Abstract
Camptocormia is an abnormal posture with marked flexion of thoracolumbar spine that abates in the recumbent position. Camptocormia has been described in various neurological (Parkinsonism), muscular (myopathy), psychogenic or orthopedic disorders. There are several hypotheses that can explain this impaired posture but they are usually related to the concomitant pathologies. We report the first case of a patient with confirmed myotonic dystrophy addressed to our medical center for impaired posture who underwent extensive medical exams and explorations because of a myotonic hand. Axial weakness and muscle atrophy, validated by CT-scan imaging, are discussed independent of the concomitant pathology (Parkinson, myopathy).
Keywords: Posture, Camptocormia, Myotonic dystrophy, Muscular atrophy
Introduction
Camptocormia is an abnormal posture with marked flexion of thoracolumbar spine that abates in the recumbent position. Several reports suggest that this bent posture may result from Parkinson disease [2], dystonia [9], focal myopathy [20], or other neuromuscular disorders [5, 6, 16, 24]. We report an original case of confirmed myotonic dystrophy type 1 with axial predominance and associated painful camptocormia and discuss possible mechanisms underlying this forward-flexed posture.
Case report
We report the case of a 54-year-old woman with a history of progressive low-back pain for the last 5 years and progressive bent spine these past 2 years; simultaneously she has been complaining of leg stiffness and gait difficulties specially when climbing up stairs.
Her medical history is eventless, except for one miscarriage at 3.5 months of pregnancy.
At clinical examination, the trunk was flexed forward with active extension up to 60° and complete passive extension without head drop (Fig. 1). However, trunk extension was strongly limited by pain. There was no sign of evolutive radiculopathy. Hip motion was not limited, but the hip was spontaneously flexed in a standing position. However, there were signs of myotonic dystrophy: when shaking hands with our patient we clearly noticed a grip myotonia, unveiled also by percussion myotonia, bilateral asymmetrical ptosis, facial diplegia, discrete dysarthria, and frontal baldness. Muscle testing found bilateral weakness in proximal legs and arms (4/5 on MRS scale), with a positive Gowers’ sign. Amyotrophy was not found. Examination with a slit lamp found a cataract in the left eye. There was no evidence of cognitive impairment or abnormalities in sensory, cerebellar or autonomic nervous system functions.
Fig. 1.
Photograph of the patient in standing position. Patient consent was obtained for publication of this figure
Family history (mother, maternal aunt, sister and daughter) revealed similar clinical features (frontal baldness, myotonia, cataracts), suggesting a dominant inheritance.
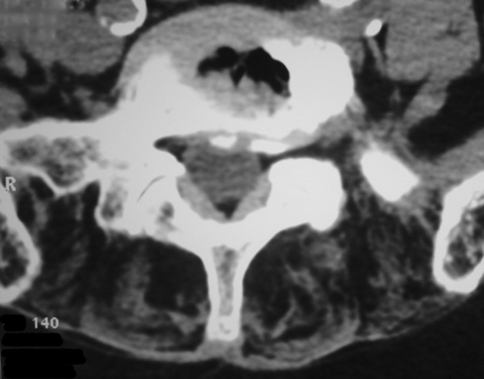
The electromyogram revealed typical myotonic patterns, in several muscles. CT scan and magnetic resonance imaging (MRI) (Fig. 2) of the entire spine revealed severe disk degeneration and massive fatty degeneration of axial thoracic and lumbar muscles. Ultrasound imaging and 24-h electrocardiograms revealed ventricular and supraventricular extrasystolia without heart block. During lung investigation we found a decrease in vital lung capacity (86%).
Fig. 2.
Disk degeneration and degenerative facet hypertrophy associated with severe paraspinal amyotrophy
The diagnosis of myotonic dystrophy type 1 (DM1) was confirmed by the presence of ~800 CTG triplet expansion on chromosome 19q13.3.
Discussion
In DM1, autosomal dominant inherited muscle disorder, weakness and amyotrophia are usually delayed and predominant in distal extremities. From this point of view, our patient is strikingly different. DM1 diagnosis was based on severe axial weakness and camptocormia at the age of 54. Camptocormia associated with weakness of proximal or axial muscles and electrical myotonia has rarely been described before [15, 22], and was never validated by genetic testing.
In the literature, several clinical cases [7, 15–18, 22] consider camptocormia as a consequence of axial myopathy. Previously described CT-scan findings of massive fat infiltration in the paraspinal muscles at lumbar and thoracic level [16, 18, 19] with muscle hypodensity [16] were found in our patient. Histological analysis revealed muscle atrophy, fat infiltration or mitochondrial changes which are nonspecific and probably age-related [16]; only the increase of fibrous tissue was specific to camptocormia and not related to age or lumbar osteoarticular lesions [16]. In the same population of forward-flexed patients, Delisle et al. did not find any histological changes in deltoid biopsies [16]. These observations of isolated paraspinal muscle disease support the idea that camptocormia is probably related to a primary involvement of the paravertebral muscles [16]. However, Seror et al. [21] described a case of complete fatty degeneration of the lumbar erector spinae without camptocormia and the extent to which this muscle degeneration with axial weakness is really responsible for the camptocormia is debated in the literature [8, 12, 15, 16, 20, 24].
Although back pain and/or degenerative facet syndrome are not explicitly described as potential risk factors for bent posture, they are frequently reported in clinical observations of camptocormia [3, 4, 13]. In 1904, Gowers developed the idea that post-traumatic back pain evolves differently over time due to a lingering impaired posture as opposed to classic back pain. In the first description of camptocormia, Souques and Rosanoff-Saloff [23] suspected two mechanisms leading to camptocormia: (1) the forward-flexed posture being a protective attitude caused by pain, (2) this persistent posture being related to pre-existing conditions (neuropathy, psychogenic…). More recently, cases of axial dystonia [11] or camptocormia [10] have been related to pain caused by disc herniation. The possible role of trunk disease seems an interesting lead worth exploring. In primary muscle dystrophy, muscle weakness is associated with lordosis in order to increase spinal stability when the articular facet joints are locked [14]. This adaptation occurs in young people with intact facet joints but probably not in older individuals with degenerative lesions. Moreover, the pelvic parameters are probably important to consider as in the case of bent posture described as a compensatory mechanism to reduce excessive anterior pelvic tilt [1]. It is reasonable to think that biomechanical adaptations of the trunk are certainly involved in this posture impairment. However Bloch et al. [3] did not find a relationship between camptocormia and a medical history of disc herniation or back surgery, but unveiled a rapidly progressing camptocormia in patients with low-back pain probably linked to mechanical adaptations of the lumbopelvic area.
Our review of the literature leads us to hypothesize that Camptocormia is probably the consequences of two or more factors: axial weakness caused by muscle disorders (myopathy or other), and lower back disorders or pelvic restrictions which cannot compensate for muscle loss.
Conclusion
DM1 has variable and atypical clinical manifestations. Clinicians should think of a DM1 diagnosis when faced with camptocormia, axial weakness and proximal muscle wasting. This original description illustrates the fundamental role of paraspinal muscular disease in this progressive forced posture and points out the possible role of trunk disease in generating camptocormia.
Conflict of interest statement
None of the authors has any potential conflict of interest.
Contributor Information
Arnaud Dupeyron, Phone: +33-466683481, FAX: +33-466683960, Email: arnaud.dupeyron@chu-nimes.fr.
Nina Stober, Email: nina.stober@web.de.
Anthony Gelis, Email: anthony.gelis@chu-nimes.fr.
Giovani Castelnovo, Email: giovani.castelnovo@chu-nimes.fr.
Pierre Labauge, Email: pierre.labauge@chu-nimes.fr.
Jacques Pélissier, Phone: +33-466683481, FAX: +33-46668396, Email: jacques.pelissier@chu-nimes.fr.
References
- 1.Abdulhadi HM, Kerrigan DC. Camptocormia: a biomechanical analysis. A case report. Am J Phys Med Rehabil. 1996;75:310–313. doi: 10.1097/00002060-199607000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Azher SN, Jankovic J. Camptocormia: pathogenesis, classification, and response to therapy. Neurology. 2005;65:355–359. doi: 10.1212/01.wnl.0000171857.09079.9f. [DOI] [PubMed] [Google Scholar]
- 3.Bloch F, Houeto JL, Tezenas du Montcel S, et al. Parkinson’s disease with camptocormia. J Neurol Neurosurg Psychiatry. 2006;77:1223–1228. doi: 10.1136/jnnp.2006.087908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouzgarou E, Dupeyron A, Castelnovo G, Boudousq V, Collombier L, Labauge P, Pelissier J. Camptocormia disclosing Parkinson’s disease. Ann Readapt Med Phys. 2007;50:55–59. doi: 10.1016/j.annrmp.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 5.Charpentier P, Dauphin A, Stojkovic T, et al. Maladie de Parkinson, camptocormie et myosite focale paraspinale. Rev Neurol (Paris) 2005;161:459–463. doi: 10.1016/s0035-3787(05)85077-5. [DOI] [PubMed] [Google Scholar]
- 6.Seze MP, Creuze A, Seze M, Mazaux JM. An orthosis and physiotherapy programme for camptocormia: a prospective case study. J Rehabil Med. 2008;40:761–765. doi: 10.2340/16501977-0252. [DOI] [PubMed] [Google Scholar]
- 7.Delcey V, Hachulla E, Michon-Pasturel U, et al. Camptocormie: un signe de myopathie axiale. A propos de 7 observations. Rev Med Interne. 2002;23:144–154. doi: 10.1016/S0248-8663(01)00530-6. [DOI] [PubMed] [Google Scholar]
- 8.Diederich NJ, Goebel HH, Dooms G, Bumb A, Huber F, Kompoliti K, Meinck HM. Camptocormia associated with focal myositis in multiple-system atrophy. Mov Disord. 2006;21:390–394. doi: 10.1002/mds.20686. [DOI] [PubMed] [Google Scholar]
- 9.Djaldetti R, Mosberg-Galili R, Sroka H, Merims D, Melamed E. Camptocormia (bent spine) in patients with Parkinson’s disease—characterization and possible pathogenesis of an unusual phenomenon. Mov Disord. 1999;14:443–447. doi: 10.1002/1531-8257(199905)14:3<443::AID-MDS1009>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 10.Duman I, Baklaci K, Tan AK, Kalyon TA. Unusual case of camptocormia triggered by lumbar-disc herniation. Clin Rheumatol. 2007;27:525–527. doi: 10.1007/s10067-007-0763-2. [DOI] [PubMed] [Google Scholar]
- 11.Ghika J, Nater B, Henderson J, Bogousslavsky J, Regli F. Delayed segmental axial dystonia of the trunk on standing after lumbar disk operation. J Neurol Sci. 1997;152:193–197. doi: 10.1016/S0022-510X(97)00186-X. [DOI] [PubMed] [Google Scholar]
- 12.Gomez-Puerta JA, Peris P, Grau JM, Martinez MA, Guanabens N. Camptocormia as a clinical manifestation of mitochondrial myopathy. Clin Rheumatol. 2007;26:1017–1019. doi: 10.1007/s10067-006-0259-5. [DOI] [PubMed] [Google Scholar]
- 13.Haig AJ, Tong HC, Kendall R. The bent spine syndrome: myopathy + biomechanics = symptoms. Spine J. 2006;6:190–194. doi: 10.1016/j.spinee.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Kerr TP, Lin JP, Gresty MA, Morley T, Robb SA. Spinal stability is improved by inducing a lumbar lordosis in boys with Duchenne Muscular Dystrophy: a pilot study. Gait Posture. 2008;28:108–112. doi: 10.1016/j.gaitpost.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Kocaaga Z, Bal S, Turan Y, Gurgan A, Esmeli F. Camptocormia and dropped head syndrome as a clinic picture of myotonic myopathy. Jt Bone Spine. 2008;75:730–733. doi: 10.1016/j.jbspin.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Laroche M, Delisle MB, Aziza R, Lagarrigue J, Mazieres B. Is camptocormia a primary muscular disease? Spine. 1995;20:1011–1016. doi: 10.1097/00007632-199505000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Linazasoro G, Suarez JA. Myopathic camptocormia in a patient with levodopa unresponsive parkinsonism. Neurologia. 2002;17:162–164. [PubMed] [Google Scholar]
- 18.Mahjneh I, Marconi G, Paetau A, Saarinen A, Salmi T, Somer H. Axial myopathy—an unrecognised entity. J Neurol. 2002;249:730–734. doi: 10.1007/s00415-002-0701-9. [DOI] [PubMed] [Google Scholar]
- 19.Oerlemans WG, Visser M. Dropped head syndrome and bent spine syndrome: two separate clinical entities or different manifestations of axial myopathy? J Neurol Neurosurg Psychiatry. 1998;65:258–259. doi: 10.1136/jnnp.65.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schabitz WR, Glatz K, Schuhan C, et al. Severe forward flexion of the trunk in Parkinson’s disease: focal myopathy of the paraspinal muscles mimicking camptocormia. Mov Disord. 2003;18:408–414. doi: 10.1002/mds.10385. [DOI] [PubMed] [Google Scholar]
- 21.Seror P, Krahn M, Laforet P, Leturcq F, Maisonobe T. Complete fatty degeneration of lumbar erector spinae muscles caused by a primary dysferlinopathy. Muscle Nerve. 2008;37:410–414. doi: 10.1002/mus.20910. [DOI] [PubMed] [Google Scholar]
- 22.Serratrice J, Weiller PJ, Pouget J, Serratrice G. Une cause méconnue de camptocormie: la myopathie myotonique proximale. Presse Med. 2000;29:1121–1123. [PubMed] [Google Scholar]
- 23.Souques A, Rosanoff-Saloff I. La camptocormie; incurvation du tronc, consecutive aux traumatismes du dos et des lombes; considerations morphologiques. Rev Neurol (Paris) 1915;28:937–939. [Google Scholar]
- 24.Wunderlich S, Csoti I, Reiners K, Gunthner-Lengsfeld T, Schneider C, Becker G, Naumann M. Camptocormia in Parkinson’s disease mimicked by focal myositis of the paraspinal muscles. Mov Disord. 2002;17:598–600. doi: 10.1002/mds.10110. [DOI] [PubMed] [Google Scholar]