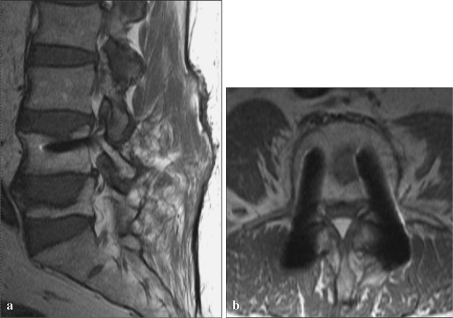
Other MRI sequences are less susceptible to intravoxel dephasing. Spin-echo (SE) sequences use refocusing 180° radiofrequency pulses that correct for static/fixed magnetic field inhomogeneities, resulting in a dramatic reduction in intravoxel dephasing signal loss (Figs. 3a, b, 4a, b). Misregistration artifacts in the vicinity of a metallic implant cause distinctive signal alterations, including signal voids and loss and signal increases resulting in hypointense and hyperintense signal artifacts around the implanted metal. Misregistration is a major source of metal-related signal artifacts on SE and FSE imaging sequences. The magnitude of local misregistration artifact is also inversely proportional to the frequency encoding (readout) gradient strength used in the imaging acquisition [25]. Misregistration artifacts occur only in the frequency encoding direction and are not seen in the phase encoding direction [4, 16, 26–28]. Orientation of the frequency and phase encoding gradients can be selected such that misregistration artifacts are directed away from areas of anticipated clinical diagnostic interest. The magnitude of misregistration artifact is inversely proportional to frequency encoding gradient strength, and increasing the frequency encoding gradient strength also may decrease these artifacts. This can be achieved practically on MRI acquisitions by widening or increasing the receiver bandwidth.
Fig. 3.
a Sagittal T2 FSE sequence, b Axial T2 FSE sequence
Fig. 4.
a Sagittal T1 FSE sequence, b Axial T1 FSE sequence
The presence of large magnetic field inhomogeneities adjacent to ferromagnetic materials also results in increased dephasing per unit distance of travel in randomly diffusing spinning protons. This artifact determines signal loss, represented as a dark or black region around the orthopedic hardware on the final images. This diffusion-related signal loss is most pronounced on long TE (T2-weighted) acquisition sequences and, in contrast to intravoxel dephasing signal loss, is not recoverable with an 180° refocusing pulse and affects GRE, SE, and FSE sequences [23, 24]. FSE imaging sequences employ multiple sequential refocusing 180° pulses in acquiring multiple lines of information (K-space data) within a single repetition time (TR): it refocuses spins at a faster interval than with conventional spin echo imaging, causing a small reduction in diffusion-related signal loss. This effect may be increased further, resulting in artifact reduction, with the use of smaller interecho spacing [16, 29, 30]. FSE also reduces artifact arising from malrotation [31], which is a relatively small contributor to signal loss around metal.
Frequency-selective fat saturation relies on the different resonance frequencies of hydrogen protons within water and fat. Fat signal suppression is achieved with the application of a narrow-bandwidth radiofrequency pulse limited to the spectral frequency of fat, and the magnetic field must be homogeneous within the imaging volume in order to obtain uniform fat suppression within the field of view. Variation of the regional magnetic field surrounding metallic devices or debris creates an inhomogeneous magnetic field, with resultant areas of suboptimal fat saturation [32]. Short inversion time inversion recovery (STIR) imaging is an effective alternative method of fat signal suppression and is less dependent on the homogeneity of the main magnetic field [4, 14, 23, 32–34]. The major disadvantage in STIR sequencing is decreased signal-to-noise ratio, resulting in a grainy appearance to the final images with loss of tissue signal resolution (Fig. 5).
Fig. 5.
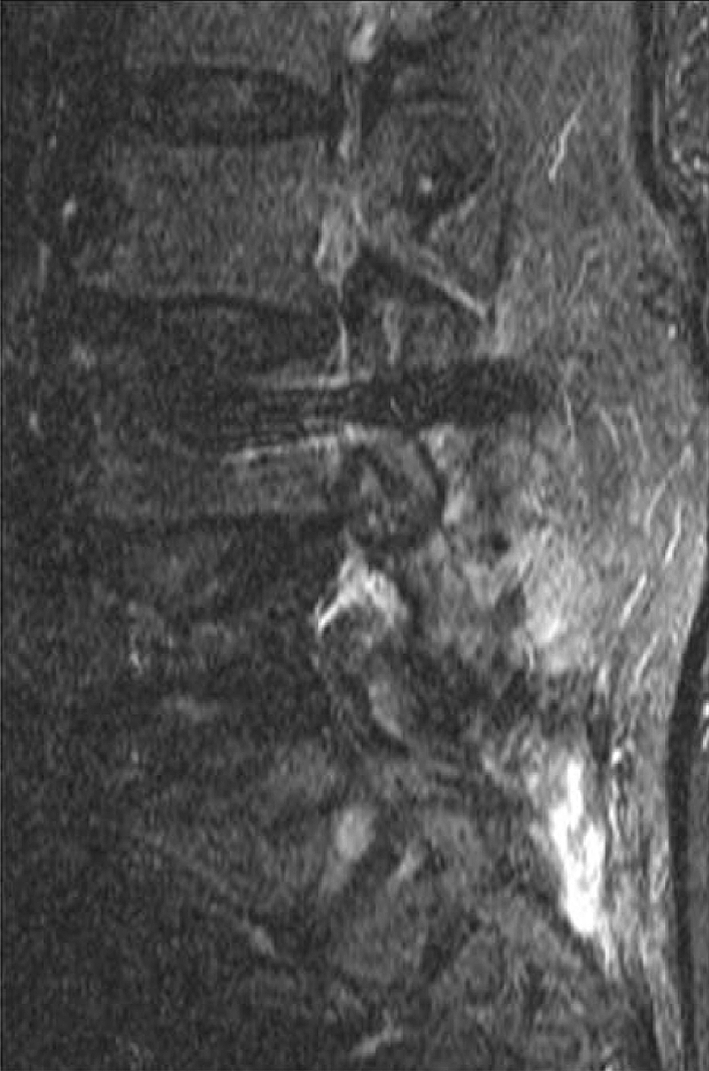
Sagittal STIR sequence
An additional technique, view angle tilting, has been advocated more recently to decrease metallic artifacts [35, 36]. It technically involves application of a “compensatory gradient” during imaging acquisitions, correcting for inhomogeneous perturbations in the local magnetic field in the vicinity of a metallic device. It should be used in conjunction with increased receiver bandwidth, readout gradient strength, and reduced voxel size. View angle tilting results in image blurring across the entire imaging field of view, which must be partially compensated for by imaging parameter alterations (increases in the phase and frequency encoding gradients). The major drawback of this new MRI sequence is a resultant low image signal-to-noise ratio, although this has been shown not to compromise overall image interpretation [16, 35, 36].
There is an additional MRI method, single-point imaging (SPI). SPI is immune to the susceptibility artifacts observed with conventional MRI, which require several milliseconds for signal preparation and acquisition, failing to image metal materials because signal from these systems vanishes before it can be recorded completely. In SPI methods, this problem is solved by acquiring only one point of the free induction decay as soon as possible after excitation. SPI requires large gradient amplitudes and long scanning times; these problems are being addressed with good results [2, 16].
Sequence parameters
Slice thickness variations may be complex and account for significant signal loss and image distortion, particularly adjacent to metal devices with a complex three-dimensional geometry [26]. For imaging in the vicinity of a metal device, slice thickness should be minimized as much as possible because small voxel size in MRI in the vicinity of metal increases image quality. Small voxel size increases the spatial definition of metal-induced artifacts, reducing the apparent size of signal void present [4, 24, 25, 37]. As mentioned previously, smaller voxel size additionally may help decrease the degree of diffusion-related signal loss [16, 23, 24]. Voxel size may also be reduced without increasing examination time by increasing the number of frequency encoding steps used. Decreasing voxel size through this method is associated, however, with a tradeoff of progressive loss of image signal-to-noise ratio, which may require a compensatory increase in the number of excitations with associated increased imaging time.
Clinical applications of high-field strength MRI of evaluation of instrumented spine
Instrumented postero-lateral, or less frequently anterior, spinal fusion was performed with pedicular screws, longitudinal bars and cages to treat degenerative pathology (ostheoarthritis, spondylolisthesis, disc herniations, spinal stenosis), fractures, or severe scoliosis.
The major complications imaged are prosthesis loosening, periprosthetic fracture, and infection. Other complications that can be investigated by MRI include heterotopic ossification and prosthetic fracture. Adjacent or surrounding structures can also be evaluated, with common pathologies such as avascular necrosis, tumor recurrence, and internal derangement of joints potentially identified.
Prosthesis loosening shows fluid collections in the setting of mechanical loosening that had low T1 signal intensity and high T2 signal intensity surrounding the femoral stem with no enhancement. MRI findings of small particle disease consist of focal periprosthetic intraosseous masses. These masses are intermediate signal on proton density sequences [15]; low signal intensity on T1 sequences; and heterogeneous, predominately low to intermediate signal intensity, on T2 sequences with rim enhancement (occasional internal enhancement also can be seen) [23]. Pyogenic infection shows fluid signal on T1 and T2, peripheral rim enhancement, and surrounding edema (high T2 signal within adjacent tissues). Heterotopic ossification on MRI is visualized as areas or masses with well-circumscribed hypointense cortices and internal adipose signal intensity.
Conclusion
Multichannel CT allows faster scanning times, resulting in reduced motion artifacts; thinner sections, with which it is possible to create a scanned volume of isotropic voxels with equivalent image resolution in all planes; and the generation of a higher X-ray tube current, which may result in better penetration of metal hardware and reduction of artifacts. The acquisition of multiplanar reformatted images in the axial, sagittal, coronal, and oblique planes and of three-dimensional volume-rendered images optimizes image interpretation. Wide window settings are best for reviewing images when hardware is present. The integrity of hardware is best assessed with multiplanar average intensity projection. Soft-tissue structures are best visualized by interactively varying the window width and level settings. A kilovolt peak of 140 is recommended for the imaging of spines with orthopedic hardware. Exceptions are made for the imaging of cervical spine and in patients with small cross-sectional areas, in which cases 120 kVp may be used. In general, imaging of smaller body parts such as the cervical spine benefits from the use of the narrowest section thickness.
To reduce metal-related artifacts at MR imaging, orthopedic hardware should be positioned to parallel as closely as possible the direction of the main magnetic field. With respect to hardware composition, a titanium alloy produces less severe artifacts than does stainless steel. The fast SE pulse sequence is the best MRI sequence for artifact reduction, and the GRE sequence is the least beneficial. For purposes of suppressing the signal from fat while avoiding severe metal-related artifacts, the STIR pulse sequence is preferable to frequency-selective fat saturation. Use of lower magnetic field strength is desirable; however, if a currently available clinical MR imaging system with a high-field-strength magnet is used, the imaging parameters chosen (e.g., small field of view, high-resolution image matrix, thin sections, increased echo train length, and higher gradient strength for small voxel sizes) may help to reduce metal-related artifacts.
Acknowledgments
Conflict of interest statement None of the authors has any potential conflict of interest.
References
- 1.White LM, Buckwalter KA. Technical considerations: CT and MR imaging in the postoperative orthopedic patient. Semin Musculoskelet Radiol. 2002;6:5–17. doi: 10.1055/s-2002-23160. [DOI] [PubMed] [Google Scholar]
- 2.Barrett JF, Keat N. Artifacts in CT: recognition and avoidance. RadioGraphics. 2004;24:1679–1691. doi: 10.1148/rg.246045065. [DOI] [PubMed] [Google Scholar]
- 3.Yazdi M, Gingras L, Beaulieu L. An adaptive approach to metal artifact reduction in helical computed tomography for radiation therapy treatment planning: experimental and clinical studies. Int J Radiat Oncol Biol Phys. 2005;62:1224–1231. doi: 10.1016/j.ijrobp.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 4.Lee MF, Kim S, Lee SA, et al. Overcoming artifacts from metallic orthopedic implants at high-field-strength mr imaging and multidetector CT. RadioGraphics. 2007;27:791–803. doi: 10.1148/rg.273065087. [DOI] [PubMed] [Google Scholar]
- 5.Douglas-Akinwande AC, Buckwalter KA, Rydberg J, et al. Multichannel CT: evaluating the spine in postoperative patients with orthopaedic hardware. RadioGraphics. 2006;26:S97–S110. doi: 10.1148/rg.26si065512. [DOI] [PubMed] [Google Scholar]
- 6.Robertson DD, Weiss PJ, Fishman EK, et al. Evaluation of CT techniques for reducing artifacts in the presence of metallic orthopaedic implants. J Comput Assist Tomogr. 1988;12:236–241. doi: 10.1097/00004728-198803000-00012. [DOI] [PubMed] [Google Scholar]
- 7.White LM, Buckwalter KA. Technical considerations: CT and MR imaging in the postoperative orthopedic patient. Semin Musculoskelet Radiol. 2002;6:5–17. doi: 10.1055/s-2002-23160. [DOI] [PubMed] [Google Scholar]
- 8.Robertson DD, Magid D, Poss R, Fishman EK, et al. Enhanced computed tomographic techniques for the evaluation of total hip arthroplasty. J Arthroplasty. 1989;4:271–276. doi: 10.1016/S0883-5403(89)80024-5. [DOI] [PubMed] [Google Scholar]
- 9.Haramati N, Staron RB, Mazel-Sperling K, et al. CT scans through metal scanning technique versus hardware composition. Comput Med Imaging Graph. 1994;18:429–434. doi: 10.1016/0895-6111(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 10.Wang JC, Yu WD, Sandhu HS, et al. A comparison of magnetic resonance and computed tomographic image quality after the implantation of tantalum and titanium spinal instrumentation. Spine. 1998;23:1684–1688. doi: 10.1097/00007632-199808010-00014. [DOI] [PubMed] [Google Scholar]
- 11.Ohashi K, El-Khoury GY, Bennett DL, et al. Orthopedic hardware complication diagnosed with multi-detector row CT. Radiology. 2005;237:570–577. doi: 10.1148/radiol.2372041681. [DOI] [PubMed] [Google Scholar]
- 12.Lorenzen M, Wedegartner U, Weber C, et al. Clinical relevance of multislice CT of the spine after osteosynthesis. Rofo. 2005;177(11):1540–1544. doi: 10.1055/s-2005-858555. [DOI] [PubMed] [Google Scholar]
- 13.Schroder RJ, Noor J, Pflugmacher R, et al. Short-term CT findings after osteosynthesis of fractures of the vertebral spine. Rofo. 2004;176(5):694–703. doi: 10.1055/s-2004-812952. [DOI] [PubMed] [Google Scholar]
- 14.Viano AM, Gronemeyer SA, Haliloglu M, et al. Improved MR imaging for patients with metallic implants. Magn Reson Imaging. 2000;18:287–295. doi: 10.1016/S0730-725X(99)00135-6. [DOI] [PubMed] [Google Scholar]
- 15.Ludeke KM, Roschmann P, Tischler R. Susceptibility artefacts in NMR imaging. Magn Reson Imaging. 1985;3:329–343. doi: 10.1016/0730-725X(85)90397-2. [DOI] [PubMed] [Google Scholar]
- 16.Harris CA, White LM. Metal artifact reduction in musculoskeletal magnetic resonance imaging. Orthop Clin N Am. 2006;37:349–359. doi: 10.1016/j.ocl.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Hassfeld S. Artefacts in magnetic resonance imaging caused by dental material. MAGMA. 2005;18:103–111. doi: 10.1007/s10334-005-0101-0. [DOI] [PubMed] [Google Scholar]
- 18.Suh JS, Jeong EK, Shin KH, et al. Minimizing artifacts caused by metallic implants at MR imaging: experimental and clinical studies. AJR Am J Roentgenol. 1998;171:1207–1213. doi: 10.2214/ajr.171.5.9798849. [DOI] [PubMed] [Google Scholar]
- 19.Ganapathi M, Joseph J, Savage R, et al. MRI susceptibility artefacts related to scaphoid screws: effect of screw type, screw orientation and imaging parameters. J Hand Surg Br. 2002;27:165–170. doi: 10.1054/jhsb.2001.0717. [DOI] [PubMed] [Google Scholar]
- 20.Guermazi A, Miaux Y, Zaim S, et al. Metallic artefacts in MR imaging: effects of main field orientation and strength. Clin Radiol. 2003;58:322–328. doi: 10.1016/S0009-9260(02)00540-8. [DOI] [PubMed] [Google Scholar]
- 21.Mueller PR, Stark DD, Simeone JF, et al. MR guided aspiration biopsy: needle design and clinical trials. Radiology. 1986;161:605–609. doi: 10.1148/radiology.161.3.3786706. [DOI] [PubMed] [Google Scholar]
- 22.Wendt RE, Wicott MR, Nitz W, et al. MR imaging of susceptibility-induced magnetic field inhomogeneities. Radiology. 1988;168:837–841. doi: 10.1148/radiology.168.3.3406413. [DOI] [PubMed] [Google Scholar]
- 23.White LM, Kim JK, Mehta M, et al. Complication of total hip arthroplasty: MR imaging initial experience. Radiology. 2000;215:254–262. doi: 10.1148/radiology.215.1.r00ap11254. [DOI] [PubMed] [Google Scholar]
- 24.Port JD, Pomper MG. Quantification and minimization of magnetic susceptibility artifacts on GRE images. J Comput Assist Tomogr. 2000;23:958–964. doi: 10.1097/00004728-200011000-00024. [DOI] [PubMed] [Google Scholar]
- 25.Peterslige CA, Lewin JS, Duerk JL, et al. Optimizing imaging parameters for MR evaluation of the spine with titanium pedicle screws. AJR Am J Roentgenol. 1996;166:1213–1218. doi: 10.2214/ajr.166.5.8615272. [DOI] [PubMed] [Google Scholar]
- 26.Bakker CJG, Bhagwandien R, Moerland MA, et al. Susceptibility artifacts in 2DFT spin-echo imaging: the cylinder model revisited. Magn Reson Imaging. 1993;11:539–548. doi: 10.1016/0730-725X(93)90473-Q. [DOI] [PubMed] [Google Scholar]
- 27.Schenck JF. The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatibility of the first and second kinds. Med Phys. 1996;23:815–850. doi: 10.1118/1.597854. [DOI] [PubMed] [Google Scholar]
- 28.Frazzini VI, Kagetsu Nk, Johnson CE, et al. Internally stabilized spine: optimal choice of frequency-encoding gradient direction during MR imaging minimizes susceptibility artifact from titanium vertebral body screws. Radiology. 1997;204:268–272. doi: 10.1148/radiology.204.1.9205258. [DOI] [PubMed] [Google Scholar]
- 29.Tartaglino LM, Flanders AE, Vitinski S, et al. Metallic artifacts on MR images of the postoperative spine: reduction with fast spin-echo techniques. Radiology. 1994;190:565–569. doi: 10.1148/radiology.190.2.8284417. [DOI] [PubMed] [Google Scholar]
- 30.Hilfiker P, Zanetti M, Debatin JF, et al. Fast spinecho inversion-recovery imaging versus fast spin echo imaging in bone marrow abnormalities. Invest Radiol. 1995;30:110–114. doi: 10.1097/00004424-199502000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Czerny C, Krestan C, Imhof H, et al. Magnetic resonance imaging of the postoperative hip. Top Magn Reson Imag. 1999;10:214–220. doi: 10.1097/00002142-199908000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell DG, Cohen MS. Transverse magnetization and T2 contrast. In: Mitchell DG, Cohen MS, editors. MRI principles. 2. New York: Springer; 2000. pp. 35–47. [Google Scholar]
- 33.Olsen RV, Munk PL, Lee MJ, et al. Metal artifact reduction sequence: early clinical applications. Radiographics. 2000;20:699–712. doi: 10.1148/radiographics.20.3.g00ma10699. [DOI] [PubMed] [Google Scholar]
- 34.Chang SD, Lee MJ, Munk PL, et al. MRI of spinal hardware: comparison of conventional T1-weighted sequence with a new metal artifact reduction sequence. Skeletal Radiol. 2001;20:213–218. doi: 10.1007/s002560100331. [DOI] [PubMed] [Google Scholar]
- 35.Lee MJ, Janzen DL, Munk PL, et al. Quantitative assessment of an MR technique for reducing metal artifact: application to spin-echo imaging in a phantom. Skeletal Radiol. 2001;30:398–401. doi: 10.1007/s002560100332. [DOI] [PubMed] [Google Scholar]
- 36.Potter HG, Nestor BJ, Bryan J, et al. Magnetic resonance imaging after total hip arthroplasty: evaluation of periprosthetic soft tissue. J Bone Joint Surg Am. 2004;86:1947–1954. doi: 10.2106/00004623-200409000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Suh JS, Jeong EK, Shin KH, et al. Minimizing artifacts caused by metallic implants at MR imaging: experimental and clinical studies. AJR Am J Roentgenol. 1998;171:1207–1213. doi: 10.2214/ajr.171.5.9798849. [DOI] [PubMed] [Google Scholar]