Abstract
SWISSspine is a so-called pragmatic trial for assessment of safety and efficiency of total disc arthroplasty (TDA). It follows the new health technology assessment (HTA) principle of “coverage with evidence development”. It is the first mandatory HTA registry of its kind in the history of Swiss orthopaedic surgery. Its goal is the generation of evidence for a decision by the Swiss federal office of health about reimbursement of the concerned technologies and treatments by the basic health insurance of Switzerland. During the time between March 2005 and 2008, 427 interventions with implantation of 497 lumbar total disc arthroplasties have been documented. Data was collected in a prospective, observational multicenter mode. The preliminary timeframe for the registry was 3 years and has already been extended. Data collection happens pre- and perioperatively, at the 3 months and 1-year follow-up and annually thereafter. Surgery, implant and follow-up case report forms are administered by spinal surgeons. Comorbidity questionnaires, NASS and EQ-5D forms are completed by the patients. Significant and clinically relevant reduction of low back pain VAS (70.3–29.4 points preop to 1-year postop, p < 0.0001) leg pain VAS (55.5–19.1 points preop to 1-year postop, p < 0.001), improvement of quality of life (EQ-5D, 0.32–0.73 points preop to 1-year postop, p < 0.001) and reduction of pain killer consumption was revealed at the 1-year follow-up. There were 14 (3.9%) complications and 7 (2.0%) revisions within the same hospitalization reported for monosegmental TDA; there were 6 (8.6%) complications and 8 (11.4%) revisions for bisegmental surgery. There were 35 patients (9.8%) with complications during followup in monosegmental and 9 (12.9%) in bisegmental surgery and 11 (3.1%) revisions with new hospitalization in monosegmental and 1 (1.4%) in bisegmental surgery. Regression analysis suggested a preoperative VAS “threshold value” of about 44 points for increased likelihood of a minimum clinically relevant back pain improvement. In a short-term perspective, lumbar TDA appears as a relatively safe and efficient procedure concerning pain reduction and improvement of quality of life. Nevertheless, no prediction about the long-term goals of TDA can be made yet. The SWISSspine registry proofs to be an excellent tool for collection of observational data in a nationwide framework whereby advantages and deficits of its design must be considered. It can act as a model for similar projects in other health-care domains.
Keywords: Total disc arthroplasty, Health technology assessment, Registry, Outcome, SWISSspine
Introduction
For the greatest part, spinal surgery deals with problems of degenerative spinal diseases with pain and/or radiculopathy. When pharmacological and functional treatment strategies fail, surgical interventions remain as ultima ratio in many cases. The fusion of affected lumbar segments in surgical candidates with chronic low back pain has been the standard surgical procedure for almost 50 years and remains the gold standard until today [6]. There are, however, well-known problems with the technique [7].
Total disc arthroplasty (TDA) is, for what we know today, a promising complement to fusion, although, choosing the right patient for the right procedure probably is the key to success. The goals of TDA must nowadays certainly be listed as pain reduction over time, functional improvement, durability of the implant, safety at the time of implantation, safety during the life time of the implant, and safety in case of revision. The protection from adjacent segment disease (ASD) seems to be a more theoretical and biomechanical concept since recent clinical and radiographic long-term studies in patients after spinal fusion conclude that ASD is more likely to be determined by individual factors than by the fusion itself [12]. Interestingly, whilst mid-term results are promising, long-term results over 10 years are less favourable [13]. Therefore, TDA is one of the most controversially discussed motion preserving concepts of today. Various types of prosthesis are currently available but rising costs and their containment are increasingly important issues in all modern health-care systems. Orthopaedic surgery is a discipline under special focus. Many orthopaedic interventions are expensive and do not directly save lives. Nevertheless, in cases like, e.g. joint arthroplasty their impact on patients’ quality of life, regained mobility and independence are undisputed. Spinal surgery is the fastest growing orthopaedic subspecialty. Scientific evidence, however, is lacking for many interventions and innovations in this sector. Short-term results are supported by studies with a high level of evidence. Mid- and long-term evidence, however, is still poorly available [1]. Randomized controlled trials for new implants and indications and rigorous long-term post-market surveillance for adverse events is called for [5]. The Swiss Federal Office of Health decided to conduct a nationwide observational study according to the principle of “coverage with evidence development” before taking the final decision about reimbursement of TDA by the basic health insurance [11].
This article reports the methodology and implementation of the SWISSspine registry and the early results of the cases with lumbar TDA. The analysis is based on the specifications that the Swiss Federal Office of Public Health (SFOPH) demanded for the health technology assessment (HTA) reports of the registry.
Materials and methods
Initiated in March 2005, the SWISSspine registry is ongoing until today. The preliminary timeframe for the registry was 3 years and has meanwhile been extended for at least another year. Given the new restrictions for market release of medical innovations in Switzerland, the implant industry had approached the decision-making political bodies and the Swiss Society for Spinal Surgery (SGS) with the aim to form a task force for implementing a medical device registry for collecting data and providing the evidence of the safety and what Archie Cochrane described as “efficiency” of TDA, i.e. its performance in the clinical setting [3]. In other parts of the world this term is sometimes also referred to as “efficacy”. The Institute for Evaluative Research in Orthopedic Surgery at the University of Bern (IEFO), an international leader in the field of registry implementation was mandated to serve as technology provider and organizer of the SWISSspine registry. In a working group consisting of stakeholders of industry, the Swiss Spine Society (SGS) and the IEFO, the different tasks and duties were assigned. An expert committee of the society generated the basic hypotheses and worked up the medical content accordingly. Industry partners provided funding according to market share and support for device-related questions, and the IEFO consulted and finally implemented all questionnaires in an online and paper-based version for optical mark reader scanning on its scientific documentation portal www.memdoc.org. Hereby, all content needed to be available in three of the four official Swiss languages: German, French, and Italian. While each participant can make use of its own data, the data pool is owned by the SGS that can delegate data analysis to individual surgeons or to a neutral academic data clearing centre like the IEFO.
A unique manoeuvre in the Swiss medical profession policy making was the formation of an SGS expert group that decided about certification of spine surgeons. In order to obtain it, a formal application with proof of qualification and infrastructure had to be submitted by all Swiss spine surgeons who intended to perform TDA. Along with the approval went the written consent to participate in the registry. The certification can be withdrawn if data analysis shows an unacceptably high number of complications or if the proportion of documented interventions is too low. For the latter, the industry partners deliver their sales figures to the SGS registry group and the IEFO delivers the numbers of documented interventions.
Content and follow-up schemes
Documentation forms and outcome instruments that are used to achieve the documentation standards for the SWISSspine registry are listed as follows:
Primary intervention forms for TDA (surgeon administered)
Implant form (for TDA barcode stickers)
Follow-up form for TDA (surgeon administered)
Euroqol-5D (patient assessment)
NASS (patient assessment)
Comorbidity questionnaire (patient assessment)
Two patient consent forms (one remains at the treatment centre, one at IEFO)
One annotation form about the registry and its purpose (also signed by patient)
At the time of surgery, primary intervention and implant forms are completed by the surgeon. Informed written consent about participation in the registry has to be given by the patient as well as a completed set of EQ-5D, NASS and comorbidity questionnaire. During follow-ups, scheduled at 3 months, 1 year and annually thereafter, follow-up questionnaires are completed by the surgeon. Patients are again asked to complete the EQ-5D and NASS questionnaires. Validated translations of all patient-based instruments needed to be available for all the three languages. With one exception only, all surgeons outsourced data entry to the technical staff at IEFO. Paper questionnaires are sent to the institute by mail where data is punched or questionnaires are scanned. Surgeons can autonomously view, print or analyze their data via the online interface after the data has been entered into the MEMdoc database.
Statistical analysis
Wilcoxon rank-sum test was used for comparisons between baseline and follow-up examinations of continuous variables such as pain VAS. When comparing proportions, the χ2 test was used.
In 2003, Hägg et al. [10] reported the minimum clinically relevant improvement in low back pain to be 18–19 points on VAS. Based on this finding, we defined the desired outcomes for the 1-year follow-up as:
achievement of back pain relief ≥18 points on VAS
achievement of leg pain relief ≥18 points on VAS
For the EQ-5D an improvement ≥0.25 points was arbitrarily set as desired outcome.
Multiple logistic regression models were built in order to identify possible covariates with a significant influence on the three outcomes.
Prosthesis model, patient sex, patient age, preoperative pain levels, preoperative EQ-5D score, surgical volume of centre of intervention, number of levels of intervention, the level of intervention and pharmacologically treated depression were included as covariates in the initial models.
For the surgical volume, clinics were categorized into four classes: <10 operations, 10–20 operations, 21–30 operations, and >30 operations, respectively. The patient age was categorized into four age groups according to the quartiles of the underlying age distribution: 18.5–37.0 years, 37.1–41.8 years, 41.9–47.2 years and 47.3–64.7 years, respectively. Using backward elimination of covariates with a p > 0.05, a list of significant variables was received. For the analysis of courses of pain alleviation, quality of life improvement and for calculation of threshold values linear regression models were built. The necessary preconditions of linear relationship between the two respective variables, constant error variance around the regression line and normally distributed data were sufficiently given. This level of significance was used throughout the complete study. The stability of the multiple logistic regression models was assessed using Hosmer and Lemeshow Goodness-of-Fit test. All statistical analyses were conducted using SAS 9.1 (SAS Institute Inc, Cary, NC).
Sample characteristics
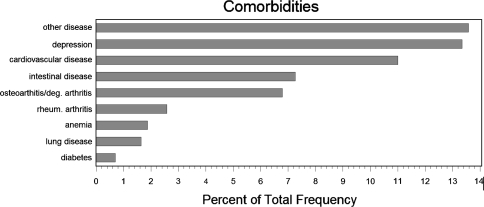
In March 2008, 36 months after registry launch, there were 427 patients with 497 lumbar TDAs recorded in the database, which corresponds with an about 80% documentation rate when compared with the sales figures. There were 241 females (56.4%) and 186 males (43.6%). Mean ages were 41 years (range 18.5–64.7 years) and 43.4 years (range 19.6–64 years), respectively, at the time of surgery. 19.6% of operations were bisegmental. Distribution of comorbidities revealed that about 13.4% of patients had a depression and 10.5% regularly took medication for it (Fig. 1). In total, 896 lumbar TDA follow-up records from 11 to 915 days (about 2.5 years) postoperative were completed and stored in the database. Also 1,253 (360 preoperative, 893 follow-up) EQ-5D forms and 1,242 NASS (365 preop, 877 followup) forms for evaluation of general and disease-specific quality of life were available for evaluation. For the regression analysis, we used the last available followup in postoperative year one; that way 365 records with a mean follow-up time of 6 months could be analyzed. Also, 342 comorbidity questionnaires were assessed. Between 30 and 40 patients had no followup information recorded on either the NASS and/or EQ-5D and/or surgeon-based follow-up forms. These patients were not any different to the patients with follow-up information with respect to their demographics, preoperative pain levels or quality of life. However, 17.1% of patients without followups had indicated a pharmacologically managed depression before surgery compared with 10.4% in the patient group with followups.
Fig. 1.
Preoperative comorbidities of the study sample
Results
Pain relief
Low back pain
One of the main advantages of lumbar TDA is the fast and efficacious pain reduction. Measurement of pain was conducted with VAS scores on the patient administered NASS questionnaire.
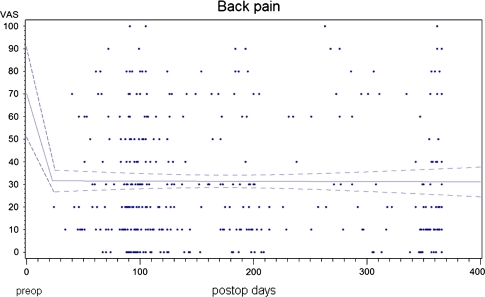
The mean preoperative back pain was 70.3 points. At the 3 months follow-up (88.2% follow-up rate), it was reduced to a mean of 30.5 points and to 29.4 points 1-year postoperative (69% followup rate) (p < 0.001 for both follow-up intervals) (Fig. 2). An exploratory analysis of the available 2-year follow-ups in 83 patients revealed a mean back pain score of 27.1 points. However, some patients had worsening of back pain or did not achieve a minimum clinically relevant back pain alleviation (Fig. 3).
Fig. 2.
Course of pre- to postoperative back pain alleviation over the first postoperative year. The linear regression shows the average pain over the postoperative time. The dotted lines are 95% confidence intervals
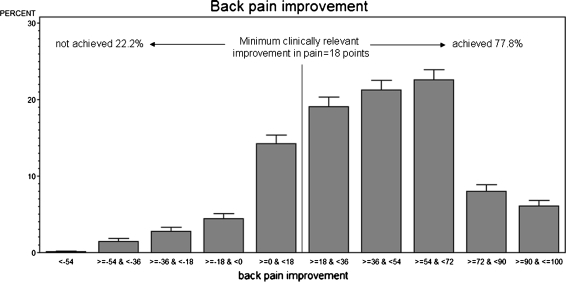
Fig. 3.
Distribution of patients with back pain improvement/worsening
Leg pain
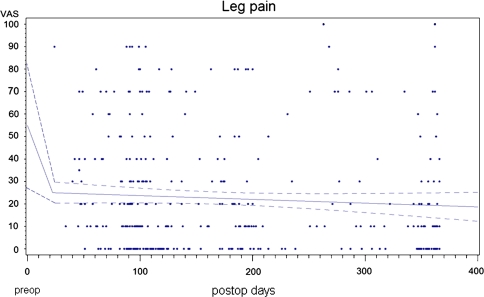
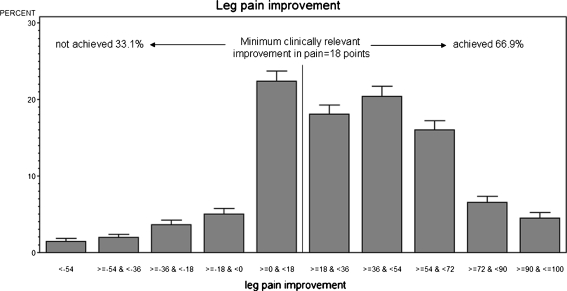
The mean preoperative leg pain was 55.5 points. A reduction to 23.3 points after 3 months followed by a decrease to 19.9 points at the 1-year postoperative follow-up (p < 0.001 for both followup intervals) was observed (Fig. 4). The same follow-up rates as for back pain apply. An exploratory analysis of the available 2-year follow-ups in 83 patients revealed a mean leg pain score of 17.4 points. As with back pain, some patients had worsening of leg pain or did not achieve a minimum clinically relevant leg pain alleviation (Fig. 5).
Fig. 4.
Course of pre- to postoperative leg pain alleviation over the first postoperative year. The linear regression shows the average pain over the postoperative time. The dotted lines are 95% confidence intervals
Fig. 5.
Distribution of patients with leg pain improvement/worsening
Reduction of pain medication
The consumption of pain killers decreased significantly. The amount of patients who did not need any pain medication increased from 2.5% preoperative to 65.6% at the 3 months follow-up and to 61.8% at 1 year postoperative (p < 0.001 for both follow-up intervals). The proportion of patients consuming NSAIDs decreased from 69.6% before the intervention to 28.8% at 3 months postoperative and 29.4% at the 1-year follow-up (p < 0.001 for both follow-up intervals). Morphine and morphine derivates were needed by 28% of patients before surgery. This number was reduced to 5.6% at the 3 months follow-up and showed a slight increase to 8.8% at 12 months postoperative (p < 0.001 for both follow-up intervals).
Quality of life improvement
Improved quality of life was achieved by significant pain reduction and consequently decreased pain killer consumption. Values of the EQ-5D range from 1 (best possible QoL) to −0.6 (QoL worse than death). On preoperative examination, the mean EQ-5D score was 0.32 points. It improved to 0.72 points at 3 months (88.5% follow-up rate) and to 0.73 points at the 1-year follow-up (72.3% follow-up rate) (p < 0.0001 for both intervals). 27.1% of patients indicated a preoperative QoL below zero. This percentage was reduced to 6.4% at the 1-year follow-up (p < 0.0001). An exploratory analysis of the available 2-year follow-ups in 83 patients revealed a mean EQ-5D score of 0.81 points.
Factors influencing pain relief and QoL improvement
Assessing factors with a possible significant influence on pain alleviation or quality of life improvement in the first year after surgery, we included the above described covariates into the regression models.
Of all analyzed covariates, preoperative back pain (p < 0.001) and leg pain (p = 0.048) had an influence on the postoperative back pain relief. The higher the preoperative back pain values were, the higher was the chance for a clinically relevant back pain alleviation; the lower the preoperative pain levels the lower was the chance for a relative pain improvement. The odds ratio for preoperative back pain was 1.53 (95% CI 1.3–1.79) per additional 10 points on VAS. This means that each increase of preoperative back pain by 10 points implies an increase of the chance for a clinically relevant back pain alleviation by 53%. The opposite influence was seen with preoperative leg pain. The odds ratio was 0.89 (95% CI 0.78–0.99), which means that a decrease of preoperative leg pain by 10 points implies an increase of the chance for a clinically relevant back pain alleviation by 12%. The stability of the model was confirmed by the Hosmer and Lemeshow Goodness-of-Fit test (p > 0.05).
Preoperative leg pain (p < 0.001) had an influence on the postoperative leg pain relief. The odds ratio for preoperative leg pain was 3.54 (95% CI 2.23–5.62) per 10 points on VAS. This means that an increase of preoperative leg pain by 10 points implies a 3.5 times higher chance for a clinically relevant leg pain alleviation. The model was also stable (p > 0.05).
The preoperative EQ-5D score (p < 0.001) and a pharmacologically treated depression (p = 0.042) had a significant influence on the postoperative quality of life improvement. The odds ratio for the preoperative EQ-5D score was 0.95 (95% CI 0.94–0.96) per 0.1 point preoperative score increase. This means that a decrease of the preoperative EQ-5D score by 0.1 points implies an increase of the chance for a clinically relevant score improvement by 5%. The odds ratio for pharmacologically treated depression was 3.3 (95% CI 1.14–9.33). Hence, the patients without depression had a 3.3 times higher chance for a clinically relevant EQ-5D score improvement. The stability of the model was confirmed (p > 0.05). A sensitivity analysis with an EQ-5D score improvement of either 0.15 points or of 0.35 points as desired outcome did not reveal other significant covariates.
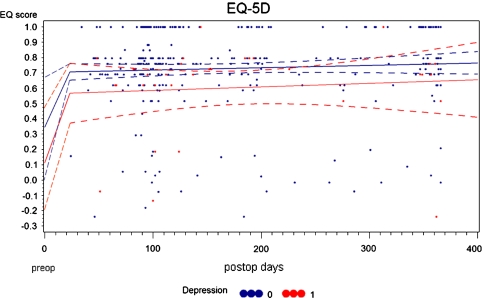
Stratifying patients with a pharmacologically treated depression and those without, the following differences in pre- and postoperative QoL were revealed: non-depressed patients had a mean preop Qol of 0.34 points versus 0.12 points in the depressed group. At 3 months postop, these differences were 0.73 (nd) versus 0.53 (depr) and at 1-year postop they were 0.77 (nd) versus 0.6 (depr) (Fig. 6).
Fig. 6.
Differences in pre- to postoperative QoL in non-depressed and depressed patients. The linear regression shows the average scores for both samples over the postoperative time. The dotted lines are 95% confidence intervals
There was no difference in pain relief between the different prostheses models, the various treatment centres, the levels of intervention or in single level versus bisegmental interventions in the registry. Also, patient age or sex did not influence pain relief or QoL improvement.
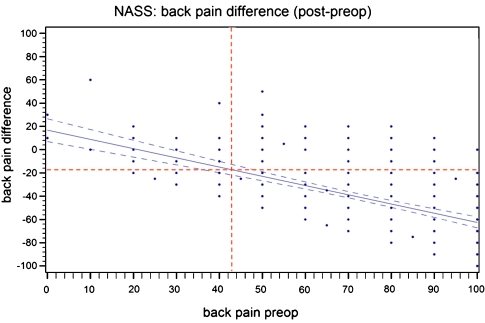
We examined the allocation of patients in terms of the minimum clinically relevant back pain improvement of 18 points versus preoperative back pain and performed a linear regression analysis. Evaluating the interception for the MCRPI with the regression-graph, a “threshold-value” of 43.8 points for preoperative back pain was revealed (Fig. 7). Analyzing the percentage of patients below the preoperative pain threshold, 10.1% were below the back pain threshold; 14.3% were above the back pain threshold but did not achieve the MCRPI.
Fig. 7.
Threshold values for clinically relevant back pain relief of 18 points on VAS
Complications and revisions
Complications were assessed by the surgeons themselves and marked as vascular lesion, ureteral lesion, vertebral body fracture, sintering of prosthesis into vertebral body and dura lesion, as predefined on the surgery questionnaire, or marked as “other complication” with written specification. On the follow-up forms, they were described as (new) complications—none, implant related, access/surgery related. Specification of complications: perfusion problems after vessel injury, sympathectomy effect, new radiculopathy level 1, new radiculopathy level 2, retrograde ejaculation, other. Specification of perfusion problems: disappeared since … (date), still present but improved, still present but unchanged. Same for sympathectomy effect, retrograde ejaculation, radiculopathy level 1, radiculopathy level 2.
Intraoperative complications/revisions
Analyzing 357 monolevel interventions, intraoperative complications occurred 14 times (3.9%) during one level surgery. Seven revisions (2%) within the same hospital stay were recorded.
The 70 bisegmental lumbar interventions revealed six intraoperative complications (8.6%) and eight revisions (11.4%) within the same hospital stay (Table 1).
Table 1.
Intraoperative complications and revisions during hospital stay
| Intraoperative complications | Monosegmental TDA (N = 357) | Bisegmental TDA (N = 70) |
|---|---|---|
| Blood vessel injury | 10 | 3 |
| Ureter injury | 1 | – |
| Vertebral body fracture | 1 | 1 |
| Sintering of implant | 1 | 1 |
| Dura lesion | 1 | 1 |
| Total | 14 (3.9%) | 6 (8.6%) |
| Revisions during hospitalization | 7 (2%) | 8 (11.4%) |
Complications/revisions during FU
There were 44 patients with 47 recorded complications during followup: 35 patients (9.8%) after a monosegmental operation and 9 patients (12.9%) with 12 complications after bisegmental surgery (Table 2).
Table 2.
Complications recorded during followup
| Type of complication | Overall frequency (percentage) | Monosegmental TDA (N = 357) | Bisegmental TDA (N = 70) |
|---|---|---|---|
| Delayed wound healing/wound infection | 3 | 3 | – |
| Incision hernia/abdominal hernia | 2 | 2 | – |
| Cutaneal nerve irritation | 1 | 1 | – |
| Abdominal pain | 2 | 1 | 1 |
| Testicular pain | 1 | 1 | – |
| Recurring pain | 2 | 2 | – |
| Sympathectomy effects | 8 | 6 | 2 |
| Retrograde ejaculation | 2 | 1 | 1 |
| Urethral problem | 1 | 1 | – |
| Radiculopathy | 7 | 6 | 1 |
| Drop foot | 2 | – | 2 |
| Psychogenic foot paralysis | 1 | 1 | – |
| OA facet joint | 1 | – | 1 |
| Residual disc sequester | 1 | 1 | – |
| Fx endplate | 2 | 2 | – |
| Dislocation | 3 | 1 | 2 |
| Spondylolisthesis | 1 | 1 | – |
| Foot pain intermittent | 1 | – | 1 |
| Functional foot paralysis | 1 | 1 | – |
| Unspecified | 5 | 4 | 1 |
| Total | 47 (11%) | 35 (9.8%) | 12 (17%) |
| Revision after discharge | 12 (2.8%) | 11 (3.1%) | 1 (1.4%) |
Additionally, there were 11 revisions with a second hospitalization for monolevel surgeries (3.1%) as well as one revision for a bilevel surgery (1.4%). The indications for monosegmental revisions were: one implant removal, three ventral spondylodesis, two cases of lumbar pain, one dorsal spondylodesis, one spondylodesis without specification, one decompression, one wound revision, and one vertebral body endplate fracture. The indication for the revision of the single patient with bilevel operation stayed unspecified; however, DIAM stabilization of the lower adjacent level was performed.
Length of stay
The mean length of stay for patients with a monosegmental cervical intervention was 7.8 days; that of patients with bisegmental TDA was one half day longer, i.e. 8.3 days.
Discussion
Since its implementation in the year 2005, the SWISSspine registry can meanwhile be considered a successful endeavour. It is the first nationwide project of its kind. The collaboration of all participants—surgeons, the Swiss Spinal Society, the Institute for Evaluative Research in Orthopedic Surgery and the implant suppliers—helped making the creation of a national HTA registry under the mandate of the Swiss Federal Office of Public Health a positive experience. A relatively high number of observational datasets could be acquired in a short period of time. The main intentions of this study, proof of safety and efficiency of TDA, were fulfilled from the short-term outcome perspective and a foundation for long-term data collection and evaluation was built.
Significant reduction of back and leg pain at the 1-year follow-up were shown which increased the quality of life after TDA to a great extent. Applying the observations of the Swedish lumbar spine study group about a minimum necessary VAS improvement of 18 points for a clinically relevant outcome we could show that patients should not have a preoperative back pain “threshold value” below approximately 40 points. For the treatment of back and/or leg pain, TDA seems an efficient and relatively safe option so far. Since its initiation few major complications and a small number of revisions were recorded in the SWISSspine database. In view of the difficulty and the dangers inherent to anterior revision, in particular at the L4/L5 level, future results must yet be expected.
The search for predictors of a good outcome revealed four covariates with a significant influence on pain alleviation and quality of life improvement. Preoperative back and leg pain as well as the EQ-5D score correlated with their own postoperative outcome. A pharmacologically treated depression showed a negative influence on postoperative quality of life improvement. Analyzing other covariates such as the number of treated levels, surgical volume of the participating centres, types of prostheses or patient sex and age had no influence on postoperative outcome.
With an observational study like the SWISSspine registry, an evidence level of no more than three can be reached. The lack of a control group is one of the most compromising factors, but the increased documentation burden for an established and routinely reimbursed comparator procedure like, e.g. an anterior stand-alone fusion, was considered unacceptable on a nationwide level. Therefore, our findings cannot be considered as conclusive results but must be interpreted on the background of the problems that are inherent in this prospective multicenter case-series. Although good results were shown so far, it is not possible to predict the mid and long-term outcomes of TDA. A 1-year follow-up period is very short for the treatment of a vertebral segment with a mechanical implant that may suffer from wear, corrosion or loosening, while the segment itself may become ankylosed, undergo late infection or further degradation of the considered level (facet joints) or the adjacent levels. Of course, reoccurrence of pain for unknown reasons may also happen after some time. No conclusion on none of these aspects can be drawn after such a short follow-up.
When the registry was initiated, surgeons not only had to cope with their surgical learning curve and the task of reorganising established workflows, also an extensive documentation had to be integrated into the day-to-day routines. The necessary changes to the content of the documentation forms during the first year may be an additional reason for an only slowly improving discrepancy between sales and documentation figures. Due to this fact, the quality of the recorded information has probably been affected, especially during year one of the registry life cycle. The relatively low number of documented complications and revisions could be a possible consequence. Undoubtedly, the lack of a pilot phase must be seen as a major deficit during project initiation. Time concerns from the point of view of the industry partners should not be a legitimation for leaving out a testing and pre-evaluation phase. A pilot also serves as a learning programme for training all participants before the actual registry start. During a pilot, study content and data collection instruments can be tested under routine conditions in the various clinical settings.
On the other hand, a possible advantage with a register like this is the potential for external validity. All Swiss surgeons performing TDA and therefore patients from all over Switzerland were involved in the current investigation. Thus, there is a low probability for selection bias in the results. Further, the collaboration between implant industry and documentation centre for comparing sales versus documentation figures for each individual participant is a sophisticated and cost-effective monitoring mechanism for a nationwide observational study. The figures were compared by a neutral legislative clearing office and only communicated to the SGS steering group for issues of confidentiality and competition laws. Countries that aim at adopting such a study design need to consider and respect the essential value of a strong collaboration between all stakeholders for guaranteeing a similar amount of transparency. In the case of SWISSspine a fully operational registry had to be accomplished in a minimum amount of time, now showing the merit of all involved. An expert institution in study set-up and implementation together with a readily available technical infrastructure are necessary to turn such a venture into a success.
The significant and clinically relevant pain relief we revealed is also reported by other authors investigating the success of lumbar TDA [2, 14]. We have not found comparable analyses about preoperative threshold values for minimum clinical relevant pain alleviation in the literature. After the regression analysis had excluded other influential factors on pain alleviation but the preoperative pain levels itself, we aimed at investigating the relationship between the preoperative pain levels and the relative postoperative improvement. The use of the minimum necessary 18-point improvement for a clinically relevant result, as suggested by Hägg et al. [10], revealed a threshold value of about 40 points on the VAS. This result can not be interpreted as a golden rule or exclusion criterion for future treatments but it may serve as rule of thumb for assessing the likelihood of clinical relevant pain alleviation for TDA patients. The thresholds could be considered as a minimum requirement and the further patients are above the preoperative VAS of 40 points, the more likely is a clinically relevant pain alleviation. The regression showed that of all evaluated factors preoperative back pain and preoperative leg pain had an influence on back pain relief, and preoperative leg pain had an influence on leg pain relief; furthermore, the preoperative EQ-5D score and pharmacologically treated depression had an influence on postoperative general quality of life. Other factors not assessed in the SWISSspine registry may have an influence as well. The potential impact of depressive diseases on chronic low back pain and the insufficient investigation of such comorbidities by many orthopaedic surgeons is described by some authors [4, 8]. Correlations of these factors with the treatment outcome have to be further investigated, but surgeons should have an increased awareness for their impact on the treatment results. Contradicting our findings, results from the Swedish lumbar spine study group showed that a depressive disorder did not have a negative influence on outcome after lumbar fusion surgery for chronic low back pain [9]. The authors did, however, state that these results might be based on a rather successful selection of patients since the prevalence of major depression was lower than in other patient samples. Hence, the negative influence we found in the current analysis may disappear in future assessments of SWISSspine data since surgeons may become more critical and careful in selecting candidates for lumbar TDA.
Published rates for complications range from 6 to 39% [4] and the complication rates in SWISSspine are relatively low. A possible explanation is the framework of SWISSspine where only surgeons with a proven expertise in spinal surgery received certification for the intervention. In addition, there may have been a benefit from advancements in instrumentation and access as well as surgical techniques that the first movers of the technology did not have yet. Finally, it is possible that the surgeon-based reporting of complications underrepresents the complication rates from the patients’ point of view. In the Swedish “SweSpine” registry as well as in the European Spine Tango registry the patient questionnaires ask some predefined questions about postoperative use of antibiotics, return to hospital or new spine surgeries indicating eventual complications from the index operation. Moreover, patients can indicate in written any other event that they considered a complication. Such a form of complication reporting may lead to higher complication rates than the current ones but also improve the external validity of results. Therefore, future revision and complication rates need to be awaited.
As opposed to cervical disc prostheses, mid and long-term outcomes of the lumbar implants have been under observation for many more years. Nevertheless long-term outcomes are still rarely reported [13]. The few available investigations are rather critical in their conclusions as opposed to the reported more promising mid and short-term results [4]. Further investigations are undoubtly needed.
The results and knowledge obtained by the SWISSspine registry show that a nationwide registry with a relatively large documentation burden can be implemented in a short period of time and conducted if the consequences of action are of sufficient importance for all stakeholders. Surgeons and industry joined forces in order to produce evidence for the safety, and especially for the efficiency of the intervention and patients agreed to read and complete consent forms and patient questionnaires in order to receive TDA. An essential ingredient against wearing-out participants and loosing the generated momentum and data collection activities is a feed back loop to the user community. Data evaluation is not only performed and communicated by the MEM-Research-Center as organizing central institution but due to the good accessibility of the MEMdoc-database every participating clinical department is able to monitor their individual progress and status quo and to compare themselves against the pool of participating centres with online statistical tools. Finally, the Swiss Federal Office of Public Health has an evidence based background for defining the framework for future TDA reimbursement.
The set-up and organization of the SWISSspine registry has introduced a new attitude amongst its participants. The need for constant documentation and commitment to the “rules” of the registry brakes previous long rehearsed manners. Transparency and information exchange is required and will be of increasing importance in future considerations, also in other medical subspecialties. For some colleagues this may be a “cultural clash”. However, the potential quality of the generated evidence makes such new approaches worth being considered. These results may not only verify or falsify previously made findings of other investigators but can also generate new information about the field of investigation like the so far unreported pain thresholds. Especially in those cases were a surgical alternative like lumbar fusion exists, the findings, though not considerable as guidelines, may serve as decision-making aid for a careful and conservative use of the new technologies.
As previously mentioned, there are a number of questions which can not be answered with the SWISSspine registry, others do only evolve from its results. To continue the established registry and further evaluate its data pool is the challenge of the future. Other study designs may be needed. A logical and scientifically desirable development could be the creation and integration of a second study arm receiving fusion. With such a study design, a matched prospective cohort study would become realizable and higher quality evidence for or against lumbar TDA generated. Implementation of randomized control trials leading to the gold standard of scientific results is not feasible, nor payable in a national set-up. Moreover, the SPORT trial has shown that there may be such a high rate of cross-over to the other treatment arm, that the study suffers from a severe bias and that the resulting “noise” in the results leads to unclear findings [15].
Conclusion
Evaluation of the SWISSspine registry showed that TDA is efficient in short-term back and leg pain reduction. Along with it goes an improved quality of life and moderate rates of complications and revisions. Certain preoperative pain threshold values correlate with postoperative outcome. The results may provide help in every day clinical decision making for a further optimized surgical treatment. With the collected data, SWISSspine further accomplishes one of its main objectives which is providing information about the safety and efficiency of lumbar TDA. The registry can be seen as a foundation for other data collection and evaluation projects in Swiss health technology assessment. It can also act as a feasibility model for comparable studies in other countries.
Acknowledgments
We are indebted to Prof. M. Aebi and all staff members of the Institute for Evaluative Research in Orthopaedic Surgery involved in the SWISSpine project. Without his belief in the absolute necessity of outcome documentation, the vision of an academic data clearing house with proprietary data collection system and the endurance of turning this vision into reality in the past 8 years, the SWISSspine registry would not have become a successful postmarket surveillance project within such a short time frame. We thank Daniel Dietrich, PhD, for statistical consulting in all analyses presented in the current article. We are thankful to SGS and the SWISSspine registry group who made this research possible by populating the database with their valuable and much appreciated entries. Aebi M, Baerlocher C, Baur M, Berlemann U, Binggeli R, Boos N, Boscherini D, Cathrein P, Etter C, Favre J, Forster T, Grob D, Hasdemir M, Hausmann O, Heini P, Huber J, Jeanneret B, Kast E, Kleinstueck F, Kroeber M, Lattig F, Lutz T, Maestretti G, Marchesi D, Markwalder T, Martinez R, Min K, Morard M, Otten P, Payer M, Porchet F, Ramadan A, Renella R, Richter H, Schaeren S, Schizas C, Schwarzenbach O, Selz T, Sgier F, Stoll T, Tessitore E, Van Dommelen K, Vernet O, Wernli F.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s00586-009-0971-3
References
- 1.Baur-Melnyk A, Birkenmaier C, Reiser MF. Lumbale Bandscheibenendoprothesen: Indikationen, Biomechanik, Typen und radiologische Kriterien. Radiologe. 2006;46:768–778. doi: 10.1007/s00117-006-1356-9. [DOI] [PubMed] [Google Scholar]
- 2.Bertagnoli R, Yue JJ, Shah RV, Nanieva R, Pfeiffer F, Fenk-Mayer A, Kershaw T, Husted DS. The treatment of disabling single-level lumbar discogenic low back pain with total disc arthroplasty utilizing the prodisc prosthesis: a prospective study with 2-year minimum follow-up. Spine. 2005;30:2230–2236. doi: 10.1097/01.brs.0000182217.87660.40. [DOI] [PubMed] [Google Scholar]
- 3.Cochrane AL. Archie Cochrane in his own words. Selections arranged from his 1972 introduction to “Effectiveness and Efficiency: Random Reflections on the Health Services” 1972. Control Clin Trials. 1989;10:428–433. doi: 10.1016/0197-2456(89)90008-1. [DOI] [PubMed] [Google Scholar]
- 4.Kleuver M, Oner FC, Jacobs WC. Total disc replacement for chronic low back pain: background and a systematic review of the literature. Eur Spine J. 2003;12:108–116. doi: 10.1007/s00586-002-0500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deyo RA, Nachemson A, Mirza SK. Spinal-fusion surgery—the case for restraint. N Engl J Med. 2004;350:722–726. doi: 10.1056/NEJMsb031771. [DOI] [PubMed] [Google Scholar]
- 6.Errico TJ. Lumbar disc arthroplasty. Clin Orthop Relat Res. 2005;435:107–117. doi: 10.1097/01.blo.0000165718.22159.d9. [DOI] [PubMed] [Google Scholar]
- 7.Fritzell P, Hagg O, Nordwall A. Complications in lumbar fusion surgery for chronic low back pain: comparison of three surgical techniques used in a prospective randomized study. A report from the Swedish Lumbar Spine Study Group. Eur Spine J. 2003;12:178–189. doi: 10.1007/s00586-002-0493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grevitt M, Pande K, O’Dowd J, Webb J. Do first impressions count? A comparison of subjective and psychologic assessment of spinal patients. Eur Spine J. 1998;7:218–223. doi: 10.1007/s005860050059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hägg O, Fritzell P, Ekselius L, Nordwall A. Predictors of outcome in fusion surgery for chronic low back pain. A report from the Swedish Lumbar Spine Study. Eur Spine J. 2003;12:22–33. doi: 10.1007/s00586-002-0465-z. [DOI] [PubMed] [Google Scholar]
- 10.Hägg O, Fritzell P, Nordwall A. The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur Spine J. 2003;12:12–20. doi: 10.1007/s00586-002-0464-0. [DOI] [PubMed] [Google Scholar]
- 11.Hutton J, Trueman P, Henshall C. Coverage with evidence development: an examination of conceptual and policy issues. Int J Technol Assess Health Care. 2007;23:425–432. doi: 10.1017/S0266462307070651. [DOI] [PubMed] [Google Scholar]
- 12.Pellise F, Hernandez A, Vidal X, Minguell J, Martinez C, Villanueva C. Radiologic assessment of all unfused lumbar segments 7.5 years after instrumented posterior spinal fusion. Spine. 2007;32:574–579. doi: 10.1097/01.brs.0000256875.17765.e6. [DOI] [PubMed] [Google Scholar]
- 13.Putzier M, Funk JF, Schneider SV, Gross C, Tohtz SW, Khodadadyan-Klostermann C, Perka C, Kandziora F. Charite total disc replacement—clinical and radiographical results after an average follow-up of 17 years. Eur Spine J. 2006;15:183–195. doi: 10.1007/s00586-005-1022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tropiano P, Huang RC, Girardi FP, Marnay T. Lumbar disc replacement: preliminary results with ProDisc II after a minimum follow-up period of 1 year. J Spinal Disord Tech. 2003;16:362–368. doi: 10.1097/00024720-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein JN, Lurie JD, Tosteson TD, Skinner JS, Hanscom B, Tosteson AN, Herkowitz H, Fischgrund J, Cammisa FP, Albert T, Deyo RA. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA. 2006;296:2451–2459. doi: 10.1001/jama.296.20.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]