Abstract
We report the clinical and radiological results on the safety and efficacy of an unusual surgical strategy coupling anterior cervical discectomy and fusion and total disc replacement in a single-stage procedure, in patients with symptomatic, multilevel cervical degenerative disc disease (DDD). The proposed hybrid, single-stage, fusion–nonfusion technique aims either at restoring or maintaining motion where appropriate or favouring bony fusion when indicated by degenerative changes. Twenty-four patients (mean age 46.7 years) with symptomatic, multilevel DDD, either soft disc hernia or different stage spondylosis per single level, with predominant anterior myeloradicular compression and absence of severe alterations of cervical spine sagittal alignment, have been operated using such hybrid technique. Fifteen patients underwent a two-level surgery, seven patients received a three-level surgery and two a four-level procedure, for a total of 59 implanted devices (27 disc prostheses and 32 cages). Follow-up ranged between 12 and 40 months (mean 23.8 months). In all but one patient clinical follow-up (neurological examination, Nurick scale, NDI, SF-36) demonstrated significant improvement; radiological evaluation showed functioning disc prostheses (total range of motion 3–15°) and fusion through cages. None of the patients needed revision surgery for persisting or recurring symptoms, procedure-related complications or devices dislocations. To the authors’ best knowledge, this is the first study with the longest available follow-up describing a different concept in the management of cervical multilevel DDD. Although larger series with longer follow-up are needed, in selected cases of symptomatic multilevel DDD, the proposed surgical strategy appears to be a safe and reliable application of combined arthroplasty and arthrodesis during a single surgical procedure.
Keywords: Anterior cervical discectomy and fusion, Arthroplasty, Degenerative disc disease, Hybrid, Spondylosis, Total disc replacement
Introduction
Total disc replacement (TDR) is an increasingly accepted and diffusing technique used to treat cervical degenerative disc disease (DDD). Reported short- and intermediate-term clinical and radiological results are encouraging [1–6].
Symptomatic patients with soft disc hernia or moderate DDD can be considered for TDR. Few studies on the application of TDR in cervical spondylosis have been published [7–11], but concern remains on the rationale, efficacy and safety of disc prostheses in patients with multilevel spondylotic disease of the cervical spine, because of concomitant facet joints alterations and segmental bony degenerative changes.
In cases of single- or double-level spondylotic disease, anterior cervical discectomy and fusion (ACDF) remains the current most largely accepted procedure with satisfactory clinical outcome and proven radiological fusion ranging from 90 to 100% [3, 12]. Patients with symptomatic, multilevel, spondylotic myeloradiculopathy are usually treated either by anterior decompression and fusion [13], with or without plating [14] or by posterior decompression with or without lateral mass screw fixation [15].
This study presents a different concept in the management of cervical, symptomatic, multiple level DDD. The accuracy, efficacy and safety of a hybrid, single-stage, technique coupling TDR and ACDF were analysed, according to the peculiarity of the disease at each level, in order to restore or maintain motion where appropriate and promote intervertebral fusion of most degenerated levels.
Clinical material and methods
From November 2004 to March 2007, 24 patients (15 males) ranging in age from 35 to 65 years (mean age 46.7 years), with two- to four-level symptomatic DDD, causing anterior neural compression leading to radiculopathy and/or myelopathy (in 14 patients) associated in all but six with neck pain, were offered surgery using the hybrid, single stage, fusion–nonfusion technique.
None of the patients had undergone previous cervical spine surgery nor had pending socioeconomic litigations. With the exception of spondylotic changes, other exclusion criteria included the same currently accepted for cervical TDR.
Preoperative clinical evaluation included neurological examination, grading of myelopathy according to the Nurick scale, the Short Form 36 (SF-36) and the Neck Disability Index (NDI) questionnaires. In all patients imaging was based on antero-posterior (AP) and lateral X-rays, with flexion–extension views, computerized tomography (CT), with bony algorithm and magnetic resonance (MR). In every patient multilevel DDD sustaining symptoms, with one or more levels more severely affected, with osteophytes and/or facet joints alterations and uncoarthrosis, was revealed.
Patients were informed about the procedure’s rationale and agreed, signing an informed consent, that the final decision on whether to implant disc prostheses (Prodisc-C, Synthes Spine, Paoli, PA, USA; Prestige LP or Bryan disc, Medtronic Sofamor Danek, Memphis, TN, USA) or intervertebral cages (carbon fiber reinforced polymer—CFRP-, DePuy, Leeds, UK or Cornerstone CFC cages, Medtronic Sofamor Danek, Memphis, TN, USA) at each level treated could have been changed intra-operatively according to surgical findings. None of the patients received supplemental anterior cervical plating instrumentation with the cage fusion surgery.
Fifteen patients underwent a two-level surgery, seven a three-level and two a four-level procedure, respectively. In the three-level cases two disc prostheses and one cage were used in two patients, and two cages and one artificial disc in the remaining five patients. In one of these the arthroplasty was performed at C4/C5 and cages were implanted, respectively, at C3/C4 and C5/C6 (Fig. 1). Prestige LP prostheses were used in two patients, Prodisc-C in 17 cases and Bryan discs in five cases. Disc prostheses were implanted either at the disc level above or below the one(s) receiving a cage (Figs. 2, 3). A total of 59 devices (19 Prodisc-C, 3 Prestige LP, 5 Bryan, 27 CFRP cages and 5 Cornerstone CFC) have been implanted. Clinical data and surgical procedures are summarized in Table 1.
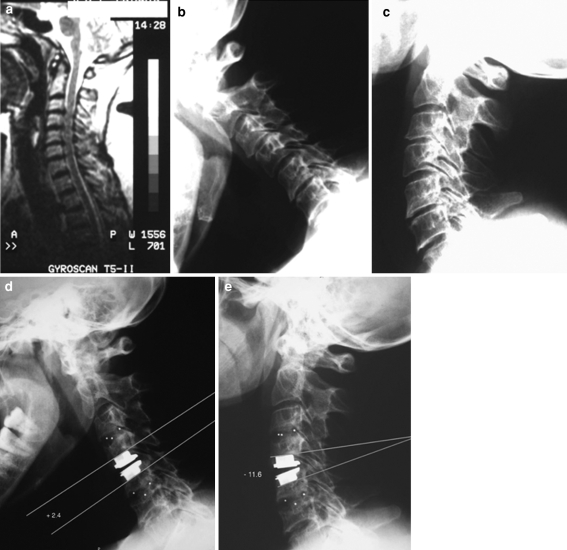
Fig. 1.
Case 9. Sagittal, T2-weighted MRI (a) and flexion-extension X-rays (b, c) demonstrating spondylosis at C3/C4 and C5/C6 and soft disc hernia at C4/C5. Postoperative dynamic X-rays (d, e) showing arthroplasty at C4/C5 and arthrodesis at C3/C4 and C5/C6. Total artificial disc motion at C4/C5 is 14°
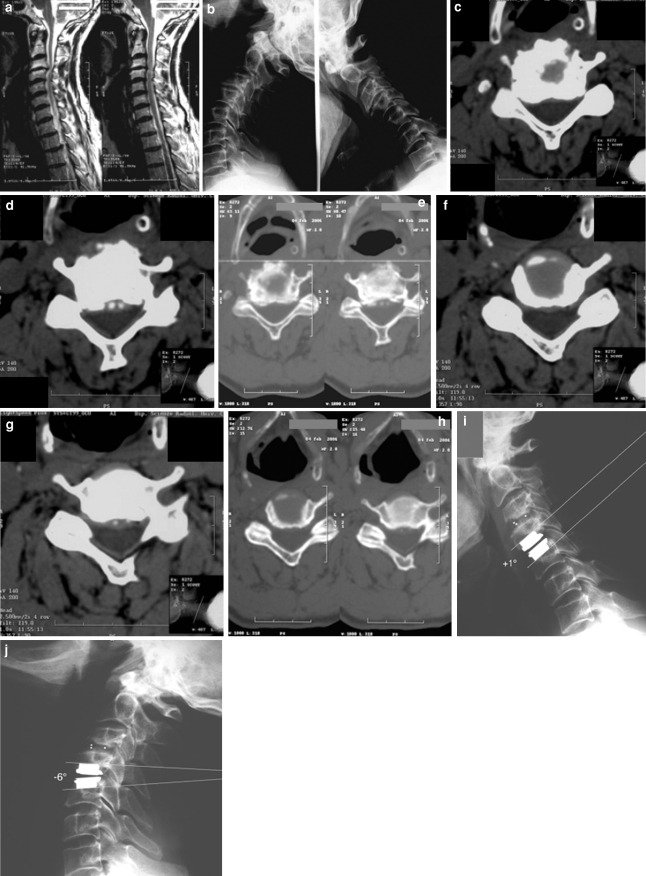
Fig. 2.
Case 10. Sagittal, T2-weighted MRI (a) and flexion–extension X-rays (b) demonstrating clear spondylotic changes with reduced disc height at C3/C4, and soft disc hernia at C4/C5. Axial CT scan, with bony algorithm, confirming the presence of spondylotic changes involving the facet joints at C3/C4 (c, d, e), and degenerative disc disease with preserved facet joints at C4/C5 (f, g, h). Postoperative flexion–extension X-rays (i, j) reveal a ROM of 7°
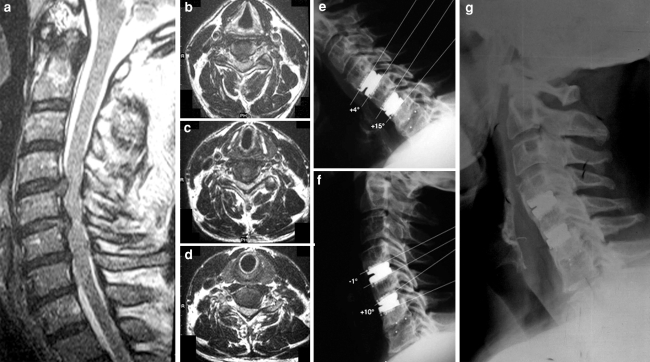
Fig. 3.
Case 1. Preoperative sagittal (a) and axial MRI showing disc herniation at C4/C5 (b), at C5/C6 (c) and spondylotic disc degeneration at C6/C7 (d). Postoperative lateral flexion (e) and extension (f) radiographs showing Prestige LP prostheses at C4/C5, C5/C6 and a CFRP cage at C6/C7, with moving artificial disc prostheses and fusion through the cage. Lateral X-ray (g) at 18-month-follow-up showing diffuse heterotopic ossification at C4/C5 and C5/C6 (see text)
Table 1.
Summary of demographic and clinical data, surgical procedures and postoperative ROM
| Case no. | Age (years), sex | Symptoms | Pathology per disc level | Procedure used per disc level | Total postoperative ROM at arthroplasty level (mean value) at final follow-up |
|---|---|---|---|---|---|
| 1 | 48, M | Neck pain myeloradiculopathy | SDH C4–C5, C5–C6 SPD C6–C7 | Arthroplasty at C4–C5, C5–C6; arthrodesis at C6–C7 | 0° (C4–C5) <3° (C5–C6) |
| 2 | 53, M | Neck pain myeloradiculopathy | SPD C5–C6, C6–C7 | Arthrodesis at C5–C6, arthroplasty at C6–C7 | 3° |
| 3 | 55, M | Neck pain myeloradiculopathy | SPD C4–C5, C5–C6, C6–C7 | Arthroplasty at C4–C5, arthrodesis at C5–C6, C6–C7 | 5° |
| 4 | 37, M | Neck pain, radiculopathy | SDH C5–C6 SDH-SPD C6–C7 | Arthroplasty at C5–C6, arthrodesis at C6–C7 | 4° |
| 5 | 54, M | Myeloradiculopathy | SPD C5–C6, C6–C7 | Arthroplasty at C5–C6, arthrodesis at C6–C7 | 6° |
| 6 | 45, F | Neck pain, radiculopathy | SPD C3–C4, C5–C6 | Arthrodesis at C3–C4, arthroplasty at C5–C6 | 15° |
| 7 | 37, M | Neck pain, radiculopathy | SPD C5–C6, C6–C7 | Arthrodesis at C5–C6, arthroplasty at C6–C7 | 9° |
| 8 | 54, F | Neck pain, myeloradiculopathy | SPD C4–C5, C5–C6 | Arthroplasty at C4–C5, arthrodesis at C5–C6 | 13° |
| 9 | 49, F | Neck pain, myeloradiculopathy | SPD C3–C4, C5–C6 SDH C4–C5 | Arthroplasty at C4–C5, arthrodesis at C3–C4, C5–C6 | 14° |
| 10 | 64, M | Neck pain, myeloradiculopathy | SPD C3–C4, C4–C5 | Arthrodesis at C3–C4, arthroplasty at C4/C5 | 7° |
| 11 | 66, M | Myeloradiculopathy | SPD C3–C4, C4–C5, C5/C6 | Arthroplasty at C3–C4, C4–C5; arthrodesis at C5–C6 | 7.5° (C3–C4) 8° (C4–C5) |
| 12 | 38, M | Neck pain, myeloradiculopathy | SDH C3–C4 SPD C5–C6, C6–C7 | Arthroplasty at C3–C4 arthrodesis at C5–C6, C6–C7 | 12.6° |
| 13 | 46, F | Neck pain, myeloradiculopathy | SPD C3–C4, C4–C5, C5–C6, C6–C7 | Arthroplasty at C3–C4, C4–C5; arthrodesis at C5–C6, C6–C7 | 6° (C3–C4) 9.3° (C4–C5) |
| 14 | 45, M | Neck pain, myeloradiculopathy | SPD C3–C4, C4–C5, C5–C6, C6–C7 | Arthroplasty at C3–C4; arthrodesis at C4–C5, C5–C6, C6–C7 | 7.6° |
| 15 | 42, F | Neck pain, myeloradiculopathy | SDH C4–C5 SDH/SPD C5–C6, C6–C7 | Arthroplasty at C4–C5; arthrodesis at C5–C6, C6–C7 | 8.6° |
| 16 | 51, M | Neck pain, myelopathy | SDH C5–C6 SPD C6–C7 | Arthroplasty at C5–C6; arthrodesis at C6–C7 | 8.2° |
| 17 | 46, M | Neck pain, myeloradiculopathy | SPD C3–C4, C4–C5, C5–C6 | Arthroplasty at C4–C5; arthrodesis at C3–C4, C5–C6 | 7.6° |
| 18 | 36, F | Neck pain, myeloradiculopathy | SPD C4–C5, C5–C6 | Arthroplasty at C4–C5; arthrodesis at C5–C6 | 10° |
| 19 | 52, M | Neck pain, myeloradiculopathy | SDH C5–C6, SPD C6–C7 | Arthroplasty at C5–C6; arthrodesis at C6–C7 | 7.3° |
| 20 | 37, M | Radiculopathy | SDH C5–C6, C6–C7 | Arthroplasty at C5–C6; arthrodesis at C6–C7 | 12° |
| 21 | 42, F | Radiculopathy | SPD C5–C6, C6–C7 | Arthroplasty at C5–C6; arthrodesis at C6–C7 | 8° |
| 22 | 37, F | Radiculopathy | SPD C5–C6, C6–C7 | Arthroplasty at C5–C6; arthrodesis at C6–C7 | 7° |
| 23 | 38, F | Radiculopathy | SPD C4–C5, C5–C6 | Arthrodesis at C4–C5; arthroplasty at C5–C6 | 11° |
| 24 | 41, M | Radiculopathy | SDH C5–C6, C6–C7 | Arthroplasty at C5–C6; arthrodesis at C6–C7 | 9° |
SDH soft disc herniation, SPD spondylosis, ROM range of motion
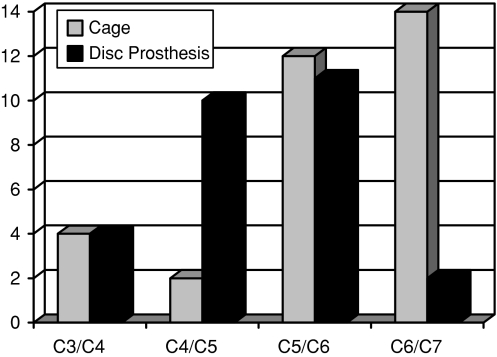
C5/C6 was the most frequently treated level, with a cage implanted in 12 patients and a disc prosthesis in 11 cases. C4/C5 was treated in 12 patients and disc prostheses were used in 10 of them. The C3/C4 disc was replaced four times with a disc prosthesis and in other four cases with cages. Sixteen C6/C7 disc levels were operated (14 cages and 2 artificial discs; Fig. 4).
Fig. 4.
Implanted cages and disc prostheses stratified per disc level
All patients were mobilized the day after surgery and discharged home 48–72 h post-surgery, after being advised to wear a soft collar for a month; anti-inflammatory drugs were not routinely administered during the first 6 weeks and oral analgesics were only used when required.
Surgical technique
A right-sided, cervical microsurgical discectomy was used in all cases to perform the neural decompression and prepare the disc space and the vertebral endplates for positioning of either a cage or disc prosthesis, taking care to choose a device of proper height in order to avoid overdistraction of facet joints. A standard technique as described by manufacturers was used to implant the different devices.
All cages were filled in with demineralized bone matrix (Grafton DBM, Osteotech, Inc.).
Outcome measures and statistical analysis
Clinical and radiological outcome measures were collected 6 weeks, 3, 6, 12, 18 and 24 months postoperatively, and then at regular intervals. Follow-up ranged between 12 and 40 months (mean 23.8 months).
The SF-36 (physical component summary—PCS- and mental component summary—MCS-) and NDI questionnaires were used for patient self-assessment and to compare pre- and postoperative clinical conditions. The Nurick scale was used to evaluate changes in myelopathic patients.
Radiographic evaluation included AP and lateral X-rays obtained postoperatively at the same time intervals detailed above, including flexion–extension views. A specifically designed computer software (OsiriX Medical Imaging Software) was used to measure angular intervertebral disc motion on digitalized images.
Measurements of SF-36 and NDI questionnaires scores were statistically compared using a paired two-sided Student’s t test for P < 0.05.
Results
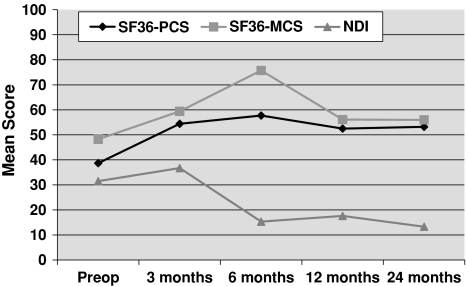
An attending neurosurgeon, not involved in the surgical procedures and blinded to patients’ clinical conditions, analysed the collected data. Questionnaire results showed that mean PCS score was 38.7 before surgery and 53.2 after surgery; mean MCS score rose from 48.2, preoperatively, to 56 postoperatively. Mean NDI score was modified from 31.5 to 13.3 after surgery. Both the SF-36 and NDI values confirmed statistically significant postoperative improvements (P < 0.05; Fig. 5).
Fig. 5.
SF-36 and NDI mean scores. Asterisk indicates paired two-sided Student’s t test with P < 0.05. SF-36 short form 36, PCS physical component summary, MCS mental component summary, NDI neck disability index
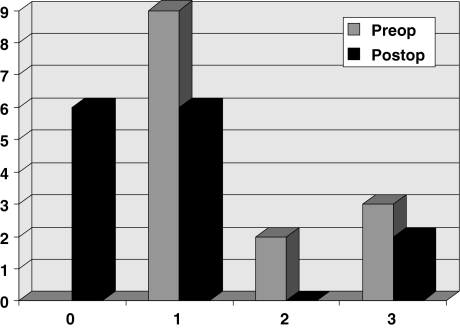
An obvious improvement was also registered in all but three myelopathic patients. Before surgery nine patients presented Nurick grade 1 myelopathy, two grade 2 and three grade 3 myelopathy, respectively. Postoperatively, six patients were grade 0, six were grade 1 and two grade 3. Only three patients, who were grade 1 pre-operatively, remained unchanged after surgery (Fig. 6).
Fig. 6.
Grading of myelopathic patients according to the Nurick Scale 3 grade-1 patients unchanged postoperatively
This clinical improvement is in keeping with the reported consistent reduction of medications (anti-inflammatories and analgesics) used postoperatively. All patients confirmed their satisfaction stating that they would have undergone again the same surgical procedure.
Neuroimaging studies were reviewed by an independent radiologist blinded to clinical outcome, in order to analyse evidence of stability, range of angular disc prosthesis motion, fusion through the cages, presence of heterotopic ossification (HO), device subsidence and effectiveness of neural decompression. All angular movement measurements were repeated three times, per single level, and a mean value was determined. Flexion–extension X-rays showed a present disc prosthesis motion in all but one patient. Case no. 1 presented a McAfee grade 2 HO at C4/C5 and C5/C6 at 12-month follow-up. This progressed to a complete fusion (McAfee grade 4) at C4/C5 and to a fusion significantly impairing movement (McAfee grade 3) at C5/C6 6 months later (Fig. 3). Table 1 shows the postoperative range of motion of arthroplasty levels.
With the exception of the above McAfee grade 4 HO and of a grade 2 HO in another case, no other complications either related to the approach and the surgical procedure or to the implanted devices were encountered. Neither implant dislocation nor subsidence nor loosening, or failure, of artificial disc components, was registered. Vertebral stability and fusion progression through cages were satisfactory in all cases. Our radiologically relevant HO rate is 8.3%. However, when clinically analysed, it comes down to the actual value of 0 (i.e. none of the two patients complaining of persisting or recurring symptoms).
Discussion
Anterior cervical discectomy and fusion, a safe and reliable technique, is regarded as the gold standard procedure for single- or multilevel cervical spondylosis leading to radiculopathy and/or myelopathy. Nonetheless, a large body of studies have analysed both in vitro [16–18] and in vivo [19–26] the role of ACDF in the onset, or progression, of DDD involving the adjacent segments (adjacent segment disease—ASD) and requiring further surgery [19, 22, 27]. Indeed, it is not clear whether or not ASD is due to the physiological ageing of the spine or to alterations induced by previous fusion [21]. Furthermore, fusion can be associated with other problems like pseudoarthrosis [28], graft-donor site complications and alterations of the adjacent motion segments’ biomechanics.
This increasingly recognised evidence has stimulated the seeking for other treatment options aiming at preserving segmental motion of the cervical spine, avoiding long-segment fusions and preventing further long-term adjacent-level degenerative disease, while restoring or maintaining sagittal alignment and stability [29, 30].
Over the past decade an increasing number of reports on the short- and intermediate-term outcome of cervical arthroplasty [1, 2, 4–6, 9–11, 26, 31–37] have been published. It is beyond our goal to discuss the indications for cervical arthroplasty [38, 39], in young or middle-aged patients with soft disc hernia or moderate DDD currently being accepted indications. Although few studies have reported favourable outcomes either after de novo TDR in spondylotic patients [8, 10; R. Assietti et al., personal communication and K. Daniel Riew K et al., personal communication at the 6° Congress of the Spinal Arthroplasty Society, Montreal, Canada, 2006] or with second-stage TDR in previously operated patients [26, 35], radiculopathy or myeloradiculopathy secondary to multilevel degenerative disease, including spondylotic degeneration, is not currently regarded as an “ideal” indication for arthroplasty.
We have used the hybrid, single stage,fusion–nonfusion technique in patients affected by multilevel DDD (soft disc hernia and/or spondylosis) causing anterior compression of the neural structures, and without severe alterations of the cervical sagittal alignment. The rationale behind our choice was based on a different and novel concept: we tried to tailor what we considered the most suitable treatment we could provide at each level according to several factors, to preserve motion, where appropriate (also aiming at avoiding further degeneration of other adjacent segments), without inducing unstable or painful conditions by trying to mobilize segments with severe spondylotic changes, either in the anterior or posterior columns, or already partially fused. The reason to use such single-stage hybrid technique relies on the obvious clinical consideration that multilevel degenerative disease, both in the cervical and lumbar spine, does not always entail the same type and degree of alterations at each level. Therefore, it is not certain that all disc levels necessarily need fusing, if alternative and equally safe and effective treatments can be performed.
Whilst cervical spondylosis is a naturally progressing phenomenon, it remains to be proven whether “segmental nonfusion” coupled with fusion in multilevel diseased spines, particularly in young or middle-aged patients, is associated with changes in the rate and degree of evolving degenerative changes or in the long-term clinical picture.
Selection criteria for TDR or ACDF
In order to define precise selection criteria for choosing ACDF or TDR at each level the first author (G.M.V.B.) developed a decision-making algorithm (G. Barbagallo et al., personal communication at the 8° Congress of the Spinal Arthroplasty Society, Miami, FL, USA, 2008) (Fig. 7). This considers the following factors:
- Type of DDD (soft disc hernia—SDH vs spondylosis—SPD) per single level
- SDH: can be treated with TDR.
- SPD: Either ACDF or TDR can be used according to the presence of angular motion (>3°) on preoperative flexion–extension X-rays and the rate of degenerative changes (see below). In cases of <3° angular motion ACDF is preferred.
- Degree of spondylotic vertebral body/facet joints degeneration
- We choose ACDF when CT bony algorithm shows clear signs either of advanced vertebral body spondylosis or facet joints’ degeneration, or pre-operative dynamic X-rays fail to show a viable angular movement (>3°). Conversely, we use TDR when angular movement is seen on X-rays and no advanced signs of DDD, particularly of posterior facet joints, are obvious.
- Amount of bone removal needed to decompress the neural structures
- When a significant amount of drilling is required to remove large osteophytes or gross bony abnormalities, because of the increased risk of vertebral body weakening or of inducing HO, ACDF is preferred.
- Shape of the inferior endplate of the cranial vertebra at the disc level involved
- A cage is implanted in cases requiring significant endplate remodelling for artificial disc insertion, in order to avoid increased risks of device subsidence or split fractures secondary to endplate and/or cortical weakening, particularly when using prostheses with keels or rails
- Presence of adjacent already degenerated discs not needing surgical treatment at the time of index procedure
- In these cases arthroplasty when feasible according to the above criteria, is considered a better option than fusion, in order to reduce increased stress on adjacent degenerated segments.
Fig. 7.
Decision-making algorithm used per single disc level
We did not decide any age as cut-off for such hybrid technique vs more conventional procedures (ACD and ACDF): type and degree of alterations per single level were assumed to be more useful elements in analysing the strategy to use, keeping in mind the natural history of cervical DDD. However, young and middle-aged patients having a less severely spondylotic cervical spine and a reasonable life expectancy and, therefore, likely to undergo degeneration of adjacent segments over years, were considered suitable candidates.
Preoperative radiological assessment of the facet joints condition and segmental range of motion were two important elements in the evaluation process leading to the final decision on whether to choose arthroplasty or arthrodesis. Although no reliable and validated CT or MRI-based grading systems for cervical facet joint degeneration are currently available [40], we considered the presence of osteophytes on the facet joint’s surface, of subchondral sclerosis or of irregularity of articular rim, or partial fusion, visualized on CT bony algorithm, as signs of significant facet joints degeneration and indeed a contraindication to arthroplasty at that level.
Such reasoning is the rationale guiding our initial experience with this new surgical strategy. It also explains the use of ACDF at C4/C5 or C5/C6 (physiologically the most mobile levels) in those cases where necessary conditions (i.e. not severely degenerated or fused facet joints and still functioning motion segment) were no longer present.
In one of the three-level patients (case 9) presenting spondylosis at C3/C4 and C5/C6 and a soft disc hernia in between, a prosthesis was implanted at C4/C5 and CFRP cages, respectively, at C3/C4 and C5/C6, aiming at preserving the range of motion still present at C4/C5 and avoiding a long segment fusion (three levels; Fig. 1). Different combinations of cages and artificial discs were used according to the discussed elements (Figs. 1, 2, 3). Overall, more cages than disc prostheses were implanted (32 and 27, respectively). Anterior cervical plates were never used.
In all but one patient, analysis of postoperative flexion–extension views confirmed the presence of functioning artificial discs, with a mean total motion ranging from 3° to 15° (Table 1), and the presence of fusion through cages. Whether a different postoperative ROM at the same disc level, i.e. C4/C5, reflects a less severe underlying disease or a consequence of other factors related to each patient (age, sex, number of treated levels, condition of facet joints, biomechanical features), can not be determined yet. However, the artificial discs ROM compares favourably with that reported in the literature [1, 4, 7, 33, 37]. Three levels (two patients) show HO. We cannot explain whether this is due to the DDD itself in these two patients, or to surgical/technical reasons. At the time of surgery in those two cases we did not routinely use bone wax to seal the vertebral body anterior edges. Moreover, in case 1 (grade 3 and 4 HO) slightly larger prostheses might have been used, to cover thoroughly the endplates as far as the anterior margin. This latter technical adjunct would seem to contribute to the reducing of the rate of clinically relevant HO.
Neurological follow-up confirms resolution of radiculopathy in all patients (this being the result of the nerve root decompression rather than the specific technique –ACDF vs TDR- used), and the postoperative Nurick score demonstrates improvement of myelopathy (Fig. 6). The SF-36 and NDI scores show a statistically significant improvement (Fig. 5).
It could be hypothesized that the proposed hybrid fusion–nonfusion technique could increase biomechanical stress on disc prostheses, due to their placement adjacent to a fused level, and determine malfunctioning and/or device dislocation. So far, our clinical experience rules these complications out, although we are aware that a longer follow-up is necessary for accurate evaluation. DiAngelo et al. analysed in a multi-level human cadaveric cervical spine the biomechanical response of an artificial disc adjacent to a fused level and found that arthroplasty did not alter the overall biomechanical integrity of the operated spine [D.J. DiAngelo et al., personal communication at the 6° Congress of the Spinal Arthroplasty Society, Montreal, QC, Canada, 2006]. Recently, other biomechanical in vitro studies have demonstrated a positive role of artificial discs adjacent to fused levels [A. Faizan et al., personal communication at the 8° Congress of the Spinal Arthroplasty Society, Miami, FL, USA, 2008].
So far, none of our patients needed surgery again because of persisting/recurrent symptoms or procedure/device-related complications. It is also worth highlighting that cages did not interfere with the normal function of artificial disc prostheses, and these did not halt the fusion process through cages.
To the best of our knowledge, there are no published studies in the literature describing the hybrid technique combining cervical arthrodesis and arthroplasty during the same surgical procedure in accordance with the above specified criteria. Yoon et al. reported that in cases of double-level pathology they preferred combining arthrodesis and arthroplasty instead of multiple levels of arthroplasty, due to the lack of results of TDR outcome [41]. This decision-making process appears to be clearly different from the rationale supporting our strategy.
Conclusions
We submit that the hybrid, single stage, fusion–nonfusion technique appears to be a promising and valid option in the treatment of symptomatic multilevel cervical DDD with prevalent anterior myeloradicular compression and different severity per single level, particularly in younger patients. It allows preserving or restoring movement in some motion segments without determining iatrogenic spine instability or painful conditions secondary to induced or forced mobilization of severely degenerated levels.
Long-term follow-up on larger series of patients is necessary to confirm our positive follow-up results, the longest available in the literature, on this simple, safe and effective surgical strategy, which might become a viable option in the daily practice.
Acknowledgments
The Authors would like to thank Professor Denis DiAngelo, Joint—Implant Biomechanics Laboratory, School of Biomedical Engineering, The University of Tennessee, USA, for his inputs and for kindly reviewing the manuscript. Sincere thanks also to Professor Giancarlo Ettorre, Chairman of the Radiology Department at the Azienda Ospedaliero-Universitaria Policlinico “G. Rodolico”, Catania, and to Ms Marisa Valastro, Radiographer, for her valuable technical support during this study.
Financial disclosure: none of the Authors has any financial interest in the products cited in this study. No funding from grant-giving organizations or other sources has been or will be received for any aspect of this work.
Conflict of interest satement None of the authors has any potential conflict of interest.
References
- 1.Bertagnoli R, Yue JJ, Pfeiffer F, et al. Early results after ProDisc-C cervical disc replacement. J Neurosurg Spine. 2005;2:403–410. doi: 10.3171/spi.2005.2.4.0403. [DOI] [PubMed] [Google Scholar]
- 2.Duggal N, Pickett GE, Mitsis DK, et al. Early clinical and biomechanical results following cervical arthroplasty. Neurosurg Focus. 2004;17(3):62–68. doi: 10.3171/foc.2004.17.3.9. [DOI] [PubMed] [Google Scholar]
- 3.Mummaneni PV, Burkus JK, Haid RW, et al. Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: a randomized controlled clinical trial. J Neurosurg Spine. 2007;6(3):198–209. doi: 10.3171/spi.2007.6.3.198. [DOI] [PubMed] [Google Scholar]
- 4.Pickett GE, Mitsis DK, Sekhon LH, et al. Effects of a cervical disc prosthesis on segmental and cervical spine alignment. Neurosurg Focus. 2004;17(3):30–35. doi: 10.3171/foc.2004.17.3.5. [DOI] [PubMed] [Google Scholar]
- 5.Porchet F, Metcalf NH. Clinical outcomes with the Prestige II cervical disc: preliminare results from a prospective randomized clinical trial. Neurosurg Focus. 2004;17(3):36–43. doi: 10.3171/foc.2004.17.3.6. [DOI] [PubMed] [Google Scholar]
- 6.Robertson JT, Metcalf NH. Long-term outcome after implantation of the Prestige I disc in an end-stage indication: 4-year results from a pilot study. Neurosurg Focus. 2004;17(3):69–71. doi: 10.3171/foc.2004.17.3.10. [DOI] [PubMed] [Google Scholar]
- 7.Bryan VE. Cervical motion segment replacement. Eur Spine J. 2002;11(Suppl 2):S92–S97. doi: 10.1007/s00586-002-0437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lafuente J, Casey AT, Petzold A, et al. The Bryan cervical disc prosthesis a san alternative to arthrodesis in the treatment of cervical spondylosis. J Bone Joint Surg Br. 2005;87(4):508–512. doi: 10.1302/0301-620X.87B4.15436. [DOI] [PubMed] [Google Scholar]
- 9.Pimenta L, McAfee PC, Cappuccino A, et al. Superiority of multilevel cervical arthroplasty outcomes versus single-level outcomes: 229 consecutive PCM prostheses. Spine. 2007;32(12):1337–1344. doi: 10.1097/BRS.0b013e318059af12. [DOI] [PubMed] [Google Scholar]
- 10.Sekhon LHS. Cervical arthroplasty in the management of spondylotic myelopathy: 18-month results. Neurosurg Focus. 2004;17(3):55–61. doi: 10.3171/foc.2004.17.3.8. [DOI] [PubMed] [Google Scholar]
- 11.Sekhon LHS. Two-level artificial disc placement for spondylotic cervical myelopathy. J Clin Neurosci. 2004;11(4):412–415. doi: 10.1016/j.jocn.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser MG, Haid RW, Jr, Subach BR, et al. Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurgery. 2002;50:229–238. doi: 10.1097/00006123-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Matz PG, Pritchard PR, Hadley MN. Anterior cervical approach for the treatment of cervical myelopathy. Neurosurgery. 2007;60(Suppl):64–70. doi: 10.1227/01.NEU.0000215399.67006.05. [DOI] [PubMed] [Google Scholar]
- 14.Ashkenazi E, Smorgick Y, Rand N, et al. Anterior decompression combined with corpectomies and discectomies in the management of multilevel cervical myelopathy: a hybrid decompression and fixation technique. J Neurosurg Spine. 2005;3:205–209. doi: 10.3171/spi.2005.3.3.0205. [DOI] [PubMed] [Google Scholar]
- 15.Wiggins GC, Shaffrey CI. Dorsal surgery for myelopathy and myeloradiculopathy. Neurosurgery. 2007;60(Suppl):71–81. doi: 10.1227/01.NEU.0000215379.90537.0B. [DOI] [PubMed] [Google Scholar]
- 16.DiAngelo DJ, Robertson JT, Metcalf NH, et al. Biomechanical testing of an artificial cervical joint and an anterior cervical plate. J Spinal Disord Tech. 2003;16:314–323. doi: 10.1097/00024720-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 17.DiAngelo DJ, Foley KT, Morrow BR, et al. In vitro biomechanics of cervical disc arthroplasty with Prodisc-C total disc implant. Neurosurg Focus. 2004;17(3):44–54. doi: 10.3171/foc.2004.17.3.7. [DOI] [PubMed] [Google Scholar]
- 18.DiAngelo DJ, Foley KT. An improved biomechanical testing protocol for evaluating spinal arthroplasty and motion preservation devices in a multilevel human cadaveric cervical model. Neurosurg Focus. 2004;17(3):22–29. [PubMed] [Google Scholar]
- 19.Goffin J, Geusens E, Vantomme N, et al. Long-term follow-up after interbody fusion of the cervical spine. J Spinal Disord Tech. 2004;17:79–85. doi: 10.1097/00024720-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Hilibrand AS, Carlson GD, Palumbo MA, et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519–528. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4:190S–194S. doi: 10.1016/j.spinee.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Katsuura A, Hukuda S, Saruhashi Y, et al. Kyphotic malalignment after anterior cervical fusion s one of the factors promoting the degenerative process in adjacent intervertebral levels. Eur Spine J. 2001;10:320–324. doi: 10.1007/s005860000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulkarni V, Rajshekhar V, Raghuram L. Accelerated spondylotic changes adjacent to the fused segments following central cervical corpectomy: magnetic resonance imaging study evidence. J Neurosurg Spine. 2004;1(100):2–6. doi: 10.3171/spi.2004.100.1.0002. [DOI] [PubMed] [Google Scholar]
- 24.Robertson JT, Papadopoulos SM, Traynelis VC. Assessment of adjacent-segment disease in patients treated with cervical fusion or arthroplasty: a prospective 2-year study. J Neurosurg Spine. 2005;3:417–423. doi: 10.3171/spi.2005.3.6.0417. [DOI] [PubMed] [Google Scholar]
- 25.Wang JC, McDonough PW, Endow K. The effect of cervical plating on single-level anterior cervical discectomy and fusion. J Spinal Disord. 1999;12:467–471. doi: 10.1097/00002517-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Wigfield C, Gill S, Nelson R, et al. Influence of an artificial cervical joint compared with fusion on adjacent-level motion in the treatment of degenerative cervical disc disease. J Neurosurg Spine. 2002;1(96):17–21. doi: 10.3171/spi.2002.96.1.0017. [DOI] [PubMed] [Google Scholar]
- 27.Gore DR, Sepic SB. Anterior discectomy and fusion for painful cervical disc disease. A report of 50 patients with an average follow-up of 21 years. Spine. 1998;23:2047–2051. doi: 10.1097/00007632-199810010-00002. [DOI] [PubMed] [Google Scholar]
- 28.Albert TJ, Eichenbaum MD. Goals of cervical disc replacement. Spine J. 2004;4:292S–293S. doi: 10.1016/j.spinee.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 29.Oskouian RJ, Whitehill R, Samii A, et al. The future of spinal arthroplasty: a biomaterial perspective. Neurosurg Focus. 2004;17(3):10–14. doi: 10.3171/foc.2004.17.3.2. [DOI] [PubMed] [Google Scholar]
- 30.Smith HE, Wimberley DW, Vaccaro AR. Cervical arthroplasty: material properties. Neurosurg Focus. 2004;17(3):15–21. doi: 10.3171/foc.2004.17.3.3. [DOI] [PubMed] [Google Scholar]
- 31.Coric D, Finger F, Boltes P. Prospective randomized controlled study of the Bryan Cervical Disc: early clinical results from a single investigational site. J Neurosurg Spine. 2006;4:31–35. doi: 10.3171/spi.2006.4.1.31. [DOI] [PubMed] [Google Scholar]
- 32.Cummins BH, Robertson JT, Gill SS. Surgical experience with an implanted artificial cervical joint. J Neurosurg. 1998;88:943–948. doi: 10.3171/jns.1998.88.6.0943. [DOI] [PubMed] [Google Scholar]
- 33.Goffin J, Casey A, Kehr P, et al. Preliminary clinical experience with the Bryan cervical disc prosthesis. Neurosurgery. 2002;51:840–847. doi: 10.1097/00006123-200209000-00048. [DOI] [PubMed] [Google Scholar]
- 34.Goffin J, Calenbergh F, Loon J, et al. Intermediate follow-up after treatment of degenerative disc disease with the Bryan Cervical Disc Prosthesis: single-level and bi-level. Spine. 2003;28:2673–2678. doi: 10.1097/01.BRS.0000099392.90849.AA. [DOI] [PubMed] [Google Scholar]
- 35.Sekhon LHS, Sears W, Duggal N. Cervical arthroplasty after previous surgery: results of treating 24 discs in 15 patients. J Neurosurg Spine. 2005;3:335–341. doi: 10.3171/spi.2005.3.5.0335. [DOI] [PubMed] [Google Scholar]
- 36.Traynelis VC. The Prestige cervical disc replacement. Spine J. 2004;4:310S–314S. doi: 10.1016/j.spinee.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 37.Wigfield CC, Gill SS, Nelson RJ, et al. The new Frenchay artificial cervical joint: results from a two-year pilot study. Spine. 2002;27:2446–2452. doi: 10.1097/00007632-200211150-00006. [DOI] [PubMed] [Google Scholar]
- 38.Le H, Thongtrangan I, Kim DH. Historical review of cervical arthroplasty. Neurosurg Focus. 2004;17(3):1–9. doi: 10.3171/foc.2004.17.3.1. [DOI] [PubMed] [Google Scholar]
- 39.McAfee PC. The indications for lumbar and cervical disc replacement. Spine J. 2004;4:177S–181S. doi: 10.1016/j.spinee.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Kettler A, Wilke H-J. Review of existing grading systems for cervical and lumbar disc and facet joint degeneration. Eur Spine J. 2006;15:705–718. doi: 10.1007/s00586-005-0954-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon DH, Yi S, Shin HC, et al. Clinical and radiological results following cervical arthroplasty. Acta Neurochir (Wien) 2006;148(9):943–950. doi: 10.1007/s00701-006-0805-6. [DOI] [PubMed] [Google Scholar]