Abstract
Delayed complications following lumbar spine fusion may occur amongst which is adjacent segment degeneration (ASD). Although interspinous implants have been successfully used in spinal stenosis to authors’ knowledge such implants have not been previously used to reduce ASD in instrumented lumbar fusion. This prospective controlled study was designed to investigate if the implantation of an interspinous implant cephalad to short lumbar and lumbosacral instrumented fusion could eliminate the incidence of ASD and subsequently the related re-operation rate. Groups W and C enrolled initially each 25 consecutive selected patients. Group W included patients, who received the Wallis interspinous implant in the unfused vertebral segment cephalad to instrumentation and the group C selected age-, diagnosis-, level-, and instrumentation-matched to W group patients without interspinous implant (controls). The inclusion criterion for Wallis implantation was UCLA arthritic grade <II, while the exclusion criteria were previous lumbar surgery, severe osteoporosis or degeneration >UCLA grade II in the adjacent two segments cephalad to instrumentation. All patients suffered from symptomatic spinal stenosis and underwent decompression and 2–4 levels stabilization with rigid pedicle screw fixation and posterolateral fusion by a single surgeon. Lumbar lordosis, disc height (DH), segmental range of motion (ROM), and percent olisthesis in the adjacent two cephalad to instrumentation segments were measured preoperatively, and postoperatively until the final evaluation. VAS, SF-36, and Oswestry Disability Index (ODI) were used. One patient of group W developed pseudarthrosis: two patients of group C deep infection and one patient of group C ASD in the segment below instrumentation and were excluded from the final evaluation. Thus, 24 patients of group W and 21 in group C aged 65+ 13 and 64+ 11 years, respectively were included in the final analysis. The follow-up averaged 60 ± 6 months. The instrumented levels averaged 2.5 + 1 vertebra for both groups. All 45 spines showed radiological fusion 8–12 months postoperatively. Lumbar lordosis did not change postoperatively. Postoperatively at the first cephalad adjacent segment: DH increased in the group W (P = 0.042); ROM significantly increased only in group C (ANOVA, P < 0.02); olisthesis decreased both in flexion (P = 0.0024) and extension (P = 0.012) in group W. The degeneration or deterioration of already existed ASD in the two cephalad segments was shown in 1 (4.1%) and 6 (28.6%) spines in W and C groups, respectively. Physical function (SF-36) and ODI improved postoperatively (P < 0.001), but in favour of the patients of group W (P < 0.05) at the final evaluation. Symptomatic ASD required surgical intervention was in 3 (14%) patients of group C and none in group W. ASD remains a significant problem and accounts for a big portion of revision surgery following instrumented lumbar fusion. In this series, the Wallis interspinous implant changed the natural history of ASD and saved the two cephalad adjacent unfused vertebra from fusion, while it lowered the radiographic ASD incidence until to 5 years postoperatively. Longer prospective randomized studies are necessary to prove the beneficial effect of the interspinous implant cephalad and caudal to instrumented fusion. We recommend Wallis device for UCLA degeneration I and II.
Keywords: Adjacent segment degeneration, Wallis, Lumbar fusion, Disc degeneration
Introduction
Lumbar fusion has been used to reduce persistent conservative treatment axial back pain and/or segmental instability. Decompression and fusion in symptomatic degenerative spondylolisthesis (grades I, II) has been followed by very good functional results [49]. Furthermore, wide decompression for significant symptomatic spinal stenosis often associated with the loss of segmental lumbar lordosis may jeopardise segmental stability of the lumbar spine that makes an additional stabilization mandatory.
Although instrumented lumbar spine fusion is a commonly performed procedure, its role remains debated, and moreover, delayed complications may occur, amongst which is adjacent segment degeneration (ASD).
Adjacent segment degeneration describes nearly any abnormal process that develops in the mobile segment next to a spinal fusion and although the exact mechanism remains uncertain, altered biomechanical stresses (hypermobility, olisthesis, disc height (DH) loss, instability) appear to play a key role in its development [4, 16, 19, 30, 33, 34, 39, 47]. Although several clinical and radiological criteria have been introduced to define segmental spinal instability there is no consensus as regard its definition.
Although most often the criteria to determine ASD are based solely on radiographic findings [2, 7, 19, 28, 30, 34, 39, 41, 59] reporting an ASD incidence ranging from 8 to 100%, the symptomatic incidence of ASD is significantly lower ranging from 5.2 to 18.5% [7, 28, 31], while the rate of re-operation rate for symptomatic ASD ranges from 2.7 to 20% [16, 58].
There is a controversy regarding the risk factors involved in the development of ASD [2, 11, 12, 22, 28, 40, 41, 47, 57–59]. Non-rigid, dynamic or flexible instrumentations for lumbar spine have been developed to reduce ASD [17, 18, 27]. These implants are either fixed in the pedicles, or secured between the spinous processes of adjacent vertebrae [38, 48]. Long-term results showed several significant drawbacks and implant-related complications in the non-rigid pedicle fixed instrumentations [18, 54].
The interspinous process implants, that are currently used for the treatment of neurogenic Claudicatio, reduce pathologic extension at the symptomatic spinal levels and intradiscal pressure and facet load, preventing narrowing of the spinal canal and neural foramens [36, 46, 59, 61].
Amongst these interspinous process implants a “second” generation implant for non-rigid stabilization of lumbar segments, called Wallis system, has been developed [48]. A recent in vitro biomechanical and finite-element analysis of the Wallis showed that this implant reduces motion without suppressing it and lowers stress in the disc fibres and annulus matrix [32].
The hypothesis of this prospective randomized comparative study was if the Wallis interspinous implant, inserted in the unfused segment cephalad to instrumented lumbar fusion, could reduce the incidence of ASD.
Materials and methods
From May 2001 to March 2002, we carried out a prospective controlled study comparing two consecutive homogenous groups of 25 consecutive patients each, who underwent surgery for degenerative spinal stenosis, spondylolisthesis, loss of segmental lordosis or combined in the same period. Group W included patients who received the Wallis implant in the unfused segment cephalad to pedicle screw instrumentation and group C patients without interspinous implant, who were selected subsequently to match the characteristics of the patients of group W and were used as controls. All 50 patients, who were initially enrolled in this study were treated with the wide decompression and posterior transpedicular rigid fixation and fusion. All surgeries were performed by the first author who is a senior spine surgeon. The surgeon was unaware preoperatively that patient was going to be included in each group to avoid bias in patient’s selection. The ethics committee of this institution approved this study.
The inclusion criteria were the following degenerative spine disease (spinal stenosis, spondylolisthesis, loss of segmental lordosis), 2–4 instrumented vertebrae and fusion in the lumbar and lumbosacral spine, modified arthritic UCLA scale grade ≤II [15] without olisthesis or lytic lesion in the cephalad the instrumentation segment, and informed consensus.
The exclusion criteria were the following: severe osteoporosis, loss of lumbar lordosis [28], previous lumbar surgery–fracture, lack of motion (ankylosis), UCLA > II arthritic grade in the adjacent segment cephalad to instrumentation, spondylolisthesis, and acquired spinous process insufficiency.
The radiographic criteria for ASD in the cephalad segment above to instrumentation were the development of olisthesis, disc collapse, increased segmental range of motion (ROM), deterioration (>grade II) of modified UCLA arthritic grade (Table 1) [15, 19, 30, 33, 47].
Table 1.
Arthritic grade for intervertebral disc degeneration
| UCLA grading for intervertebral space degeneration | |||
|---|---|---|---|
| Disc space narrowing | Osteophytes | Endplate sclerosis | |
| I | (−) | (−) | (−) |
| II | (+) | (−) | (−) |
| III | (+/−) | (+/−) | (−) |
| IV | (+/−) | (+/−) | (+) |
Grade is based upon the most severe radiographic evident on plain radiographs
(+ present, − absent, +/− either present or absent)
The clinical criteria for ASD were the worsening of low back pain, despite radiographic solid fusion in the instrumentation area and the absence of any surgery-related complication. In this study, the vertebral segment cephalad to instrumented fusion was selected for several documented reasons: (1) this is the most frequent localisation of ASD [3]; (2) symptomatic ASD in the lower lumbar and lumbosacral instrumented fusion is very rare (<3%) [15]; and (3) with exception of few studies, all clinical and biomechanical studies address cranial segment degeneration following the rigid lumbar/lumbosacral instrumentation [39].
All patients were clinically assessed preoperatively and postoperatively with the SF-36 (physical function domain) and Oswestry Disability Index (ODI) score and the pain magnitude with Visual Analogue Scale (0–10 scale, VAS). The preoperative radiological work-up included conventional standing whole spine roentgenograms (lumbar lordosis, and DH and UCLA grading in the cephalad segment), sitting lateral dynamic bending films to measure olisthesis and ROM and supine oblique views for spinal fusion determination. The radiological parameters that were measured preoperatively to the latest evaluation included: T12–S1 lumbar lordosis, ROM (flexion and extension) in degrees of the vertebral segment immediately cephalad to instrumentation, olisthesis of the vertebra cephalad instrumentation (in flexion and extension), anterior and posterior standing DH.
As instability was defined as any sagittal translation of the adjacent vertebral body above fusion greater than 3 mm and/or angle change greater than 10° between two adjacent vertebrae.
CT scans, MRI were made in most, but not in all cases and thus were not included in the evaluation of the ASD changes as others also quite recently did [52]. The radiographic changes were evaluated by a senior orthopaedic radiologist and spine surgeon who did not participate in surgery and thus did not know to which group each patient belong.
Surgical technique and Wallis interspinous implant
The second generation Wallis implant, that was used in this study is a interspinous blocker, which is made of PEEK (polyetheretherketone). Due to its shape (Fig. 1) and the properties of PEEK, the implant has much greater elasticity (30 times less rigid than titanium) than the first generation titanium implant. In addition, the implant includes two ligaments made of woven Dacron that are wrapped around the spinous processes and fixed under tension to the blocker. Wallis (Abbot, USA) is fixed to the spine by two polyester bands looped around the proximal and distal spinous processes of the instrumented level and reattached to the spacer by means of two clips that are visible on plain radiographs. Four implant sizes (10, 12, 14, and 16 mm) are available to fit individual interspinous distances. While during the surgical procedure, the smallest size that had sufficient stability on the two laminae is chosen to avoid reduction of lumbar lordosis [42]. Wallis confers substantial mechanical advantages [32]: when the spinal column is submitted to loading, the interspinous blocker displaces the mechanical constraints dorsally and reduces the load upon the disc and the facet joint system. The Dacron ligaments resist traction of 200 daN and stretch approximately 20% before failure by overloading. The overall implant constitutes a “floating” system with no permanent fixation in the vertebral bone, which might otherwise expose in the risk of loosening. Mechanical human cadaver studies [48] have shown that Wallis permits a reduction in the mobility of intervertebral segments previously destabilized by discectomy and that it achieves an increase in the rigidity of the destabilized segment beyond normal values. There is no implant for the L5/S1 segment and thus it cannot be used below a L4/L5 fusion [63].
Fig. 1.
The Wallis implant
Rigid pedicle screw instrumentation was used in this series for both groups. Care was taken to avoid to harm the facet joints adjacent to instrumentation (avoidance opening of facet joint capsule and lateral insertion of the pedicle screws. For technical reasons (too narrow space between spinous process and pedicle screw tulips), the Wallis device was inserted immediately after pedicle screw insertion and decompression; then the longitudinal rods were assembled after appropriate contouring. In all patients were used autogenous local bone derived from decompression and decortication mixed with coralline HA (50:50) for posterior and intertransverse fusion.
One-way ANOVA was used to show the changes of each parameter in a group and t test for differences in a parameter between the two groups.
Results
Four patients were excluded from the final evaluation for different reasons: one patient of group W for pseudarthrosis; two patients of group C for deep infection that required re-operation and one patient of group C for ASD in the segment below instrumentation.
Twenty-four patients of group W and 21 patients of group C, who showed radiological fusion 8–12 months postoperatively were included in the final evaluation. The age of the patients of groups W and C averaged 65 ± 13 years (range 32–72 years) and 64 ± 11 years (range 33–71 years), respectively.
The instrumented levels in both groups averaged 2.5 ± 1 (range 2–4).
The Wallis was most often inserted in the L3/L4 segment in 14/24 cases of group W, while the adjacent segment cephalad to instrumentation was the L3/L4 in 15/21 cases of C group.
The follow-up observation averaged 54 ± 6 months.
T12–S1 lordosis (Fig. 2) did not postoperatively change until the final evaluation in any group, while no difference between individuals of different groups was shown in all periods of observation (W group, ANOVA, P = 0.35 vs. C group, ANOVA, P = 0.41).
Fig. 2.
Comparative plotting of T12–S1 lordosis changes preoperative to the last evaluation
Standing anterior DH (Fig. 3) did not postoperatively change in W group (ANOVA, P = 0.26) and C group (ANOVA, P = 0.69).
Fig. 3.
Changes of anterior disc height (mm) preoperatively and postoperatively to the latest evaluation
Standing posterior DH (Fig. 4) increased immediately postoperatively (P = 0.042) in the W group, while it did not change in the C group (P = 0.44).
Fig. 4.
Changes of posterior disc height (mm) preoperatively and postoperatively to the latest evaluation
The ROM (Fig. 5) at the cephalad to the instrumentation segment did not significantly postoperatively change (ANOVA, P = 0.5) in the W group, while it significantly increased at final evaluation (ANOVA, P < 0.02) in the spines of the group C.
Fig. 5.
Range of motion in the cephalad segment above instrumentation
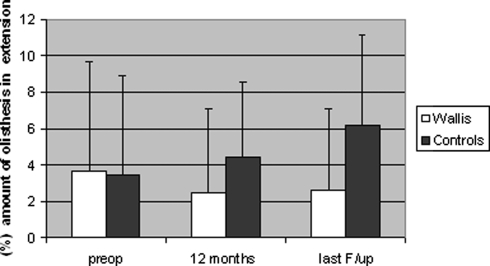
Wallis decreased significantly (P = 0.0024) the percent amount of olisthesis in flexion of the vertebra cephalad to instrumented spinal segments a year postoperatively, while it insignificantly (P = 0.31) decreased at the final evaluation (Fig. 6).
Fig. 6.
Percent amount of olisthesis in flexion preoperatively to the last evaluation. Wallis decreased significantly (P = 0.0024) the percent amount of olisthesis
Wallis decreased postoperatively significantly (P = 0.012) the amount of olisthesis in extension (retrolisthesis) of the cephalad instrumentation vertebra one-year follow-up postoperatively (Fig. 7), while it remained unchanged at the final evaluation (P = 0.28).
Fig. 7.
Percent amount of olisthesis in extension preoperatively to the last evaluation. Wallis decreased (P = 0.012) the amount of olisthesis in extension
Physical function domain (SF-36) improved (P < 0.001) postoperative (1 year) in an equal amount in the patients of both groups (Fig. 8). At the last evaluation, there was a statistically significant (P = 0.05) difference in favour of the patients of W group.
Fig. 8.
SF-36 (physical function domain) changes preoperative until the final evaluation
Oswestry Disability Index decreased significantly (P < 0.005) in an equal amount in the patients of both groups a year postoperatively (Fig. 9). At the final observation, there was a significant difference (P < 0.05) in ODI score in favour of the patients of W group.
Fig. 9.
ODI changes preoperatively to the last evaluation
Visual Analogue Scale score (lumbar spine) averaged preoperatively 7.2 ± 2.1 and 7.4 ± 3 in the patients of groups W and C, respectively, and improved postoperatively to 3 ± 2 and 3.6 ± 3 in groups W and C, respectively.
Degeneration or deterioration of already existed low-grade degeneration (UCLA ≤ II) in the adjacent segment cephalad to instrumentation was shown in 1 (4.1%) and 6 (28.6%) spines in W and C groups, respectively. The levels involved in ASD are shown in Table 2.
Table 2.
Levels of ASD in above instrumentation segments
| Group | Segment | ||
|---|---|---|---|
| L2–L3 | L3–L4 | L4–L5 | |
| Wallis | 0a | 1a, 1b | 0a |
| Controls | 1a, 1b | 4a, 4b | 1a, 1b |
aOne level above instrumentation
bTwo levels above instrumentation
Symptomatic ASD required surgical intervention was shown in 3 (14%) patients of group C with UCLA grade-IV degeneration (disc space narrowing, osteophytes and endplate sclerosis) (Table 1; Figs. 10, 11, 12) in the first cephalad segment, while no patient from group W needed intervention (Figs. 13, 14, 15) (Table 3).
Fig. 10.
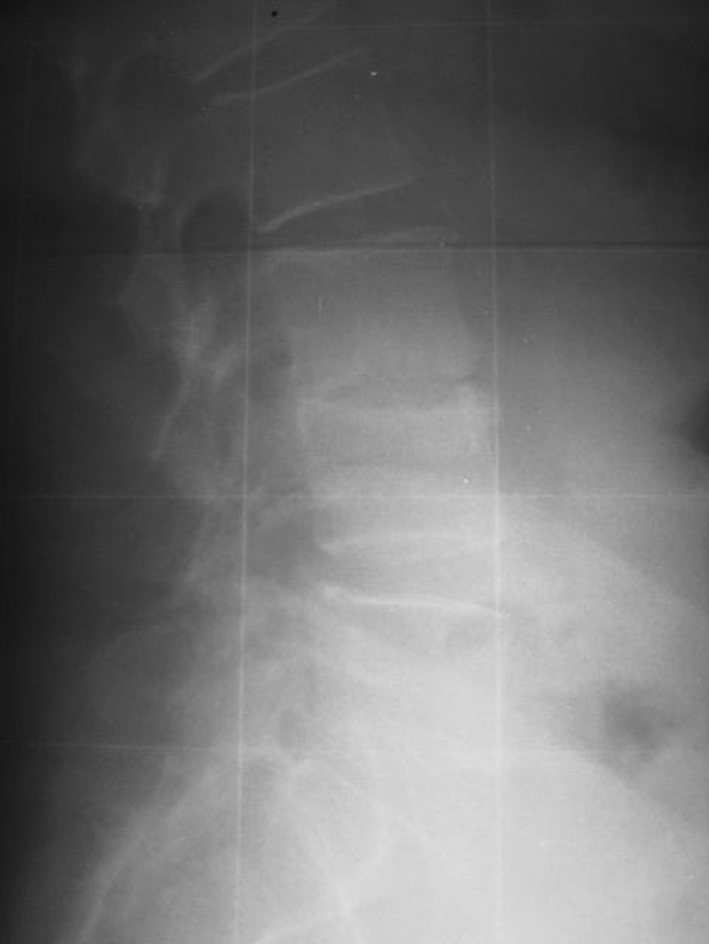
Standing lateral roentgenogram of a 60-year-old female patient suffering from degenerative disc disease L3/L4
Fig. 11.
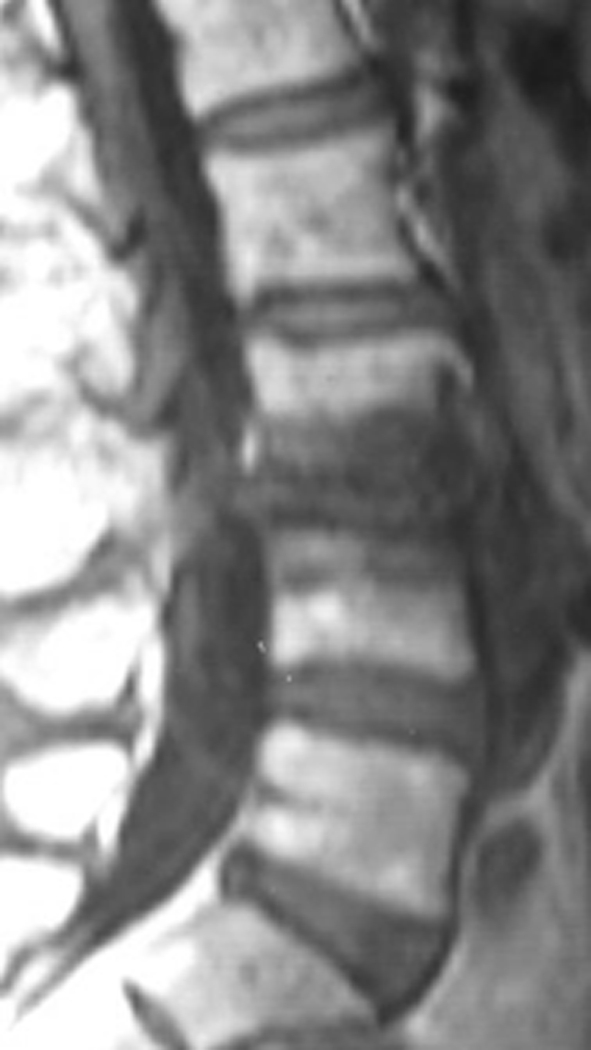
Lateral MRI view showing severe degeneration in the segment L3/L4 of the patient of Fig. 10
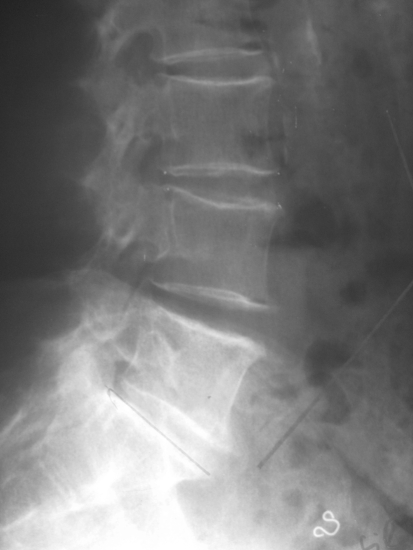
Fig. 12.
Lateral roentgenogram of the patient of Figs. 10 and 11, 34 months postoperatively showing collapse of this disc. No interspinous implant was inserted at the L2/L3 segment. This patient was revised because of intractable pain 38 months postoperatively
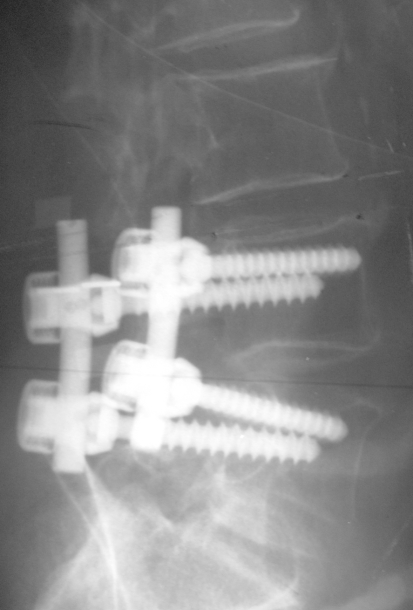
Fig. 13.
Lateral roentgenogram of a 58-year-old female patient with degenerative disc disease and disc herniation at the segment L4/L5. UCLA degeneration II at the L3/L4 segment
Fig. 14.
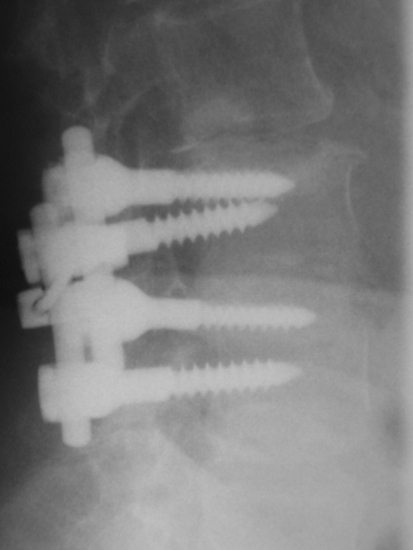
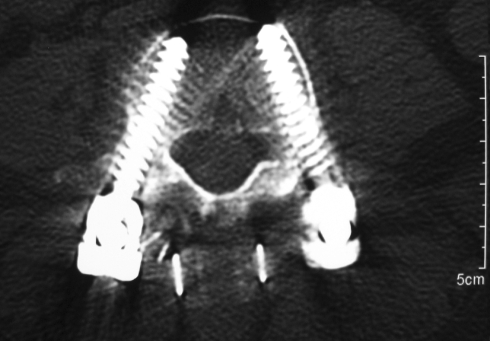
Lateral roentgenogram of the patient of Fig. 13, 57 months after L4/L5 laminectomy and discectomy plus instrumented fusion. At the segment L3/L4, a Wallis has been inserted (arrow). Note the normal height of the disc L3/L4
Fig. 15.
Axial CT view of the patient of Figs. 13 and 14 showing the correct position of the Wallis implant close to the spinous process
Table 3.
Arthritic grade for intervertebral disc degeneration in first cephalad segment
| UCLA+ | Wallis groupa | Control groupa | ||
|---|---|---|---|---|
| Grading | Preoperatively | Postoperatively | Preoperatively | Postoperatively |
| I | 17 | 17 | 16 | 15 |
| II | 7 | 6 | 5 | 2 |
| III | 0 | 1 | 0 | 1 |
| IV | 0 | 0 | 0 | 3 |
| 24 | 24 | 21 | 21 | |
Grade is based upon the most severe radiographic evident on plain radiographs
aNumber of patients listed according to UCLA grading
Complications
In one patient of group W and two patients of group C accidentally occurred intraoperatively dural violation that was immediately sutured without further problems.
Two patients of group C developed deep infection in the early postoperative phase (6–12 days postoperation) that required re-operation (wound drainage and continuous irrigation plus intravenous antibiotics).
In one patient of group W and one of group C, there were observed incidentally at the final observation remote simple osteoporotic compression fractures (AO type A1.1.1) 4 and 5 levels cephalad to instrumentation. These fractures were not linked to any known trauma and were clinically silent.
Discussion
Lumbar spine fusion is a common procedure in spine surgery to improve the pain and clinical outcome of patients with failed conservative treatment for lumbar degenerative disease and degenerative spondylolisthesis by eliminating segmental instability, which is recognised as a cause of low back pain. Indications comprise degenerative disorders and spondylolisthesis (grades I, II) [20, 24, 49]. During the last years, lumbar fusion has been increasingly criticised [6], while clinical studies have shown similar long-term follow-up results with conservative treatment [8, 14]. Side effects of lumbar fusion include ASD, pseudarthrosis, bone-graft morbidity, high rates of re-operation, implant failure, and sagittal spinal imbalancing. More specifically, spinal fusion alters the biomechanics of the spine and the loss of motion at the fused levels is at least theoretically compensated by increased motion at other unfused segments resulting in ASD [33].
Adjacent segment degeneration is a common long-term sequela or complication of spinal fusion surgery. The exact aetiology is uncertain but alterations in facet loading, hypermobility, and increased intradiscal pressure at the segments adjacent to fusion mass is believed to play a key role [15, 21, 35, 44, 51, 60]. Superior segment facet violation or laminectomy has recently shown in vitro to destabilize the adjacent level in transpedicular fixation [9].
Radiological disc degeneration (ASD) is all too common (5.2–100%) complication that the initial good results following a posterior spinal fusion degrade as adjacent mobile segments proximal to the fusion degenerate over time compromising the late outcome of many short and mid-term successes [1, 16, 26, 33, 43, 53]. There is an increasing concern, regarding the long-term consequences of these asymptomatic changes; however, correlation of ASD and clinical outcome is still unclear [46, 52].
The reported incidence of symptomatic ASD is significantly lower (5.2–18.5%) than the radiologic ASD. ASD incidence is higher in patients with transpedicular instrumentation (12.2–18.5%) compared with patients fused with other forms of instrumentation or with no instrumentation (5.2–5.6%). Evidence of radiographic degeneration, however, does not necessarily lead to a poor clinical outcome of surgery [21, 52]. A recent clinical study [52] showed that the incidence of ASD (DH reduction) in the first cephalad adjacent segment 10 years following 360° instrumented lumbar fusion averaged 21%, while in the second adjacent level averaged 16%. In our series, the incidence of ASD both in the first and second cephalad adjacent segment following posterolateral transpedicular fixation without Wallis averaged 28.6%, while in the Wallis group it was 4.1%. Thus, although different fusion methods and imaging techniques were used in Schulte’s and ours series it seems that the addition of Wallis protected not only the first but also the second cephalad segment from ASD. In our series, the incidence of radiographic ASD cephalad to instrumentation was 4.1% in the patients who received interspinous implant, significantly lower compared with patients without spacer in which it was 28.6%. Although the most common segment involved in ASD was the L3/L4 in 5/7 spines, no conclusion can be drawn regarding correlation between levels involved in ASD and clinical outcome because of small sample of patients. In the present study, the incidence of symptomatic ASD in the cephalad segment that required surgical intervention was 14% and was limited only in the patients without Wallis (group C).
However, to solve the complication of ASD several flexible or even dynamic devices have been used with controversial results [17, 43, 50, 52]. It has been proposed that non-fusion motion preservation surgery may prevent accelerated ASD because of the protective effects of continuing segmental motion. Dynesys have been used for motion preservation since 1994 to allow mobile load transfer, and provide controlled motion, thereby off-loading the facet joints and posterior disc [51]. Because of the rigidity of Dynesys some authors have doubted the protective effect of this construct on adjacent segment [23, 51, 55, 56]. A recent prospective clinical study with 2 years of follow-up showed with the use of MRI that disc degeneration at the bridged and cranial adjacent segment continue (20%) despite Dynesys dynamic stabilization [29]. Others [27] compared three posterior pedicle-screw instrumentations (rigid, semi-rigid and dynamic) and found no differences in the incidence of ASD after a follow-up of 4 years.
With the exception of a few studies, all of the biomechanical and clinical studies address cranial segment degeneration [15, 39], because ASD caudal to a fusion is significantly less common [10]. The explanation for this is that in the adjacent segment cephalad to a fusion there is increased mobility compared with the adjacent caudal segment [5]. A recent clinical study showed that ASD occurred in 89% of the cases cephalad to lumbar fusion, 3.7% of the cases caudal and combined cephalad and caudal in 7.5% of the cases [10]. For these reasons, in our study, we investigated the mobility and associated degenerative signs only the vertebral segment cephalad to instrumented lumbar fusion. In our series of 45 followed up patients only one (2.2%) developed ASD caudal to instrumentation.
Most of the previous relative studies have correlated “static” radiographic criteria (DH, traction spurs, osteophytes, etc) with clinical symptoms [3, 37, 45]. Others have additionally used advanced imaging techniques as computed tomography (CT), magnetic resonance imaging (MRI) [13, 25, 53, 63]. In this study, we used the “statistic” radiographic criteria (UCLA arthritic grading system) that has been successfully used by others [15] along with dynamic motion parameters for the cephalad ASD (ROM, olisthesis). Schulte recently used DH reduction on plain roentgenograms as a measure of ASD. In the present study, we were able to show that the increased ROM and olisthesis in the control group should be responsible for the higher incidence of radiological incidence of ASD in this group when compared with the Wallis group.
To reduce the incidence of ASD by preserving motion, several implants of non-rigid or even dynamic stabilization of lumbar intervertebral segments have been developed. Some of them (Graf, Dynesys) were secured to the spine by pedicle screw fixation systems [17], while other implants are secured in the interspinous space [38, 48]. Although early results of pedicle-screw systems of flexible intervertebral stabilization have been encouraging [17] some long-term results have revealed possible drawbacks [18, 54], including increased lumbar lordosis, stretching of the Dacron parts, and malpositioning and loosening of pedicle screws leading to increased re-operation rate.
Recently, several implants have been developed with non-bony fixation, some connecting spinous processes, and laminae [5, 42] other connecting two adjacent spinous processes [38].
Amongst these implants is the Wallis, a “second” generation PEEK implant for non-rigid interspinous stabilization of lumbar segments, which was used in our series cephalad to the uppermost instrumented lumbar vertebra to preserve motion and reduce ASD incidence in this transitional unfused segment. A recent comparative biomechanical study showed that Wallis reduced the ROM and load on the disc and articular processes stresses, while it increased loads transmitted through the spinous processes [43]. In our series, Wallis implant controlled the ROM of the cephalad not fused vertebra and restored the DH at this segment without reduction of the global lumbar lordosis and sagittal balance until the latest observation 60 months after index surgery.
Using an MRI-based classification, some investigators inserted the Wallis implant to treat disc degeneration grades II–IV [33, 62]. In our study, we used the Wallis implant also in patients with UCLA I and II degeneration in the segment cephalad to instrumentation.
Senegas reported 7% re-operation rate, within 3 months postoperatively due to the loosening of the previous generation implant in a discectomy population that was treated with the Wallis implant because of persistent low back pain. No loosening or re-operation of the second generation Wallis was shown in the present series.
There are limitations to our study, as there are several inherent difficulties in studying ASD. First, the definition of ASD, not to mention the specific definition of radiographic ASD and clinical ASD, differs from study to study. In this study, we studied clear static radiographic along with dynamic parameters. The latter seems to be in accordance with others (Schulte), who recently used plain radiological criteria (DH) to evaluate ASD. Second, MRI was not used to define the degree of ASD in the segment cephalad instrumentation; however, there are no evidence-based studies to support any link between pain and MR-positive signal. Third, ASD in the “unprotected” segment cephalad to rigid fixation may be a physiological process and not the results of stress concentration even after short fusion? Finally, the outcome evaluation questionnaires (VAS, SF-36 and ODI) specific enough to differentiate the origin of pain (ASD degeneration vs. other aetiology of postoperative pain).
In accordance with previous observations, the incidence of clinically important ASD was significantly less than that of the radiographic ASD. Thus, surgeons should be aware that radiographic evidence of disc space narrowing and degenerative changes do not necessarily correlate with symptoms [43].
In this series, the Wallis interspinous implant changed the natural history of ASD in the free segment cephalad to 2–4 levels instrumented rigid lumbar fusion and reduced until to 5 years postoperatively in an equal rate the incidence of the radiographic and symptomatic ASD in the two adjacent segments cephalad to instrumented lumbar fusion. The remote fractures in the thoracolumbar spine seem not to be related to spine surgery but to natural history of the degenerative disease and ageing process.
We recommend the use of interspinous implants such as Wallis in UCLA I and II grades to protect the two adjacent cephalad to short (2–4 vertebrae) rigid fixation segments in the lumbar spine. However, for more severe arthritic changes (UCLA ≥ II) we strongly recommend inclusion of degenerated segments into the fusion.
Prospective randomized comparative studies with greater number of patients, more levels of instrumentation and longer follow-up are necessary to definitively support the conclusions of this study and to determine the usefulness of the Wallis to protect adjacent unfused mobile lumbar segments.
References
- 1.Adams MA, et al. Biomechanics of spinal implants. In: Szpalski M, Gunzburg R, Spengler DM, et al., editors. Instrumented fusion of the degenerative lumbar spine: state of the art, questions, and controversies. Philadelphia: Lippincott-Raven; 1996. [Google Scholar]
- 2.Aota Y, Kumano K, Hirabayashi S. Postfusion instability at the adjacent segments after rigid pedicle screw fixation for degenerative lumbar spinal disorders. J Spinal Disord. 1995;8:464–473. doi: 10.1097/00002517-199512000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Axelsson P, Johnsson R, Stromqvist B. The spondylolytic vertebra and its adjacent segment. Mobility measured before and after posterolateral fusion. Spine. 1997;22:414–422. doi: 10.1097/00007632-199702150-00012. [DOI] [PubMed] [Google Scholar]
- 4.Bastian L, Lange U, Knop C, et al. Evaluation of the mobility of adjacent segments after posterior thoracolumbar fixation: a biomechanical study. Eur Spine J. 2001;10:295–300. doi: 10.1007/s005860100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boden SD, Davis DO, Dina TS, et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. J Bone Joint Surg Am. 1990;72:403–408. [PubMed] [Google Scholar]
- 6.Bono CM, Lee CK. Critical analysis of treads in fusion for degenerative disc disease over the past 20 years: influence of technique on fusion rate and clinical outcome. Spine. 2004;29:455–463. doi: 10.1097/01.BRS.0000090825.94611.28. [DOI] [PubMed] [Google Scholar]
- 7.Booth KC, Bridwell KH, Eisenberg BA, et al. Minimum 5-year results of degenerative spondylolisthesis treated with decompression and instrumented posterior fusion. Spine. 1999;24:1721–1727. doi: 10.1097/00007632-199908150-00014. [DOI] [PubMed] [Google Scholar]
- 8.Brox IJ, Sorensen R, Friis A, et al. Randomized clinical trial of lumbar instrumented fusion and cognitive intervention and exercises in patients with chronic low back pain and disc degeneration. Spine. 2003;28:1913–1921. doi: 10.1097/01.BRS.0000083234.62751.7A. [DOI] [PubMed] [Google Scholar]
- 9.Cardoso JM, Dmitriev EA, Helgeson M, et al. Does superior-segment facet violation or laminectomy destabilize the adjacent level in lumbar transpedicular fixation? Spine. 2008;26:2868–2873. doi: 10.1097/BRS.0b013e31818c63d3. [DOI] [PubMed] [Google Scholar]
- 10.Cheh G, Bridwell KH, Lenke L, et al. Adjacent segment disease following lumbar/thoracolumbar fusion with pedicle screw instrumentation: a minimum 5-year follow-up. Spine. 2007;32(20):2253–2257. doi: 10.1097/BRS.0b013e31814b2d8e. [DOI] [PubMed] [Google Scholar]
- 11.Chen CS, Cheng CK, Liu CL, et al. Stress analysis of the disc adjacent to interbody fusion in lumbar spine. Med Eng Phys. 2001;23:483–491. doi: 10.1016/S1350-4533(01)00076-5. [DOI] [PubMed] [Google Scholar]
- 12.Esses SI, Doherty BJ, Crawford MJ, et al. Kinematic evaluation of lumbar fusion techniques. Spine. 1996;21:676–684. doi: 10.1097/00007632-199603150-00003. [DOI] [PubMed] [Google Scholar]
- 13.Farfan HF. Mechanical disaorders of the low back. Philadelphia: Lea & Febiger; 1973. [Google Scholar]
- 14.Fritzell P, Hagg, Nordwall A (2004) 5–10 years follow up in the Swedish lumbar spine study. Spine Week Porto, Portugal, 30 May 05 June 2004
- 15.Ghiselli G, Wang JC, Hsu WK, Dawson EG. L5–S1 segment survivorship and clinical outcome analysis after L4–L5 isolated fusion. Spine. 2003;12:1275–1280. doi: 10.1097/00007632-200306150-00011. [DOI] [PubMed] [Google Scholar]
- 16.Gillet P. The fate of the adjacent motion segments after lumbar fusion. J Spinal Disord Tech. 2003;16:338–345. doi: 10.1097/00024720-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Grevitt MP, Gardner AD, Spilsbury J, et al. The Graf stabilisation system: early results in 50 patients. Eur Spine J. 1995;4:169–175. doi: 10.1007/BF00298241. [DOI] [PubMed] [Google Scholar]
- 18.Grob D, Benini A, Junge A, et al. Clinical experience with the Dynesys semirigid fixation system for the lumbar spine: surgical and patient-oriented outcome in 50 cases after an average of 2 years. Spine. 2005;30:324–331. doi: 10.1097/01.brs.0000152584.46266.25. [DOI] [PubMed] [Google Scholar]
- 19.Hambly MF, Wiltse LL, Raghavan N, et al. The transition zone above a lumbosacral fusion. Spine. 1998;23:1785–1792. doi: 10.1097/00007632-199808150-00012. [DOI] [PubMed] [Google Scholar]
- 20.Hanley EN, Jr, David SM. Lumbar arthrodesis for the treatment of back pain. J Bone Joint Surg Am. 1999;81:716–730. doi: 10.2106/00004623-199905000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4:190S–194S. doi: 10.1016/j.spinee.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Hsu K, Zucherman J, White A, et al. Deterioration of motion segments adjacent to lumbar spine fusions. Ortho Transact. 1988;12:605–606. [Google Scholar]
- 23.Huang RC, Wright TM, Panjabi MM, et al. Biomechanics of nonfusion implants. Orthop Clin North Am. 2005;36:271–280. doi: 10.1016/j.ocl.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Ishihara H, Osada R, Kanamori M, Kawaguchi Y, Ohmori K, Kimura T, Matsui H, Tsuji H. Minimum 10-year follow-up study of anterior lumbar interbody fusion for Isthmic spondylolisthesis. J Spinal Disord. 2001;14:91–99. doi: 10.1097/00002517-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Kimura S, Steinbach GC, Watenpaugh DE, Hargens AR. Lumbar spine disc height and curvature responses to an axial load generated by a compression device compatible with magnetic resonance imaging. Spine. 2001;26:2596–2600. doi: 10.1097/00007632-200112010-00014. [DOI] [PubMed] [Google Scholar]
- 26.Kleiner JB, Odom JA, Jr, Moore MR, et al. The effect of instrumentation on human spinal fusion mass. Spine. 1995;20:90–97. doi: 10.1097/00007632-199501000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Korovessis P, Papazisis Z, Koureas G, Lambiris E. Rigid, Semirigid versus dynamic instrumentation for degenerative lumbar spinal stenosis. A correlative radiological and clinical analysis of short-term results. Spine. 2004;29:735–742. doi: 10.1097/01.BRS.0000112072.83196.0F. [DOI] [PubMed] [Google Scholar]
- 28.Korovessis P, Stamatakis M, Baikousis A. Segmental Roentgenographic analysis of vertebral inclination on sagittal plane in asymptomatic versus chronic low back pain patients. J Spinal Disord Tech. 1999;12:131–137. [PubMed] [Google Scholar]
- 29.Kumar A, Beastall J, Hughes J, et al. Disc changes in the bridged and adjacent segments after dynamic stabilization system after two years. Spine. 2008;33(26):2909–2914. doi: 10.1097/BRS.0b013e31818bdca7. [DOI] [PubMed] [Google Scholar]
- 30.Kumar MN, Baklanov A, Chopin D. Correlation between sagittal plane changes and adjacent segment degeneration following lumbar spine fusion. Eur Spine J. 2001;10:314–319. doi: 10.1007/s005860000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar MN, Jacquot F, Hall H. Long-term follow-up of functional outcomes and radiographic changes at adjacent levels following lumbar spine fusion for degenerative disc disease. Eur Spine J. 2001;10:309–313. doi: 10.1007/s005860000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuslich SD, Danielson G, Dowdle JD, et al. Four-year follow-up results of lumbar spine arthrodesis using the Bagby and Kuslich lumbar fusion cage. Spine. 2000;25:2656–2662. doi: 10.1097/00007632-200010150-00018. [DOI] [PubMed] [Google Scholar]
- 33.Lafage V, Gangnet N, Senegas J, et al. New interspinous implant evaluation using an in vitro biomechanical study combined with a finite-element analysis. Spine. 2007;32(16):1706–1713. doi: 10.1097/BRS.0b013e3180b9f429. [DOI] [PubMed] [Google Scholar]
- 34.Lee CK. Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine. 1988;13:375–377. doi: 10.1097/00007632-198803000-00029. [DOI] [PubMed] [Google Scholar]
- 35.Lee CK, Langrana NA. Lumbosacral spine fusion. A biomechanical study. Spine. 1984;9:574–581. doi: 10.1097/00007632-198409000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Leong JC, Chun SY, Grange WJ, et al. Long-term results of lumbar intervertebral disc prolapse. Spine. 1983;8:793–799. doi: 10.1097/00007632-198310000-00018. [DOI] [PubMed] [Google Scholar]
- 37.Lindsey DP, Swanson KE, Fuchs P, et al. The effects of an interspinous implant on the kinematics of the instrumented and adjacent levels in the lumbar spine. Spine. 2003;28:2192–2197. doi: 10.1097/01.BRS.0000084877.88192.8E. [DOI] [PubMed] [Google Scholar]
- 38.MacNab I. The traction spur: an indicator of segmental instability. J Bone Joint Surg Am. 1971;53:663–670. [PubMed] [Google Scholar]
- 39.Minns RJ, Walsh WK. Preliminary design and experimental studies of a novel soft implant for correcting sagittal plane instability in the lumbar spine. Spine. 1997;22:1819–1825. doi: 10.1097/00007632-199708150-00004. [DOI] [PubMed] [Google Scholar]
- 40.Miyakoshi N, Abe E, Shimada Y, et al. Outcome of one-level posterior lumbar interbody fusion for spondylolisthesis and postoperative intervertebral disc degeneration adjacent to the fusion. Spine. 2000;25:1837–1842. doi: 10.1097/00007632-200007150-00016. [DOI] [PubMed] [Google Scholar]
- 41.Nagata H, Schendel MJ, Transfeldt EE, et al. The effects of immobilization of long segments of the spine on the adjacent and distal facet force and lumbosacral motion. Spine. 1993;18:2471–2479. doi: 10.1097/00007632-199312000-00017. [DOI] [PubMed] [Google Scholar]
- 42.Nakai S, Yoshizawa H, Kobayashi S. Long-term follow-up study of posterior lumbar interbody fusion. J Spinal Disord. 1999;12:293–299. doi: 10.1097/00002517-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Papp T, Porter RW, Aspden RM, et al. An in vitro study of the biomechanical effects of flexible stabilization on the lumbar spine. Spine. 1997;22:151–155. doi: 10.1097/00007632-199701150-00005. [DOI] [PubMed] [Google Scholar]
- 44.Park P, Garton HJ, Gala VC, et al. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine. 2004;29:1938–1944. doi: 10.1097/01.brs.0000137069.88904.03. [DOI] [PubMed] [Google Scholar]
- 45.Pfirrmann CWA, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:4873–4878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 46.Pope MH, Hanley EN, Matteri RE, et al. Measurement of intervertebral disc space height. Spine. 1977;2:282–286. doi: 10.1097/00007632-197712000-00007. [DOI] [Google Scholar]
- 47.Richards JC, Majumdar S, Lindsey DP, et al. The treatment mechanism of an interspinous process implant for lumbar neurogenic intermittent claudication. Spine. 2005;30:744–749. doi: 10.1097/01.brs.0000157483.28505.e3. [DOI] [PubMed] [Google Scholar]
- 48.Rigby MC, Selmon GP, Foy MA, et al. Graft ligament stabilisation: mid- to long-term follow-up. Eur Spine J. 2001;10:234–236. doi: 10.1007/s005860100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rousseau MA, Lazennec JY, Saillant G. Predictors of outcomes after posterior decompression and fusion in degenerative spondylolisthesis. Eur Spine J. 2006;15:8–15. doi: 10.1007/s00586-005-0935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schlegel JD, Smith JA, Schleusener RL. Lumbar motion segment pathology adjacent to thoracolumbar, lumbar, and lumbosacral fusions. Spine. 1996;21:970–981. doi: 10.1097/00007632-199604150-00013. [DOI] [PubMed] [Google Scholar]
- 51.Schnake KJ, Schaeren S, Jeanneret B. Dynamic stabilization in addition to decompression for lumbar stenosis with degenerative spondylolisthesis. Spine. 2006;31:442–449. doi: 10.1097/01.brs.0000200092.49001.6e. [DOI] [PubMed] [Google Scholar]
- 52.Schulte LT, Leistra F, Bullmann V, Osada N, Vieth V, Marquardt B, Lerner T, Liljenqvist U, Hachenberg L. Disc height reduction in adjacent segments and clinical outcome 10 years after lumbar 360° fusion. Eur Spine J. 2007;16:2152–2158. doi: 10.1007/s00586-007-0515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Senegas J. Mechanical supplementation by non-rigid fixation in degenerative intervertebral lumbar segments: the Wallis system. Eur Spine J. 2002;11(Suppl 2):164–169. doi: 10.1007/s00586-002-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sénégas J, Vital JM, Guerin J, et al. Stabilisation lombaire souple, instabilite vertebrales lombaires. Expans Sci Fr. 1997;12:4–32. [Google Scholar]
- 55.Sengupta DK. Point of view: dynamic stabilization in addition to decompression for lumbar spine stenosis with degenerative spondylolisthesis. Spine. 2006;31:450. doi: 10.1097/01.brs.0000200051.24623.33. [DOI] [PubMed] [Google Scholar]
- 56.Singh K, An HS. Motion preservation technologies: alternatives to spinal fusion. Am J Orthop. 2006;35:411–416. [PubMed] [Google Scholar]
- 57.Swanson KE, Lindsey DP, Hsu KY, et al. The effects of an interspinous implant on intervertebral disc pressures. Spine. 2003;28:26–32. doi: 10.1097/00007632-200301010-00008. [DOI] [PubMed] [Google Scholar]
- 58.Umehara S, Zindrick MR, Patwardhan AG, et al. The biomechanical effect of postoperative hypolordosis in instrumented lumbar fusion on instrumented and adjacent spinal segments. Spine. 2000;25:1617–1624. doi: 10.1097/00007632-200007010-00004. [DOI] [PubMed] [Google Scholar]
- 59.Whitecloud TS, III, Davis JM, Olive PM. Operative treatment of the degenerated segment adjacent to a lumbar fusion. Spine. 1994;19:531–536. doi: 10.1097/00007632-199403000-00007. [DOI] [PubMed] [Google Scholar]
- 60.Weihofer SL, Guyer RD, Herbert M, et al. Intradiscal pressure measurements above an instrumented fusion. A cadaveric study. Spine. 1995;20:526–531. doi: 10.1097/00007632-199503010-00004. [DOI] [PubMed] [Google Scholar]
- 61.Wiltse LL, Radecki SE, Biel HM, et al. Comparative study of the incidence and severity of degenerative change in the transition zones after instrumented versus noninstrumented fusions of the lumbar spine. J Spinal Disord. 1999;12:27–33. doi: 10.1097/00002517-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 62.Wiseman C, Lindsey DP, Fredrick AD, et al. The effect of an interspinous process implant on facet loading during extension. Spine. 2005;15(30):903–907. doi: 10.1097/01.brs.0000158876.51771.f8. [DOI] [PubMed] [Google Scholar]
- 63.Zhu SH, McCarthy ID, McGregor AH, Coombs RR, Hughes SP. Geometrical dimensions of the lower lumbar vertebra. Eur Spine J. 2000;9(3):242–248. doi: 10.1007/s005860000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zucherman JF, Hsu KY, Hartjen CA, et al. A prospective randomized multi-center study for the treatment of lumbar spinal stenosis with the X STOP interspinous implant: 1-year results. Eur Spine J. 2004;13:22–31. doi: 10.1007/s00586-003-0581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]