Summary
The groundbreaking discovery about arterial and venous expression of ephrinB2 and EphB4, respectively, in early embryonic development has led to a new paradigm for vascular research, providing compelling evidence that arterial and venous endothelial cells are established by genetic mechanisms before circulation begins. For arterial specification, vascular endothelial growth factor (VEGF) induces expression of Notch signaling genes, including Notch1 and its ligand, Delta-like 4 (Dll4), and Foxc1 and Foxc2 transcription factors directly regulate Dll4 expression. Upon activation of Notch signaling, the Notch downstream genes, Hey1/2 in mice or gridlock in zebrafish, further promote arterial differentiation. On the other hand, the orphan nuclear receptor COUP-TFII is a determinant factor for venous specification by inhibiting expression of arterial specific genes, including Nrp1 and Notch. After arterial and venous endothelial cells differentiate, a subpopulation of venous endothelial cells is thought to become competent to acquire lymphatic endothelial cell fate by progressively expressing the transcription factors Sox18 and Prox1 to differentiate into lymphatic endothelial cells. Therefore, it has now evident that arterial-venous cell fate determination and subsequent lymphatic development are regulated by the multi-step regulatory system associated with the key signaling pathways and transcription factors. Furthermore, new signaling molecules as additional regulators in these processes have recently been identified. As the mechanistic basis for a link between signaling pathways and transcriptional networks in arterial, venous and lymphatic endothelial cells begins to be uncovered, it is now time to summarize the literature on this exciting topic and provide perspectives for future research in the field.
Keywords: Arterial-venous specification, lymphatic specification, VEGF, Notch, Fox
Introduction
During vascular development, angioblasts, which are multipotent endothelial progenitors originating from the mesoderm, coalesce and undergo vasculogenesis to form the primitive capillary plexus. Angiogenesis is the subsequent process of vascular remodeling and gives rise to a mature network of blood vessels including arteries and veins. This process is regulated in part by hemodynamic forces. However, recent studies in zebrafish and mice clearly demonstrate that in the developing embryo, arterial and venous identity is established by genetic mechanisms, including VEGF, Notch and ephrinB2, before circulation begins (Aitsebaomo et al., 2008; Hong et al., 2008; Swift and Weinstein, 2009). After arteriovenous diversification, a subpopulation of the venous cells acquires lymphatic cell fate by progressively expressing Sox18 and Prox1 and differentiates into lymphatic endothelial cells. This process leads to the formation of the second vascular network, the lymphatic vasculature. Despite recent advances toward understanding genetic programming in vascular development, there are many important questions that remain unanswered. In particular, the mechanisms underlying differential gene regulation and critical signaling pathways that are crucial for arterial, venous and lymphatic specification need to be determined. In this review, I will summarize the current understanding of endothelial cell specification during early embryonic development. A comprehensive summary of critical factors involved in arterial, venous and lymphatic specification during vascular development is given in Table 1.
Table 1.
Factors involved in regulating arterial, venous and lymphatic cell fates
| Factor | Phenotype/Role | References |
|---|---|---|
| Arterial identity | ||
| Shh | Loss of Shh results in lack of arterial identity in zebrafish. Shh acts upstream of VEGF. | Lawson et al., 2002 |
| VEGF | VEGF acts downstream of Shh signaling to activate Notch via the PLCγ/ERK pathway in zebrafish. Mutant mice expressing only VEGF188 lack arterial differentiation. |
Lawson et al., 2002; Stalmans et al., 2002 Hong et al., 2006; Covassin et al., 2009 |
| Nrp1 | Null mice display impaired arterial differentiation. Nrp1 is involved in a positive feedback loop of VEGF signaling. |
Jones et al., 2008; Mukouyama et al., 2005 |
| Notch | Notch acts downstream of Shh and VEGF signaling in zebrafish. Notch1; Notch4 mutant mice have abnormal vascular development. |
Lawson et al., 2001; Lawson et al., 2002 Krebs et al., 2000 |
| Dll4 | Null mice lack arterial specification. |
Duarte et al., 2004; Gale et al., 2004; Krebs et al., 2004 |
| Dll1 | Null mice fail to maintain arterial identity. | Sorensen et al., 2009 |
| Hey1/2 (Grl) |
Null mice lack arterial specification. Lack of grl in zebrafish results in loss of arterial specification. |
Zhong et al., 2001; Fisher et al., 2004 Kokubo et al., 2005 |
| Foxc1/c2 | Foxc1; Foxc2 mutant mice lack arterial specification. Foxc1 and Foxc2 directly regulate Dll4 and Hey2 expression. Foxc1 and Foxc2 are also involved in lymphatic vessel development. |
Kume et al., 2001; Seo et al., 2006 Hayashi and Kume, 2008 |
| Sox7/18 | Lack of Sox7/18 results in loss of arterial identity in zebrafish. |
Cermenati et al., 2008; Herpers et al., 2008 Pendeville et al., 2008 |
| Snrk-1 | Snrk-1 acts downstream or parallel to Notch signaling in zebrafish. | Chun et al., 2009 |
| Dep1 | Dep1 acts upstream of PI3K in arterial specification in zebrafish. | Rodriguez et al., 2008 |
| Crlr | Shh regulates VEGF activity by controlling crlr expression in zebrafish. | Nicoli et al., 2008 |
| EphrinB2 | Null mice lack boundaries between arteries and veins. EphrinB2 is involved in lymphatic vascular remodeling and maturation. |
Wang et al., 1998; Adams et al., 1999; Gerety and Anderson, 2002; Makinen et al., 2005 |
| Venous identity | ||
| COUP-TFII | COUP-TFII suppresses arterial cell fate by inhibiting Nrp1 and Notch. COUP-TFII also interacts with Prox1 to regulate lymphatic gene expression. |
You et al., 2005; Lee et al., 2009 Yamazaki et al., 2009 |
| EphB4 | Null mice lack boundaries between arteries and veins. | Gerety et al., 1999 |
| Lymphatic identity | ||
| Sox18 | Null mice fail to specify lymphatic endothelial cells. Sox18 induces Prox1 expression. | Francois et al., 2008 |
| Prox1 | Prox1 induces lymphatic markers and maintains lymphatic cell identity. |
Wigle and Oliver, 1999; Wigle et al., 2002 Hong et al., 2002; Petrova et al., 2002 Johnson et al., 2008 |
EphrinB2/EphB4
Given that arteries and veins are morphologically and physiologically distinct, it was, until recently, believed that they are formed from primitive blood vessels according to hemodynamic changes and physiological factors. However, seminal studies demonstrate that ephrinB2 and its receptor EphB4, members of the ephrin-Eph receptor tyrosine kinase family, are differentially expressed in arterial and venous endothelial cells of the early mouse embryo, respectively (Wang et al., 1998). Surprisingly, the expression of ephrinB2 and EphB4 is distinctively detected in the primary vascular plexus before the onset of circulation in the developing embryo. This provides the first evidence that arterial-venous identity is genetically predetermined, although several lines of evidence suggest there is local environments (e.g. hemodynamic forces) influences plasticity of arterial-venous identity (Moyon et al., 2001; Othman-Hassan et al., 2001; le Noble et al., 2004).
Consistent with the notion that ephrin-Eph signaling can be bidirectional, mice deficient for ephrinB2 and EphB4 have similar vascular defects in remodeling of primary capillary vessels into a mature vascular network composed of arteries and veins (Wang et al., 1998; Adams et al., 1999; Gerety et al., 1999; Gerety and Anderson, 2002). Importantly, these mutant mice do not have clear boundaries between arterial and venous vessels, indicating that ephrinB2-EphB4 signaling is required for the establishment and maintenance of arterial-venous interaction. However, the lack of ephrinB2 or EphB4 does not impair the specification of arteries and veins per se, and this observation led to the identification of other factors that act upstream of ephrinB2-EphB4 signaling in arterial-venous cell determination, as discussed below.
Arterial specification
Hedgehog
Sonic hedgehog (Shh) is a member of the Hedgehog family and is involved in numerous aspects of embryonic development. Shh acts as a secreted molecule and transmit its signal into recipient cells via the transmembrane receptor Patched (Ptc) and the G protein-coupled receptor Smoothened (Smo). Studies in the zebrafish have shown that Shh secreted by the notochord at the midline of the developing embryo induce arterial cell fate in angioblasts (Lawson et al., 2002). Zebrafish embryos lacking Shh signaling show loss of arterial expression of ephrinB2 and have a single axial vessel expressing venous markers, suggesting that Shh is required for arterial identity. On the other hand, exogenous expression of Shh in the zebrafish embryo induces ectopic formation of arterial endothelial cells expressing ephrinB2 within the trunk vessels. The effect of Shh on arterial identity in zebrafish is indirect by inducing expression of vascular endothelial growth factor (VEGF) in the adjacent somites. As described below, VEGF signaling acts downstream of Shh and in turn activates Notch signaling in endothelial cells to promote the arterial program (Lawson et al., 2001; Lawson et al., 2002; Lawson et al., 2003) (Fig. 1).
Fig. 1.
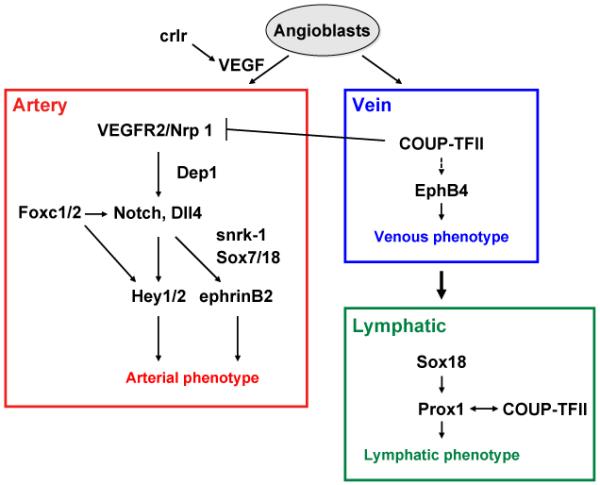
A model for molecular pathway for arterial, venous and lymphatic cell fate determination in vertebrate embryo. The VEGF-VEGFR2/Nrp1 pathway activates Notch signaling in angioblasts, leading to the specification of arterial cell fate, while crlr controls VEGF expression. Dep1 acts upstream of the VEGF-activated PI3K pathway. Foxc1 and Foxc2 directly regulate Dll4 and Hey2 expression, presumably downstream of VEGF signaling. Sox7/18 and snrk-1 may act upstream of grl (the Hey2 homolog in zebrafish). EphrinB2 is a downstream target of Notch. In venous endothelial progenitors, COUP-TFII suppresses Nrp1 and Notch activation and promotes venous cell fate. A subpopulation of venous endothelial cells subsequently expresses Sox18 and Prox1, thereby inducing lymphatic cell fate. COUP-TFII also contributes to lymphatic gene expression by interacting with Prox1.
Although the role of Shh signaling in arterial specification has been well demonstrated in zebrafish, it is still unclear whether the mammalian homologues have similar functions during development. Shh mutant mice do not exhibit severe vascular defects, although they show reduced vascularization in the developing lung (van Tuyl et al., 2007). Smo mutant mice have defects in formation of the dorsal aorta and remodeling of the yolk sac vasculature (Byrd et al., 2002; Vokes et al., 2004). Although further examinations of the vascular phenotype in Smo mutants need to be performed, it is possible that murine Shh signaling is dispensable for arterial-venous specification.
VEGF
VEGF signaling is the best-known pathway that regulates the formation and morphogenesis of blood and lymphatic vessels in development and disease (Lohela et al., 2009). In zebrafish, loss of VEGF results in lack of arterial expression of ephrinB2, consistent with the observation that loss of Shh signaling leads to lack of VEGF expression in the somites. Importantly, overexpression of VEGF in the zebrafish embryo induces ectopic expression of arterial markers as seen in embryos overexpressing Shh and can rescue arterial expression in embryos lacking Shh signaling (Lawson et al., 2002). Zebrafish experiments including forward genetic screening have further revealed critical pathways mediated by VEGF in arterial cell fate determination. While the zebrafish VEGF receptor Kdrl plays a major role in arterial development (Covassin et al., 2006; Covassin et al., 2009), phospholipase C (PLC)-γ1, an immediate downstream component of the VEGF receptors, is required for transducing VEGF signaling (Lawson et al., 2003; Covassin et al., 2009). Furthermore, phenotype-based small-molecule chemical screening on zebrafish gridlock mutant embryos described below have provided evidence that a crosstalk between VEGF-activated intracellular signaling pathways, the phosphoinositide 3-kinase (PI3K) and the extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) signaling pathways, antagonistically regulate arterial-venous specification during development (Peterson et al., 2004; Hong et al., 2006; Hong et al., 2008).
Homozygous and heterozygous mutant mice for VEGF display embryonic lethality associated with cardiovascular defects, including abnormal blood vessel formation (Carmeliet et al., 1996; Ferrara et al., 1996). Interestingly, mutant mice expressing only the matrix-associated VEGF188 isoform show impaired arterial differentiation in the retina, suggesting that the lower molecular weight isoforms (diffusible VEGF120 and intermediate VEGF166) are required for arterial development in the retina (Stalmans et al., 2002). Consistent with this finding, neuropilin-1 (Nrp1), a co-receptor for VEGF164 to facilitate VEGF signaling in concert with VEGF receptor-2 (VEGFR2), is expressed in arterial endothelial cells (Herzog et al., 2001;Moyon et al., 2001; Mukouyama et al., 2002; le Noble et al., 2004). In fact, Nrp1 mutant mouse embryos show impaired arterial differentiation, independent of blood flow patterns (Jones et al., 2008). Mukouyama et al. have also demonstrated that a critical step for arterial differentiation is the induction of Nrp1 expression by VEGF signaling as a positive feedback loop (Mukouyama et al., 2005). Thus, the VEGF-VEGFR2/Nrp1 pathway is critical for arterial specification during vascular development (Fig. 1).
Notch
The Notch signaling pathway directs cell fate decisions during embryonic development. Upon activation of a Notch receptor (Notch1 to Notch4 in mammals) by binding to one of ligands (Jagged-1 to -2 or Delta-like [Dll]1 to -3 in mammals), a proteolytic cleavage results in the release of the Notch intracellular domain (NICD) into the cytoplasm. The transcriptional regulator Suppressor of Hairless [Su(H)] then interacts with the translocated NICD in the nucleus, leading to the induction of the bHLH transcription factors, including Hey1 and Hey2 in mouse and gridlock (grl) in zebrafish. Notch receptors (Notch1 and Notch4 in mouse) and Notch ligands (Jagged1, Jagged2 and Dll4 in mouse) are specifically expressed in arterial endothelial cells of the early embryo. Importantly, studies in zebrafish have first provided compelling evidence that Notch signaling acts downstream of the Shh and VEGF pathways in arterial specification (Lawson et al., 2001; Lawson et al., 2002).
Compound homozygous mutant mice for Notch1 and Notch4 exhibit abnormal vascular development (Krebs et al., 2000), whereas mice lacking Dll4 show defects in arterial specification in a dosage dependent manner (Duarte et al., 2004; Gale et al., 2004; Krebs et al., 2004). While before embryonic day (E) 13.5, Dll1 is not expressed in blood vessels of the developing mouse embryo, its expression is restricted to arterial endothelial cells beginning at E 13.5. Analysis of hypomorphic and endothelial-specific Dll1 mutant mice reveals that Dll1 is required for the maintenance of arterial identity (Sorensen et al., 2009). Interestingly, although these Dll1 mutant mice display reduced expression of VEGFR2 and Nrp1 and upregulation of the venous marker COUP-TFII, Dll4 is still expressed in Dll1-mutant endothelial cells (Sorensen et al., 2009). These results suggest that Dll4 is essential for initiating the arterial program, but is dispensable for maintaining arterial identity in the late stage of embryonic development. It is also worth noting that Notch signaling activated by Dll1 regulates the Nrp1 promoter, indicating direct control of VEGF responsiveness by Notch (Sorensen et al., 2009).
During vascular development, Hey1/2 in mouse and grl in zebrafish act downstream of the Notch signaling pathway. Like Dll4 mutant mice (Duarte et al., 2004), compound Hey1 and Hey2 mutant mice fail to express arterial markers such as ephrinB2 in endothelial cells and exhibit fusions between arteries and veins (arteriovenous malformations) (Fischer et al., 2004; Kokubo et al., 2005). Grl is a zebrafish ortholog of mammalian Hey2 and is also involved in arterial specification in the zebrafish embryo (Zhong et al., 2001). Overexpression of grl results in suppression of venous markers, but no ectopic expression of arterial markers. It is still unclear whether grl is a direct downstream target of Notch in zebrafish because grl expression is detected in zebrafish embryos defective of Notch signaling (Lawson et al., 2001). Consistently, it has been shown that Hey2 expression is Notch-independent during cardiac development (Rutenberg et al., 2006; Timmerman et al., 2004).
Although critical signaling pathways including Shh, VEGF and Notch in arterial fate determination have now been identified, the regulatory mechanisms associated with the key signaling pathways and transcriptional networks need to be elucidated. As described below, recent evidence demonstrates that Fox and Sox transcription factors play major roles in arterial cell fate determination during embryonic development.
Foxc1 and Foxc2 transcription factors
Members of the Forkhead/Fox transcription factor family have been implicated in cardiovascular development and disease (Papanicolaou et al., 2008). In particular, two closely related proteins in the Foxc subfamily, Foxc1 and Foxc2, play overlapping roles in cardiovascular development. Compound Foxc1; Foxc2 homozygous mouse mutants show defective vascular remodeling of primitive blood vessels and abnormal vascular connections, arteriovenous malformations (Kume et al., 2001; Seo et al., 2006). It is important to note that arteriovenous malformations similarly develop in endothelial cells of mutant mice and zebrafish in which Notch signaling is defective (Lawson et al., 2001; Zhong et al., 2001; Duarte et al., 2004; Krebs et al., 2004; Kokubo et al., 2005). Endothelial cells of compound Foxc1; Foxc2 homozygous mutants fail to express arterial-specific genes including Dll4 and Hey2, whereas venous markers such as COUP-TFII and EphB4 are normally expressed in these mutant embryos (Seo et al., 2006). Most significantly, Foxc1 and Foxc2 can directly activate the Dll4 promoter via a Foxc-binding element (FBE), suggesting that Foxc1 and Foxc2 act upstream of Notch signaling in arterial cell specification (Seo et al., 2006) (Fig. 1).
A recent study has further demonstrated that Foxc1 and Foxc2 directly regulate expression of the Notch target gene, Hey2, by activating its promoter in endothelial cells (Hayashi and Kume, 2008). The Hey2 promoter includes two FBEs that are adjacent to a binding site for Su(H). Of significance is the finding that Foxc2, but not Foxc1, directly binds to Su(H) and forms a protein complex with Su(H) and NICD to induce the Hey2 promoter. Thus, Foxc2 functionally interacts with the Notch pathway to induce Hey2 expression in endothelial cells (Hayashi and Kume, 2008).
Foxc-induced promoter activity of Dll4 and Hey2 is significantly enhanced by VEGF in endothelial cells (Hayashi and Kume, 2008). Consistent with the report that the VEGF-mediated PI3K pathway induces the transcription of Notch1 and Dll4 in vitro (Liu et al., 2003), modulation of Foxc activity by VEGF is enhanced by the PI3K pathway or inhibited by the ERK/MAPK pathway. By contrast, in the zebrafish embryo, the VEGF-activated PI3K pathway inhibits the stimulation of the ERK signaling cascade, leading to the suppression of arterial differentiation (Hong et al., 2006). Although reasons for the discrepancy between the in vitro and in vivo results remain unclear, one possible explanation is that these in vitro experiments were not conducted in uncommitted endothelial progenitor cells (Liu et al., 2003; Hayashi and Kume, 2008). Alternatively, given evidence that endothelial cells have plasticity even after specification (Moyon et al., 2001; Othman-Hassan et al., 2001; le Noble et al., 2004), it is also possible that epigenetic regulation in vivo such as hemodynamic force and blood flow may affect genetic programming in arterial-venous specification of the developing embryo (Swift and Weinstein, 2009).
In in vitro differentiation of arterial and venous endothelial cells using mouse embryonic stem (ES) cells, high VEGF concentration (50 ng/ml) induces arterial marker genes, whereas low and intermediate VEGF concentrations (2 and 10 ng/ml, respectively) preferentially upregulate expression of the venous marker COUP-TFII (Lanner et al., 2007). This observation implies that graded VEGF signaling could govern preferential activation of either PI3K or ERK pathway. Since Foxc1 and Foxc2 are expressed in both arteries and veins in the mouse embryo (Seo et al., 2006), it is possible that VEGF-mediated posttranslational modifications, such as phosphorylation, are critical for the activation of Foxc1 and Foxc2 proteins in the induction of arterial-specific genes. Taken together, these findings suggest that Foxc transcriptional factors control multiple steps of the VEGF-Notch/Dll4-Hey2 molecular cascade, thereby promoting arterial cell determination.
Sox transcription factors
Three Sox genes, Sox7, Sox17 and Sox18, encode proteins that belong to a subgroup (SoxF) of the Sox transcription factor family. All three genes are expressed in vascular endothelial cells during development, while only Sox18 is expressed in lymphatic vessel development (Hosking et al., 2009). Consistent with their expression patterns, Sox7 and Sox18 have a cooperative role in the specification of arterial-venous identity in zebrafish development (Cermenati et al., 2008; Herpers et al., 2008; Pendeville et al., 2008), whereas compound Sox17; Sox18 mutant mice display defects in cardiovascular development and postnatal neovascularization (Matsui et al., 2006; Sakamoto et al., 2007). Although the mechanistic basis for the function of Sox7/18 in arterial-venous specification remains unclear, it is noteworthy that the phenotypic similarities are observed between Sox7/18-deficient embryos and grl mutants and that Sox7/18-deficient embryos lack grl expression. Thus, Sox7 and Sox18 may regulate arterial-venous specification, at least in part, by acting upstream of grl. As described below, Sox18 also is an essential regulator of early lymphatic development. Recent studies demonstrate that Sox7 and Sox17, as genetic modifiers, can be induced in lymphatic endothelial progenitors in the absence of Sox18 in certain mouse strains and compensate the lymphatic phenotype of Sox18 mutant mice (Hosking et al., 2009). Therefore, the three Sox transcription factors cooperatively act in multiple processes of endothelial specification during development (Fig. 1).
Novel signaling molecules in arterial specification
Sucrose nonfermenting-related kinase-1 (snrk-1), a relatively novel serine/threonine kinase that belongs to the class of AMP-activated protein kinases (AMPKs), has recently been implicated in arterial-venous specification in zebrafish (Chun et al., 2009). Snrk-1 is specifically expressed in zebrafish vasculature and acts downstream or parallel to Notch signaling (Fig. 1). Importantly, snrk-1 counteracts with the function of dual-specific phosphatase-5 (dusp-5) in vascular development, and mutations in human snrk-1 and dusp-5 are identified in venous or lymphatic malformation patients (Pramanik et al., 2009). As described below, lymphatic endothelial cells are derived from the venous lineage. Thus, protein phosphorylation/dephosphorylation mediated by snrk-1 and dusp-5 may be critical for the lineage determination of artery, vein and lymphatic vessels.
Another phosphatase recently reported to be involved in arterial specification is the protein tyrosine phosphatase Dep1. Zebrafish Dep1a and Dep1b are both expressed in vascular endothelial cells, as well as in other embryonic tissues. Knockdown of both Dep1a and Dep1b in zebrafish results in reduced expression of arterial markers (notch5, grl and eprhinB2) and an expansion of venous markers (ephB4 and dab2) in the vasculature (Rodriguez et al., 2008). By contrast, the PI3K inhibitor LY294002 as well as overexpression of NICD or grl can rescue the vascular phenotype of embryos with double knockdown of Dep1a/Dep1b. These findings suggest that Dep1 is required for arterial-venous specification by acting upstream of the PI3K pathway (Fig. 1). Although Dep1 has been shown to interact with VEGFR2-mediated signaling in endothelial cell-cell contacts (Grazia Lampugnani et al., 2003), further studies need to be performed to determine whether Dep1 is directly associated with VEGFR2 signaling in the arterial program during development.
The calcitonin receptor-like receptor (crlr) is a 7-transmembrane G protein-coupled receptor and can function as a receptor for adrenomedullin. Crlr is expressed in the somite and arterial progenitors during zebrafish development. Crlr-deficient zebrafish embryos show loss of arterial gene expression such as ephrinB2 and notch5 (Nicoli et al., 2008). While lack of crlr results in a reduction in VEGF expression in the somites, VEGF overexpression can rescue impaired arterial differentiation of crlr-deficient embryos (Nicoli et al., 2008), suggesting that crlr functions upstream of VEGF in arterial specification. On the other hand, Shh signaling regulates crlr expression in the somite. These results indicate that Shh modulates VEGF activity by controlling crlr expression in zebrafish. Consistent with the role of crlr in zebrafish embryos, analysis of the in vitro differentiation system of mouse ES cells has also revealed that adrenomedullin signaling coordinates with the VEGF and Notch pathways in arterial differentiation (Yurugi-Kobayashi et al., 2006).
Venous Specification
According to a number of studies in the zebrafish embryo, it was thought that venous cell fate is a default status during early embryonic development and that the activation of Notch signaling mediated by the Shh/VEGF pathways augments arterial specification. However, You et al. have demonstrated that chicken ovalbumin upstream promoter-transcription factor (COUP-TFII, which is specifically expressed in venous endothelial cells, is a genetic determinant factor of venous specification by acting upstream of EphB4 in mouse (You et al., 2005) (Fig. 1). COUP-TFII belongs to the orphan nuclear receptor family, although it has recently been shown to be a retinoic acid-activated receptor (Kruse et al., 2008). COUP-TFII mutant mice show ectopic expression of arterial markers such as Nrp1, Notch1 and eprinB2 in veins, while mis-expression of COUP-TFII in endothelial cells results in reduced expression of Nrp1 and Jagged1 in arteries (You et al., 2005). It should be noted that the lack of COUP-TFII leads to a slight reduction in EphB4 expression in veins, suggesting that COUP-TFII is not a sole factors, but may cooperate with additional factor(s) in venous cell fate determination. Unlike arterial specification, the molecular mechanisms controlling venous specification remain largely unknown. Therefore, although COUP-TFII suppresses arterial cell fate by inhibiting expression of Nrp1 and Notch (You et al., 2005), it is important to define the mechanistic basis for the action of COUP-TFII during arterial-venous specification. Significantly, after the process of the determination of arterial and venous cell fates, COUP-TFII is also critical for lymphatic gene expression by interacting with Prox1 (see below). This observation supports evidence that the event of arterial-venous determination is progressively followed by lymphatic specification during embryonic development. Consistently, Nrp2 is expressed in venous and lymphatic endothelial cells (Herzog et al., 2001; Yuan et al., 2002), while VEGFR3 expression is initially detected in blood vessels of the early embryo but becomes restricted to venous and then lymphatic endothelial cells at the late stages of development (Kaipainen et al., 1995; Kukk et al., 1996).
Lymphatic specification
The mammalian lymphatic vascular system originates solely from the venous endothelial cells (Srinivasan et al., 2007). After arterial and venous endothelial cells differentiate, a subpopulation of venous endothelial cells is thought to become competent to acquire lymphatic endothelial cell fate by progressively expressing the transcription factors Sox18 and Prox1 to differentiate into lymphatic endothelial cells (Adams and Alitalo, 2007; Kiefer and Adams, 2008; Maby-El Hajjami and Petrova, 2008). VEGF-C, a VEGFR3 ligand, is expressed mainly in mesenchymal cells surrounding embryonic veins (Karkkainen et al., 2004). Prox1/VEFR3-positive lymphatic endothelial progenitors subsequently bud and migrate from the veins via paracrine VEGF-C/VEGFR3 signaling, leading to the formation of the lymphatic network, a process called (developmental) lymphangiogenesis (Adams and Alitalo, 2007; Kiefer and Adams, 2008; Maby-El Hajjami and Petrova, 2008).
Sox18 is first detected in a subpopulation of the cardinal vein and precedes the onset of Prox1 expression. Although Sox18 homozygous mutant mice on a mixed background do not have lymphatic defects because of genetic compensation by Sox7 and Sox17 (Hosking et al., 2009), these mutant embryos on a C57BL/6 genetic background show a complete blockade of lymphatic endothelial differentiation, accompanied by the lack of Prox1 expression in the cardinal vein (Francois et al., 2008). Furthermore, Sox18 can directly induce Prox1 expression via two Sox18-binding sites on the Prox1 promoter (Francois et al., 2008). These data indicate that Sox18 directly acts upstream of Prox1 in the specification of lymphatic cell fate during embryonic development (Fig. 1).
Compelling evidence shows that Prox1 is a master regulator of lymphatic endothelial identity. Prox1 mutant mice fail to specify lymphatic endothelial cells and lack the expression of lymphatic markers such as VEGFR3 and LYVE1 (Wigle and Oliver, 1999; Wigle et al., 2002). Indeed, endothelial cells sprouted from the cardinal vein of Prox1-deficient embryo express blood endothelial markers. These results demonstrate that the loss of Prox1 in mouse results in an arrest of the lymphatic program. Consistent with the lymphatic phenotype in Prox1 mutant mice, Prox1 play a pivotal role in controlling lymphatic gene expression (Hong et al., 2002; Petrova et al., 2002). Recent studies further reinforce the importance of Prox1 function in lymphatics (Johnson et al., 2008). Prox1 suppresses blood endothelial cell identity and promotes and maintains lymphatic cell identity even in adult mice. Remarkably, analysis of inducible ablation of Prox1 in mouse reveals that the loss of Prox1 leads to reprogramming of the lymphatic cell phenotype to the blood endothelial cell phenotype. Together these results clearly demonstrate that the two-endothelial identities are reversible depending on Prox1 activity (Johnson et al., 2008), although it remains unclear whether Prox1-deficient lymphatic endothelial cells can acquire venous or unspecified endothelial identity. It should be noted that Sox18 seems essential for the induction of lymphatic differentiation, but dispensable for the maintenance of the lymphatic phenotype. Besides lymphatic endothelial identity, Prox1 also controls the migration of lymphatic endothelial cells toward VEGF-C by directly inducing the expression of FGFR3, VEGFR3 and integrin α9 (Shin et al., 2006; Mishima et al., 2007).
Implications for regulators of arterial-venous identity in lymphatic vessel development
Since the lymphatic vasculature is derived from venous endothelial cells in mammals, it is, not surprisingly, important to state that several key factors involved in arterial-venous identity play important roles in lymphatic vessel development. For instance, ephrinB2 plays a role in remodeling the lymphatic capillary plexus into a mature lymphatic network as well as lymphatic valve formation (Makinen et al., 2005). Notch1 and Notch4 have been shown to be expressed in normal and pathological lymphatics in mouse and human (Shawber et al., 2007). Given evidence that Notch signaling directly upregulates expression of ephrinB2 and VEGFR3 by activating their promoters in blood endothelial cells (Grego-Bessa et al., 2007; Shawber et al., 2007), it is plausible to consider the possibility that the Notch pathway may control ephrinB2 and VEGFR3 expression in lymphatic endothelial cells. Yet no lymphatic abnormalities have been reported on mutant mice for Notch signaling genes (Gridley, 2007), possibly because these Notch mutant mice die earlier than the onset of lymphatic vessel development.
The venous determinant factor COUP-TFII is reported to be expressed in lymphatic endothelial cells, and COUP-TFII and Prox1 physically interact with each other (Lee et al., 2009; Yamazaki et al., 2009). COUP-TFII and Prox1 cooperatively control lymphatic gene expression such as VEGFR3 and FGFR3 (Lee et al., 2009), supporting the idea that venous cell identity is a prerequisite for lymphatic specification. Interestingly, COUP-TFII negatively and positively regulates VEGFR3 expression in blood and lymphatic endothelial cells, respectively (Yamazaki et al., 2009). COUP-TFII also suppresses Prox1-induced cyclin E1 expression and cell proliferation (Yamazaki et al., 2009). Thus, it is likely that COUP-TFII differentially functions by interacting with other transcriptional regulators such as Prox1 in venous and lymphatic endothelial cells.
Compound Foxc1+/−; Foxc2−/− mutant mice have a reduction in the number of Prox1+ lymphatic endothelial cells sprouting from the cardinal vein (Seo et al., 2006). Notably, while expression domains of Foxc1 and Foxc2 are overlapped with those of VEGF-C in the mesenchyme, compound Foxc1+/−; Foxc2−/− mutants exhibit significant reduction in VEGF-C expression. Therefore, Foxc transcription factors are likely to regulate lymphatic vessel development in a paracrine manner. Foxc2 homozygous mutant mice show defective lymphatic valve formation and abnormal pericyte recruitment of lymphatic vessels, and Foxc2 and VEGFR3 act through a common genetic pathway in lymphatic vessel development (Petrova et al., 2004). Recent evidence further demonstrates that during lymphatic valve formation, Foxc2 interacts with another transcription factor, NFATc, which has previously been implicated in the formation of cardiac valves from the endocardium (Norrmen et al., 2009). These results provide evidence that like COUP-TFII, Foxc2 is a critical regulator in multiple stages of vascular formation, including arterial specification and lymphatic vessel development.
Concluding remarks and future directions
Studies in zebrafish and mouse have made significant progress toward understanding arterial, venous and lymphatic cell fate determination. For example, it has become evident the developing embryo progressively acquires lymphatic specification after the arterial-venous fate decisions in vascular endothelial cells. Yet the mechanistic basis for a link between the key signaling pathways and transcriptional networks in the three similar, but distinct, endothelial cell populations is still not completely understood. Although new molecules recently identified have been discussed in this review, additional factors and signaling pathways may need to be discovered to full understand the complex process of endothelial cell fates. In particular, the molecular hierarchies that act at the nexus of arterial, venous and lymphatic specification/differentiation remains to be elucidated. Addressing this fundamental question will lead to a more complete understanding of plasticity and/or reprogramming of one endothelial cell type to another and will also be clinically relevant to vascular disorders in humans.
Acknowledgements
This work was supported by an NIH grant (HL074121) to T.K.
References
- Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell. Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- Aitsebaomo J, Portbury AL, Schisler JC, Patterson C. Brothers and sisters: molecular insights into arterial-venous heterogeneity. Circ. Res. 2008;103:929–939. doi: 10.1161/CIRCRESAHA.108.184937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd N, Becker S, Maye P, Narasimhaiah R, St-Jacques B, Zhang X, McMahon J, McMahon A, Grabel L. Hedgehog is required for murine yolk sac angiogenesis. Development. 2002;129:361–372. doi: 10.1242/dev.129.2.361. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Cermenati S, Moleri S, Cimbro S, Corti P, Del Giacco L, Amodeo R, Dejana E, Koopman P, Cotelli F, Beltrame M. Sox18 and Sox7 play redundant roles in vascular development. Blood. 2008;111:2657–2666. doi: 10.1182/blood-2007-07-100412. [DOI] [PubMed] [Google Scholar]
- Chun CZ, Kaur S, Samant GV, Wang L, Pramanik K, Garnaas MK, Li K, Field L, Mukhopadhyay D, Ramchandran R. Snrk-1 is involved in multiple steps of angioblast development and acts via notch signaling pathway in artery-vein specification in vertebrates. Blood. 2009;113:1192–1199. doi: 10.1182/blood-2008-06-162156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covassin LD, Villefranc JA, Kacergis MC, Weinstein BM, Lawson ND. Distinct genetic interactions between multiple Vegf receptors are required for development of different blood vessel types in zebrafish. Proc. Natl. Acad. Sci. U. S. A. 2006;103:6554–6559. doi: 10.1073/pnas.0506886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covassin LD, Siekmann AF, Kacergis MC, Laver E, Moore JC, Villefranc JA, Weinstein BM, Lawson ND. A genetic screen for vascular mutants in zebrafish reveals dynamic roles for Vegf/Plcg1 signaling during artery development. Dev. Biol. 2009;329:212–226. doi: 10.1016/j.ydbio.2009.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, Bekman E, Costa L, Henrique D, Rossant J. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 2004;18:2474–2478. doi: 10.1101/gad.1239004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004;18:901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M, Davidson T, Tutt D, Cheah KS, Stacker SA, Muscat GE, Achen MG, Dejana E, Koopman P. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456:643–647. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc. Natl. Acad. Sci. U. S. A. 2004;101:15949–15954. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol. Cell. 1999;4:403–414. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- Gerety SS, Anderson DJ. Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development. 2002;129:1397–1410. doi: 10.1242/dev.129.6.1397. [DOI] [PubMed] [Google Scholar]
- Grazia Lampugnani M, Zanetti A, Corada M, Takahashi T, Balconi G, Breviario F, Orsenigo F, Cattelino A, Kemler R, Daniel TO, Dejana E. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J. Cell Biol. 2003;161:793–804. doi: 10.1083/jcb.200209019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grego-Bessa J, Luna-Zurita L, del Monte G, Bolos V, Melgar P, Arandilla A, Garratt AN, Zang H, Mukouyama YS, Chen H, Shou W, Ballestar E, Esteller M, Rojas A, Pérez-Pomares JM, de la Pompa JL. Notch signaling is essential for ventricular chamber development. Dev. Cell. 2007;12:415–429. doi: 10.1016/j.devcel.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134:2709–2718. doi: 10.1242/dev.004184. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Kume T. Foxc transcription factors directly regulate Dll4 and Hey2 expression by interacting with the VEGF-Notch signaling pathways in endothelial cells. PLoS ONE. 2008;3:e2401. doi: 10.1371/journal.pone.0002401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpers R, van de Kamp E, Duckers HJ, Schulte-Merker S. Redundant roles for sox7 and sox18 in arteriovenous specification in zebrafish. Circ. Res. 2008;102:12–15. doi: 10.1161/CIRCRESAHA.107.166066. [DOI] [PubMed] [Google Scholar]
- Herzog Y, Kalcheim C, Kahane N, Reshef R, Neufeld G. Differential expression of neuropilin-1 and neuropilin-2 in arteries and veins. Mech. Dev. 2001;109:115–119. doi: 10.1016/s0925-4773(01)00518-4. [DOI] [PubMed] [Google Scholar]
- Hong CC, Peterson QP, Hong JY, Peterson RT. Artery/Vein Specification Is Governed by Opposing Phosphatidylinositol-3 Kinase and MAP Kinase/ERK Signaling. Curr. Biol. 2006;16:1366–1372. doi: 10.1016/j.cub.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CC, Kume T, Peterson RT. Role of crosstalk between phosphatidylinositol 3-kinase and extracellular signal-regulated kinase/mitogen-activated protein kinase pathways in artery-vein specification. Circ. Res. 2008;103:573–579. doi: 10.1161/CIRCRESAHA.108.180745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YK, Harvey N, Noh YH, Schacht V, Hirakawa S, Detmar M, Oliver G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev. Dyn. 2002;225:351–357. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- Hosking B, Francois M, Wilhelm D, Orsenigo F, Caprini A, Svingen T, Tutt D, Davidson T, Browne C, Dejana E, Koopman P. Sox7 and Sox17 are strain-specific modifiers of the lymphangiogenic defects caused by Sox18 dysfunction in mice. Development. 2009;136:2385–2391. doi: 10.1242/dev.034827. [DOI] [PubMed] [Google Scholar]
- Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, Oliver G. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev. 2008;22:3282–3291. doi: 10.1101/gad.1727208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EA, Yuan L, Breant C, Watts RJ, Eichmann A. Separating genetic and hemodynamic defects in neuropilin 1 knockout embryos. Development. 2008;135:2479–2488. doi: 10.1242/dev.014902. [DOI] [PubMed] [Google Scholar]
- Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl. Acad. Sci. U. S. A. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Adams RH. Lymphatic endothelial differentiation: start out with Sox--carry on with Prox. Genome Biol. 2008;9:243. doi: 10.1186/gb-2008-9-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubo H, Miyagawa-Tomita S, Nakazawa M, Saga Y, Johnson RL. Mouse hesr1 and hesr2 genes are redundantly required to mediate Notch signaling in the developing cardiovascular system. Dev. Biol. 2005;278:301–309. doi: 10.1016/j.ydbio.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004;18:2469–2473. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse SW, Suino-Powell K, Zhou XE, Kretschman JE, Reynolds R, Vonrhein C, Xu Y, Wang L, Tsai SY, Tsai MJ, Xu HE. Identification of COUP-TFII orphan nuclear receptor as a retinoic acid-activated receptor. PLoS Biol. 2008;6:e227. doi: 10.1371/journal.pbio.0060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, Alitalo K. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development. 1996;122:3829–3837. doi: 10.1242/dev.122.12.3829. [DOI] [PubMed] [Google Scholar]
- Kume T, Jiang H, Topczewska JM, Hogan BL. The murine winged helix transcription factors, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis. Genes Dev. 2001;15:2470–2482. doi: 10.1101/gad.907301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanner F, Sohl M, Farnebo F. Functional arterial and venous fate is determined by graded VEGF signaling and notch status during embryonic stem cell differentiation. Arterioscler. Thromb. Vasc. Biol. 2007;27:487–493. doi: 10.1161/01.ATV.0000255990.91805.6d. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev. Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Mugford JW, Diamond BA, Weinstein BM. phospholipase C gamma-1 is required downstream of vascular endothelial growth factor during arterial development. Genes Dev. 2003;17:1346–1351. doi: 10.1101/gad.1072203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Noble F, Moyon D, Pardanaud L, Yuan L, Djonov V, Matthijsen R, Breant C, Fleury V, Eichmann A. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development. 2004;131:361–375. doi: 10.1242/dev.00929. [DOI] [PubMed] [Google Scholar]
- Lee S, Kang J, Yoo J, Ganesan SK, Cook SC, Aguilar B, Ramu S, Lee J, Hong YK. Prox1 physically and functionally interacts with COUP-TFII to specify lymphatic endothelial cell fate. Blood. 2009;113:1856–1859. doi: 10.1182/blood-2008-03-145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, Fairman RM, Velazquez OC, Herlyn M. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol. Cell Biol. 2003;23:14–25. doi: 10.1128/MCB.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin. Cell Biol. 2009;21:154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Maby-El Hajjami H, Petrova TV. Developmental and pathological lymphangiogenesis: from models to human disease. Histochem. Cell Biol. 2008;130:1063–1078. doi: 10.1007/s00418-008-0525-5. [DOI] [PubMed] [Google Scholar]
- Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Kanai-Azuma M, Hara K, Matoba S, Hiramatsu R, Kawakami H, Kurohmaru M, Koopman P, Kanai Y. Redundant roles of Sox17 and Sox18 in postnatal angiogenesis in mice. J. Cell Sci. 2006;119:3513–3526. doi: 10.1242/jcs.03081. [DOI] [PubMed] [Google Scholar]
- Mishima K, Watabe T, Saito A, Yoshimatsu Y, Imaizumi N, Masui S, Hirashima M, Morisada T, Oike Y, Araie M, Niwa H, Kubo H, Suda T, Miyazono K. Prox1 induces lymphatic endothelial differentiation via integrin alpha9 and other signaling cascades. Mol. Biol. Cell. 2007;18:1421–1429. doi: 10.1091/mbc.E06-09-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyon D, Pardanaud L, Yuan L, Breant C, Eichmann A. Plasticity of endothelial cells during arterial-venous differentiation in the avian embryo. Development. 2001;128:3359–3370. doi: 10.1242/dev.128.17.3359. [DOI] [PubMed] [Google Scholar]
- Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Mukouyama YS, Gerber HP, Ferrara N, Gu C, Anderson DJ. Peripheral nerve- derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development. 2005;132:941–952. doi: 10.1242/dev.01675. [DOI] [PubMed] [Google Scholar]
- Nicoli S, Tobia C, Gualandi L, De Sena G, Presta M. Calcitonin receptor-like receptor guides arterial differentiation in zebrafish. Blood. 2008;111:4965–4972. doi: 10.1182/blood-2007-10-118166. [DOI] [PubMed] [Google Scholar]
- Norrmen C, Ivanov KI, Cheng J, Zangger N, Delorenzi M, Jaquet M, Miura N, Puolakkainen P, Horsley V, Hu J, Augustin HG, Ylä-Herttuala S, Alitalo K, Petrova TV. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J. Cell Biol. 2009;185:439–457. doi: 10.1083/jcb.200901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman-Hassan K, Patel K, Papoutsi M, Rodriguez-Niedenfuhr M, Christ B, Wilting J. Arterial identity of endothelial cells is controlled by local cues. Dev. Biol. 2001;237:398–409. doi: 10.1006/dbio.2001.0383. [DOI] [PubMed] [Google Scholar]
- Papanicolaou KN, Izumiya Y, Walsh K. Forkhead transcription factors and cardiovascular biology. Circ. Res. 2008;102:16–31. doi: 10.1161/CIRCRESAHA.107.164186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendeville H, Winandy M, Manfroid I, Nivelles O, Motte P, Pasque V, Peers B, Struman I, Martial JA, Voz ML. Zebrafish Sox7 and Sox18 function together to control arterial-venous identity. Dev. Biol. 2008;317:405–416. doi: 10.1016/j.ydbio.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Shaw SY, Peterson TA, Milan DJ, Zhong TP, Schreiber SL, MacRae CA, Fishman MC. Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat. Biotechnol. 2004;22:595–599. doi: 10.1038/nbt963. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Makinen T, Makela TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Yla-Herttuala S, Alitalo K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Yla-Herttuala S, Miura N, Alitalo K. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat. Med. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- Pramanik K, Chun CZ, Garnaas MK, Samant GV, Li K, Horswill MA, North PE, Ramchandran R. Dusp-5 and Snrk-1 coordinately function during vascular development and disease. Blood. 2009;113:1184–1191. doi: 10.1182/blood-2008-06-162180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez F, Vacaru A, Overvoorde J, den Hertog J. The receptor protein-tyrosine phosphatase, Dep1, acts in arterial/venous cell fate decisions in zebrafish development. Dev. Biol. 2008;324:122–130. doi: 10.1016/j.ydbio.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Rutenberg JB, Fischer A, Jia H, Gessler M, Zhong TP, Mercola M. Developmental patterning of the cardiac atrioventricular canal by Notch and Hairy- related transcription factors. Development. 2006;133:4381–4390. doi: 10.1242/dev.02607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto Y, Hara K, Kanai-Azuma M, Matsui T, Miura Y, Tsunekawa N, Kurohmaru M, Saijoh Y, Koopman P, Kanai Y. Redundant roles of Sox17 and Sox18 in early cardiovascular development of mouse embryos. Biochem. Biophys. Res. Commun. 2007;360:539–544. doi: 10.1016/j.bbrc.2007.06.093. [DOI] [PubMed] [Google Scholar]
- Seo S, Fujita H, Nakano A, Kang M, Duarte A, Kume T. The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Dev. Biol. 2006;294:458–470. doi: 10.1016/j.ydbio.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Shawber CJ, Funahashi Y, Francisco E, Vorontchikhina M, Kitamura Y, Stowell SA, Borisenko V, Feirt N, Podgrabinska S, Shiraishi K, Chawengsaksophak K, Rossant J, Accili D, Skobe M, Kitajewski J. Notch alters VEGF responsiveness in human and murine endothelial cells by direct regulation of VEGFR-3 expression. J. Clin. Invest. 2007;117:3369–3382. doi: 10.1172/JCI24311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JW, Min M, Larrieu-Lahargue F, Canron X, Kunstfeld R, Nguyen L, Henderson JE, Bikfalvi A, Detmar M, Hong YK. Prox1 promotes lineage-specific expression of fibroblast growth factor (FGF) receptor-3 in lymphatic endothelium: a role for FGF signaling in lymphangiogenesis. Mol. Biol. Cell. 2006;17:576–584. doi: 10.1091/mbc.E05-04-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen I, Adams RH, Gossler A. DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood. 2009;113:5680–5688. doi: 10.1182/blood-2008-08-174508. [DOI] [PubMed] [Google Scholar]
- Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, Oliver G. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21:2422–2432. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalmans I, Ng YS, Rohan R, Fruttiger M, Bouche A, Yuce A, Fujisawa H, Hermans B, Shani M, Jansen S, Hicklin D, Anderson DJ, Gardiner T, Hammes HP, Moons L, Dewerchin M, Collen D, Carmeliet P, D’Amore PA. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J. Clin. Invest. 2002;109:327–336. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift MR, Weinstein BM. Arterial-venous specification during development. Circ. Res. 2009;104:576–588. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, de la Pompa JL. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18:99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tuyl M, Groenman F, Wang J, Kuliszewski M, Liu J, Tibboel D, Post M. Angiogenic factors stimulate tubular branching morphogenesis of sonic hedgehog- deficient lungs. Dev. Biol. 2007;303:514–526. doi: 10.1016/j.ydbio.2006.11.029. [DOI] [PubMed] [Google Scholar]
- Vokes SA, Yatskievych TA, Heimark RL, McMahon J, McMahon AP, Antin PB, Krieg PA. Hedgehog signaling is essential for endothelial tube formation during vasculogenesis. Development. 2004;131:4371–4380. doi: 10.1242/dev.01304. [DOI] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Yoshimatsu Y, Morishita Y, Miyazono K, Watabe T. COUP-TFII regulates the functions of Prox1 in lymphatic endothelial cells through direct interaction. Genes Cells. 2009;14:425–434. doi: 10.1111/j.1365-2443.2008.01279.x. [DOI] [PubMed] [Google Scholar]
- You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- Yuan L, Moyon D, Pardanaud L, Breant C, Karkkainen MJ, Alitalo K, Eichmann A. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129:4797–4806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]
- Yurugi-Kobayashi T, Itoh H, Schroeder T, Nakano A, Narazaki G, Kita F, Yanagi K, Hiraoka-Kanie M, Inoue E, Ara T, Nagasawa T, Just U, Nakao K, Nishikawa S, Yamashita JK. Adrenomedullin/cyclic AMP pathway induces Notch activation and differentiation of arterial endothelial cells from vascular progenitors. Arterioscler. Thromb. Vasc. Biol. 2006;26:1977–1984. doi: 10.1161/01.ATV.0000234978.10658.41. [DOI] [PubMed] [Google Scholar]
- Zhong TP, Childs S, Leu JP, Fishman MC. Gridlock signalling pathway fashions the first embryonic artery. Nature. 2001;414:216–220. doi: 10.1038/35102599. [DOI] [PubMed] [Google Scholar]


