Abstract
The classification system of spondylolisthesis proposed by Marchetti and Bartolozzi is the most practical regarding prognosis and treatment and includes the description of both low- and high-dysplastic developmental spondylolisthesis (HDDS). Unfortunately, it does not provide strict criteria on how to differentiate between these two subtypes. The accepted treatment for HDDS is surgical. However, there is no consensus on how to surgically stabilize this subtype of spondylolisthesis, and although the concept of reducing spinal deformity before fusion is attractive, the issue of surgical reduction versus in situ fusion remains controversial, especially for HDDS (Meyerding Grades III and IV). The purpose of this study was (1) to describe the severity index (SI) as a simple method that can be used in the identification of low-dysplastic developmental spondylolisthesis from HDDS allowing earlier surgical stabilization to prevent slip progression, (2) to provide guidelines for using the unstable zone for the inclusion of L4 in stabilization, and (3) to describe a surgical technique in the reduction and stabilization of this challenging surgical entity in an attempt to decrease the risk of iatrogenic L5 neurologic injury. The concepts of SI and unstable zone in the evaluation and treatment of HDDS are relatively new. In our study, patients with an SI value >20% were classified as having HDDS and surgical stabilization was offered. In addition, all vertebrae that were contained in the defined unstable zone were surgically instrumented and fused with attempts at anatomic reduction. This case series involved the retrospective radiological review of 25 consecutive patients surgically treated for HDDS between April 2000 and September 2004 by two senior surgeons. All 25 patients had a minimum 3-year follow-up. Reduction of slip, lumbosacral kyphosis, sacral inclination, fusion rate, maintenance of reduction, and iatrogenic L5 neurologic injury were evaluated. Twenty-two patients underwent a single-level L5–S1 fusion. Three patients had extension of the L5–S1 fusion to include L4 because it fell into the unstable zone. Slip improved from 67.2 to 13.6%, focal L5–S1 kyphosis improved from +17.5° to −6.4°. There were no pseudoarthroses and all patients had radiographic evidence of solid bony fusion at latest follow-up. To date, there have been no re-operations secondary to progression of deformity or loss of fixation. Two re-operations were performed, one for a superficial wound infection, the other for further laparoscopic decompression for continued L5 nerve root symptoms after the index surgery. One patient developed an iatrogenic L5 radiculopathy with dysaesthesiae 3 days postoperatively which completely resolved over 6 weeks. HDDS is best treated surgically. Early identification and stabilization of this challenging surgical entity could prevent the progression of slip and deformity making the index surgery less technically demanding. Vertebrae that are contained in the unstable zone can be instrumented and stabilized so that progression of the deformity and re-operation might be avoided. The authors suggested surgical technique can provide a way to restore sagittal balance, provide an environment for successful fusion, and decrease the risk of iatrogenic L5 neurologic injury.
Keywords: Spondylolisthesis, Reduction, PLIF, ALIF
Introduction
Two of the more commonly used classification systems for spondylolisthesis were proposed by Wiltse et al. [37] and Marchetti and Bartolozzi [21]. Wiltse et al. categorized spondylolisthesis into six types. Type I, or dysplastic, involves congenital dysplasia of the sacrum or L5 neural arch, with pars elongation or lysis which can develop later. Type II, or isthmic, is a defect in the pars interarticularis, with pars lysis (type IIA), pars elongation (type IIB), or acute pars fracture (type IIC). Type III, or degenerative, is a result of facet arthrosis leading to subluxation. Type IV, or traumatic, is secondary to acute fracture of the posterior elements other than the pars. Type V, or pathologic, is associated with incompetence of the posterior elements secondary to a systemic or local bony pathologic process. Type VI, or post-surgical, is a result of loss of posterior elements due to surgery. This classification system combines both anatomical and etiological factors and it is not always easy to distinguish type I (congenital dysplastic) from type II (isthmic) since the latter can also be congenital. In addition, it does not provide any guidelines for treatment.
The most practical classification system in terms of prognosis and therapy is that of Marchetti and Bartolozzi [21]. In this system, spondylolisthesis is divided into two major groups, developmental or acquired. Developmental spondylolisthesis is further divided into two types, both with lysis and elongation: low dysplastic and high dysplastic, depending on the severity of the bony dysplastic changes present on the L5 and S1 vertebrae and on the risk of slip progression. The low-dysplastic type is described as having a relatively normal lumbosacral profile, a normal appearing rectangular L5 vertebra, a normal S1 superior end plate, no pelvic retroversion or hyperlordosis, and very low risk of slip progression (up to 50%). Conversely, high-dysplastic spondylolisthesis is associated with lumbosacral kyphosis, a trapezoidal L5 vertebra, a dome-shaped superior end plate of S1, pelvic retroversion and hyperlordosis, and very high risk of slip progression (up to spondyloptosis). In order to decide on the best course of treatment, it is important to distinguish between low- and high-dysplastic spondylolisthesis, since the accepted treatment of the latter is surgical. Although Marchetti and Bartolozzi were the first to introduce the concept of low- and high-dysplastic developmental spondylolisthesis (HDDS), they did not include strict criteria to differentiate these two subtypes. We believe that the key difference between low- and high-dysplastic spondylolisthesis is the pelvic retroversion present only in high-dysplastic subtype.
In addition to the bony morphologic changes seen in high-dysplastic spondylolisthesis, spinopelvic balance plays an important role in the development and progression of spondylolisthesis [36]. Abnormal spinopelvic balance can alter the biomechanical stresses at the lumbosacral junction and compensatory mechanisms used to maintain adequate posture and gait.
Severity index
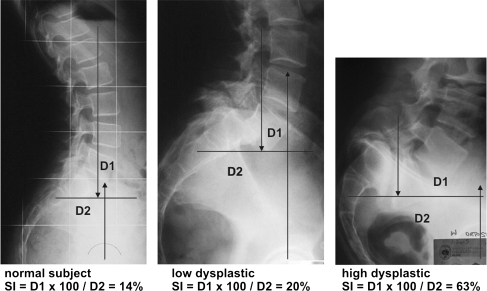
Vidal and Marnay [36] studied that, in normal subjects, a gravitational line, whose path from the external auditory meatus travels through L5–S1 and the centre of femoral heads, is continuous. Sometimes, even in normal subjects, this alignment is lost at the L5–S1 level due to a slight anterior displacement of the hips, and in this event a minor torque (two opposing forces which produce rotation) is created (Fig. 1).
Fig. 1.
In normal subjects, the standing lateral radiograph demonstrates a minor torque. Also in low-dysplastic spondylolisthesis patients, a minor torque is present. In high-dysplastic spondylolisthesis patients, an abnormal spinopelvic alignment creates a major torque
In patients with HDDS (Fig. 1), the alignment is always lost and the torque is of much greater force. The gravitational force passing through L5–S1 is counteracted by the ground reaction force passing through the centre of the femoral heads. Pelvic retroversion, resulting in verticalization of the sacrum and anterior displacement of the centre of the hips, results in abnormal spinopelvic sagittal balance. As the distance between the opposing gravitational and ground reaction forces increases, an increasing torque results (Fig. 1). The severity index (SI) is a calculation of this torque and consequently of the pelvic retroversion. Unlike Vidal and Marnay’s Index C (couple-charnière) [36], SI is directly proportional to the severity of the spondylolisthesis.
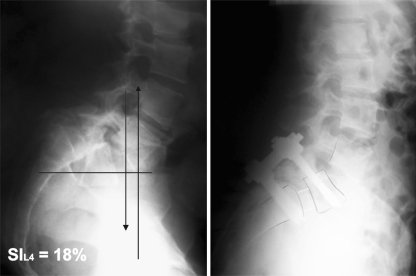
As in the Index C calculation, a horizontal line is drawn through the centre of S2 on a standing lateral radiograph of the lumbar spine that includes the hips. A vertical line is drawn through the centre of the femoral heads. A second vertical is drawn through the middle of L5 inferior end plate. The distance from the centre of S2 to the vertical of the centre of the femoral heads is D2; the distance from the vertical of the middle of L5 inferior end plate to the vertical of the centre of the femoral heads is D1. The SI is calculated as follows: SI = D1 × 100/D2. SI is a direct measure of pelvic retroversion. With increasing pelvic retroversion, there is an anterior displacement of the femoral heads and an increase in D1 (Fig. 1).
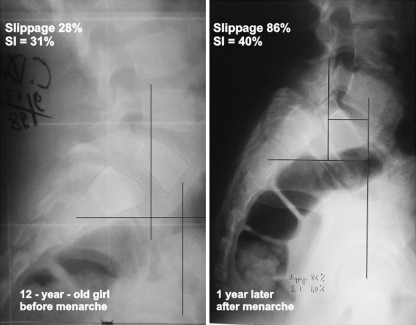
As demonstrated by Vidal and Marnay, SI is <20% in normal subjects, and it is also <20% in low-dysplastic spondylolisthesis patients since there is no pelvic retroversion (Fig. 1). SI calculation is a simple criteria in the characterization and assessment of slip progression of spondylolisthesis, when treating young subjects distinguishing the low dysplastic and high dysplastic can be very difficult (Fig. 2).
Fig. 2.
In this 12-year-old patient, the diagnosis of high-dysplastic spondylolisthesis can be difficult without SI calculation. One year later, a severe evolution occurs
Unstable zone
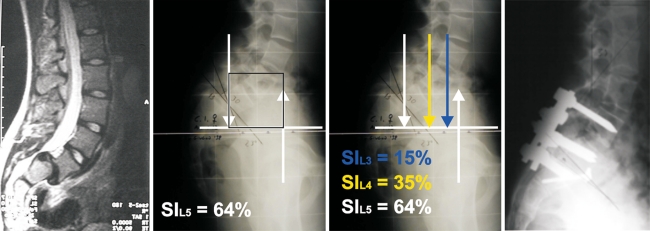
A controversial aspect in HDDS treatment is whether or not to include L4 in the instrumented area. For this reason, it is necessary to define the unstable zone in spondylolisthesis. As previously described by Lamartina [20], the unstable zone in spondylolisthesis can be defined on a standing lateral radiograph of the lumbar spine that includes the hips. A square, whose area is identified by a segment of the horizontal line that passes through the centre of S2, defines the unstable zone. The limits of this segment are the point where this horizontal line is intersected by the gravity line (the vertical passing through the midpoint of the inferior end plate of L5) and a line representing the ground reaction force (the vertical passing through the centre of the femoral head). The area in this square represents the unstable zone. All vertebrae that fall into this unstable zone should be included in the instrumentation and fusion when reduction of the L5 spondylolisthesis is being performed (Fig. 3).
Fig. 3.
MRI and standing lateral radiograph showing high-dysplastic developmental spondylolisthesis. The square indicates the unstable zone and includes both L5 and L4. The unstable zone always includes the slipped L5 and sometimes L4, if there is a large torque (SI >20%) on L4 due to high pelvic retroversion. Postoperative radiograph showing reduction of the L5 slip and the inclusion of L4 in the instrumented fusion
The concept of an unstable zone can be applied and can assist the surgeon in the decision to extend the instrumentation and fusion beyond L5–S1 during the preoperative planning.
Reduction
The treatment of the high-grade (>50% slip) spondylolisthesis (HDDS) is controversial. There is agreement among many authors that the treatment of this condition is surgical. Many studies include a mixture of various types and grades of spondylolisthesis, making it difficult to determine the result of treatment of the specific entity of this spondylolisthesis.
Recent years have seen the development of surgical techniques and instrumentations, which provide spinal surgeons with the means to substantially reduce spondylolisthetic deformity and restore spinal balance [2, 15, 17, 31]. Although the concept of reducing spinal deformity before fusion is attractive, the issue of surgical reduction versus in situ fusion remains controversial, especially for high-grade spondylolisthesis (Meyerding Grades III and IV) [5, 7, 9, 10, 14, 18, 29, 30].
In situ posterolateral fusion is a well-known technique for which many surgeons have reported satisfactory clinical outcomes in low-grade spondylolisthesis (Grades I and II) [8, 16, 22, 26]. In high-grade spondylolisthesis, the technique is prone to a significant rate of nonunion or subsequent slip progression. For both low- and high-grade spondylolisthesis, in situ fusion runs contrary to the principle of restoring physiological alignment and balance, and may have long-term implications on adjacent segment disease [1, 35].
Reduction of the spondylolisthetic condition may lessen the incidence of nonunion. Reduction of the slip angle (lumbosacral kyphosis) allows direct neural decompression and improves the sagittal lumbosacral orientation. This partial correction of the lumbosacral kyphosis not only places the fusion mass in more compression but also improves the global sagittal balance and cosmetic appearance by spontaneous correction of the thoracic hypokyphosis and lumbar hyperlordosis. Overall sagittal balance is then improved, allowing the patients to stand fully upright. Multiple techniques have been proposed and attempted. Traction and cast reduction was used by several authors with variable results [3, 6]. Problems with this method include incomplete reduction, motor deficits, and prolonged treatment. Posterior distraction reduction techniques were popular but often necessitated fusion to the upper lumbar spine. The main argument against reduction by distraction is that it involves more extensive surgery, the hypolordosis and an increase risk for neurologic injury. The incidence of neurologic complications with reduction by distraction has been reported to be as high as 31%.
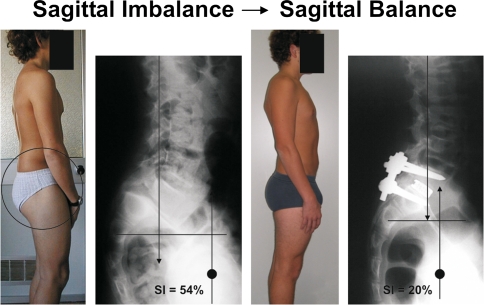
We believe that reduction in low-dysplastic developmental spondylolisthesis is not necessary since there is no pelvic retroversion and consequently sagittal unbalance. Conversely, reduction of pelvic retroversion is the key point of treatment of HDDS in order to balance the spine and the pelvis (Fig. 4).
Fig. 4.
In this 13-year-old patient, preoperative imbalance is due to the pelvic retroversion, which causes hip and knee flexion and a high torque (SI = 54%). After pelvic retroversion reduction, no hip and knee flexion and the SI is normal
If the decision is taken to correct a spondylolisthetic deformity, it is necessary to support the reduction with an interbody implant and bone grafting. Failure to do so will result in a high rate of loss of correction with hardware failure and nonunion [4, 11, 33]. The interbody fusion may be done using a posterior lumbar interbody fusion (PLIF) technique or with an anterior lumbar interbody fusion (ALIF) technique [29, 31, 32].
Once surgical stabilization is indicated, the goals of surgery are restoration of the normal anatomy with minimal functional restriction. Reduction of the spondylolisthesis may enhance the rate of fusion by increasing the bony contact and the area in compression, reducing the stress across the fusion mass. The authors’ reduction technique accomplishes these goals and allows a minimal distraction of the L5–S1 segment, thus reducing the risk of neurological injury (Fig. 5).
Fig. 5.
In this 26-year-old patient with spondyloptosis, SI is normal in L4; for this reason, the instrumented and fusion area includes only L5. Reduction technique allows a minimal distraction of the L5–S1 segment
Materials and methods
Study design
This is a case series that involved the retrospective radiologic review of 25 consecutive patients surgically treated for HDDS between April 2000 and September 2004 by two senior surgeons. All 25 patients had a minimum 3-year follow-up. Reduction of slip, lumbosacral kyphosis, sacral inclination, SI, fusion rate, maintenance of reduction, and iatrogenic L5 neurologic injury were evaluated.
Patient sample
The average patient age was 14.8 years (range 11.4–31.9 years). There were 7 males and 18 females. Twenty-two patients underwent a single-level L5–S1 fusion. Three patients had fusions that included L4–L5–S1, because L4 fell into the unstable zone. Twenty patients had posterior pedicle instrumentation and PLIF with cages, and five ALIF with cage.
Surgical indications
The principal indication for surgery was relief of low back pain and radicular pain which was secondary to foraminal stenosis. All 25 patients complained of low back pain which worsened under flexion and extension of the lumbar spine. There were 16 patients who complained of sciatic pain; L5 sensory deficits were present in eight patients; four patients had combined L5 sensory and motor deficits.
Deformity measures
Spondylolisthesis was measured according to the Taillard technique [34] and Meyerding Grade [23]. In addition, all patients had a SI >20% and were categorized as high-dysplastic with an increased risk of progression of slip and deformity and offered surgical stabilization. Six objective radiographic measurements of the lumbosacral junction were obtained. These included percent slip or displacement, slip angle, lumbosacral segmental angle L5–S1, sacral inclination, SI, and the unstable zone. In addition, total lumbar lordosis as well as the relation of the vertical line from the odontoid to the posterior border of the sacrum (sagittal balance) was measured. The position of the hips was included on the lumbar lateral radiograph so as to calculate the SI and unstable zone. The lumbosacral angle was measured from the upper end plate of L5 and, therefore, differs from the slip angle because of the trapezoid shape of L5 in HDDS.
Average preoperative slip was 73.2% (range 51–100%). Eleven were Meyerding Grade III, nine were Grade IV, and five were spondyloptotic. Slip angle averaged 34.2° before surgery (range 13°–54°). Lumbosacral angle averaged +17.5° before surgery (range −2° to +43°). Preoperative sacral inclination averaged 32.3° (range from 8° to 41°). Preoperative SI averaged 41% (range 32–64%). The unstable zone was measured in all 25 patients. Three patients had the vertebra of L4 fall into the unstable zone and thus it was included in the instrumented fusion.
Surgical technique
Preoperative patient positioning is a critical step in the procedure. With the patient on the modified Jackson frame, the hips are positioned in maximum extension. This is occasionally accomplished with the patients’ legs suspended above the operating room table. If the L5–S1 segment is mobile, this allows a partial reduction of the pelvic retroversion and slip angle.
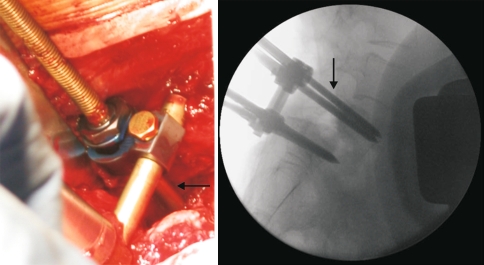
The exposure of the L5 transverse processes is often very difficult, but this step is crucial for L5 pedicle screw insertion; a Gill procedure with exposure of both the L5 and the S1 roots is then performed. The L5 nerve roots are exposed as far laterally as possible to ensure adequate visualization during the reduction. Two pedicle screws are placed bicortically in the sacrum with the screws emerging at the sacral promontory. Two double-threaded Schanz screws are then placed bicortically in the L5 pedicles with the tips of the screws just exiting the anterior cortex of the L5. In order to reduce the risk of pull-out during the reduction manoeuvre, all screws are inserted in a convergent fashion. Intraoperative fluoroscopic check is obtained to confirm placement. Two rods are tightened to the sacral screws and mounted to Schanz screw clamps without tightening. A partial reduction is performed by translating L5 back onto S1 using the double-threaded Schanz screws. All these allow better visualization for the L5–S1 discectomy and fusion.
A lumbosacral discectomy is completed using a combination of disc space shavers, curettes and rasps. This is preformed posteriorly using a root retractor to protect the dura as the discectomy is performed in a sequential fashion. In order to reduce the risk of cage subsidence, care is taken not to violate the bony end plates. The mobility obtained from the discectomy may afford additional reduction with further hip extension. The interbody spreader can be helpful in freeing the slipped L5 vertebra with gentle progressive manoeuvres to enhance the correction of the spondylolisthesis. The distance between the Schanz clamps and the L5 transverse processes reflects the residual slippage (Fig. 6). The reduction is then completed using L5 double-threaded Schanz screws. Morselized autologous iliac crest bone graft is then placed in the anterior portion of the disc space. Structural interbody cages (titanium or PEEK cages filled with morselized iliac graft) are placed bilaterally in the disc space. During reduction, the L5 roots are visualized to avoid any compression or over-distraction.
Fig. 6.
Two rods are mounted to L5 Schanz screws and locked to S1 screws. The arrows show the distance between the clamps and the L5 transverse process
If the L5 roots appear to be distracted, an ALIF with cage is performed in the same procedure (Fig. 7). Finally, the pedicle screws are compressed to restore lumbar lordosis in L5–S1 and reduce pelvic retroversion. A posterolateral fusion is completed with decortication and grafting of the transverse processes and sacrum.
Fig. 7.
In this 27-year-old patient with spondyloptosis, since SI is normal in L4 the instrumented and fusion area includes only L5. ALIF with cage was performed due to the risk of L5 root over distraction during the posterior cages insertion
A stable construct is obtained by the reduction of the slip with concurrent reduction of lumbosacral kyphosis and restoration of spinopelvic balance.
Outcome measures
Patient demographics, surgical indications, operative details, and complications (operative and postoperative) were recorded. Patients completed preoperative surveys recording visual analogue pain scores (VAS), low back outcome scores (LBOS), and short form (SF)-12 physical and mental measures. Postoperative clinical outcome surveys recording VAS, LBOS, and SF-12 physical and mental measures were administered along with patient satisfaction questionnaires at 4 weeks, 4, 12, 24 months, and further if available.
Patient satisfaction surveys recorded five-point patient subjective outcome scores [13] (worse, unchanged, fair, good, and excellent) and included two questions: “Did you consider the surgery was worthwhile?” and “Under the same circumstances, would you have the surgery again?” Retrospective radiographic measurements of slip reduction, lumbosacral kyphosis, sacral inclination, SI, evidence of fusion, and maintenance of reduction were obtained. In addition, lumbar lordosis of the segments above the fusion and the sacral plumb line (sagittal balance) [19] were available on all patients and were measured and reviewed independently.
Statistical analysis
The differences between the pre- and postoperative measures of VAS, LBOS, and SF-12 (mental component summary and physical component summary), reduction of slip, lumbosacral kyphosis, sacral inclination, lumbar lordosis, and the sacral plumb line (sagittal balance) were analysed using a two-tailed, paired t test.
Results
Clinical
Preoperative VAS improved from 6.2 ± 2.5 to 2.1 ± 3.2 at last follow-up (P = 0.01) and LBOS from 23.2 ± 14.5 to 43.7 ± 21.6 (P = 0.001). The mean preoperative SF-12 measures, PCS and MCS, were 23.7 ± 13.1 and 39.8 ± 12.4, respectively. At the latest follow-up, they measured 41.3 ± 14.7 (P = 0.01) and 53.2 ± 10.8 (P = 0.01).
At final follow-up, 25 of 25 patients considered the surgery to have been worthwhile and indicated that they would have the surgery again under the same circumstances. Twenty-one (84%) considered their outcome to be excellent, three good.
Operative time and blood loss
For a single posterior instrumented fusion, the mean surgical time was 236 min (range 245–320 min) and the mean operative blood loss was 450 ml (range 150–1,200 ml). For the posterior and anterior approach, the mean surgical time was 365 min (range 330–395 min).
Operative complications
In this small series, there were no serious intraoperative or interbody implant-related complications. There were no dural tears. Difficulty with pedicle screw placement was encountered in two patients secondary to the local lumbosacral kyphosis in spondyloptosis.
Early and late postoperative complications
There was no pseudoarthrosis. All patients had a solid bony fusion at latest follow-up, without any progression of deformity compared to immediate postoperative radiographs. There was one superficial wound infection that was successfully treated with early operative debridement. Two patients had signs of a L5 root lesion after surgery. The first patient had continued preoperative L5 root symptoms which responded to revision laparoscopic decompression of the L5 roots. The second patient underwent reduction of an 84% L5 slip back to 8% developed a delayed L5 radiculopathy with dysaesthesiae 3 days after surgery. The patient’s symptoms completely resolved over 6 weeks.
Deformity correction
Pre- and postoperative radiographs were available for analysis of deformity correction in all patients with a minimum follow-up of 3 years (Table 1). Significant improvements were noted in both the degree of slip, reducing from 73.2 to 13.6% (range 0–26%; P = 0.001) and lumbosacral angle from +17.5° to −6.4° at the latest follow-up (range −14.3° to −2.2°; P = 0.001). Slip angle passed from 34.2° to −2.2° (range −7.3° to −1.2°; P = 0.001), sacral inclination from 32.3° to 41.3° (range 35°–61°; P = 0.001), SI from 41 to 18% (range 16–20%; P = 0.001). Although overall lumbar lordosis (L1–S1) did not change, there was a significant decrease in the mean lordosis above the spondylolisthesis after correction of the focal deformity (P = 0.02). The plumb line from the centre of C2 in relation to the upper posterior edge of S1 was 53.7 mm before surgery (range 3–104) and 42.8 mm at latest follow-up (range 0–87). More relevant after L5/S1 fusion is the plumb line in relation to the upper posterior edge of L5, which was 26.5 mm at latest follow-up (range −2 to 63).
Table 1.
Radiographic and clinical improvement after surgical correction (min.–max.)
| Preoperative | Last follow-up | P | |
|---|---|---|---|
| Slip | 73.2% (51/100) | 13.6% (0/26) | 0.001 |
| Slip angle | 34.2° (13/54) | −2.2° (−7.3/−1.2) | 0.001 |
| Lumbosacral angle | +17.5° (−2/+43) | −6.4° (−14.3/−2.2) | 0.001 |
| Sacral inclination | 32.3° (8/41) | 41.3° (35/61) | 0.001 |
| Severity index | 41% (32/64) | 18% (16/20) | 0.001 |
| VAS | 6.2 ± 2.5 | 2.1 ± 3.2 | 0.01 |
| LBOS | 23.2 ± 14.5 | 43.7 ± 21.6 | 0.001 |
| PCS | 23.7 ± 13.1 | 41.3 ± 14.7 | 0.01 |
| MCS | 39.8 ± 12.4 | 53.2 ± 10.8 | 0.01 |
Discussion
The optimal surgical management of HDDS remains controversial. Although the primary goal of surgery remains the relief of pain and neurological compression, the importance of correcting associated deformity and spinal imbalance is attracting increasing attention in the literature [5, 9, 24, 27].
HDDS is basically characterized by one main pathologic condition: the retroversion of the pelvis. Besides the sacrum shows a dome-shaped deformity, L5 vertebra is trapezoidally deformed with a concave lower end plate, and there is L5–S1 kyphosis. Local deformity affects the overall posture of the patient; retroversion of the pelvis causes flexion of the hip and knee joints, and lumbosacral kyphosis leads to compensatory hyperlordosis of the adjacent lumbar segments. There is a great torque in L5–SI. Ideally, surgery should restore normal anatomy with minimal functional restriction. This process entails complete correction of the local deformity with the shortest possible fusion. Complete reduction of the L5 slippage with restoration of segmental lordosis and correction of the pelvic retroversion allows for normalization of the overall sagittal profile. The load distribution in the adjacent segments is normalized, and thus potential adjacent disc degeneration is avoided.
The technique of reduction described in the present paper meets these requirements. Reduction of the slippage is achieved by a combination of two principles. First, the reduction of the stiffness of the slipped vertebra is the removal of the most of the kinematic constraints which fix the deformity. The wide mobilization of the slipped vertebra is achieved by the extended posterior decompression, the careful release of the roots far lateral from the foramen, and the complete excision of the disc except for the outermost lamellas of the annulus. Second, the combined movement of rotation and translation is applied to the sacrum and the L5 vertebra. The hyperextension of the hips forces the anterior rotation of the pelvis, pushes the sacrum to be less vertical and reduces the sacral slope. The progressive traction force applied to the screws in L5 is able to overcome the residual stiffness of the vertebral body and to translate the L5 posteriorly.
Even more important than reduction of olisthesis is the correction of pelvic retroversion, and consequently the lumbosacral kyphosis. In our technique, correction of pelvic retroversion and L5–S1 kyphosis is achieved by posterior compression against an anterior support. The anterior cages act as a pivot, and the posteriorly applied compression force creates lordosis. To avoid distraction of the L5 roots, only small cages are used. Besides, posterior compression increases sacrum inclination and reduces L5 root stress. A further advantage of the cages is that they allow the reduction of the L5 acting as an inclined plane and resist shear forces exponentially better than bone on bone. Correction of pelvic retroversion and lumbosacral kyphosis has an enormous effect on the overall sagittal profile. Monosegmental fusion at L5/S1 has a major impact on the pelvic alignment as well as on lumbar lordosis and thoracic kyphosis. Sacral inclination increases, thereby reducing flexion of the hip joints. L5 incidence and L5 slope decrease, reducing shear forces at the lower lumbar discs. Lumbar lordosis decreases, thoracic kyphosis increases, and gravity line is normalized.
The potential benefits of restoring balance must be weighted against the risks of the reduction. In the case of high-grade slips, the risk of causing neurological deficit is the principal concern. Review of the neurological complications reported in a number of published case series of patients undergoing substantial reduction and fusion for high-grade spondylolistheses [2, 4, 6, 9, 10, 12, 17, 24, 25, 27–29] indicates the total number of patients [28 of 224 (12.5%)] with neurological deficits. Twenty of these deficits were transient or relatively minor (e.g. weakness of extensor hallucis longus), and the rate of significant postoperative radiculopathy was 3.6% (eight patients). In the present series, the only complication that could be referred specifically to the surgical procedure was the development of L5 transient radiculopathy in 2 patients of 25 (8%). The low incidence of neurological complications in this series could be related to the technique of reduction which mainly uses posterior translation and minimizes distraction stress on the roots.
The current results appear to compare favourably with those reported using other techniques. The rotation/translation technique is attractive in that it enables the correction of deformity in the sagittal and coronal planes with support of the anterior column and high fusion rates but without the need for excessive dural retraction or risk to neurological structures. The dural retraction required for insertion of the spreaders, implants and bone graft is similar to that required during a standard lumbar discectomy.
Conclusions
Although the long-term role of deformity correction and restoration of lumbar spinal balance in cases of spondylolisthesis is yet to be determined, the current study of HDDS appears to indicate that satisfactory short-term clinical outcomes can be achieved combining rotation/translation reduction of the deformity with PLIF with cages technique. There appeared to be low levels of serious complications, and substantial deformity correction was achieved. Key point is to normalize the torque in lumbosacral area correcting the pelvic retroversion which is the main characteristic of HDDS.
Acknowledgments
Conflict of interest statement None of the authors has any potential conflict of interest.
References
- 1.Akamaru T, Kawahara N, Yoon ST, et al. Adjacent segment motion after a simulated lumbar fusion in different sagittal alignments. Spine. 2003;28:1560–1566. doi: 10.1097/00007632-200307150-00016. [DOI] [PubMed] [Google Scholar]
- 2.Ani N, Keppler L, Biscup RS, Steffee AD. Reduction of high-grade slips (grades III–V) with VSP instrumentation: report of a series of 41 cases. Spine. 1991;16(Suppl):302S–310S. doi: 10.1097/00007632-199106001-00025. [DOI] [PubMed] [Google Scholar]
- 3.Bartolozzi P, Sandri A, Cassini M, Ricci M. One-stage posterior decompression-stabilisation and trans-sacral interbody fusion after partial reduction for severe L5–S1 spondylolisthesis. Spine. 2003;28:1135–1141. doi: 10.1097/00007632-200306010-00010. [DOI] [PubMed] [Google Scholar]
- 4.Boos N, Marchesi D, Zuber K, Aebi M. Treatment of severe spondylolisthesis by reduction and pedicular fixation. A 4–6 year follow-up study. Spine. 1993;18:1655–1661. doi: 10.1097/00007632-199309000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Boxall D, Bradford DS, Winter RB, Moe JH. Management of severe spondylolisthesis in children and adolescents. J Bone Joint Surg Am. 1979;61:479–495. [PubMed] [Google Scholar]
- 6.Bradford DS. Closed reduction of spondylolisthesis: an experience in 22 patients. Spine. 1988;13:580–587. doi: 10.1097/00007632-198805000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Bradford DS, Gotfried Y. Staged salvage reconstruction of grade IV and V spondylolisthesis. J Bone Joint Surg Am. 1987;69:191–202. [PubMed] [Google Scholar]
- 8.Bridwell KH, Sedgewick TA, O’Brien MF, Lenke LG, Baldus C. The role of fusion and instrumentation in the treatment of degenerative spondylolisthesis with spinal stenosis. J Spinal Disord. 1993;6:461–472. doi: 10.1097/00002517-199306060-00001. [DOI] [PubMed] [Google Scholar]
- 9.DeWald RL, Faut MM, Taddonio RF, Neuwrith MG. Severe lumbosacral spondylolisthesis in adolescents and children: reduction and staged circumferential fusion. J Bone Joint Surg Am. 1981;63:619–626. [PubMed] [Google Scholar]
- 10.Dick WT, Schnebel B. Severe spondylolisthesis. Reduction and internal fixation. Clin Orthop Relat Res. 1988;232:70–79. [PubMed] [Google Scholar]
- 11.Edwards C. Reduction of spondylolisthesis. In: Bridwell K, DeWald R, editors. The textbook of spinal surgery. Philadelphia: JB Lippincott; 1991. pp. 605–634. [Google Scholar]
- 12.Fabris DA, Costantini S, Nena U. Surgical treatment of severe L5–S1 spondylolisthesis in children and adolescents. Results of intraoperative reduction, posterior interbody fusion, and segmental pedicle fixation. Spine. 1996;21:728–733. doi: 10.1097/00007632-199603150-00014. [DOI] [PubMed] [Google Scholar]
- 13.Greenough CG, Peterson MD, Hadlow S, Fraser RD. Instrumented posterolateral lumbar fusion. Spine. 1998;23:479–486. doi: 10.1097/00007632-199802150-00015. [DOI] [PubMed] [Google Scholar]
- 14.Harris IE, Weinstein SL. Long-term follow-up of patients with grade III and IV spondylolisthesis. Treatment with and without posterior fusion. J Bone Joint Surg Am. 1987;69:960–969. [PubMed] [Google Scholar]
- 15.Hashimoto T, Shigenobu K, Kanayama M, et al. Clinical results of single-level posterior lumbar interbody fusion using the Brantigan I/F carbon cage filled with a mixture of morselised bone and bioactive ceramic granules. Spine. 2002;27:258–262. doi: 10.1097/00007632-200202010-00011. [DOI] [PubMed] [Google Scholar]
- 16.Herkowitz H, Kurz L. Degenerative lumbar spondylolisthesis with spinal stenosis. J Bone Joint Surg Am. 1991;73:802–807. [PubMed] [Google Scholar]
- 17.Hu SS, Bradford DS, Transfeldt EE, Cohen M. Reduction of high-grade spondylolisthesis using Edwards instrumentation. Spine. 1996;21:367–371. doi: 10.1097/00007632-199602010-00023. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JR, Kirwan EO. The long-term results of fusion in situ for severe spondylolisthesis. J Bone Joint Surg Br. 1983;65:43–46. doi: 10.1302/0301-620X.65B1.6822600. [DOI] [PubMed] [Google Scholar]
- 19.Kawakami M, Tamaki T, Ando M, Yamada H, Hashizume H, Yoshida M. Lumbar sagittal balance influences the clinical outcome after spondylolisthesis. Spine. 2002;27:59–64. doi: 10.1097/00007632-200201010-00014. [DOI] [PubMed] [Google Scholar]
- 20.Lamartina C. A square to indicate the unstable zone in severe spondylolisthesis. Eur Spine J. 2001;10(5):444–448. doi: 10.1007/s005860100284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marchetti PG, Bartolozzi P. Classification of spondylolisthesis as a guideline for treatment. In: Bridwell K, DeWald R, editors. The textbook of spinal surgery. 2. Philadelphia: Lippincott-Raven; 1997. pp. 1211–1254. [Google Scholar]
- 22.Mardjetko S, Connolly P, Shott S. Degenerative spondylolisthesis: a meta-analysis of the literature 1970–1993. Spine. 1994;10:2256S–2265S. [PubMed] [Google Scholar]
- 23.Meyerding HW. Spondylolisthesis. Surg Gynecol Obstet. 1932;54:371–378. [Google Scholar]
- 24.Molinari RW, Bridwell KH, Lenke LG, Baldus C. Anterior column support in surgery for high-grade, isthmic spondylolisthesis. Clin Orthop Relat Res. 2002;394:109–120. doi: 10.1097/00003086-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Molinari RW, Bridwell KH, Lenke LG, Ungacta FF, Riew KD. Complications in the surgical treatment of pediatric high-grade isthmic, dysplastic spondylolisthesis: a comparison of three surgical approaches. Spine. 1999;24:1701–1711. doi: 10.1097/00007632-199908150-00012. [DOI] [PubMed] [Google Scholar]
- 26.Moller H, Hedlund R. Surgery versus conservative management in adult isthmic spondylolisthesis: a prospective randomized study: part 1. Spine. 2000;25:1711–1715. doi: 10.1097/00007632-200007010-00016. [DOI] [PubMed] [Google Scholar]
- 27.Muschik M, Zippel H, Perka C. Surgical management of severe spondylolisthesis in children and adolescents: anterior fusion in situ versus anterior spondylodesis with posterior transpedicular instrumentation and reduction. Spine. 1997;22:2036–2043. doi: 10.1097/00007632-199709010-00020. [DOI] [PubMed] [Google Scholar]
- 28.O’Brien JP, Mehdian H, Jaffray D. Reduction of severe lumbosacral spondylolisthesis: a report of 22 cases with a ten-year follow-up period. Clin Orthop Relat Res. 1994;300:64–69. [PubMed] [Google Scholar]
- 29.Poussa M, Schlenzka D, Seitsalo S, Ylikoski M, Hurri H, Osterman K. Surgical treatment of severe isthmic spondylolisthesis in adolescents: reduction or fusion in situ. Spine. 1993;18:894–901. doi: 10.1097/00007632-199306000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Seitsalo S, Osterman K, Hyvarinen H, Tallroth K, Schlenzka D, Poussa M. Progression of spondylolisthesis in children and adolescents. A long term follow-up of 272 patients. Spine. 1991;16:417–421. doi: 10.1097/00007632-199104000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Spruit M, Pavlov PW, Leitao J, Kleuver M, Anderson PG, Boer F. Posterior reduction and anterior lumbar interbody fusion in symptomatic low-grade adult isthmic spondylolisthesis: short term radiological and functional outcome. Eur Spine J. 2002;11:428–433. doi: 10.1007/s00586-002-0450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suk KS, Jeon CH, Park MS, Moon SH, Kim NH, Lee HM. Comparison between posterolateral fusion with pedicle screw fixation and anterior interbody fusion with pedicle screw fixation in adult spondylolytic spondylolisthesis. Yonsei Med J. 2001;42(3):316–323. doi: 10.3349/ymj.2001.42.3.316. [DOI] [PubMed] [Google Scholar]
- 33.Suk SI, Lee CK, Kim WJ, Lee JH, Cho KJ, Kim HG. Adding posterior lumbar interbody fusion to pedicle screw fixation after decompression in spondylolytic spondylolisthesis. Spine. 1997;22:210–219. doi: 10.1097/00007632-199701150-00016. [DOI] [PubMed] [Google Scholar]
- 34.Taillard W. Le spondylolisthesis chez l’enfant et l’adolescent (etude 50 cas) Acta Orthop Scand. 1954;24:115. [PubMed] [Google Scholar]
- 35.Umehara S, Zindrick MR, Patwardhan AG, et al. The biomechanical effect of postoperative hypolordosis in instrumented lumbar fusion on instrumented and adjacent spinal segments. Spine. 2000;25:1617–1624. doi: 10.1097/00007632-200007010-00004. [DOI] [PubMed] [Google Scholar]
- 36.Vidal J, Marnay T. Morphology and anteroposterior body equilibrium in spondylolisthesis L5/S1. Rev Chir Orthop. 1983;69(1):17–28. [PubMed] [Google Scholar]
- 37.Wiltse LL, Newman PH, Macnab I. Classification of spondylolysis and spondylolisthesis. Clin Orthop Relat Res. 1976;117:23–29. [PubMed] [Google Scholar]