Abstract
The aim of our prospective non-randomized clinical study was to analyze operative data, short-term results, safety, efficacy, complications, and prognostic factors for single-level total lumbar disc replacement (TLDR), and to compare results between different levels (L4–L5 vs. L5–S1). Thirty-six patients with single-level L4–L5 or L5–S1 TLDR, with 1-year minimum follow-up (FU), had complete clinical [SF36, visual analog scale (VAS), Oswestry Disability Index (ODI)] and radiological data, and were included in our study. Mean FU was 38.67 ± 17.34 months. Replaced level was L4–L5 in 12 (33.3%) cases, and L5–S1 in 24 cases (66.7%). Mean age at diagnosis was 41.17 ± 7.14 years. 24 (66.7%) were females and 12 (33.3%) were males. Statistical analyses were assessed using t tests or Mann–Whitney test for continuous variables and Chi-square test or Fisher’s exact test analyses for categorical variables. Univariate linear regression and binary logistic regression analyses were utilized to evaluate the relationship between surgical outcomes and covariates (gender, age, etiology, treated level, pre-operative SF36, ODI, and VAS). Mean operative time was 147.03 ± 30.03 min. Mean hospital stay was 9.69 ± 5.39 days, and mean return to ambulation was 4.31 ± 1.17 days. At 1-year FU, patients revealed a statistical significant improvement in VAS pain (P = 0.000), ODI lumbar function (P = 0.000), and SF36 general health status (P = 0.000). Single-level TLDR is a good alternative to fusion for chronic discogenic low back pain refractory to conservative measures. Our study confirmed satisfactory clinical results for monosegmental L4–L5 and L5–S1 disc prosthesis, with no difference between the two different levels for SF36 (P = 0.217), ODI (P = 0.527), and VAS (P = 0.269). However, replacement of the L4–L5 disc is affected by an increased risk of complication (P = 0.000). There were no prognostic factors for intraoperative blood loss or return to ambulation. Age (P = 0.034) was the only prognostic factor for operative time. Hospital stay was affected by level (P = 0.036) and pre-op VAS (P = 0.006), while complications were affected by the level (P = 0.000) and pre-op ODI (P = 0.049). Complete pre-operative assessment (in particular VAS and ODI questionnaires) is important because more debilitating patients will have more hospital stay and higher complications or complaints. Patients had to be informed that complications, possibly severe, are particularly frequent (80.6%).
Keywords: Total disc replacement, Spine arthroplasty, Different levels, Results, Complications, Prognostic factors
Introduction
Discogenic low back pain, or symptomatic degenerative disc disease (DDD), is an endemic problem in our modern society, with important social and economic impacts [40]. Currently, there are no clear and consensual guidelines for the treatment of DDD, which usually responds well to various conservative treatment measures [1, 24]. Pharmacological, physical, massage, and manipulation therapies are associated with good clinical results [12]. Only after conservative measures fail, and with persistent and increasing pain, is surgery usually necessary [24].
When surgical treatment is necessary, the gold standard technique is spinal fusion. Segmental fusion has had good clinical results, but with significant medium- and long-term-related problems, such as pseudoarthrosis (~16%), pain at the iliac crest donor site (~9%), junctional pathology (up to 79% at 10 years), reduction in lumbar motion, and modification of sagittal balance [7, 11, 24, 26, 30–32, 50]. From a recent meta-analysis [8], clinical success for fusion is ~75%, but with a very slow return to normality and work (mean of 15 months).
Disc arthroplasty, or total lumbar disc replacement (TLDR), after more than 15,000 implantations in over 30 countries worldwide [42], has been shown to be a good alternative to fusion. Its aim is to preserve motion, especially in adjacent levels. After initially encouraging TLDR reports [2, 14, 15, 18, 25, 37, 54], there is now a more reflexive approach to this procedure. With medium-term follow-up (FU) results, it is clear that correct indications and contraindications are the keys for the success [6, 20, 21, 35, 44, 53, 55].
Results of TLDR from clinical trials are not clear. Efficacy of single-level TLDR is well known, with good or excellent results in 75–99% of cases [2, 4, 6, 17, 18, 36, 38, 54]. The indications for multilevel TLDR are still controversial. In some prospective clinical studies, randomized and non-randomized [28, 49, 55], double-level TLDR has similar results to single-level TLDR, while arthroplasty of three or more levels has not. In others studies, monosegmental TLDR has better clinical results than multisegmental TLDR [13, 14, 16, 45, 46], with results being influenced by age [45] and the replaced disc’s motion [13]. Even the use of disc arthroplasty below a long-segment fusion is a viable alternative to performing fusion to additional motion segments, with good clinical results in some case reports [3, 33].
To our knowledge, only one previous study [46] had specifically focused on different outcomes of TLDR for different levels, showing better results for L4–L5 versus L5–S1. Little is still known about prognostic factors.
The aim of our prospective non-randomized clinical study was to analyze operative data, short-term results, safety, efficacy, complications, and prognostic factors for single-level TLDR, and to compare results between different levels (L4–L5 vs. L5–S1).
Materials and methods
Between October 2001 and December 2006, 84 TLDRs in 62 patients were performed in our Spine Unit. Data were prospectively collected and patient selection was non-randomized. The minimum FU period was 12 months, mean 38.67 ± 17.34 months. Five patients were lost to FU. Among the remaining 57 patients (with 78 TLDRs), 36 (63.2%) were mono-level, 15 (26.4%) bi-level, 3 (5.2%) triple-level, 3 (5.2%) hybrids. Therefore, 36 of 62 patients were qualified for this study. The replaced level was L4–L5 in 12 (33.3%) cases, and L5–S1 in 24 cases (66.7%). Medical indications were DDD (52.8%), post-discectomy disc disease (36.1%), disc herniation (5.55%), and junctional pathology (5.55%; Table 1). All patients had failure of conservative treatment for at least 6 months (lumbosacral brace, drugs, and various kind of manipulative or physical therapies). The mean age at diagnosis was 41.17 ± 7.14 years (range 30–56). 24 (66.7%) were females and 12 (33.3%) were males. The first 17 (47.2%) prostheses were Prodisc-II (Synthes Spine Solutions Inc., West Chester, PA, USA), the other 19 (52.8%) were Maverick (Medtronic Sofamor Danek, Memphis, TN, USA).
Table 1.
Demographic, pre-operative and peri-operative data in the overall population, and in the two groups L4–L5 (A) and L5-S1 (B)
| Total (n = 36) | L4–L5 (n = 12) | L5–S1 (n = 24) | P value | |
|---|---|---|---|---|
| Etiology | ||||
| Degenerative | 19 (52.8%) | 58.3% (7) | 50.0% (12) | 0.132 |
| Post-discectomy | 13 (36.1%) | 25.0% (3) | 41.7% (10) | 0.429 |
| Disc herniation | 2 (5.55%) | 0.0% (0) | 8.3% (2) | – |
| Junctional pathology | 2 (5.55%) | 16.7% (2) | 0.0% (0) | – |
| Prosthesis | ||||
| Maverick | 19 (52.8%) | 58.3% (7) | 50.0% (12) | 0.384 |
| Prodisc | 17 (47.2%) | 41.7% (5) | 50.0% (12) | 1.000 |
| Gender (M) | 12 (33.3%) | 50.0% (6) | 25.0% (6) | 0.253 |
| Age (years) | 41.17 ± 7.14 | 41.20 ± 7.79 | 0.962 | |
| Operative time (min) | 147.03 ± 30.03 | 155.00 ± 35.99 | 141.43 ± 27.26 | 0.332 |
| Intraoperative blood loss (ml) | 284.72 ± 284.06 | 313 ± 162 | 212 ± 89 | 0.075 |
| Return to ambulation (days) | 4.31 ± 1.17 | 4.82 ± 1.25 | 4.13 ± 1.01 | 0.604 |
| Hospitalization stay (days) | 9.69 ± 5.39 | 10.45 ± 5.47 | 8.22 ± 5.57 | 0.185 |
| Pre-op SF36 | 37.17 ± 13.64 | 37.27 ± 12.75 | 36.09 ± 14.10 | 0.650 |
| Pre-op VAS | 75.00 ± 15.31 | 74.55 ± 15.88 | 75.22 ± 15.99 | 0.883 |
| Pre-op ODI | 40.72 ± 15.54 | 38.55 ± 16.15 | 40.96 ± 13.18 | 0.594 |
| Subjective satisfaction | 28 (77.78%) | 9 (75%) | 19 (79.1%) | 0.687 |
Comparative statistical analysis (P) was performed between the two groups A and B
Pre-operative diagnosis was made on the basis of the clinical signs and symptoms, lumbar standard and dynamic radiographs (anteroposterior, lateral, flexion and extension view images), lumbar MRI, lumbar CT with 3D reconstruction of abdominal prevertebral vessels. Discography was rarely used, since its role is debated and unclear in identifying discogenic pain and in distinguishing symptomatic level [5, 9, 10]. Pre-operative clinical questionnaires examined health status (SF36), lumbar function (Oswestry Disability Index, ODI), and lumbar visual analog scale (VAS) pain. Operative and rehabilitative data collected were operating time, intraoperative blood loss, return to ambulation, duration of hospitalization. Demographic and peri-operative clinical data are summarized in Table 1.
Clinical questionnaires (SF36, ODI, VAS), complications, and lumbar standard and dynamic radiographs were used to collect data in the post-operative period (1, 3, 6, 12 months). Patient data were accumulated prospectively, and retrospectively reviewed.
Patients were divided into two groups: (A) L4–L5 TLDR and (B) L5–S1 TLDR. The two groups were compared by demographic data (Table 1), clinical questionnaires’ results (Tables 1, 2, 3; Fig. 1), and complications (Table 4).
Table 2.
Comparison of clinical questionnaires between pre-operative and 1-year follow-up in the overall population and in the two groups
| Total | P value | L4–L5 | P value | L5–S1 | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | 1 year | Pre | 1 year | Pre | 1 year | ||||
| SF36 | 37.17 ± 13.64 | 60.17 ± 20.84 | 0.000 | 37.27 ± 12.75 | 58.20 ± 22.55 | 0.003 | 36.09 ± 14.10 | 59.96 ± 21.09 | 0.000 |
| VAS | 75.00 ± 15.31 | 35.00 ± 26.87 | 0.000 | 74.55 ± 15.88 | 35.00 ± 27.18 | 0.001 | 75.22 ± 15.99 | 35.87 ± 28.31 | 0.000 |
| ODI | 40.72 ± 15.54 | 22.23 ± 15.18 | 0.000 | 38.55 ± 16.15 | 22.20 ± 17.90 | 0.002 | 40.96 ± 13.18 | 21.91 ± 14.57 | 0.000 |
There was statistical significant improvement of quality of life, lumbar pain, and lumbar function in all groups
Table 3.
Comparison of clinical questionnaires’ improvement between pre-op and 1-year FU in the two groups
| ΔRelative | L4–L5 | L5–S1 | P value |
|---|---|---|---|
| SF36 | −0.43 ± 0.53 | −0.74 ± 0.66 | 0.217 |
| VAS | 0.64 ± 0.31 | 0.49 ± 0.32 | 0.269 |
| ODI | 0.53 ± 0.34 | 0.44 ± 0.30 | 0.527 |
There was no statistical significant difference in SF36 health status (P = 0.217), in VAS lumbar pain (P = 0.269), and in ODI lumbar function (P = 0.527)
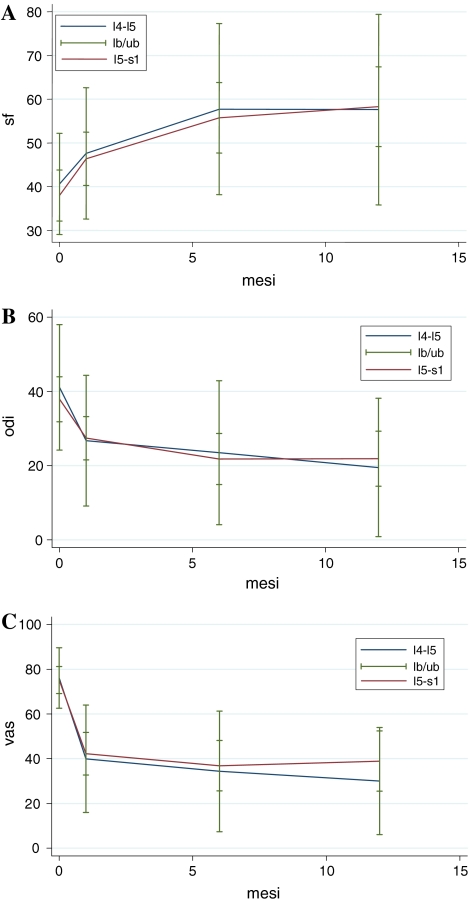
Fig. 1.
Comparison of SF36, VAS, and ODI clinical outcomes for TLDR at different levels. a SF36 health survey (P = 0.003 in L4–L5, and P = 0.000 in L5–S1), b ODI lumbar function (P = 0.002 in L4–L5, and P = 0.000 in L5–S1), and c VAS lumbar pain (P = 0.001 in L4–L5, and P = 0.000 in L5–S1) showed statistical significant improvement between pre-op and 1-year FU. There was no statistical significance difference between the two different levels (P = 0.217 for SF36; P = 0.527 for ODI; P = 0.269 for VAS)
Table 4.
Complications in our single-level total lumbar disc replacement groups
| Single-level TLDR | |||
|---|---|---|---|
| Total (n = 36) | L4–L5 (n = 12) | L5–S1 (n = 24) | |
| Surgical approach | |||
| Laparocele | 4 | 2 | 2 |
| Persistent abdominal pain | 2 | 2 | – |
| Wound dehiscence | 2 | 2 | – |
| Transitory urinary disorders | 1 | 1 | – |
| L1–L2 paresthesia | 1 | – | 1 |
| Prosthesis | |||
| Junctional pathology | |||
| Surgery | 1 | – | 1 |
| No | 4 | 3 | 1 |
| Persistent radiculitis | |||
| Surgery | 4 | 1 | 3 |
| No | 2 | 1 | 1 |
| Facet degeneration | 4 | 1 | 3 |
| Persistent back pain | 2 | 1 | 1 |
| Transitory radiculitis | 2 | 1 | 1 |
| Ossification | – | – | – |
| 29 | 15 | 14 | |
| 80.6% | 125.0% | 58.3 | |
| P = 0.000 | |||
Mean complication rate (80.6%) was frequent. Complications were particularly frequent in L4–L5 group (125%) than in L5–S1 group (58.3%) (P = 0.000)
The presence of prognostic factors (such as gender, age, etiology, level, and clinical questionnaires) in TLDR was studied using covariates as operating time, intraoperative blood loss, time to return to ambulation, hospitalization duration, and complications (Table 5).
Table 5.
Univariate linear regression and binary logistic regression were utilized to evacuate the relationship between surgical outcomes (operative time, intraoperative blood loss, return to walk, hospital stay, and complications) and covariates as gender, age, etiology, treated level, pre-op values of SF36, VAS, and ODI
| Operative time (min) | Intraoperative blood loss (ml) | Return to walk (days) | Hospital stay (days) | Complications (n/%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 147.03 ± 30.03 | 284.72 ± 284.06 | 4.31 ± 1.17 | 9.69 ± 5.39 | 29/36 (80.6%) | |||||
| B | P value | B | P value | B | P value | B | P value | B | P value | |
| Gender (F) | −21.3 | 0.095 | −52.78 | 0.316 | −0.71 | 0.181 | 0.58 | 0.822 | 0.24 | 0.134 |
| Age | 12.03 | 0.034 | 4.76 | 0.843 | 0.08 | 0.727 | −0.99 | 0.368 | 2.00 | 0.609 |
| Etiology | 14.37 | 0.280 | −73.02 | 0.185 | −0.24 | 0.667 | 2.53 | 0.353 | 1.39 | 0.737 |
| Level (L5–S1) | −14.45 | 0.280 | −100.7 | 0.055 | −0.21 | 0.725 | −5.38 | 0.036 | 1.29 | 0.000 |
| Pre-op SF36 | 0.31 | 0.584 | 1.45 | 0.519 | −0.01 | 0.588 | 0.03 | 0.591 | 0.94 | 0.112 |
| Pre-op VAS | −0.21 | 0.585 | 1.18 | 0.479 | 0.03 | 0.133 | 0.20 | 0.006 | 1.08 | 0.067 |
| Pre-op ODI | −0.46 | 0.304 | −2.45 | 0.170 | 0.02 | 0.368 | −0.07 | 0.426 | 1.08 | 0.049 |
This comparison outlined that operative time was influenced by age (P = 0.034); hospital stay was influenced by level (P = 0.036) and pre-op VAS (P = 0.006); while complications were influenced by level (P = 0.000) and pre-op ODI (P = 0.049)
Statistically significant values are in bold
No comparison was made between the two different prostheses.
Continuous data were expressed as mean ± SD, categorical variables as percentage. Differences between treated levels were assessed using t tests or the Mann–Whitney test for continuous variables and Chi-square test or Fisher’s exact test analysis for categorical variables.
Univariate linear regression and binary logistic regression analyses were utilized to evaluate the relationship between surgical outcomes and covariates. The statistical significance was defined as P value < 0.05.
Analyses were performed using STATA statistical software, version 9.2 (Stata Corp.), and SPSS version 12.0 (SPSS Inc.).
Results
Overall results are outlined in Table 1. A total of 36 TLDRs were performed in a period of 63 months. 12 (33.3%) were in L4–L5, 24 (66.7%) in L5–S1. The majority of operations were performed for DDD (52.8%), sometimes for post-discectomy disc disease (36.1%), rarely for disc herniation (5.55%), or junctional pathology (5.55%). Patients were more frequently females (66.7%).
Mean FU period was 38.67 ± 17.34 months (range 12–68). Mean operative time was 147.03 ± 30.03 min (range 85–210). There was no difference between the two groups (P = 0.332). Mean intraoperative blood loss was 284.72 ± 284.06 ml (range 100–1,800). There was no difference between the two groups (P = 0.075). Mean return to walk was 4.31 ± 1.17 days (2–8). There was no difference between the two groups (P = 0.604). Mean hospital stay was 9.69 ± 5.39 days (range 6–37). There was no difference between the two groups (P = 0.185; Table 1). Overall subjective patient satisfaction rate was 77.78% (28/36). It was slightly higher in L5–S1 group (79.1%) versus L4–L5 (75%), but with no statistical significance difference (P = 0.687).
Mean pre-operative SF36 was 37.17 ± 13.64 (17–71). There was no difference between the two groups (P = 0.650). Mean pre-operative VAS was 75.00 ± 15.31 (40–100). There was no difference between the two groups (P = 0.883). Mean pre-operative ODI was 40.72 ± 15.54 (10–80). There was a statistical significance improvement of SF36, VAS, and ODI after 1-year FU both in L4–L5 (P = 0.003 for SF36, P = 0.002 for ODI, P = 0.001 for VAS) and in L5–S1 (P = 0.000 for SF36, P = 0.000 for ODI, P = 0.000 for VAS) groups (Table 2). There was no statistical difference between improvement after 1-year FU of SF36 (P = 0.217), ODI (P = 0.527), and VAS (P = 0.269) between the two study groups (Table 3; Fig. 1).
All complications are outlined in Table 4. In our overall population, the complication rate was 80.6%. Complications were particularly frequent in L4–L5 group (125%) than in L5–S1 group (58.3%) (P = 0.000). This was especially for complications secondary to the surgical approach (58.33% in L4–L5 vs. 12.5% in L5–S1) than for complication due to the prosthesis itself (66.7 vs. 45.8%; Table 4). Complications secondary to the surgical approach were laparoceles (4/36 = 11.1%), persistent abdominal pain (2/36 = 5.55%), wound dehiscence (2/36 = 5.55%), transitory urinary disorder (1/36 = 2.8%), and L1–L2 paresthesia (1/36 = 2.8%). Between the complications related to the prosthesis itself, frequents were persistent radiculitis (6/36 = 16.7%, 66.7% of which need surgery), junctional pathology (5/36 = 13.9%, 80% of which need surgery), and posterior facet degeneration (4/36 = 11.1%); less frequents were persistent back pain (2/36 = 5.55%) and transitory radiculitis (2/36 = 5.55%). There was no case of ossification of the replaced disc, both for L4–L5 and for L5–S1.
Regarding the study (Table 5) on prognostic factors (gender, age, etiology, level, and pre-operative clinical questionnaires) for surgical outcomes (operating time, intraoperative blood loss, return to walk, hospitalization’s length) and complications, our data outlined that operative time was influenced by age (P = 0.034), but not by gender (P = 0.095), etiology (P = 0.280), level (P = 0.280), pre-op SF36 (P = 0.584), pre-op ODI (P = 0.304), and pre-op VAS (P = 0.585). Intraoperative blood loss was not influenced by gender (P = 0.316), age (P = 0.843), etiology (P = 0.185), level (P = 0.055), pre-op SF36 (P = 0.519), pre-op ODI (P = 0.170), and pre-op VAS (P = 0.479). Even return to walk was not influenced by gender (P = 0.181), age (P = 0.727), etiology (P = 0.667), level (P = 0.725), pre-op SF36 (P = 0.588), pre-op ODI (P = 0.368), and pre-op VAS (P = 0.133). Hospital stay was influenced by level (P = 0.036) and pre-op VAS (P = 0.006), while not by gender (P = 0.822), age (P = 0.368), etiology (P = 0.353), pre-op SF36 (P = 0.591), and pre-op ODI (P = 0.426). Complications were influenced by level (P = 0.000) and pre-op ODI (P = 0.049), not by gender (P = 0.134), age (P = 0.609), etiology (P = 0.737), pre-op SF36 (P = 0.112), and pre-op VAS (P = 0.067; Table 5).
Discussion
Disc arthroplasty (TLDR) had been shown to be a good alternative to fusion, but long-term prospective results are lacking. In particular, efficacy of single-level TLDR is well known, with good or excellent results in 75–99% of cases [2, 4, 6, 17, 18, 36, 38, 54]. Success of the procedure depends upon following the proper indications and contraindications [6, 20, 21, 35, 44, 53, 55].
The indication for TLDR [3, 19, 34] is DDD in one or two discs (usually not more than two) between L3 and S1 in patients 18–60-year old with back pain/sciatica, after at least 6 months of conservative treatment failure. This should be associated with lumbar functional impairment over 40% (ODI > 20/50), integrity of posterior facets, and radiographic evidence of DDD (reduction of disc height >2 mm; segmental instability with >3 mm translation or >5° angulation, but no slipping; external disc thickened or dehydrated on MRI; contained disc herniation; disc vacuolization).
Contraindications [3, 19, 34] can be general or specific. General contraindications include osteoporosis or other osteopathies that reduce load-bearing capacity of the vertebral body endplate (increased risk of implant slipping); spinal deformities (degenerative scoliosis, kyphosis, etc.); vertebral fracture; suspected vertebral tumor; acute or chronic spondylodiscitis; general signs of infection; fever; pregnancy; severe obesity; foreign body sensitivity to implant materials; dependency on pharmaceutical drugs; drug abuse or alcoholism; psychosocial factors; previous major abdominal surgery or irradiation.
Specific contraindications include: disc herniation with predominant radicular symptoms or signs of cauda equina compression; posterior element pathology (such as Fujiwara’s III–IV° facet spondylarthrosis, spinal canal stenosis, postlaminectomy, failed back surgery syndrome with significant epidural fibrosis); translational forward instability (isthmic or degenerative listhesis); severe endplate irregularities (e.g., large Schmorl’s nodes).
In our 36-patient single-level TLDR group study (Table 1), subjective satisfaction rate was 77.78% (28/36), with a statistical significance improvement after a mean of 3-year FU of the SF36 health status (P = 0.000), ODI lumbar function (P = 0.000), and VAS lumbar pain (P = 0.000) (Tables 2, 3; Fig. 1).
Despite a large number of studies certifying the efficacy of TLDR, still few and absolutely necessary are studies about predictive outcomes in TLDR [27]. In a recent study, Siepe et al. [46] proved that monosegmental L4–L5 TLDR had better clinical results than L5–S1, but with a higher complication rate.
In our group, results revealed that there was no difference in monosegmental disc replacement between L4–L5 (Fig. 2) and L5–S1 (Fig. 3), for SF36 (P = 0.217), ODI (P = 0.527), and VAS (P = 0.269) (Table 3). However, disc replacement at L4–L5 had a higher complication rate (P = 0.000) (Table 4). The two groups were similar for etiology, model of prosthesis, gender (P = 0.253), age (P = 0.926), and pre-operative clinical parameters (SF36, ODI, VAS) (Table 1). Surgery showed a reduced operative time (about 2 h and a half), with little intraoperative blood loss (less than 300 ml). There was no difference in operative parameters between different levels (Table 1).
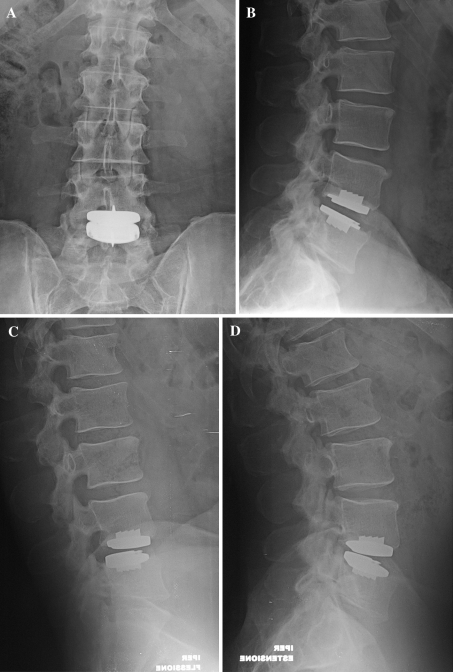
Fig. 2.
Five-year follow-up (FU) anteroposterior (a) and lateral (b) standard X-rays of a 48-year-old man with a L4–L5 TLDR. He was completely satisfied, with an important improvement in SF36 health status (from 56 pre-op to 93 at 5-year FU), in ODI lumbar function (from 50 pre-op to 0 at 5-year FU), and VAS lumbar pain (14 pre-op, 0 5-year FU). Dynamic X-rays (c, d) showed a prosthetic disc ROM of 18° in flexion–extension
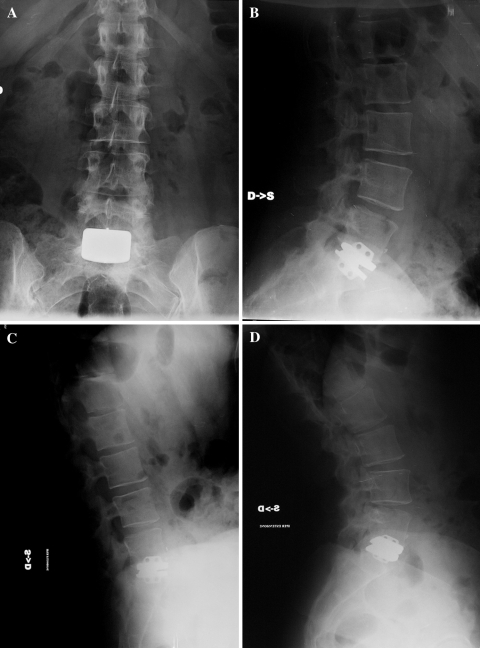
Fig. 3.
After 1 year from L5–S1 TLDR (a, b), a 35-year-old woman was completely satisfied. She had an improvement in SF36 health status (from 71 pre-op to 94 at 1-year FU), in ODI lumbar function (40 pre-op, 6 1-year FU), and VAS lumbar pain (90 pre-op, 0 1-year FU). Flexion–extension L5–S1 prosthetic disc ROM was 6° at dynamic radiographs (c, d)
For some authors, rehabilitation begins with a good pre-operative educational program [23]. Post-operatively, rapid mobilization provides better clinical results. Excessive extension and rotation should be avoided for at least 3 weeks [14, 42]. If there are no complications, sport activity can be resumed in 3 months [23].
With a mean hospitalization of less than 10 days, our patients showed a mean return to ambulation in about 4 days (Table 1). There was no difference between prostheses at L4–L5 or L5–S1 (P = 0.604). Our patients wore a soft lumbar brace for a period of 3–4 weeks post-operatively. This was followed by an abdominal elastic fascia for 6 months (as suggested by the Vascular Surgeon).
In considering prognosis, we searched potentially predictive factors (gender, age, etiology, level, and pre-operative clinical questionnaires) for surgical outcomes and complications. Our data demonstrated that operative time was influenced only by age (P = 0.034). Hospital stay was influenced by level (P = 0.036) and pre-op VAS (P = 0.006). Complications were influenced by level (P = 0.000) and pre-op ODI (P = 0.049). Intraoperative blood loss and return to ambulation had no predictive outcomes (Table 5).
Complication rates in TLDR are very variable in the literature. In 2005, Le Huec [34] reported complications in 39 of his 64 patients (61%). Seventy-two percent of these were major. Complications for TLDR could be secondary to the anterior abdominal surgical approach, or due to the prosthesis itself, and are divided into immediate and late. Late complications are still not well known, since this method is relatively new, and the vast majority of the study is short- or medium-term FU [22]. Anterior abdominal surgery complications include paralytic ileum, small intestine occlusion, retrograde ejaculation secondary to sympathetic hypogastric plexus injury, caval vein injury, left iliac vein lesion, left uretheral lesion, left iliac vein thrombosis, pelvic phlebitis, infection, deep hematoma, and laparocele [42]. Vascular or general surgical assistance, retroperitoneal approach, uretheral stents for revision surgery, proper hemostasis, attention to sterility and rectus abdomen fascia suture help to reduce surgical complications [42].
Acute prosthetic complications include epidural blood loss, transitory radiculitis due to excessive disc distraction [42]. Malpositioning of the implant is usually a cause of patient dissatisfaction, with frequent mobilization or sinking of the implant [42].
Late prosthetic complications include heterotrophic ossification, with a variable incidence of 4–6% at 2 years using Charité [37, 48], and 76.2% at 1 year using ProDisc [39]. To avoid this, it is recommended to avoid excessive vertebral osteotomy, and to administer adequate post-operative anti-inflammatory medications [42]. Possible causes of persistent back pain or sciatica are inadequate patient selection, suboptimal implantation, mobilization or sinking of the implant, posterior facet degeneration or ligamentous inflammation secondary to overload [41, 42], and junctional pathology (24% at 8.7 years FU, especially with motility < 10° [29]). Posterior facet pain and pain from the sacroiliac joint are frequent causes of patient dissatisfaction [47]. Salvage procedures are always possible, even for severe late complications. These could include replacement of the prosthetic disc, a posterior fusion, or a 270° fusion with removal of the disc prosthesis (more risky but has better clinical results) [38, 41].
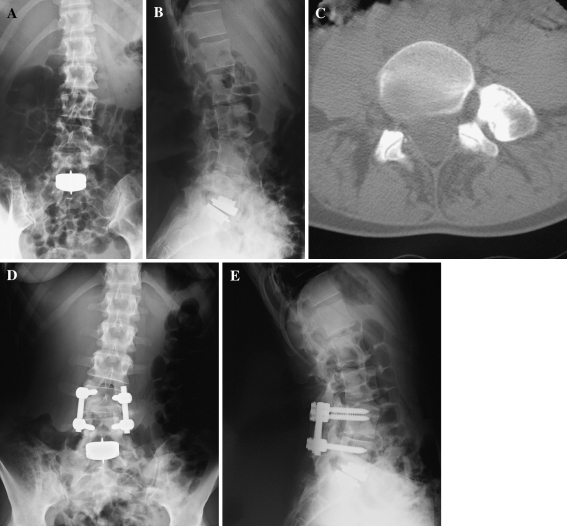
Overall, the complication risk is high, including severe complication. In our group of patients, the mean complication rate was 80.6%. The highest risk, with a complication rate of 125%, is for L4–L5 TLDR (P = 0.000), particularly for a surgical approach (laparoceles, persistent abdominal pain, and wound dehiscences; Table 4). The other risk factor for complication was the pre-op ODI (P = 0.049). The higher the pre-op lumbar dysfunction value, the higher the risk of complication. This was probably due to the difficulty of complete return to normality in a globally compromised lumbar spine, especially in severe cases (high pre-op ODI). Anatomical factors such as muscle or posterior element degeneration, and even psychological and social factors such as spinal balance, habits, or confidence, could contribute to compromising outcomes. Revision surgery was necessary in 5 (13.9%) patients: 4 (11.1%) for persistent radiculitis (Fig. 4), and 1 (2.8%) for junctional pathology (Fig. 5). After a mean FU period of more than 3 years (38.67 ± 17.34 months), the risk of symptomatic junctional pathology was 13.9% (5/36), while the overall risk of junctional pathology (symptomatic and non-symptomatic) was 22.2% (8/36). In four patients (11.1%), there was recurrence of back pain due to facet degeneration, which was treated with infiltrations. Because of the high risk of complication and considering the lack of long-term prospective randomized clinical studies, some authors today claim “it is difficult to defend the choice of a TLDR for chronic discogenic low back pain” [43, 52], and “a multidisciplinary approach is the most successful mode of treatment, while local measures aimed at the vertebral column are generally ineffective or of unproven value. Psychosocial factors are much more important than biomechanical factors in determining the outcome, but the interaction between these determinants is far more complicated than just ‘having problems’. Patients will continue to seek magic cures from ‘quacks with a knife’ as long as medical specialists are insufficiently trained to deal with unexplained somatic symptoms” [51].
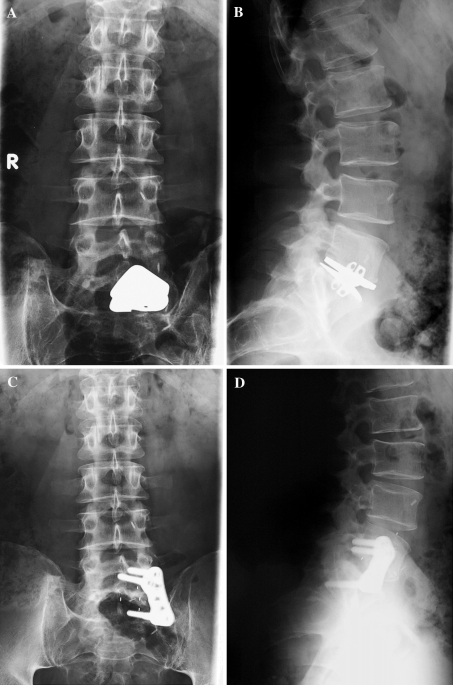
Fig. 4.
After 2 years from the L5–S1 disc replacement, a patient complained for persistent radiculitis in malpositioning of the implant (a, b). He was submitted to a 270° fusion with removal of the disc prosthesis (c, d). After 3-year FU, one from the second operation, he revealed partial but not sufficient improvement in SF36 health status (34), ODI lumbar function (66) and VAS lumbar pain (80)
Fig. 5.
A 35-year-old woman after 4 years from L5–S1 disc arthroplasty (a, b) complained for right lumbar pain (located on the right L4–L5 posterior facets space). She had a left L4–L5 bone union (a), and developed a superior junctional pathology: right L4–L5 facets degeneration secondary to overload (c). She was then submitted to L4–L5 segmental posterior fusion (d, e), with complete remission of the right lumbar pain, and the functional impairment
Conclusions
Single-level TLDR is a good alternative to fusion for chronic discogenic low back pain refractory to conservative measures. Our study confirmed satisfactory clinical results for monosegmental L4–L5 and L5–S1 disc prosthesis, with no difference between the two different levels for SF36 (P = 0.217), ODI (P = 0.527) and VAS (P = 0.269). However, replacement of the L4–L5 disc is affected by an increased risk of complication (P = 0.000). There were no prognostic factors for intraoperative blood loss or return to ambulation. Age (P = 0.034) was the only prognostic factor for operative time. Hospital stay was affected by level (P = 0.036) and pre-op VAS (P = 0.006), while complications were affected by the level (P = 0.000) and pre-op ODI (P = 0.049). Complete pre-operative assessment (in particular VAS and ODI questionnaires) is important because more debilitating patients will have more hospital stay and higher complications or complaints. Patients had to be informed that complications, possibly severe, are particularly frequent (80.6%).
Acknowledgment
Authors thank Paola Di Stefano, Ph.D., for statistical analysis.
Conflict of interest statement None of the authors has any potential conflict of interest.
References
- 1.Atlas SJ, Nardin RA. Evaluation and treatment of low back pain: an evidence-based approach to clinical care. Muscle Nerve. 2003;27:265–284. doi: 10.1002/mus.10311. [DOI] [PubMed] [Google Scholar]
- 2.Bertagnoli R, Kumar S. Indications for full prosthetic disc arthroplasty: a correlation of clinical outcome against a variety of indications. Eur Spine J. 2002;11:S131–S136. doi: 10.1007/s00586-002-0428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertagnoli R, Marnay T, Mayer HM, editors. Total disc replacement. Tuttlingen: Spine Solutions GmbH; 2003. [Google Scholar]
- 4.Bertagnoli R, Yue JJ, Shah RV, Nanieva R, Pfeiffer F, Fenk-Mayer A, Kershaw T, Husted DS. The treatment of disabling single-level lumbar discogenic low back pain with total disc arthroplasty utilizing the Prodisc prosthesis: a prospective study with 2-year minimum follow-up. Spine. 2005;30:2230–2236. doi: 10.1097/01.brs.0000182217.87660.40. [DOI] [PubMed] [Google Scholar]
- 5.Block AR, Vanharanta H, Ohnmeiss DD, Guyer RD. Discographic pain report. Influence of psychological factors. Spine. 1996;21:334–338. doi: 10.1097/00007632-199602010-00017. [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal S, McAfee PC, Guyer RD, Hochschuler SH, Geisler FH, Holt RT, Garcia R, Jr, Regan JJ, Ohnmeiss DD. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine. 2005;30:1565–1575. doi: 10.1097/01.brs.0000170587.32676.0e. [DOI] [PubMed] [Google Scholar]
- 7.Boden SD, Balderston RA, Heller JG, Hanley EN, Jr, Zigler JE. An AOA critical issue. Disc replacement: this time will we really cure low back and neck pain. J Bone Joint Surg Am. 2004;86:411–422. [PubMed] [Google Scholar]
- 8.Bono CM, Lee CK. Critical analysis of trends in fusion for degenerative disc disease over the past 20 years: influence of technique on fusion rate and clinical outcome. Spine. 2004;29:455–463. doi: 10.1097/01.BRS.0000090825.94611.28. [DOI] [PubMed] [Google Scholar]
- 9.Carragee EJ, Tanner CM, Yang B, Brito JL, Truong T. False-positive findings on lumbar discography. Reliability of subjective concordance assessment during provocative disc injection. Spine. 1999;24:2542–2547. doi: 10.1097/00007632-199912010-00017. [DOI] [PubMed] [Google Scholar]
- 10.Carregee EJ, Paragioudakis SJ, Khurana S. 2000 Volvo Award winner in clinical studies: lumbar high-intensity zone and discography in subjects without low back problems. Spine. 2000;25:2987–2992. doi: 10.1097/00007632-200012010-00005. [DOI] [PubMed] [Google Scholar]
- 11.Cauchoix J, David T. Arthrodeses lombaires: resultats apres plus de 10 ans. Rev Chir Orthop. 1985;71:263–268. [PubMed] [Google Scholar]
- 12.Cherkin DC, Sherman KJ, Deyo RA, Shekelle PG. A review of the evidence for the effectiveness, safety, and cost of acupuncture, massage therapy, and spinal manipulation for back pain. Ann Intern Med. 2003;138:898–906. doi: 10.7326/0003-4819-138-11-200306030-00011. [DOI] [PubMed] [Google Scholar]
- 13.Chung SS, Lee CS, Kang CS. Lumbar total disc replacement using ProDisc-II: a prospective study with a 2-year minimum follow-up. J Spinal Disord Tech. 2006;19:411–415. doi: 10.1097/00024720-200608000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Cinotti G, David T, Postacchini F. Results of disc prosthesis after a minimum follow-up period of 2 years. Spine. 1996;21:995–1000. doi: 10.1097/00007632-199604150-00015. [DOI] [PubMed] [Google Scholar]
- 15.David T. Lumbar disc prosthesis. Eur Spine J. 1993;1:254–259. doi: 10.1007/BF00298370. [DOI] [PubMed] [Google Scholar]
- 16.David T. Revision of a Charité artificial disc 9.5 years in vivo to a new Charité artificial disc: case report and explant analysis. Eur Spine J. 2005;14:507–511. doi: 10.1007/s00586-004-0842-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.David T. Long term results of one-level lumbar arthroplasty. Minimum 10-year follow-up of the Charité artificial disc in 106 patients. Spine. 2007;32:661–666. doi: 10.1097/01.brs.0000257554.67505.45. [DOI] [PubMed] [Google Scholar]
- 18.Delamarter RB, Fribourg DM, Kanim LE, Bae H. ProDisc artificial total lumbar disc replacement: introduction and early results from the United States clinical trial. Spine. 2003;28:S167–S175. doi: 10.1097/01.BRS.0000092220.66650.2B. [DOI] [PubMed] [Google Scholar]
- 19.Delamarter RB, Bae HW, Pradhan BB. Clinical results of ProDisc-II lumbar total disc replacement: report from the United States Clinical Trial. Orthop Clin North Am. 2005;36:301–313. doi: 10.1016/j.ocl.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Denoziere G, Ku DN. Biomechanical comparison between fusion of two vertebrae and implantation of an artificial intervertebral disc. J Biomech. 2006;39:766–775. doi: 10.1016/j.jbiomech.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 21.Dooris AP, Goel VK, Grosland NM, Gilbertson LG, Wilder DG. Load-sharing between anterior and posterior elements in a lumbar motion segment implanted with an artificial disc. Spine. 2001;26:E122–E129. doi: 10.1097/00007632-200103150-00004. [DOI] [PubMed] [Google Scholar]
- 22.Freeman BJ, Davenport J. Total disc replacement in the lumbar spine: a systematic review of the literature. Eur Spine J. 2006;15:S439–S447. doi: 10.1007/s00586-006-0186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frelinghuysen P, Huang RC, Girardi FP, Cammisa FP., Jr Lumbar total disc replacement part I: rationale, biomechanics, and implant types. Orthop Clin North Am. 2005;36:293–299. doi: 10.1016/j.ocl.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Fritzell P, Hägg O, Wessberg P, Nordwall A, Swedish Lumbar Spine Study Group 2001 Volvo Award Winner in Clinical Studies: lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine. 2001;26:2521–2532. doi: 10.1097/00007632-200112010-00002. [DOI] [PubMed] [Google Scholar]
- 25.Griffith SL, Shelokov AP, Büttner-Janz K, LeMaire J-P, Zeegers WS. A multicenter retrospective study of the clinical results of the LINK® SB Charité intervertebral prosthesis. The initial European experience. Spine. 1994;19:1842–1849. doi: 10.1097/00007632-199408150-00009. [DOI] [PubMed] [Google Scholar]
- 26.Guigui P, Lambert P, Lassale B, Deburge A. Long-term outcome at adjacent levels of lumbar arthrodesis. Rev Chir Orthop Reparatrice Appar Mot. 1997;83:685–696. [PubMed] [Google Scholar]
- 27.Hagg O, Fritzell P (2004) Re: Brox JI, Sörensen R, Friis A, et al. Randomized clinical trial of lumbar instrumented fusion and cognitive intervention and exercises in patients with chronic low back pain and disc degeneration. Spine 28:1913–1921. Spine 29:1160–1161 [DOI] [PubMed]
- 28.Hannibal M, Thomas DJ, Low J, Hsu KY, Zucherman J. ProDisc-L total disc replacement: a comparison of 1-level versus 2-level arthroplasty patients with a minimum 2-year follow-up. Spine. 2007;32:2322–2326. doi: 10.1097/BRS.0b013e3181557c06. [DOI] [PubMed] [Google Scholar]
- 29.Huang RC, Tropiano P, Marnay T, Giradi FP, Lim MR, Cammisa FPJ. Range of motion and adjacent level degeneration after lumbar total disc replacement. Spine J. 2006;6:242–247. doi: 10.1016/j.spinee.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Kumar MN, Baklanov A, Chopen D. Correlation between sagittal plane changes and adjacent segment degeneration following lumbar spine fusion. Eur Spine J. 2001;10:314–319. doi: 10.1007/s005860000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar MN, Jacquot F, Hall H. Long-term follow-up of functional outcomes and radiographic changes at adjacent levels following lumbar spine fusion for degenerative disc disease. Eur Spine J. 2001;10:309–313. doi: 10.1007/s005860000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CK. Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine. 1988;13:375–377. doi: 10.1097/00007632-198803000-00029. [DOI] [PubMed] [Google Scholar]
- 33.Lehman RA, Jr, Lenke LG. Long-segment fusion of the thoracolumbar spine in conjunction with a motion-preserving artificial disc replacement: case report and review of the literature. Spine. 2007;32:E240–E245. doi: 10.1097/01.brs.0000259211.22036.2a. [DOI] [PubMed] [Google Scholar]
- 34.Le Huec JC, Mathews H, Basso Y, Aunoble S, Hoste D, Bley B, Friesem T. Clinical results of Maverick lumbar total disc replacement: two-year prospective follow-up. Orthop Clin North Am. 2005;36:315–322. doi: 10.1016/j.ocl.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Lemaire JP, Skalli W, Lavaste F, Templier A, Mendes F, Diop A, Sauty V, Laloux E. Intervertebral disc prosthesis: results and prospects for the year 2000. Clin Orthop Relat Res. 1997;337:64–76. doi: 10.1097/00003086-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Mayer HM, Wiechert K. Microsurgical anterior approaches to the lumbar spine for interbody fusion and total disc replacement. Neurosurgery. 2002;51:159–165. doi: 10.1097/00006123-200211002-00022. [DOI] [PubMed] [Google Scholar]
- 37.McAfee PC, Fedder IL, Saiedy S, Shucosky EM, Cunningham BW. SB Charité disc replacement: report of 60 prospective randomized cases in a US center. J Spinal Disord Tech. 2003;16:424–433. doi: 10.1097/00024720-200308000-00016. [DOI] [PubMed] [Google Scholar]
- 38.McAfee PC, Geisler FH, Saiedy SS, Moore SV, Regan JJ, Guyer RD, Blumenthal SL, Fedder IL, Tortolani PJ, Cunningham B. Revisability of the Charité artificial disc replacement: analysis of 688 patients enrolled in the U.S. IDE study of the Charité artificial disc. Spine. 2006;31:1217–1226. doi: 10.1097/01.brs.0000217689.08487.a8. [DOI] [PubMed] [Google Scholar]
- 39.Mehren C, Suchomel P, Grochulla F, Barsa P, Sourkova P, Hradil J, Korge A, Mayer HM. Heterotopic ossification in total cervical artificial disc replacement. Spine. 2006;31:2802–2806. doi: 10.1097/01.brs.0000245852.70594.d5. [DOI] [PubMed] [Google Scholar]
- 40.Pai S, Sundaram LJ. Low back pain: an economic assessment in the United States. Orthop Clin North Am. 2004;35:1–5. doi: 10.1016/S0030-5898(03)00101-9. [DOI] [PubMed] [Google Scholar]
- 41.Punt IM, Visser VM, van Rhijn LW, Kurtz SM, Antonis J, Schurink GW, van Ooij A (2007) Complications and reoperations of the SB Charité lumbar disc prosthesis: experience in 75 patients. Eur Spine J doi:10.1007/s00586-007-0506-8 [DOI] [PMC free article] [PubMed]
- 42.Regan JJ. Clinical results of Charité lumbar total disc replacement. Orthop Clin North Am. 2005;36:323–340. doi: 10.1016/j.ocl.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Ross R, Mirza AH, Norris HE, Khatri M. Survival and clinical outcome of SB Charité III disc replacement for back pain. J Bone Joint Surg Br. 2007;89:785–789. doi: 10.1302/0301-620X.89B6.18806. [DOI] [PubMed] [Google Scholar]
- 44.SariAli el-H, Lemaire JP, Pascal-Mousselard H, Carrier H, Skalli W. In vivo study of the kinematics in axial rotation of the lumbar spine after total intervertebral disc replacement: long term results A 10–14 years follow-up evaluation. Eur Spine J. 2006;15:1501–1510. doi: 10.1007/s00586-005-0016-5. [DOI] [PubMed] [Google Scholar]
- 45.Siepe CJ, Mayer HM, Wiechert K, Korge A. Clinical results of total lumbar disc replacement with ProDisc-II: three-year results for different indications. Spine. 2006;31:1923–1932. doi: 10.1097/01.brs.0000228780.06569.e8. [DOI] [PubMed] [Google Scholar]
- 46.Siepe CJ, Mayer HM, Heinz-Leisenheimer M, Korge A. Total lumbar disc replacement. Different results for different levels. Spine. 2007;32:782–790. doi: 10.1097/01.brs.0000259071.64027.04. [DOI] [PubMed] [Google Scholar]
- 47.Siepe CJ, Korge A, Grochulla F, Mehren C, Mayer HM (2007) Analysis of post-operative pain patterns following total lumbar disc replacement: results from fluoroscopically guided spine infiltrations. Eur Spine J doi:10.1007/s00586-007-0519-3 [DOI] [PMC free article] [PubMed]
- 48.Tortolani PJ, Cunningham BW, Eng M, McAfee PC, Holsapple GA, Adams KA. Prevalence of heterotopic ossification following total disc replacement. A prospective, randomized study of two hundred and seventy-six patients. J Bone Joint Surg Am. 2007;89:82–88. doi: 10.2106/JBJS.F.00432. [DOI] [PubMed] [Google Scholar]
- 49.Tropiano P, Huang RC, Girardi FP, Cammisa FP, Jr, Marnay T. Lumbar total disc replacement. Seven to eleven-year follow-up. J Bone Joint Surg Am. 2005;87:490–496. doi: 10.2106/JBJS.C.01345. [DOI] [PubMed] [Google Scholar]
- 50.Turner JA, Ersek M, Herron L, Haselkorn J, Kent D, Ciol MA, Deyo R. Patient outcomes after lumbar spinal fusions. JAMA. 1992;268:907–911. doi: 10.1001/jama.268.7.907. [DOI] [PubMed] [Google Scholar]
- 51.Gijn J, Mast RC. Chronic low back pain: the failure of organic medicine. Ned Tijdschr Geneeskd. 2007;151:1553–1556. [PubMed] [Google Scholar]
- 52.Ooij A, Schurink GW, Oner FC, Verbout AJ. Findings in 67 patients with recurrent or persistent symptoms after implantation of a disc prosthesis for low back pain. Ned Tijdschr Geneeskd. 2007;151:1577–1584. [PubMed] [Google Scholar]
- 53.Zeegers WS, Bohnen LM, Laaper M, Verhaegen MJ. Artificial disc replacement with the modular type SB Charité III: 2-year results in 50 prospectively studied patients. Eur Spine J. 1999;8:210–217. doi: 10.1007/s005860050160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zigler JE, Burd TA, Vialle EN, Sachs BL, Rashbaum RF, Ohnmeiss DD. Lumbar spine arthroplasty: early results using the ProDisc II: a prospective randomized trial of arthroplasty versus fusion. J Spinal Disord Tech. 2003;16:352–361. doi: 10.1097/00024720-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 55.Zigler JE. Lumbar spine arthroplasty using the ProDisc II. Spine J. 2004;4:S260–S267. doi: 10.1016/j.spinee.2004.07.018. [DOI] [PubMed] [Google Scholar]