Abstract
The study is a retrospective case series. The objective is to review the results after off-label recombinant human BMP-2 (rhBMP-2) use in the pediatric spine after previously failed spinal fusion. Non-union in the pediatric spine is a challenging condition associated with increased morbidity due to instability, neurological impairment or multiple revision surgeries. BMP has been used with good results in the adult spine; however, information on its use in the pediatric population is still lacking. rhBMP-2 was used at our institution at revision posterior spinal surgery in three patients. Solid spinal fusion was achieved in all three cases despite underlying bone dysplasia (Hurler’s disease), instability or bony substance loss. No adverse reactions due to rhBMP-2 use were observed. rhBMP-2 should be considered as potential option to achieve spinal fusion in children with compromised bone healing due to congenital, local or systemic conditions.
Keywords: rhBMP-2, Immature spine, Bone dysplasia
Introduction
Non-union is an important issue in the pediatric spine surgery especially in the cervical spine or in the presence of underlying bone dysplasia. Instability may lead to neurological impairment and multiple surgeries may be needed in order to achieve fusion. BMPs have been proven as potent osteoinductors and are currently used in adult lumbar spine surgery [1]. However, their efficiency and the modalities of use in the pediatric population are not yet clearly determined.
Case reports
We used recombinant human bone morphogenic protein-2 (rhBMP-2, dibotermine alfa 12 mg, 1.5 mg/mL, Medtronic, Memphis, TN) with absorbable collagen sponge (ACS) carrier matrix, off-label in three pediatric patients at revision posterior spinal surgery. The indication was non-union after failed initial fusion in two patients and enhancement of fusion in one patient with significant local bone deficiency and poor compliance.
Parental consent in accordance with the institutional requirements was obtained before surgery in all three cases.
Demographic data for the patients are summarized in Table 1.
Table 1.
Summary of patient data
| Case | Age (years)/gender | Diagnosis | Neurological status pre-/post-op | Initial surgery | Revision surgery | Follow-up (months) |
|---|---|---|---|---|---|---|
| 1 | 6/male | Hurler’s disease | Lower extremity weakness, sleep apnea, myelopathy/improvement | Laminectomy posterior in situ fusion occiput-C4 ICBG, halo-jacket | Repeat posterior ICBG C0–C4, rhBMP-2, halo-jacket | 18 |
| 2 | 2/male | Congenital cervical kyphosis | Lower extremity weakness/improvement, able to ambulate | Posterior in situ fusion C3–T4, ICBG, halo-jacket | Repeat posterior ICBG C3–T4, rhBMP-2, Minerva | 11 |
| 3 | 10/male | Thoraco-lumbar kyphosis HSAN 4 | Normal | Posterior instrumentation T11–L4, ICBG anterior decompression + cage T12–L3, | Repeat posterior ICBG, rhBMP-2, TLSO brace | 13 |
ICBG Iliac crest bone graft, HSAN hereditary sensory autonomic neuropathy
Case 1
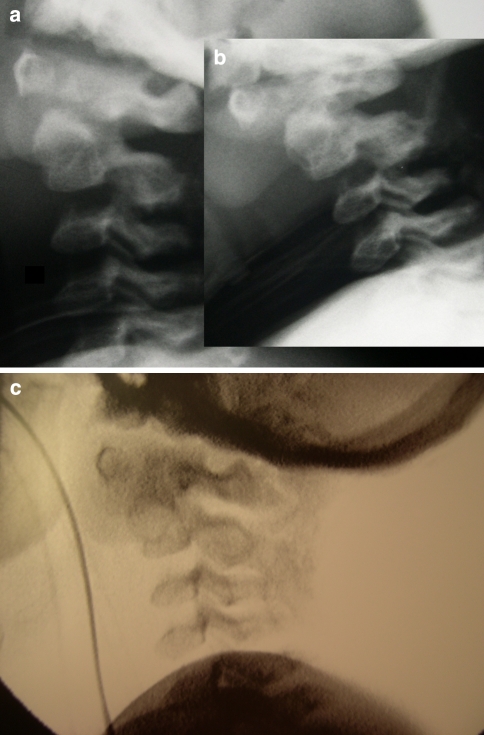
A 6-year-old patient with mucopolysaccharidosis type 1 (Hurler’s disease) presented with progressive muscle weakness in the lower extremities and sleep apnea. The MRI showed spinal cord compression in the cranio-cervical junction with myelopathy caused by C1–C2 instability and soft tissue apposition. At initial surgery, widening of the foramen magnum and C1–C2 laminectomy were performed through a posterior approach. The procedure was completed by autologous iliac crest bone grafting from the occiput to C4. The patient was immobilized in a halo-jacket. Four months after the procedure local kyphosis and anterior displacement of C1 on C2 were noticed on the lateral X-ray. Unstable non-union was confirmed with dynamic fluoroscopy (Fig. 1a). At revision surgery the fibrous pseudarthrosis tissue between C1 and C2 was removed, the posterior elements were decorticated and 12 mg rhBMP-2 was applied. The position of C1 on C2 was corrected. Halo-jacket immobilization was continued. Twelve weeks after revision surgery there was radiological evidence for solid fusion. Stability was confirmed through dynamic fluoroscopy on halo removal (Fig. 1b). At latest follow-up 18 months after revision surgery muscle weakness and sleep apnea symptoms improved. Fusion mass remained solid.
Fig. 1.
a, b Six-year-old male with Hurlers disease and cranio-cervical stenosis with myelopathy. The dynamic fluoroscopy 4 months after posterior C1–C2 decompression and in situ fusion with AICBG showed atlanto-axial instability. c Solid fusion 12 weeks after revision surgery including posterior rhBMP-2 application and repeat AICBG, evidenced on dynamic fluoroscopy. AICBG autologous iliac crest bone grafting
Case 2
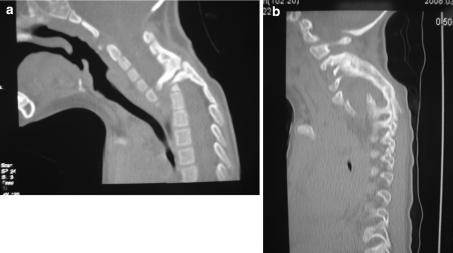
A 2-year-old male patient presented with Klippel–Feil deformity. Muscle hypotony in both lower extremities was present. MRI and CT showed fusion of C2–4, complete aplasia of the C6–7, hypoplasia of the Th1, 2, 3 bodies and fusion of the posterior elements Th2–4. There was local cervical kyphosis and anterior displacement of the upper cervical spine of 13 mm with spinal canal encroachment. There were no MRI signs of myelopathy. At initial surgery, a halo vest was applied and posterior “in situ” arthrodesis with autologous iliac crest bone graft between the fusion complex C2–4 and Th2–4 was performed. A total of eight halo pins were used and tightened with a torque of 0.25 N/m. Ten months after initial surgery a non-union was present as evidenced by thin-slice CT (Fig. 2a). Pin loosening and liquor discharge was noticed from one anterior halo pin. At revision surgery the pseudarthrotic fibrous tissue was resected. Repeat posterior autologous iliac crest bone grafting was performed and 12 mg of rhBMP-2 were applied. A Minerva cast was used for immobilization. Twelve weeks after revision solid bone fusion was confirmed with thin-slice CT (Fig. 2b). An anterior decompression and autologous strut rib grafting were subsequently performed. At latest follow-up the neurological status improved and the patient was able to ambulate.
Fig. 2.
a Frank non-union 10 months after posterior in situ fusion and AICBG in a 2-year-old patient with congenital cervical instability. b Solid fusion 12 weeks after non-union resection, posterior rhBMP-2 application and AICBG. AICBG autologous iliac crest bone grafting
Case 3
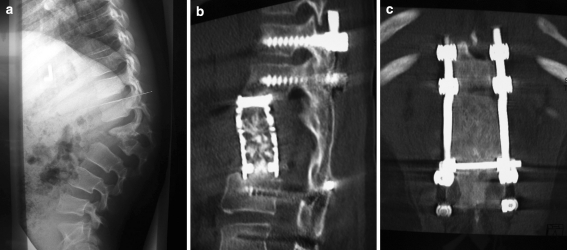
A 10-year-old patient with hereditary sensory autonomic neuropathy type IV (HSAN IV; autoaggression, anhydrosis insensitivity to pain) presented with thoraco-lumbar junctional kyphosis of 65° and a partial destruction of L1 and L2 vertebral bodies (Fig. 3a). The MRI showed spinal canal encroachment without neurological compromise. Posterior decompression of L1–2 and instrumentation from T11 to L4 was performed, followed by autologous iliac crest bone grafting. Anterior decompression of L1–2 through a ventral approach was performed in the same surgical setting. The anatomic alterations were similar to those seen in “Charcot” joints. The resected vertebral bodies of L1–2 were replaced with an anterior cage filled with autologous rib bone graft. The sagittal balance was restored. A well-moulded Risser cast was applied. This was replaced with a modified rigid thoraco-lumbar spinal brace at 6 weeks after surgery. Because of poor compliance with the brace, the underlying autoaggression and the significant bone substance deficiency concerns were raised about possible mechanical failure. After evaluation of the risk-to-benefit relationship, decision was taken to accelerate fusion by means of osteoinduction. Twelve weeks after initial surgery repeat autologous iliac crest bone grafting and application of 12 mg rhBMP-2 through posterior approach was performed. Solid fusion was confirmed with thin-slice CT 8 months after revision surgery (Fig. 3b, c).
Fig. 3.
a Thoraco-lumbar kyphosis with L1–2 partial destruction in a 10-year-old male with HSAN type IV, treated with posterior and anterior decompression L1-2. b, c Solid fusion eight months after posterior rhBMP-2 application. HSAN hereditary sensory autonomic neuropathy
Discussion
Spinal fusion in young children remains challenging. This is particularly valid for the cervical spine especially in association with underlying bone dysplasia or local substance deficiency. Diminutive osseous and ligamentous structures and anatomical variations associated with syndromic cranio-vertebral abnormalities frequently complicate the approaches and limit the use of internal fixation. Furthermore, compliance with external immobilization may be problematic.
Some authors reported satisfactory results after cervical spine fusion for instability in immature patients.
Miyoshi et al. [2] found satisfactory posterior fusion in all seven patients who sustained decompression for cranio-cervical stenosis and atlanto-axial subluxation in spondyloepiphyseal dysplasia.
Sakaura et al. [3] reported on successful posterior or antero-posterior cervical spine fusion in three children with Larsen’s syndrome.
However, pseudarthrosis with persisting instability and neurological compromise is not infrequent.
Ain et al. [4] reported on 25 patients treated for cervical instability in bone dysplasia and found 8% of pseudarthrosis and 20% of surgery-related complications.
Smith et al. [5] reviewed the outcomes in 17 patients after posterior fusion for atlanto-axial instability and found 2 with pseudarthrosis.
Rodgers et al. [6] reported 2 pseudarthroses in 23 patients treated with posterior fusion for cranio-cervical instability.
Recombinant human bone morphogenic protein 2 (rhBMP-2) has proved to be efficient in promoting de novo bone formation and is currently FDA approved as a bone graft substitute in lumbar spine surgery in the adult [7, 8]. A body of extensive clinical experience with rhBMP-2 has failed to demonstrate evidence of adverse reactions such as uncontrolled ectopic bone formation, systemic toxicity, immune responses and carcinogenicity and the BMP-2 application in the lumbar spine surgery in the adult has been evaluated as safe [9–11].
Extensive data on rhBMP-2 use in immature patients are lacking. Its effectiveness and modalities of use in terms of dosage and safety in the pediatric spine and especially in children with compromised bone healing due to local or systemic conditions are still not determined.
Lu et al. [12] and Oluigbo et al. [13] reported on rhBMP-2 application in a 4-month-old patient with Down syndrome and in a 2-year-old patient with posttraumatic C1–C2 instability, respectively. Successful fusion without side effects was obtained in both cases. However, there are some considerations when using BMP in children.
BMP-2 plays an important role in the development of the nervous system. Although exogenous rhBMP-2 has rapid clearance and has almost no systemic distribution [10], potential local side effects to the immature brain and the spinal cord due to exogenous BMP-2 are still unclear [14]. Thus, it is generally recommended, that direct contact with nervous tissue or exposed dura should be avoided [10].
Another issue is the appropriate dosage of bone morphogenic proteins. Specific guidelines for rhBMP-2 dosage are still lacking because of the limited availability of randomized controlled clinical trials and its diverse use in many spinal applications with diverse carriers. rhBMP-2 is currently used in anterior spinal surgery in the adult in a dosage varying from 0.6 to 8.4 mg per level [15, 16]. Better results have been reported with higher concentration (1.5 vs. 0.75 mg/mL) [17] and dosages of up to 40 mg per single level were reported in posterior intertransverse fusion in the adult [18]. On the other hand, higher rate of complications such as hematoma and swelling were reported with increased rhBMP-2 dosage (2.1 vs. 06 mg) in anterior adult cervical spine surgery [19].
We used rhBMP-2 off-label as a salvage procedure in revision posterior spinal surgery in three pediatric patients. The bony margins of the spinal canal were intact and the dura was not exposed, thus the spinal cord was not endangered from direct contact with BMP or from compression due to swelling. Since it was revision multilevel posterior surgery, identification of every single segment was difficult and it was not possible to apply a precise dose of BMP to a designated level. Recommendations for age, weight and level dependant dosage in children are currently not available, thus every patient received a total dosage of 12 mg rhBMP-2 in a concentration of 1.5 mg/mL on an empirical basis.
Solid fusion was achieved in all cases despite the presence of metabolic bone disease (in one patient with Hurler’s disease), initial instability (cases 1 and 2) or significant bone deficiency (case 3). No adverse reactions related to BMP application such as swelling, ectopic ossification, spinal canal or neuroforaminal encroachment were observed.
The study was limited by the fact that there was no control group, which at revision surgery received autologous bone grafting only and it was not possible to prove how bone fusion without BMP would be.
Conclusions
Recombinant human BMP-2 should be considered as viable option to achieve spinal fusion in children with compromised bone healing due to congenital, local or systemic conditions. With regard to the concerns of theoretical side effects and the limited experience, the general use of BMP-2 in immature patients is not recommended. Larger series and long-term data are needed to define the modalities for clinical use in terms of indications and safety. Guidelines for age, weight and level dependent dosage of rhBMP-2 in the pediatric population are yet to be determined.
The results suggest a role for the use of rhBMP-2 to promote fusion in selected cases in the pediatric spine surgery.
Acknowledgments
Conflict of interest statement None of the authors has any potential conflict of interest.
References
- 1.Carlisle E, Fischgrund JS. Bone morphogenetic proteins for spinal fusion. Spine J. 2005;5(6 Suppl):240S–249S. doi: 10.1016/j.spinee.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Miyoshi K, Nakamura K, Haga N, Mikami Y. Surgical treatment for atlantoaxial subluxation with myelopathy in spondyloepiphyseal dysplasia congenita. Spine. 2004;29(21):E488–E491. doi: 10.1097/01.brs.0000143621.37688.f3. [DOI] [PubMed] [Google Scholar]
- 3.Sakaura H, Matsuoka T, Iwasaki M, Yonenobu K, Yoshikawa H. Surgical treatment of cervical kyphosis in Larsen syndrome: report of 3 cases and review of the literature. Spine. 2007;32(1):E39–E44. doi: 10.1097/01.brs.0000250103.88392.8e. [DOI] [PubMed] [Google Scholar]
- 4.Ain MC, Chaichana KL, Schkrohowsky JG. Retrospective study of cervical arthrodesis in patients with various types of skeletal dysplasia. Spine. 2006;31(6):E169–E174. doi: 10.1097/01.brs.0000202758.61848.61. [DOI] [PubMed] [Google Scholar]
- 5.Smith MD, Phillips WA, Hensinger RN. Fusion of the upper cervical spine in children and adolescents. An analysis of 17 patients. Spine. 1991;16(7):695–701. doi: 10.1097/00007632-199107000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Rodgers WB, Coran DL, Emans JB, Hresko MT, Hall JE. Occipitocervical fusions in children. Retrospective analysis and technical considerations. Clin Orthop Relat Res. 1999;364:125–133. doi: 10.1097/00003086-199907000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Boden SD, Zdeblick TA, Sandhu HS, et al. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25:376–381. doi: 10.1097/00007632-200002010-00020. [DOI] [PubMed] [Google Scholar]
- 8.McKay B, Sandhu HS. Use of recombinant Human BMP-2 in spinal fusion applications. Spine. 2002;27(16S):66–85. doi: 10.1097/00007632-200208151-00014. [DOI] [PubMed] [Google Scholar]
- 9.Burkus JK, Gornet MF, Dickman CA, Zdeblick TA. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15(5):337–349. doi: 10.1097/00024720-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Poynton AR, Lane JM (2002) Safety profile for the clinical use of BMP’s in the spine. Spine 27(16S):S40–S48 [DOI] [PubMed]
- 11.Sandhu HS, Anderson DG, Anderson GBJ et al (2002) Summary statement: safety of bone morphogenic proteins for spine fusion. Spine 27(16S):39
- 12.Lu DC, Sun PP (2007) Bone morphogenetic protein for salvage fusion in an infant with Down syndrome and craniovertebral instability. Case report. J Neurosurg 106(6 Suppl):480–483 [DOI] [PubMed]
- 13.Oluigbo CO, Solanki GA. Use of recombinant human bone morphogenetic protein-2 to enhance posterior cervical spine fusion at 2 years of age: technical note. Pediatr Neurosurg. 2008;44(5):393–396. doi: 10.1159/000149907. [DOI] [PubMed] [Google Scholar]
- 14.Smith DM, Cooper GM, Mooney MP, Marra KG, Losee JE. Bone morphogenetic protein 2 therapy for craniofacial surgery. J Craniofac Surg. 2008;19(5):1244–1259. doi: 10.1097/SCS.0b013e3181843312. [DOI] [PubMed] [Google Scholar]
- 15.Baskin DS, Ryan P, Sonntag V, Westmark R, Widmayer MA (2003) A prospective, randomized, controlled cervical fusion study using recombinant human bone morphogenetic protein-2 with the CORNERSTONE-SR allograft ring and the ATLANTIS anterior cervical plate. Spine (Phila Pa 1976) 28(12):1219–1224 (discussion 1225) [DOI] [PubMed]
- 16.Burkus JK, Dorchak JD, Sanders DL. Radiographic assessment of interbody fusion using recombinant human bone morphogenetic protein type 2. Spine. 2003;28(4):372–377. doi: 10.1097/00007632-200302150-00012. [DOI] [PubMed] [Google Scholar]
- 17.Govender S, Csimma C, Genant HK et al (2002) Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Jt Surg Am 84-A(12):2123–2134 [DOI] [PubMed]
- 18.Boden SD, Kang J, Sandhu H, Heller JG, 23 Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine. 2002;27:2662–2673. doi: 10.1097/00007632-200212010-00005. [DOI] [PubMed] [Google Scholar]
- 19.Shields LB, Raque GH, Glassman SD, Campbell M, Vitaz T, Harpring J, Shields CB (2006) Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine 31(5):542–547 [DOI] [PubMed]