Abstract
The interest in cervical total disc replacement (TDR) as an alternative to the so-far gold standard in the surgical treatment of degenerative disc disease (DDD), e.g anterior cervical discectomy and fusion (ACDF), is growing very rapidly. Many authors have established the fact that ACDF may result in progressive degeneration in adjacent segments. On the contrary, but still theoretically, preservation of motion with TDR at the surgically treated level may potentially reduce the occurrence of adjacent-level degeneration (ALD). The authors report the intermediate results of an undergoing multicentre prospective study of TDR with Mobi-C® prosthesis. The aim of the study was to assess the safety and efficacy of the device in the treatment of DDD and secondary to evaluate the radiological status of adjacent levels and the occurrence of ossifications, at 2-year follow-up (FU). 76 patients have performed their 2-year FU visit and have been analyzed clinically and radiologically. Clinical outcomes (NDI, VAS, SF-36) and ROM measurements were analyzed pre-operatively and at the different post-operative time-points. Complications and re-operations were also assessed. Occurrences of heterotopic ossifications (HOs) and of adjacent disc degeneration radiographic changes have been analyzed from 2-year FU X-rays. The mean NDI and VAS scores for arm and neck are reduced significantly at each post-operative time-point compared to pre-operative condition. Motion is preserved over the time at index levels (mean ROM = 9° at 2 years) and 85.5% of the segments are mobile at 2 years. HOs are responsible for the fusion of 6/76 levels at 2 years. However, presence of HO does not alter the clinical outcomes. The occurrence rate of radiological signs of ALD is very low at 2 years (9.1%). There has been no subsidence, no expulsion and no sub-luxation of the implant. Finally, after 2 years, 91% of the patients assume that they would undergo the procedure again. These intermediate results of TDR with Mobi-C® are very encouraging and seem to confirm the efficacy and the safety of the device. Regarding the preservation of the status of the adjacent levels, the results of this unconstrained device are encouraging, but longer FU studies are needed to prove it.
Keywords: Degenerative disc disease, Cervical total disc replacement, Motion, Ossification, Adjacent level degeneration, Clinical study
Introduction
The interest in cervical total disc replacement (TDR) is growing very rapidly, and this technique is becoming more and more popular as an alternative to the so-far gold standard in the surgical treatment of degenerative disc disease (DDD), e.g anterior cervical discectomy and fusion (ACDF) [1, 30]. Actually, cervical disc arthroplasty offers several theoretical and obvious advantages compared with ACDF [7, 15, 16, 26, 32]. Above all, many authors have established the fact that in ACDF, the stresses on adjacent levels above and below the fusion site may lead to higher incidence of degeneration and segmental instability [2, 12, 17–20, 34, 36]. On the contrary, but still theoretically, preservation of motion with TDR at the surgically treated level may potentially reduce the occurrence of adjacent-level degeneration (ALD) [10]. In addition, TDR avoids common issues associated with fusion, including bone harvesting, donor site pain, adverse events with the plating, or risk of pseudarthrosis.
Several prostheses with different components and kinematic designs are now available for clinical use [30], and their early results have been published [5, 6, 8, 11, 15, 16, 27, 28, 32, 40]. Most of them present 1-year follow-up (FU) results, a very few so far presenting results with 2 or more years [35–38].
Mobi-C® (LDR Medical, Troyes, France) was designed by a group of French orthopaedic and neuro-surgeons with two main objectives: attempt to replicate the normal cervical intervertebral disc motion as much as possible and develop a device with well-known materials and wear profile which is easily placed with a simple and reliable technique. Mobi-C® was CE marked in 2004. Two Korean groups have published their preliminary results in 2007 [22] and 2008 [29].
In the prosthesis designers’ country (France), a prospective clinical and radiological multicentre study is undergoing. 335 patients were operated between November 2004 and June 2008 in nine centres. We present here the intermediate results of this study for the first consecutive 76 patients with 24-month FU.
Clinical materials and methods
Description of the device
Mobi-C® is a three-piece non-constrained articulation with a polyethylene mobile nucleus moving between two plates, which are designed to be as anatomic as possible. The Mobi-C® represents a metal on polyethylene device. It is comprised of two spinal plates consisting of cobalt, chromium, 29 molybdenum alloy (CoCrMo, ISO 5832-12) and an ultra high molecular weight polyethylene (UHMWPE) mobile insert. The mobile insert is self-centring on the inferior endplate. Each movement of the superior plate induces the mobile insert to re-position on the inferior spinal plate.
The inner contact surface of the superior plate is spherical allowing a fully congruent contact area with the convex spherical dome of the mobile insert. The inner contact surface of the inferior plate is flat and contains two lateral stops that limit the mobility of the insert by contacting its lateral surface. The lateral stops also reduce the potential for expulsion of the insert. The self-centering mobile insert is believed to potentially preserve or restore the centres of rotation, which can participate in the long-term preservation of disc degeneration cascade in the adjacent levels. Both the superior and inferior spinal plates contain two rows of inclined-shaped teeth that are located laterally on each plate to ensure the primary fixation. A titanium and hydroxyapatite plasma spray coating is applied to the bony interface surfaces of the superior and inferior plates to encourage bony on-growth.
Thirteen different plate sizes (from 13 × 15 to 19 × 19, depth × width) and four different heights (4.5–7 mm) ensure for optimal anatomic congruency.
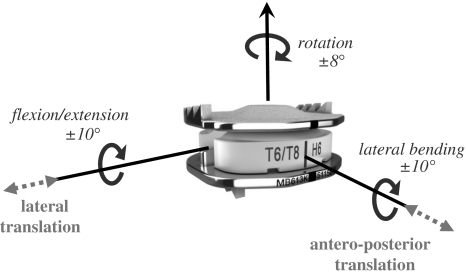
The device allows five independent degrees of freedom, two translational and three rotational, as illustrated below (Fig. 1).
Fig. 1.
The controlled motion of the Mobi-C® prosthesis. The five degrees of freedom are represented: two translational (dotted line arrows) and three rotational (full-line arrows)
Testing was conducted to determine the mechanical properties of the Mobi-C® prosthesis in accordance with applicable American Society for Testing and Materials (ASTM) standardised test methods. Durability and wear testing demonstrated a 0.08% mass loss or a total of 0.24 mm3 volumetric loss after 10 millions cycles.
All the other tests (static and dynamic axial and shear compression fatigue testing, static subsidence testing, static expulsion of mobile insert and of full device testing) were successful.
Operative technique
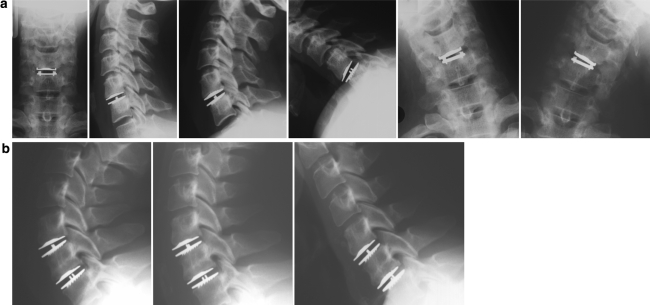
All the surgical procedures in this study were performed by senior spine surgeons. A standard approach is performed to the anterior cervical spine, with the neck in neutral position. After a thorough discectomy, the intersomatic space is distracted in a parallel way by a vertebral distractor. Once the height restoration is obtained, the distraction is maintained by the Caspar distractor, providing access to the posterior disc space. After complete disc material and osteophytes removal and neuro-foraminal decompression, the endplates are prepared without any shaping or chiselling. The debate about to resect or not the posterior longitudinal ligament (PLL) is not closed [23]. Rather than recommending systematic and complete PLL removal, we insist on performing a good release of the posterior part of the disc to ensure a parallel intervertebral space opening. Depth and width measurements allow the determination of the appropriate trial implant. The trials help to confirm the precise size of the implant, which is verified under fluoroscopic control. During this step, it is important not to exceed the height of the healthy adjacent discs in order to avoid facet joints over-distraction. The prosthesis is gently impacted into the disc space using a specific inserter. An adjustable stop allows precise adjustments of the implant anteroposterior position. The primary anchoring optimisation is obtained through compression with the Caspar distractor. An X-ray control (AP and lateral views) must confirm the adequate positioning of the implant (Fig. 2).
Fig. 2.
Neutral and dynamic post-operative X-rays of illustrative one-level C5–C6 prosthesis (a) and of a two-level C5–C6/C6–C7 case (b)
Clinical study design
This is a non-comparative, prospective and multicentre study, which aims to assess the safety and the efficacy of the Mobi-C® device in the treatment of cervical DDD. This study was performed across nine French centres, and involved 13 surgeons (orthopaedic and neuro-surgeons).
For the 335 patients enrolled to date, the indications were DDD at one or more levels between C3 and T1, leading to radiculopathy and/or myelopathy. Surgery was performed only after failure of appropriate conservative medical treatment. DDD was confirmed through cervical X-rays, CT or MRI.
Exclusion criteria are usual and include age (>65 years old), non-compliance with the study protocol, osteoporosis, metabolic bone disease, congenital or post-traumatic deformity, infection, neoplasia, instability of the intersomatic space, or a narrow canal (<12 mm).
Previous cervical spine surgery (including surgery at the index level) as well as workers’ compensations were not exclusion criteria. Patients were eligible for enrolment whatever their pre-operative NDI/VAS score (no minimal value was required). We did not exclude the learning curve cases of the study.
FU evaluation was performed at 1, 3, 6, 12, and 24 months after the index surgery. The study will be conducted for 10 years of FU.
Auto-evaluation was completed by the patient before and after the surgery at each FU visit and included visual analog scale (VAS 0–100), related to both neck and arm pain, neck disability index (NDI 0–100%) functional score, and SF-36 quality of life score. After the surgery, the patient also completed a satisfaction index.
Complications, analgesic requirements and employment status were also documented.
Statistical analysis of continuous data was made using Mann–Whitney non-parametric test. P < 0.05 was considered as significant.
Radiological evaluation considered dynamic (flexion/extension) lateral X-rays, pre-operatively and at each FU visit. Range of motion (ROM) has been measured at the index, the upper and the lower functional levels, with the SpineView® software (Surgiview, France).
The heterotopic ossifications (HOs) occurrence and the adjacent degenerative changes at 2-year FU have been evaluated on neutral and dynamic X-rays, by a senior spine surgeon (T.V), unaware of clinical outcomes and not involved in the clinical data collection during the study.
For HO, we have used the classification established by McAfee in 2003 [24, 25], and we modified it in relation to the ROM (we estimated that in classes III and IV of McAfee classification, motion is considered as blocked if ROM ≤3°).
Additionally, the occurrence of degenerative changes in the functional segments above and below the index level has been assessed 2 years after surgery, according to the Kellgren classification [21]. We report here both the occurrence of the degeneration of the initially healthy adjacent discs, and the evolution of the discs that were pre-operatively altered.
The healthy discs should have pre-operatively no ossification (Class 0 according to McAfee classification), no anterior and/or posterior osteophyte, even minimal, no hypermobility, and normal disc height relatively to other healthy discs of the spine. We considered conventionally a grade 0, which does not exist in the original Kellgren classification, for the so-called healthy discs [9]. A change from grade 0 to 1 or above was considered as a significant degenerative change for the healthy discs. For the pre-op altered discs (grade 1 or above), we considered as relevant a jump of 2 grades in the Kellgren classification.
Patients accountability
We report hereby the intermediate analysis of 76 patients, who completed their 24-month FU evaluation at the time the data bank was closed.
Occurrences of HO and of adjacent disc degeneration radiographic changes have been assessed from 2-year FU X-rays (earlier evaluation would not be pertinent for these end-points). X-rays from 68/76 patients have been collected and analysed for motion, HO and for degenerative changes at adjacent levels. Missing data include X-rays that were not available and those not measurable (off-centred films, shoulder overlay). This represents 76 analyzed segments (due to both single and double-level implantations in those 68 patients).
We consider the NDI as the primary clinical outcome criteria, and the success will be defined as an absolute improvement of the 2 years NDI score superior or equal to 15 pts from the pre-operative baseline value (if ≥30%).
Results
Demographics and surgical data
Mean age of the enrolled patients is 43.9-year-old (range 28–65), 46% (n = 35) are men and 54% (n = 41) are women.
About 86.5% of the patients had no previous cervical surgery, and 12.2% of the patients had a previous fusion.
A total of 85 prostheses have been implanted. 67/76 patients (88.2%) were operated with Mobi-C® at one level, and 9/76 patients (11.8%) at 2 levels, distributed as follows: C3–C4 (n = 1), C4–C5 (n = 6), C5–C6 (n = 32), C6–C7 (n = 27), C7–T1 (n = 1), C4–C5/C6–C7 (n = 1), C4–C5/C5–C6 (n = 2), C5–C6/C6–C7 (n = 6). 6/76 received an hybrid construct with adjacent fusion.
The mean operative duration was 88.6 min (range 45–240). The hospital stay duration averaged 3 days (range 1–6).
Clinical outcomes
-
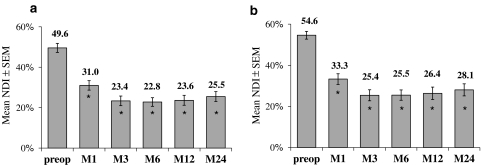
The mean NDI score was reduced significantly at each FU time-point compared to pre-operative condition (Fig. 3a) (P < 0.001). The absolute improvement at 2 years (defined as = NDIM24–NDIpre-op for each patient) averaged 24.3 pts.
Out of 76 patients 58 had a pre-operative NDI value superior or equal to 30%. The absolute improvement for these patients averaged 27 pts. In this subgroup, 42/58 patients (72%) improved their NDI score of at least 15 pts compared with pre-op baseline value, and mean scores are significantly decreased at each FU time-point versus pre-op (Fig. 3b).
-
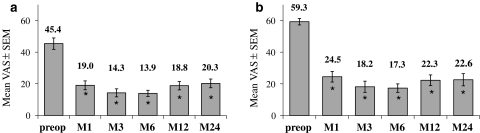
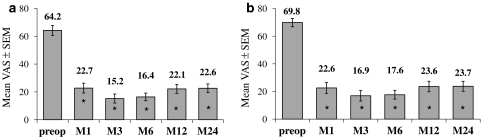
Regarding the VAS cervical pain, the mean score decreased significantly at each FU time-point compared to pre-op (Fig. 4a) (P < 0.001). The absolute improvement at 2 years (defined as = VASM24–VASpre-op for each patient) averaged 25.8 pts.
Out of 76 patients 50 had a pre-operative cervical pain VAS of at least 20 pts. The absolute improvement for these patients averaged 37 pts. In this sub-group, 36/50 patients (72%) decreased their cervical pain VAS score of at least 20 pts at 2 years compared to pre-operative baseline, and also the mean VAS score decreased significantly (P < 0.001) at all time-points compared with pre-op (Fig. 4b).
-
As for cervical pain, the mean VAS arm pain score decreased significantly at each FU time-point compared to pre-op (Fig. 5a). The absolute improvement at 2 years (defined as = VASM24–VASpre-op for each patient) averaged 41 pts.
Sixty-one out of 76 patients had a pre-operative arm pain VAS of at least 20 pts. The absolute improvement for these patients averaged 46.1 pts. In this subgroup, 47/61 patients (77%) improved their arm VAS of at least 20 pts compared to pre-operative baseline. There also, the mean VAS score decreased significantly (Fig. 5b).
The surgery thus leads to an improvement of the pain symptoms within a few weeks, and most importantly, the pain decrease is maintained over the time.
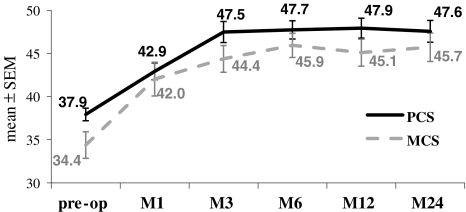
Consistent with these results on pain and function, quality of life is significantly improved after the surgery, as illustrated by SF-36 score (Fig. 6). Both physical and mental component scales (PCS and MCS, respectively) increased from early post-op, and this improvement goes on over the time course of the study.
Fig. 3.
Mean NDI score before and after the surgery (1, 3, 6, 12 or 24 months), for the 76 patients (a) and for the subgroup of patients with pre-op NDI ≥ 30% (b). Mean (±SEM) are given for each time-point. * Significant difference (P < 0.05) versus pre-op
Fig. 4.
Cervical VAS score before and after the surgery (1, 3, 6, 12 or 24 months), for the 76 patients (a) and for the subgroup of patients with pre-op cervical VAS ≥ 20 pts (b). Mean (±SEM) are given for each time-point. * Significant difference (P < 0.05) versus pre-op
Fig. 5.
Arm VAS score before and after the surgery (1, 3, 6, 12 or 24 months), for the 76 patients (a) and for the subgroup of patients with pre-op arm VAS ≥ 20 pts (b). Mean (±SEM) are given for each time-point. * Significant difference (P < 0.05) versus pre-op
Fig. 6.
SF-36 quality of life score before and after the surgery (1, 3, 6, 12 or 24 months). PCS physical component scale; MCS mental component scale. Mean (±SEM) are given for each time-point
Employment rate and analgesic use
The surgery strongly favours return to work. Employment rate (pooled partial/full-time jobs) increased after the surgery compared with pre-operative situation as shown in Table 1. Only 29.3% of the patients were at work pre-operatively versus 72.6% post-operatively at 2 years.
Table 1.
Employment rate of the patients before and 3 or 24 months after the Mobi-C® surgery
| Pre-op | 3-month FU | 24-month FU | |
|---|---|---|---|
| Out of work | 53.3% (40/75) | 32% (24/75) | 5.5% (4/73) |
| Working | 29.3% (22/75) | 54.7% (41/75) | 72.6% (53/73) |
| Inactive | 17.3% (13/75) | 13.3% (10/75) | 21.9% (16/73) |
Additionally, when returning to work, 75% of the patients keep the same job as before the surgery.
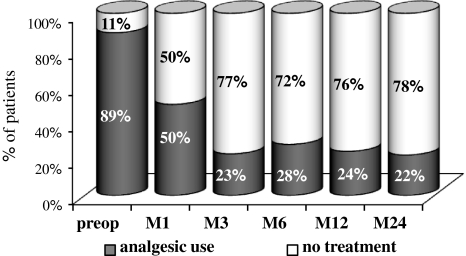
Analgesic use also strongly decreased: before the surgery 89.4% (59/66) of the patients were under medical treatment for pain, versus 22.2% (14/63) at 2-year FU (Fig. 7).
Fig. 7.
Analgesic requirement in the overall population before and after the surgery (1, 3, 6, 12 or 24 months)
Adverse events
Among the 76 patients, 10 (13.2%) meet at least one adverse event (requiring or not re-intervention, device or surgery-related, or secondary cervical surgery).
Device or surgery-related
- Without re-intervention: 8/76 (10.5%).
- Dysphagia and/or dysphonia have been reported in three patients: two were transient, but one slight dysphagia persisted 2 years after the surgery.
- One patient had persistent local sensitive troubles of the upper limbs extremities.
- One local hematoma and one cerebrospinal fluid leak have been reported without major clinical consequence or re-intervention.
- One contra-lateral shoulder girdle tendonitis, spontaneously reversible, has been noted, and was due to the per-operative patient positioning.
- In one case, planned for a 2-level arthroplasty, a vertebral body fracture occurred during distraction step: this level had to be fused, and the other level was implanted with Mobi-C®.
- One patient required foraminal infiltration at the index level.
- With re-intervention: 1/76 (1.3%)
- One local hematoma required immediate re-intervention for evacuation.
Secondary cervical surgeries
One out of 76 patients (1.3%) was re-operated at an adjacent level; he underwent arthrodesis (foraminal stenosis) at the level below Mobi-C® after 26 months. At 24-month FU, the NDI improvement of this patient was <15 pts compared to the pre-operative value; thus this patient did not meet the success criteria.
Radiographic results at 2-year follow-up
As explained above, radiological evaluation has been performed on 68 patients (corresponding to 76 implanted levels) due to not available or not measurable X-rays.
Range of motion (ROM)
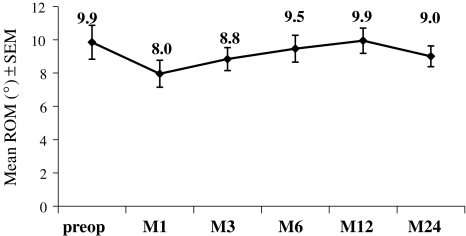
Radiological evaluation has shown at least the ability of the device to preserve the segmental motion, as flexion/extension ROM of the treated levels averaged 9.9° (range 0–24°) pre-operatively, and 9.0°(range 0–24°) at 2-year FU. The motion is maintained over the time, as illustrated in Fig. 8.
Fig. 8.
Mean range of motion (ROM) from flexion/extension at the index level before and after the surgery (1, 3, 6, 12 or 24 months). Mean (±SEM) are given for each time-point
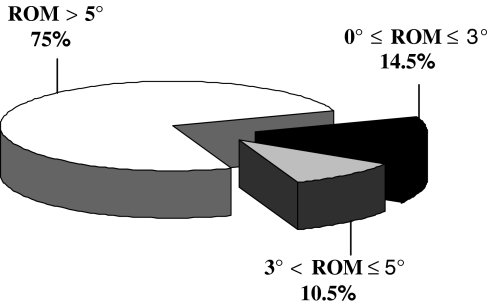
Moreover, 85.5% (65/76) of the treated levels were mobile (ROM > 3°) at 2-year FU (Fig. 9).
Fig. 9.
Distribution of the ROM at the index levels measured 2 years after the surgery. Results are expressed as percent of the 76 analyzed segments
Additionally the measurement of the ROM at the adjacent levels did not show any significant difference at 2 years versus pre-operatively. At the level above, ROM averaged 11.6 ± 1.1° and 11.6 ± 0.6° pre-operatively and after 2 years, respectively (P > 0.05). At the level below, ROM averaged 7.4 ± 1.0° and 7.6 ± 0.8° pre-operatively and at 2 years, respectively (P > 0.05).
Heterotopic ossifications (HOs) at the index level 2 years after the surgery
HO occurrence 2 years after the surgery has been assessed according to McAfee classification; 76 index levels (from 68 patients) were exploitable (Table 2).
Table 2.
Distribution of the implanted levels 2 years after the surgery according to the McAfee classification for HO
| Number of levels (%) | |
|---|---|
| Class 0 | 25 (32.9) |
| Class I | 9 (11.8) |
| Class II | 33 (43.4) |
| Class III | 3 (3.9) |
| Class IV | 6 (7.9) |
Taken together, classes 0, I, and II represent 88.1% of the index levels and 97% of these levels (65/67) have a ROM >3° at 2 years. Thus, although bone presence outside or inside the discal space, motion is not affected, even after 2 years.
Considering the classes IV (6 levels) and III (3 levels), they can be regarded as non-functional prostheses, as motion is blocked (ROM ≤3°). However, in these eight patients (9 levels), clinical outcomes are not altered compared to overall population, even at 2-year FU (data not shown).
Evolution of the adjacent levels at 2 years
In this study, radiological signs of degenerative changes at the upper and lower functional adjacent levels have been assessed using the Kellgren classification in the 68 patients. It means theoretically 136 levels to be analyzed, but because of the same technical difficulties as in HO assessment at the index levels, only 110 levels could be evaluated.
The results are the following: 5/65 upper adjacent levels show a slight degradation (4 from grade 0 to grade 1, 1 from grade 0 to grade 2) at 2-year FU, and the same was observed for 5/45 lower adjacent levels.
For those altered in pre-op, none were found to have a two-grade increase.
It means 10 new degenerative changes/110 levels at 2-year FU (9.1%).
Success rate
Seventy-two percent of the patients (42/58) at 2-year FU met the success criteria (defined as pre-op NDI superior or equal to 30% with a decrease of 15 pts or more at 2 years).
Satisfaction index
Ninety-one percent (61/67) of the patients at 2 years declared that they would undergo the surgery again (9 patients did not answer, 5 said that they would not, 1 declared he did not know).
Discussion
There is general agreement among spine surgeons to consider TDR as an efficient and reliable treatment for cervical DDD based on clinical outcomes and patient benefits.
Furthermore, this alternative to ACDF is considered by many as a potentiality to give better chances to the patient regarding adjacent disc preservation [34, 41] mostly due to the motion preservation not only at the index level but also at adjacent levels [33, 36]. Additionally, accumulating data suggests that TDR is associated with a lower complication rate than ACDF [18–20, 26].
Regarding the different devices currently available, authors generally publish encouraging results, in terms of clinical outcomes, rate of complications, and preservation of motion at the index level [5, 6, 8, 11, 15, 16, 27, 28, 32, 40]. Our study with Mobi-C® which is still undergoing shows very similar good results and confirms the encouraging clinical and radiological outcomes, obtained in a former preliminary study [3, 4, 39]. As written above, we did exclude neither the learning curve cases nor the worker’s compensation cases, nor the patients with previous arthrodesis of the cervical spine as it is the case in some studies. In that sense, we avoid the bias of “too well-selected patients”, by preserving as much as possible the conditions of recruitment as in the “real life” situation. Clinically, as shown by the VAS and the NDI scores, the clinical and functional improvements are relevant and statistically significant. They appear early in the post-operative period and are maintained over the time course of the study, up to 2-year FU.
In this study, considering the adverse event issue, we did observe neither vertebral body fracture, nor sub-luxation, nor subsidence, nor migration. This is probably in relation with the “no-keel” design of Mobi-C® which furthermore seems to allow the multilevel placement in safe conditions.
About the case with surgery at an adjacent level, the evolution was favourable; at the 3-year FU visit, pain was completely abolished, with NDI/VAS equal to 0.
There has been no re-operation at the index level in our population of 76 patients.
However, we have to report the case of a patient, who underwent prosthesis removal and arthrodesis 3 months after the initial surgery, because of untractable neck pain and on the behalf of the patient.
This patient did not perform the 24-month FU evaluation at the time the data bank was closed, and was thus not included in our radio-clinical analysis. But it has to be mentioned.
On a radiological point of view, when considering the ROM at the index level, motion is maintained at 2-year FU. The same situation is observed for the upper and lower adjacent functional levels.
Concerning the adjacent levels evolution 2 years after the surgery, the results seem to be also quite favourable. In 2005, Robertson et al. [34] compared two independent prospective clinical studies, one with ACDF (n = 158 patients), the other with TDR (n = 74 patients), both with two years FU. In those two studies, the appearance of new radiological degenerative changes or the increase of existing DDD radiological signs (new or increase narrowing of disc space, appearance or enlargement of osteophytes, new or increase ALL calcifications) were assessed at levels adjacent to a single level surgery. Only after the X-ray evaluation were the clinical symptoms related to those changes evaluated. The appearance of radiographic modifications occurred in 17.5% of patients of the TDR population and in 34.6% of the ACDF series. A new DDD was defined by this author as a narrowing of the disc space superior or equal to 30%, and he excluded the posterior osteophytes from the evaluation. In our study, was considered as a sign of a new DDD every single new calcification (McAfee class I) or anterior/posterior osteophyte, even minimal (Kellgren grade 1), that one could consider as a much more strict criteria of degenerative change. Despite this, the occurrence of degenerative change of the adjacent levels in our study is only 9.1% at 2-year FU, which seems quite encouraging preliminary results, compared to fusion, and compared to another unconstrained device for TDR. Our preliminary results suggest the ability of arthroplasty with Mobi-C® to decrease the occurrence of ALD in surgical treatment of DDD. Actually there is, for the authors, a strong belief in the ability of this unconstrained device, thanks to its controlled-mobility core and self-positioning capacities, to enforce the possibility of adjacent levels preservation, but it must be confirmed by further long-term studies.
There is a growing concern about the HO in the field of cervical TDR, but the incidence is unknown and accurate tools to evaluate it are still unavailable. Mehren et al. [25] reported results of a part of a European multicentre prospective study. In this cervical study, this author used a modified McAfee HO classification, to assess the radiographs of 54 patients with TDR (77 segments), 1 year after the surgery [25]. Clinical status was assessed with VAS and NDI scores pre- and post-operatively. This study has shown that classes 0, I, II, III, and IV represented, respectively, 33.8, 7.8, 39, 10.4, and 9.1% of the levels. The clinical outcomes improved significantly. In our study, we have a lower proportion of classes III or IV in quite close demographic situation.
Compared to the original McAfee classification, we conventionally defined more accurately the mobility of class III (the so-called “blocked” segments) by increasing its range of motion (up to 3°). By this way, we have probably over-estimated the class III population and under-estimated the class II. Despite that, we reported 9/76 class III and IV segments, versus 15/77 in the Mehren et al. report and this, at 2-year FU in our study, versus 1 year in the Mehren et al. report [25].
The aetiology of HO and its favouring factors are still unknown. Regarding the way to prevent the phenomenon, we can take into account the studies in total hip replacement [13, 14]. In our study, the choice of the use of post-operative non steroidal anti-inflammatory drugs (NSAID) was left to the attending surgeon. We found that among the 48 patients with HO of all classes at 2-year FU, 31 (64%) did not receive any NSAID therapy versus 8 (17%) who had NSAID and 9 (18.7%) for which the data are missing. On the contrary, among the 20 patients without any HO, 60% received a post-operative NSAID therapy. Those results are consistent with the Mehren study [25], and even if more accurate assessment is mandatory, this finding seems relevant and should be taken into account for future patients. The HO appears generally within the first year post-op, and does not seem to increase after 1 year of evolution. Most of all, in our study like in the limited literature on the subject [25, 31, 38], HO has no any negative influence on the clinical outcomes, pain and functional scores.
Conclusion
These intermediate results of TDR with Mobi-C® are very encouraging. Our data with 2-year FU demonstrate the efficacy and the safety of the device and confirm our preliminary results. Both pain and function are significantly improved after 2 years, and there is no pain recurrence at this last FU. Vertebral body fracture by the device, as well as device subsidence, expulsion or sub-luxation has never been reported in our experience. With 85.5% of mobile operated segments after 2 years, the Mobi-C® device maintains a physiological segmental motion in the majority of the cases. In addition, this is the most challenging issue of TDR; our study shows that motion is also maintained at adjacent levels, which are mostly preserved of DDD radiological signs occurrence. Of course, long-term studies will be needed to fully assess the future of operated spine and the ability of arthroplasty with Mobi-C® to provide the good answers to numerous questions asked by treatment of cervical DDD.
Acknowledgments
We are grateful to the following co-investigators, who participated in the study: P. DAM-HIEU, MD, Brest, France; N. GANGNET, MD, Bordeaux, France; P. MANGIONE, MD, Pessac, France; H. PERSON, MD, Brest, France; J. STECKEN, MD, Orléans, France.
References
- 1.Anderson P, Sasso RC, Riew KD. Update on cervical artificial disk replacement. Instr Course Lect. 2007;56:237–245. [PubMed] [Google Scholar]
- 2.Baba H, Furusawa N, Imura S, Kawahara N, Tsuchiya H, Tomita K. Late radiographic findings after anterior cervical fusion for spondylotic myeloradiculopathy. Spine. 1993;18:2167–2173. doi: 10.1097/00007632-199311000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Beaurain J, Bernard P, Dufour T, Fuentes JM, Hovorka I, Huppert J, Steib JP, Vital JM (2008) Mobi-C. In : Yue JJ, Bertagnoli R, McAfee P, An HS (eds) Motion preservation surgery of the spine: advanced techniques and controversies. Part III, Chap 27, Elsevier, Philadelphia, pp 231–237
- 4.Bernard P, Vital JM, Dufour T, Beaurain J, Fuentes JM, Huppert J, Hovorka I (2006) A new cervical disc prosthesis: Mobi-C. Preliminary results of a prospective study. In: Szpalski M, Gunzburg R, Le Huec JC, Brayda-Bruno M (eds) Non-fusion technologies in spine surgery. Lippincott Williams & Wilkins, pp 253–260
- 5.Bertagnoli R, Yue J, Pfeiffer F, Fenk-Mare A, Lawrence J, Kershaw T, Nanieva R. Early results after Prodisc-C cervical disc replacement. J Neurosurg Spine. 2005;2:403–410. doi: 10.3171/spi.2005.2.4.0403. [DOI] [PubMed] [Google Scholar]
- 6.Bryan V. Cervical motion segment replacement. Eur Spine J. 2002;11:S92–S97. doi: 10.1007/s00586-002-0389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummins BH, Robertson JT, Gill SS. Surgical experience with an implanted artificial cervical joint. J Neurosurg. 1998;88(6):943–948. doi: 10.3171/jns.1998.88.6.0943. [DOI] [PubMed] [Google Scholar]
- 8.Cappuccino A, Bellera F. Clinical experience with the new PCM (Cervitech) Disc. Spine J. 2004;4:315S–321S. doi: 10.1016/j.spinee.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Cote P, Cassidy JD, Yong-Hing K, Sibley J, Loewy J. Apophysial joint degeneration, disc degeneration, and sagittal curve of the cervical spine. Can they be measured reliably on radiographs? Spine. 1997;22:859–864. doi: 10.1097/00007632-199704150-00007. [DOI] [PubMed] [Google Scholar]
- 10.Dmitriev A, Cunningham B, Nianbin H, Sell G, Vigna F, McAfee P. Adjacent level intradiscal pressure and segmental kinematics following a cervical total disc arthroplasty: an in vitro human cadaveric model. Spine. 2005;30:1165–1172. doi: 10.1097/01.brs.0000162441.23824.95. [DOI] [PubMed] [Google Scholar]
- 11.Duggal N, Pickett G, Mitsis D, Keller J. Early clinical and biomechanical results following cervical arthroplasty. Neurosurg Focus. 2004;17:E9. doi: 10.3171/foc.2004.17.3.9. [DOI] [PubMed] [Google Scholar]
- 12.Eck JC, Humphreys SC, Lim TH, Jeong ST, Kim JG, Hodges SD, An HS. Biomechanical study on the effect of cervical spine fusion on adjacent-level intradiscal pressure and segmental motion. Spine. 2002;27:2431–2434. doi: 10.1097/00007632-200211150-00003. [DOI] [PubMed] [Google Scholar]
- 13.Fijn R, Koorevaar RT, Brouwers JR. Prevention of heterotopic ossification after total hip replacement with NSAIDs. Pharm World Sci. 2003;25:138–145. doi: 10.1023/A:1024830213832. [DOI] [PubMed] [Google Scholar]
- 14.Fransen M, Neal B. Non-steroidal anti-inflammatory drugs for preventing heterotopic bone formation after hip arthroplasty. Cochrane Database Syst Rev. 2004;3:CD001160. doi: 10.1002/14651858.CD001160.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Goffin J, Casey A, Kehr P, Liebig K, Lind B, Logroscino C, Pointillart V, Calenbergh F, Loon J. Preliminary clinical experience with the Bryan cervical disc prosthesis. Neurosurgery. 2002;51:840–845. doi: 10.1097/00006123-200209000-00048. [DOI] [PubMed] [Google Scholar]
- 16.Goffin J, Calenbergh F, Loon J, Casey A, Kehr P, Liebig K, Lind B, Logroscino C, Sgrambiglia R, Pointillart V. Intermediate follow-up after treatment of degenerative disc disease with the Bryan cervical disc prosthesis: single-level and bi-level. Spine. 2003;28:2673–2678. doi: 10.1097/01.BRS.0000099392.90849.AA. [DOI] [PubMed] [Google Scholar]
- 17.Gore D, Sepic S, Gardner G. Roentgenographic findings of the cervical spine in asymptomatic people. Spine. 1986;11:521–524. doi: 10.1097/00007632-198607000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Harrop JS, Youssef JA, Maltenfort M, Vorwald P, Jabbour P, Bono CM, Goldfarb N, Vaccaro AR, Hilibrand AS. Lumbar adjacent segment degeneration and disease after arthrodesis and total disc arthroplasty. Spine. 2008;33:1701–1707. doi: 10.1097/BRS.0b013e31817bb956. [DOI] [PubMed] [Google Scholar]
- 19.Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519–528. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4:190S–194S. doi: 10.1016/j.spinee.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Kettler A, Wilke H-J. Review of existing grading systems for cervical or lumbar disc and facet joint degeneration. Eur Spine J. 2006;15:705–718. doi: 10.1007/s00586-005-0954-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SH, Shin HC, Shin DA, Kim KN, Yoon DH. Early clinical experience with the Mobi-C disc prosthesis. Yonsei Med J. 2007;48:457–464. doi: 10.3349/ymj.2007.48.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAfee PC, Cunningham BW, Dmitriev A, Hu N, Woo KS, Cappuccino A, Pimenta L. Cervical disc replacement-porous coated motion prosthesis: a comparative biomechanical analysis showing the key role of the posterior longitudinal ligament. Spine. 2003;28:S176–S185. doi: 10.1097/01.BRS.0000092219.28382.0C. [DOI] [PubMed] [Google Scholar]
- 24.McAfee PC, Cunningham BW, Devine J, Williams E, Yu-Yahiro J. Classification of heterotopic ossification (HO) in artificial disk replacement. J Spinal Disord Tech. 2003;16:384–389. doi: 10.1097/00024720-200308000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Mehren C, Suchomel P, Grochulla F, Barsa P, Sourkova P, Hradil J, Korge A, Mayer HM. Heterotopic Ossification in Total Cervical Artificial Disc Replacement. Spine. 2006;31:2802–2806. doi: 10.1097/01.brs.0000245852.70594.d5. [DOI] [PubMed] [Google Scholar]
- 26.Mummaneni PV, Burkus JK, Haid RW, Traynelis VC, Zdeblick TA. Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: a randomized controlled clinical trial. J Neurosurg Spine. 2007;6:198–209. doi: 10.3171/spi.2007.6.3.198. [DOI] [PubMed] [Google Scholar]
- 27.Nabhan A, Ahlhelm F, Pitzen T, Steudel WI, Jung J, Shariat K, Steimer O, Bachelier F, Pape D. Disc replacement using Pro-Disc C versus fusion: a prospective randomised and controlled radiographic and clinical study. Eur Spine J. 2007;16:423–430. doi: 10.1007/s00586-006-0226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nabhan A, Ahlhelm F, Shariat K, Pitzen T, Steimer O, Steudel WI, Pape D. The ProDisc-C prothesis clinical and radiological experience 1 year after surgery. Spine. 2007;32:1935–1941. doi: 10.1097/BRS.0b013e31813162d8. [DOI] [PubMed] [Google Scholar]
- 29.Park JH, Roh KH, Cho JY, Ra YS, Rhim SC, Noh SW. Comparative analysis of cervical arthroplasty using Mobi-C® and anterior cervical discectomy and fusion using the solis®-cage. J Korean Neurosurg Soc. 2008;44:217–221. doi: 10.3340/jkns.2008.44.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips MF, Garfin SR. Cervical disc replacement. Spine. 2005;30:S27–S33. doi: 10.1097/01.brs.0000175192.55139.69. [DOI] [PubMed] [Google Scholar]
- 31.Pickett GE, Sekhon LH, Sears WR, Duggal N. Complications with cervical arthroplasty. J Neurosurg Spine. 2006;4:98–105. doi: 10.3171/spi.2006.4.2.98. [DOI] [PubMed] [Google Scholar]
- 32.Porchet F, Metcalf N. Clinical outcomes with the Prestige II Cervical Disc: preliminary results from a prospective randomized clinical trial. Neurosurg Focus. 2004;17:E6. doi: 10.3171/foc.2004.17.3.6. [DOI] [PubMed] [Google Scholar]
- 33.Puttlitz CM, Rousseau MA, Xu Z, Hu S, Tay BK, Lotz JC. Intervertebral disc replacement maintains cervical spine kinetics. Spine. 2004;29:2809–2814. doi: 10.1097/01.brs.0000147739.42354.a9. [DOI] [PubMed] [Google Scholar]
- 34.Robertson JT, Papadopoulos SM, Traneylis VC. Assessment of adjacent-segment disease in patients treated with cervical fusion or arthroplasty: a prospective 2-year study. J Neurosurg Spine. 2005;3:417–423. doi: 10.3171/spi.2005.3.6.0417. [DOI] [PubMed] [Google Scholar]
- 35.Robertson JT, Metcalf NH. Long-term outcome after implantation of the Prestige I disc in an end-stage indication: 4 year results from a pilot study. Neurosurg Focus. 2004;17:E10. doi: 10.3171/foc.2004.17.3.10. [DOI] [PubMed] [Google Scholar]
- 36.Sasso RC, Best NM. Cervical Kinematics after fusion and Bryan Disc Arthroplasty. J Spinal Disord Tech. 2008;21:19–22. doi: 10.1097/BSD.0b013e3180500778. [DOI] [PubMed] [Google Scholar]
- 37.Sekhon L. Cervical arthroplasty in the treatment of spondylotic myelopathy: 18-month results. Neurosurg Focus. 2004;17(3):E8. doi: 10.3171/foc.2004.17.3.8. [DOI] [PubMed] [Google Scholar]
- 38.Sola S, Hebecker R, Knoop M, Mann S. Bryan cervical disc prosthesis—three years follow-up. Eur Spine J. 2005;14(Suppl 1):38. [Google Scholar]
- 39.Vital JM, Pointillart V, Gille O, Aurouer N. Les prothèses cervicales constituent-elles un réel progrès dans la pathologie dégénérative? e-mémoires de l’Académie Nationale de Chirurgie. 2007;6(3):45–50. [Google Scholar]
- 40.Wigfield C, Gill S, Nelson R, Metcalf N, Robertson J. The new Frenchay artificial cervical joint: results from a two-year pilot study. Spine. 2002;27:2446–2452. doi: 10.1097/00007632-200211150-00006. [DOI] [PubMed] [Google Scholar]
- 41.Wigfield C, Gill S, Nelson R, Langdon I, Metcalf N, Robertson J. Influence of an artificial cervical joint compared with fusion on adjacent-level motion in the treatment of degenerative cervical disc disease. J Neurosurg. 2002;96(Suppl 1):17–21. doi: 10.3171/spi.2002.96.1.0017. [DOI] [PubMed] [Google Scholar]