Abstract
Fat embolism after long bone and pelvic fractures as well as orthopedic interventions is a well-documented phenomenon, but it is highly unusual after isolated vertebral fractures. We report a case of fatal fat embolism in a 78-year-old man after an isolated vertebral compression fracture with no related orthopedic intervention. A high index of suspicion is necessary for early diagnosis and successfully treating this unusual complication.
Keywords: Fat embolism, Vertebral compression fracture
Introduction
The relationship between fracture of long bones or orthopedic surgery and fat embolism is well documented. However, there is only one case reported in the literature of fatal fat embolism after isolated vertebral fracture, and it was related to a vertebral wedge fracture following a fall [1]. No cases of fat embolism following isolated non-wedge vertebral compression fracture have been reported. We are reporting a case of fatal diffuse bilateral fat and marrow microemboli found in post-mortem examination in an elderly patient with a compression fracture of the axial skeleton.
Case report
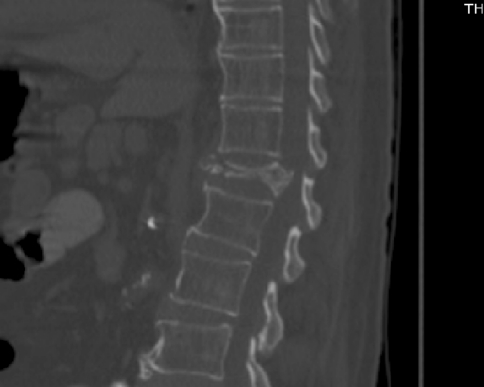
The deceased was a 78-year-old man with long-standing history of asthma that was admitted to a skilled nursing facility 2 weeks prior to admission after an isolated T12 spinal compression fracture. Computer tomography scan done at that time demonstrated a marked compression fracture of T12 with a vertebral plana and mild degenerative disease elsewhere (see Figs. 1, 2). He was found unresponsive by the staff of the facility, and emergency medical services were called, finding the patient to be in asystole. ACLS maneuvers were started in the field including intubation and transcutaneous pacemaker. Upon arrival to our medical center, the patient was unstable, hypercapnic with a pCO2 of 77 mmHg and hypoxic with a pO2 of 58 mmHg. Further workup revealed no evidence of acute myocardial infarction. Approximately 30 min after his arrival, he was found to be in pulseless electrical activity. ACLS maneuvers were resumed and continued for 30 min without response. After discussion with the family about the poor outcome, the decision was taken to discontinue cardiopulmonary resuscitation and he was disconnected from life support.
Fig. 1.
CT scan without contrast showing isolated vertebral T12 compression fracture (sagittal view)
Fig. 2.
CT scan without contrast showing isolated vertebral T12 compression fracture (axial view)
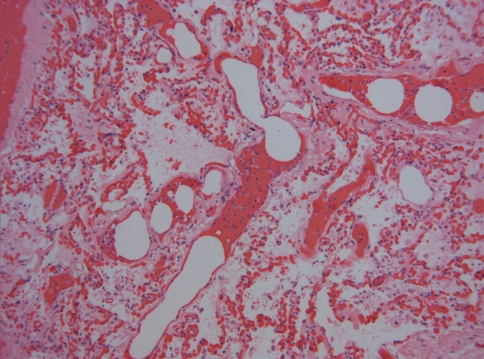
Post-mortem examination of the patient revealed gross and microscopic bilateral pulmonary congestion with bilateral diffuse fat and marrow microemboli (see Figs. 3, 4). An isolated T12 vertebral compression fracture was present. No rib fractures were evidenced. Given the sudden onset of the symptoms and the pulmonary findings described, it was concluded that the cause of death in this patient was diffuse bilateral pulmonary fat emboli most likely originating from site of vertebral T12 fracture.
Fig. 3.
Fat droplets (small arrow) and bone marrow elements (large arrow) plugging a vein in the pulmonary circulation (hematoxylin and eosin stain at ×100)
Fig. 4.
Fat droplets plugging multiple capillaries in pulmonary circulation (hematoxylin and eosin stain at ×100)
Discussion
Embolization of fat and marrow contents is a known complication of long bone and pelvic fractures, as well as certain orthopedic interventions (such as intramedullary implant insertions, total joint arthroplasty and vertebroplasty) [2, 3]. Recently, severe soft tissue trauma and spine surgery have been cited as causes for fat embolism syndrome [4, 5]. Takahashi et al. [4] described the incidence of fat embolism syndrome during spine surgery with and without instrumentation. He suggested an 80% incidence of embolic phenomena associated with pedicle instrumentation (i.e. pedicle screw).
However, up to 5% of cases of fat embolism have been related to atraumatic events such as bone marrow transplantation, sickle cell disease, diabetes mellitus and high-dose corticosteroids [2]. It is well known that pulmonary fat embolism regardless of their origin can be fatal. In traumatic events, it is characterized by the release of fat droplets and bone marrow contents into the systemic circulation after increased intramedullary pressure and its subsequent deposition in the pulmonary and cerebral circulation. It is estimated that only about 1–2% of these patients develop respiratory or neurologic symptoms [2].
Fat embolism syndrome in most often thought to be subclinical. If clinically evident, it classically comprises an asymptomatic period followed by pulmonary and neurologic manifestations combined with petechial hemorrhages. Severe pulmonary vascular obstruction with subsequent right-sided heart failure and death might ensue in a small group of patients [1, 2]. In order to make the diagnosis of fat embolism syndrome, a patient must present with a pO2 of less than 60 mmHg, a pCO2 of more than 55 mmHg and intense dyspnea with a respiratory rate over 35 respirations/min in the setting of trauma or orthopedic intervention [6].
Although the pathophysiology of this entity is not entirely clear, two different theories have been described. The first theory (mechanical theory) is simply related to the release of fat droplets from the intramedullary fat at the site of trauma into the circulation and the obstruction of capillaries by these fat droplets in end organs such as the lungs, kidneys and brain. Fat globules measuring less than 10 μm may transverse the pulmonary vasculature and embolize the systemic circulation. Larger globules measuring up to 40 μm in diameter may reach the systemic circulation in patients with probe patent foramen ovale, if the resulting pulmonary hypertension is severe enough to create a pressure gradient between the right and left atria [2]. The second theory (biochemical theory) is related to the release of free fatty acids and catecholamines after trauma or inflammatory conditions such as acute pancreatitis. Elevated C-reactive protein in these conditions, and specifically the phosphoryl choline binding site of this molecule, causes calcium-dependent intravascular agglutination of chylomicrons and fat macroglobules, forming larger fat droplets that have the potential to embolize [2, 7].
It is important to clarify that bone marrow content is frequently found in the microscopic examination of the lungs in patients that have received cardiopulmonary resuscitation and have associated rib fractures secondary to these maneuvers. However, the extent of these findings in our patient together with the presence of fat droplets plugging the vessels without associated bone marrow (see Fig. 4) and the absence of rib fractures makes this possibility unlikely in this case.
Since the most effective measure against fat embolism syndrome after long bone fractures is prophylaxis by stabilization and most treatment protocols are just supportive, a high degree of suspicion for this diagnosis must exist in any patient with bone trauma or instrumental orthopedic surgery and the symptoms described above. This is especially true in cases of vertebral compression fractures, where stabilization is not always indicated. All long bones should be stabilized as soon as the patient is medically optimized.
Conclusion
The diagnosis of fat embolism syndrome has historically been associated to patients with a history of long bone fracture or orthopedic surgery and sudden onset pulmonary or neurologic symptoms. Our case demonstrates that, although uncommon, this entity might present in patients with trauma to the axial skeleton with fatal consequences. It is, therefore, important not to limit this diagnosis to classic cases only, but to keep it in mind as a real and serious complication of vertebral fractures.
Acknowledgments
Conflict of interest statement None of the authors has any potential conflict of interest.
Contributor Information
Ricardo R. Lastra, Phone: +1-267-2419844, FAX: +1-215-8297564, Email: Ricardo.lastra@uphs.upenn.edu
Vilas Saldanha, Email: vilassaldanha@gmail.com.
Manjula Balasubramanian, Email: balasubm@einstein.edu.
References
- 1.Lewis D, Jones R, Carpenter C, et al. Fatal fat embolism following an isolated vertebral fracture. Acta Orthop Belg. 2007;73:137–139. [PubMed] [Google Scholar]
- 2.Glover P, Worthley LIG. Fat embolism. Crit Care Resusc. 1999;1:276–584. [PubMed] [Google Scholar]
- 3.Syed MI, Jan S, Patel NA, et al. Fatal fat embolism after vertebroplasty: identification of the high-risk patient. Am J Neuroradiol. 2006;27:343–345. [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi S, Kitagawa H, Ishii T. Intraoperative pulmonary embolism during spinal instrumentation surgery. J Bone Joint Surg Br. 2003;85:90–94. doi: 10.1302/0301-620X.85B1.13172. [DOI] [PubMed] [Google Scholar]
- 5.Lindeque BG, Schoeman HS, Dommisse GF, et al. Fat embolism and the fat embolism syndrome: a double-blind therapeutic study. J Bone Joint Surg Br. 1987;69:128–131. doi: 10.1302/0301-620X.69B1.3818718. [DOI] [PubMed] [Google Scholar]
- 6.Weisz G, Steiner E. The cause of death in fat embolism. Chest. 1971;59:511–516. doi: 10.1378/chest.59.5.511. [DOI] [PubMed] [Google Scholar]
- 7.Hulman G. Fat macroglobule formation from chylomicrons and non-traumatic fat embolism. Clin Chim Acta. 1988;2:173–178. doi: 10.1016/0009-8981(88)90139-8. [DOI] [PubMed] [Google Scholar]