Abstract
Glial cell line derived neurotrophic factor (GDNF) is present in adult gut although its role in the mature enteric nervous system is not well defined. The aim of the present study was to examine the role of GDNF as neuromodulator of the ascending phase of the peristaltic reflex. Colonic segments were prepared as flat sheets and placed in compartmented chambers so as to separate the sensory and motor limbs of the reflex. Ascending contraction was measured in the orad compartment and mucosal stroking stimuli (2-8 strokes) were applied in the caudad compartment. GDNF and substance P release were measured and the effects of GDNF and GDNF antibody on contraction and release were determined. Mice with reduced levels of GDNF (Gdnf+/-) and wild type littermates were also examined. GDNF was released in a stimulus-dependent manner into the orad motor but not caudad sensory compartment. Addition of GDNF to the orad motor but not caudad sensory compartment augmented ascending contraction and substance P release. Conversely, addition of GDNF antibody to the orad motor but not caudad sensory compartment reduced ascending contraction and substance P release. Similarly, the ascending contraction and substance P release into the orad motor compartment was reduced in Gdnf+/- mice as compared to wild type littermates. The results suggest that endogenous GDNF is released during the ascending contraction component of the peristaltic reflex where it acts as a neuromodulator to augment substance P release from motor neurons thereby augmenting contraction of circular muscle orad to the site of stimulation.
Keywords: colon, enteric nervous system, gastrointestinal tract, motility, neurotrophins, neuropeptides
Glial cell line derived neurotrophic factor (GDNF) has a key role in the formation of the enteric nervous system of the fetal gut. In the absence of GDNF or its receptor complex, the enteric nervous system is absent from the small bowel and colon (reviewed in Burns et al1, Kapur et al2 and Young et al3). GDNF and its receptors GFRα1 and RET, are also present in the mature mammalian gut in a variety of cells including smooth muscle, enteric neurons, and glial cells4-9. This localization of GDNF in the mature gut suggests that it might play a role in the maintenance of the enteric nervous system in adults or that GDNF may have actions in the adult unrelated to its role as a neurotrophic factor, such as a neuromodulator. This latter notion has been demonstrated for another neural growth factor brain-derived neurotrophic factor (BDNF) which augments 5-HT and calcitonin gene-related peptide (CGRP) release during peristalsis10 and increases synaptic transmission and calcium release in cultured enteric neurons11.
In the central nervous system, GDNF has been strongly associated with expression and release of several neuropeptides, including substance P. Thus, GDNF increased the survival and number of substance P-containing neurons, increased expression of substance P and mRNA of its precursor preprotachykinin A, and increased release of substance P initiated by other stimuli12-16. The expression and actions of GDNF in the central nervous system has mainly been associated with sensory neurons; however, in the gut, substance P is released from both sensory neurons and excitatory motor neurons. In the latter case, these motor neurons partly mediate the contraction of circular muscle that compromises the ascending contraction component of the peristaltic reflex17-19. Previous studies have demonstrated that in mice with a disruption of members of the GDNF family of ligands (GFL), either GDNF (GDNF+/-) or neurturin (NTN-/-) or in which there was a reduction in the GDNF receptor complex (Ret+/-,GFRα1+/-, GFRα2+/-), there is a reduction in electrically-stimulated contraction of gut circular muscle, reduction in electrically-stimulated release of substance P, reduction in substance P protein or mRNA levels, and reduced intestinal transit 7,20-23. This raised the possibility that GDNF might play a role in regulating gut motility by influencing the ascending contraction component of the peristaltic reflex.
In the present study, we tested the hypothesis that endogenous GDNF is released during the peristaltic reflex and that endogenous GDNF acts as a neuromodulator of substance P release during the peristaltic reflex to enhance the ascending contraction component of the reflex.
Materials and Methods
Measurement of the peristaltic reflex in rats and mice
The peristaltic reflex was measured in a 3-cm segment of distal colon of rat, opened to form a flat sheet and pinned mucosal side up in a two-compartment organ bath as described in detail previously10,17, 24-27. The compartments were isolated by vertical partitions sealed with vacuum grease and containing Krebs-bicarbonate medium containing in mmol L-1: 118 NaCl, 4.8 KCl, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, 25 NaH2CO3, and 11 glucose. In addition the medium contained the peptidase inhibitors 10 μmol L-1 amastatin and 1 μmol L-1 phosphoramidon, and 0.1 % bovine serum albumin. The peristaltic reflex was initiated by stroking the mucosa in the caudad compartment with a fine brush (2 to 8 strokes at a rate of 1 stroke sec-1). Previous studies26,27 using this preparation demonstrated that mucosal stroking activated a CGRP-containing intrinsic primary afferent neuron (IPAN) with mucosal projections since removal of the mucosa by blunt dissection abolished the CGRP release and peristaltic reflex elicited by mucosal stroking but did not affect the peristaltic reflex induced by muscle stretch in the circular direction. However, the ascending contraction elicited by mucosal stroking and muscle stretch was similar in magnitude and was mediated by release of substance P and acetylcholine. Ascending contraction of circular muscle was measured in the orad compartment using force-displacement transducers attached to the circular muscle layer. All preparations and experimental protocols were approved the Institutional Animal Care and Use Committee of the Virginia Commonwealth University.
Following a 60 minute equilibration period, the medium in each compartment was removed and replaced with fresh medium for a 15 minute basal period. At the end of this period, the medium was collected, aliquoted and stored at −80°C for subsequent measurement of GDNF and substance P. Each compartment was refilled with medium, and a mucosal stroking stimulus was repeated at one level of stroke stimulus 5 times at 3 minute intervals over a 15 minute period. At the end of this period, the medium was collected and stored at −80°C for subsequent measurement of neurotransmitters. Separate preparations were used for each level of stimulation. Experiments were repeated in the presence of 10 nM GDNF or a 1:200 dilution of GDNF antibody added to either the central sensory compartment or the orad or caudad compartment. In the case of GDNF there was a 10 minute pre-exposure period and in the case of the GDNF antibody, there was a 30 min pre-exposure period.
The experimental protocols and preparations were identical for rats and mice. The GDNF+/- mice were backcrossed two to four generations to C57BL/6 mice; we have previously described and characterized these mice22. The adult mice were used at 14-20 weeks of age.
Measurement of GDNF and substance P
GDNF was measured by sandwich ELISA kit following manufactures instructions (Promega; Madison, WI). The bathing medium was treated with 1N HCl for 15 min followed by neutralization with 1N NaOH to free bound GDNF. The assay is linear between 16 and 1000 pg ml-1 and the sensitivity was 30 pg ml-1. The antibody has been demonstrated to be specific for GDNF in Western blot, immunohistochemistry, and ELISA28-30. There was no cross-reactivity with vasoactive intestinal peptide, substance P, calcitonin gene-related peptide, nerve growth factor, neurotrophin-3, neurotrophin 4, brain derived neurotrophin, or neurturin. In addition in initial control studies, the GDNF antibody had no effect on the contraction of circular muscle strips of rat colon elicited by exogenous substance P or Ach.
Substance P was measured by RIA according to the directions of the manufacturer (Bachem-Peninsula; Torrance, CA) as described previously10, 17, 22-25. The sensitivity was 3 pM and the EC50 was 20 pM. There was no cross-reactivity with vasoactive intestinal peptide, somatostatin, calcitonin gene-related peptide, nerve growth factor, neurotrophin-3, neurotrophin 4, BDNF, GDNF, or neurturin.
Statistical analysis
The data were expressed as mean ± SEM. Determiniation of statistically significant difference between control and experimental groups were determined by ANOVA followed by Students t test.
Materials
GDNF, GDNF Antibody (#2791) and GDNF ELISA were purchased from Promega (Madison, WI). Substance P radioimmunoassay kit (#7451) and substance P were purchased from Bachem-Peninsula (Torrance, CA). All other chemicals and reagents were purchased from Sigma Chemicals (St. Louis, MO).
Results
Release of GDNF during ascending contraction
Basal release during the period immediately prior to stimulation was similar in the orad motor and caudad stimulation compartments. Release ranged from 1.0±0.3 fmol 100mg-min-1 in the orad motor compartment to 1.2±0.2 fmol 100mg-min-1 in the caudad sensory compartment (n=3-6). Application of mucosal strokes to the caudad sensory compartment had no effect on the release of GDNF into that compartment (range of Δ fmol 100mg-min-1 above basal: 0.10±0.05 at 4 strokes to 0.03±0.04 at 8 strokes; Fig. 1)). In contrast, mucosal stroking applied to the caudad sensory compartment cause a significant and stimulus dependent increase in GDNF release into the orad motor compartment (Fig. 1). The increase ranged from 0.38±0.05 fmol 100mg-min-1 increase above basal (p<0.01) at 2 strokes to 1.40±0.13 fmol 100mg-min-1 increase above basal (p<0.001) at 8 strokes.
Figure 1. Release of GDNF from rat colon preparation.
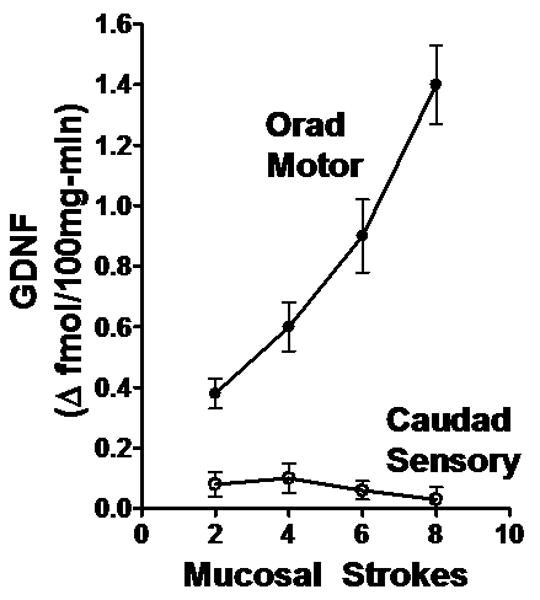
GDNF release into the caudad sensory compartment and the orad motor compartment was measured as fmol 100mg-min-1 increase above basal levels which were 1.2±0.2 and 1.0±0.3 fmol 100mg-min-1 respectively. Mucosal stroking in the caudad sensory compartment caused stimulus-dependent increase in GDNF release into the orad motor but not caudad sensory compartment. Values are mean±SE from 3-6 experiments.
Effect of exogenous GDNF on ascending contraction and concomitant release of substance P
Addition of GDNF (10 nM) to the orad motor compartment resulted in a significant increase in ascending contraction in response to mucosal stroking in the caudad sensory compartment as compared to the ascending contraction elicited in the absence of GDNF (Fig. 2 & 3). The increase in ascending contraction ranged from 192±18 % (p<0.01) at 2 strokes to 13±4% (p<0.05) at 8 strokes (n=4-5). In contrast, addition of GDNF to the caudad sensory compartment had no effect on the ascending contraction measured in the orad motor compartment (data not shown).
Figure 2. Sample tracings of ascending contraction in response to 6 strokes.
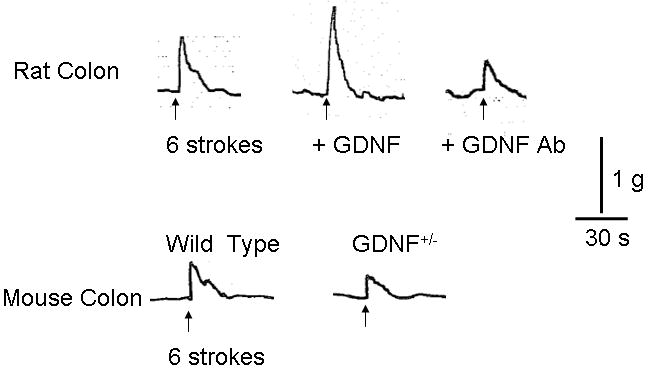
Typical tracing of ascending contraction induced by 6 strokes in rat (upper panel) and in mouse (lower panel). Exogenous GDNF (10nM) augmented (upper center) and GDNF antibody reduced (upper right) response to 6 strokes in rat. Reduction in the expression of GDNF (Gdnf+/-) in mice resulted in decreased response to 6 stroke stimulus (lower right). Vertical bar indicates 1 gram force and horizontal bar indicates 30 seconds.
Figure 3. GDNF-induced increase in ascending contraction and concomitant substance P release in rat colon.
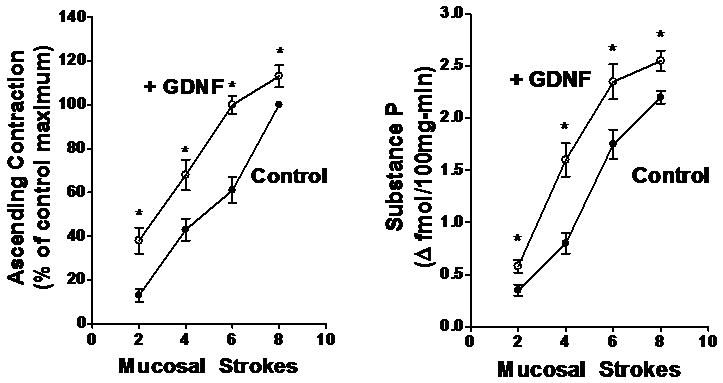
Mucosal stroking in the caudad sensory compartment caused stimulus-dependent contraction of circular muscle (left panel) and release of substance P (right panel) in the orad motor compartment. GDNF (10 nM) caused a significant increase in both ascending contraction of circular muscle and concomitant substance P release. Basal release of substance P in the orad motor compartment was 2.8±0.4 fmol 100mg-min-1. Values are mean±SE of 4-5 experiments. Asterisks indicate significance of at least p<0.05.
In the control rats in the absence of added GDNF, ascending contraction was accompanied by release of substance P. The release of substance P induced by mucosal stroking ranged from 0.35±0.05 fmol 100mg-min-1 above basal at 2 strokes to 2.20±0.06 fmol 100mg-min-1 above basal at 8 stokes; the basal level of release was 2.8±0.4 fmol 100mg-min-1. (N=4-5). Consistent with the effect of exogenous GDNF on ascending contraction of circular muscle, GDNF (10 nM) added to the orad motor compartment resulted in a significant increase in the release of the contractile transmitter substance P during ascending contraction in response to mucosal stroking in the caudad sensory compartment (Fig. 3). The GDNF-induced increase in substance P release accompanying ascending contraction ranged from 65±9 % (p<0.01) at 2 strokes to 16±5% (p<0.05) at 8 strokes. In contrast, addition of GDNF to the caudad sensory compartment had no effect on the release of substance P into the orad compartment during the ascending contraction measured in the orad motor compartment (data not shown).
Effect of GDNF antiserum on ascending contraction and concomitant release of substance P
Addition of GDNF antibody (1:200 dilution) to the orad motor compartment resulted in a significant decrease in ascending contraction in response to mucosal stroking in the caudad sensory compartment as compared to the ascending contraction elicited in the absence of GDNF antibody (Fig. 2 & 4). The decrease in ascending contraction ranged from 14±8 % (NS) at 2 strokes to 30±6% (p<0.05) at 8 strokes (n=4-5). The inhibition was significant at all levels of stimulation except at 2 strokes, where it was previously demonstrated17,24,25 that ascending contraction is largely mediated by acetylcholine. In contrast, addition of GDNF antibody to the caudad sensory compartment had no effect on the ascending contraction measured in the orad motor compartment (data not shown).
Figure 4. GDNF antibody-induced decrease in ascending contraction and concomitant substance P release in rat colon.
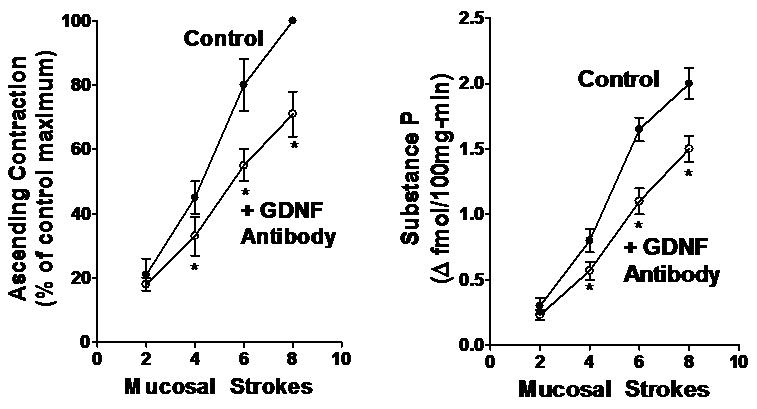
Mucosal stroking in the caudad sensory compartment caused stimulus-dependent contraction of circular muscle (left panel) and release of substance P (right panel) in the orad motor compartment. GDNF antibody (1:200 dilution) caused a significant decrease in both ascending contraction of circular muscle and concomitant substance P release. Basal release of substance P in the orad motor compartment was 2.4±0.5 fmol 100mg-min-1. Values are mean±SE of 4-5 experiments. Asterisks indicate significance of at least p<0.05.
As noted above, substance P is released from motor neurons and is a partial mediator of ascending contraction, especially at higher levels of stimulation. In the control rats in the absence of GDNF, the release of substance P induced by mucosal stroking ranged from 0.30±0.06 fmol 100mg-min-1 above basal at 2 strokes to 2.00±0.12 fmol 100mg-min-1 above basal at 8 stokes; the basal level of release was 2.4±0.5 fmol 100mg-min-1. Consistent with the effect of GDNF antiserum on ascending contraction of circular muscle, GDNF antibody added to the orad motor compartment resulted in a significant decrease in the release of substance P during ascending contraction in response to mucosal stroking in the caudad sensory compartment (Fig. 4). The decrease in substance P release accompanying ascending contraction ranged from 12±7 % (NS) at 2 strokes to 28±5% (p<0.05) at 8 strokes. In contrast, addition of GDNF antibody to the caudad sensory compartment had no effect on the release of substance P into the orad compartment during ascending contraction (data not shown).
Ascending contraction and concomitant release of substance P in GDNF+/- and wild type mice
The physiological role of GDNF in the ascending phase of the peristaltic reflex was also evaluated by examining mice heterozygous for GDNF in which the levels of GDNF are reduced (GDNF+/-) as compared to wild type littermates22. In the GDNF+/- mice, the ascending contraction elicited by mucosal stroking was reduced at all levels of stimulation (Fig. 2&5). The inhibition ranged from 20±7% (NS) at 2 stokes to 32±6% (p<0.05) at 6 stokes (n=3-4). The inhibition was statistically significant at all levels of stimulation except 2 strokes.
Figure 5. Decrease in ascending contraction and concomitant substance P release in Gdnf+/- mouse colon.
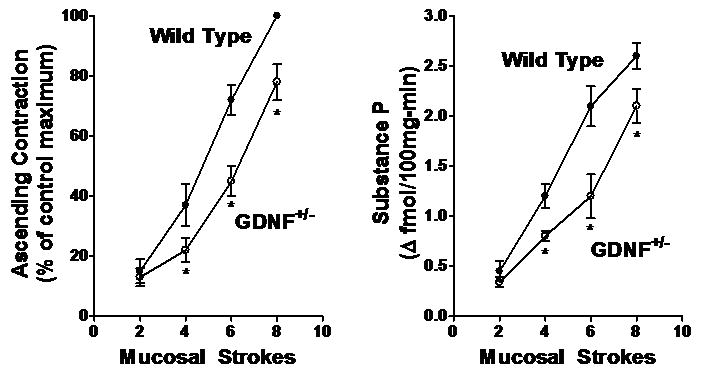
Mucosal stroking in the caudad sensory compartment caused stimulus-dependent contraction of circular muscle (left panel) and release of substance P (right panel) in the orad motor compartment in mice. The contraction of circular muscle and release of substance P was significantly less in mice in which levels of GDNF were reduced (Gdnf+/- mice). Basal release of substance P in the orad motor compartment was 3.9±0.4 fmol 100mg-min-1. Values are mean±SE of 3-4 experiments. Asterisks indicate significance of at least p<0.05.
As in the rat, substance P also mediates, in part, the ascending contraction phase of the peristaltic reflex. In wild type animals, mucosal stroking cause an increase in release of substance P above a basal level of 3.2±0.04 fmol/100mg-min. The increase ranged from 0.45 fmol 100mg-min-1 above basal at 2 strokes to 2.6 fmol 100 mg-min-1 above basal at 8 strokes. Consistent with the reduced ascending contraction in GDNF+/- mice, there was a significant decrease in the release of substance P into the orad motor compartment during ascending contraction in response to mucosal stroking in the caudad sensory compartment (Fig. 5). The decrease in substance P release accompanying ascending contraction ranged from 25±8 % (NS) at 2 strokes to 43±7% (p<0.01) at 6 strokes. There was no difference in the basal level of substance P release in the GDNF+/- mice (3.0±0.05 fmol 100mg-min-1) as compared to wild type.
Discussion
The peristaltic reflex is composed of a primary circuit of key neurons which are generally similar between species and experimental approaches. As enteric sensory neurons do not reach the lumen of the gut, the initial event requires that the luminal stimulus causes release of an agent from mucosal enteroendocrine cells, most commonly 5-HT. This agent then acts on terminals of an enteric afferent neuron which, in turn, activates ascending and descending interneuronal pathways comprised of a variety of transmitters. Ultimately, the reflex terminates by activating motor neurons orad and caudad to the site of luminal stimulation. The orad motor neurons cause contraction of circular muscle as a result of the release of acetylcholine and tachykinin, mainly substance P. The caudad motor neurons cause relaxation as a result of the release of multiple transmitters including vasoactive intestinal peptide, pituitary adenylate cyclase-stimulating peptide, nitric oxide and adenosine triphosphate. These myenteric motor neurons are the primary mediators of ascending contraction and descending relaxation regardless of whether the stimulus is of mucosal origin or due to stretch/distension of the muscularis26,27. At each point in this circuit, a variety of endogenous and exogenous neuromodulators can affect neurotransmission and release of key transmitters thereby augmenting or blunting one or more of the components of the reflex. In the present study, we have examined a neurotrophin, GDNF, to determine if it might be such an agent and have focused on the ascending contraction response to a mechanical mucosal stimulus. The results demonstrate the GDNF is released selectively in conjunction with the ascending contraction component of the reflex where it acts to enhance substance P release and thereby augment contraction of circular muscle.
There is ample reason to suspect that GDNF might play a role in the adult enteric nervous system (ENS) beyond its role in the fetal ENS. In cultures of postnatal rat myenteric ganglia, GDNF has been shown to promote neurite growth, although the effect decreases with advancing age31,32. A role in adult is also suggested by the presence of GDNF in adult gut. In intestine and colon of adult human and rat, GDNF is present in smooth muscle and glial cells4,6. These two studies suggest however, that there may be species differences in that intestinal levels of GDNF increased from fetus to adult in rat whereas in human intestine adult and fetal levels were identical. By contrast, in mouse intestine GDNF levels are highest in embryo and fall in the adult5. In this study, the levels of the related neurotrophin, neurturin (NTRN), showed the opposite pattern with increasing levels from fetal to adult intestine. The presence of GDNF in the adult gut is consistent with the present study where release of endogenous GDNF was demonstrated in response to mucosal stimulation (Figure 1).
Consistent with the effect of GDNF on enteric neurons, components of the receptor complex are present in adult enteric neurons from human, mouse, and rat4,5,7,20,21,33,34. Although GDNF primarily interacts with GFRα 1, it can also interact with the NTN- preferring GFRα 2 receptor, albeit with lower affinity. While we did not determine which of the GFRα receptor mediated the response to GDNF in the present study, it is possible that GDNF acted additionally via the GFRα 2 receptor as it is also present in adult mouse and rat intestine7,20,21,34. This possibility is further supported by studies in mice in which either the GFRα 1 or GFRα 2 receptor component was knocked out. In either case, there is a decrease in release of substance P from enteric motor neurons and decrease in intestinal motility 7,20,21-23.
The use of the compartmented preparation allows the mucosal stimulus to be applied in one compartment and the motor response to be recorded in a separate compartment. In the present study, we have used this advantage to identify the possible site of action of GDNF within the reflex circuit of the ENS. Most studies of the central nervous system have focused on the dorsal root ganglion and other sensory neurons and have shown that GDNF acts to reduce substance P expression and release in response to electrical and chemical (e.g. capsaicin) stimulation12-15. In contrast, the results of the present study strongly suggest that GDNF acts on the motor limb rather than sensory limb of the ascending contraction phase of the peristaltic reflex to augment substance P release and enhance peristalsis. This notion is supported by several findings.
First, although the mucosal stroking stimulus was applied to the sensory compartment, endogenous GDNF is released only into the orad motor compartment. In previous studies using this type of preparation, we have demonstrated that release of components of the sensory limb such as serotonin, CGRP, and BDNF are detected exclusively in the compartment where the sensory stimulus was applied10,17,24.
Second, addition of exogenous GDNF to the orad compartment caused an increase in ascending contraction and substance P release (Figure 2&3); however, addition to the caudad compartment where the stimulus was applied had no effect on ascending contraction or substance P release. This supports a site of action in the motor limb rather than sensory limb of the reflex. This is in contrast to another neurotrophin BDNF which augments the sensory limb of the reflex, exclusively10. In this case, BDNF enhances the activation of the CGRP-containing sensory neurons by augmenting serotonin release. Similarly, BDNF has been shown to enhance synaptic transmission in enteric neurons cultured from adult myenteric plexus by enhancing on-going calcium responses in enteric neurons rather than initiating responses11. Thus, where examined, neurotrophins appear to act as neuromodulators that augment activity rather than initiators of activity.
In this study, we used a concentration of 10 nM GDNF to identify the property of exogenous GDNF. While it is not possible to know the concentration of endogenous GDNF at its site of action, an estimate can be made based on the measurement of GDNF overflow into the bathing medium in the present study. The actual concentration in the bathing medium ranged up to 100pg/ml. If release into the space between the motor neurons and muscle cells where it likely acts to augment substance P release is estimated to be 1000-fold higher, then the measured overflow would extrapolate to about 3nM at tissue level. This suggests that the actions of exogenous GDNF at 10nM accurately reflect a physiological action. This notion is supported by the studies with GDNF antibody as outlined below.
Third, addition of GDNF antibody to the orad compartment caused a reduction in ascending contraction and substance P release (Figure 2&4); however, addition to the caudad compartment where the stimulus was applied had no effect on ascending contraction or substance P release. This strongly suggests that the endogenous GDNF released during peristalsis (Figure 1) acts at a site in the motor limb rather than sensory limb of the reflex. Although not able to elucidate the site of action, the studies in mice with reduced levels of GDNF (Gdnf+/-) also support a functional role of endogenous GDNF as a neuromodulator of ascending contraction and substance P release (Figure 2&5). This is consistent with previous studies using these mice demonstrating decreased levels of GDNF and reduced electrically-induced contraction and substance P release22.
One noteworthy aspect of the effect of GDNF antibody and the studies in GDNF+/- mice is the differential effect at high and low levels of stimulation. Previous studies had shown that the response to low levels of stimulation are largely mediated by acetylcholine whereas the role of substance P becomes more evident at higher levels of stimulation17,19,24,25. Consistent with this notion, the inhibitory effects of the GDNF antibody on ascending contraction and substance P release were most pronounced at the higher levels of stimulation whereas the inhibition at the lowest level of stimulation did not achieve statistical significance. Similarly, the reduction in ascending contraction and in substance P release in the Gdnf+/- mice was also most pronounced at the higher levels of stimulation. Although these do not rule out an effect of GDNF on acetycholine, they do suggest that the substance P component is strongly augmented by GDNF. Alternatively, it is possible that since there is much less GDNF released at the lower levels of stimulation as compared to higher levels of stimulation (Figure 1), the role of GDNF is less at these lower levels or that there is less GDNF for the GDNF antibody to neutralize. The fact that Gdnf+/- mice demonstrate decreased substance P release in response to electrical stimulation suggests that it is most likely that the GDNF-induced augmentation of substance P release during ascending contraction is mediated by a direct effect of GDNF on tachykinin neurons22. It is also possible that there is secondary indirect augmentation of substance P release via the ascending interneuron circuits. GDNF has been shown to increase expression of neuropeptide Y (NPY) in enteric neurons and the survival promoting effects of GDNF are reduced in NPY-/- mice35. NYP has also been shown to be present in ascending interneuronal pathways where it acts to enhance release of substance P from enteric motor neurons and thereby augment the ascending contraction36. Thus, GDNF may also act by increasing the NYP component of the ascending pathways which, in turn, would augment substance P release and the ascending contraction component of the peristaltic reflex.
In summary, GDNF is released in response to mucosal mechanical stimulation in association with the motor but not sensory limb of the peristaltic reflex. Exogenous GDNF was shown to augment the ascending contraction of circular muscle and to augment the release of substance P that accompanies ascending contraction. A physiological role for GDNF was supported by the demonstration that (i) GDNF antiserum reduces ascending contraction and substance P release in rat colon, and (ii) the ascending contraction and release of substance P is blunted in mice in which GDNF levels are reduced (Gdnf+/-). Thus, the present study demonstrates that the neurotrophin, GDNF, acts as an endogenous neuromodulator of the ascending contraction component of the peristaltic reflex.
Acknowledgments
JRG was supported by grant DK34153 from NIDDKD, ROH was supported by grants DK57038 and 64592 from NIDDKD, JFK was supported by grant DK49691 from NIDDKD, and KSM was supported by grants NIDDKD 15564 and DK34153 from NIDDKD.
Footnotes
DISCLOSURES: There are no conflicts of interest to disclose. Part of this data has been published in abstract form (DDW 2004).
References
- 1.Burns AJ, Pasricha PJ, Young HM. Enteric neural crest-derived cells and neural stem cells: biology and therapeutic potential. Neurogastroenterol Motil. 2004;16 1:3–7. doi: 10.1111/j.1743-3150.2004.00466.x. [DOI] [PubMed] [Google Scholar]
- 2.Kapur RP, Gershon MD, Milla PJ, Pachnis V. The influence of HOx gene and three intracellular signaling pathways on enteric neuromuscular development. Neurogastroenterol Motil. 2004;16 1:8–13. doi: 10.1111/j.1743-3150.2004.00467.x. [DOI] [PubMed] [Google Scholar]
- 3.Young HM, Anderson RB, Anderson CR. Guidance cues involved in the development of the peripheral autonomic nervous system. Autonom Neurosci. 2004;112:1–14. doi: 10.1016/j.autneu.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Bar KJ, Facer P, Williams NS, Tam PKH, Anand P. Glial-derived neurotrophic factor in human adult and fetal intestine and in Hirschsprung's disease. Gastroenterology. 1997;112:1381–1385. doi: 10.1016/s0016-5085(97)70154-9. [DOI] [PubMed] [Google Scholar]
- 5.Golden JP, DeMaro JA, Osborne PA, Milbrandt J, Johnson EM. Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exptl Neurol. 1999;158:504–528. doi: 10.1006/exnr.1999.7127. [DOI] [PubMed] [Google Scholar]
- 6.Peters RJ, Osinski MA, Hongo JA, Bennett GL, Okragly AJ, Haak-Frendscho M, Epstein ML. GDNF is abundant in the adult rat gut. J Auton Nerv Syst. 1998;70:115–122. doi: 10.1016/s0165-1838(98)00044-7. [DOI] [PubMed] [Google Scholar]
- 7.Rossi J, Herzig KH, Voikar V, Hiltunen PH, Segerstrale M, Airaksinen MS. Alimentary tract innervation deficits and dysfunction in mice lacking GDNF family receptor Alpha 2. J Clin Invest. 2003;112:707–716. doi: 10.1172/JCI17995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du F, Wang L, Qian W, Liu S. Loss of enteric neurons accompanied by decreased expression of GDNF and PI3K/Akt pathway in diabetic rats. Neurogastroenterol Motil. 2009;21:1229–1234. doi: 10.1111/j.1365-2982.2009.01379.x. [DOI] [PubMed] [Google Scholar]
- 9.Von Boyen GB, Steinkamp M, Geerling I, Reinshagen M, Schafer KH, Adler G, Kirsch J. Proinflammatory cytokines induce neurotrophic factor expression in enteric glia: a key to the regulation of epithelial apoptosis in Crohn's disease. Inflamm Bowel Dis. 2006;12:346–354. doi: 10.1097/01.MIB.0000219350.72483.44. [DOI] [PubMed] [Google Scholar]
- 10.Grider JR, Piland BE, Gulick M, Qiao LY. Brain-derived neurotrophic factor augments the peristaltic reflex by augmenting serotonin and calcitonin gene-related peptide release. Gastroenterology. 2006;130:771–780. doi: 10.1053/j.gastro.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 11.Boesmans W, Gomes P, Janssens J, Tack J, Vanden Berghe P. Brain-derived neurotrophic factor amplifies neurotransmitter responses and promotes synaptic communication in the enteric nervous system. Gut. 2007;57:314–311. doi: 10.1136/gut.2007.131839. [DOI] [PubMed] [Google Scholar]
- 12.Skoff AM, Resta C, Swamydas M, Adler JE. Nerve growth factor (NGH) and glial cell line-derived neurotrophic factor (GDNF) regulate substance P release in adult spinal sensory neurons. Neurochem Res. 2003;28:847–854. doi: 10.1023/a:1023211107073. [DOI] [PubMed] [Google Scholar]
- 13.Adler JE. Age-dependent differential regulation of sensory neuropeptides by glial cell line-derived neurotrophic factor. J Neurochem. 1998;71:170–177. doi: 10.1046/j.1471-4159.1998.71010170.x. [DOI] [PubMed] [Google Scholar]
- 14.Ogun-Muyiwa P, Heilliwell R, McIntyre P, Winter J. Glial cell line derived neurotrophic factor (GDNF) regulates VR1 and substance P in cultured sensory neurons. Neuroreport. 1999;10:2107–2111. doi: 10.1097/00001756-199907130-00021. [DOI] [PubMed] [Google Scholar]
- 15.Alberch j, perez-navarro E, Canals JM. Neuroprotection by neurotrophins and GDNF family members in the excitotoxic model of Hunting's disease. Brain Res Bulletin. 2002;57:817–822. doi: 10.1016/s0361-9230(01)00775-4. [DOI] [PubMed] [Google Scholar]
- 16.Humpel C, Marksteiner J, Saria A. Glial cell line-derived neurotrophic factor enhances biosynthesis of substance P in striatal neurons in vitro. Cell Tiss Res. 1996;286:249–255. doi: 10.1007/s004410050694. [DOI] [PubMed] [Google Scholar]
- 17.Grider JR. Neurotransmitters mediating the intestinal peristaltic reflex in the mouse. J Pharmacol Exptl Therap. 2003;307:460–467. doi: 10.1124/jpet.103.053512. [DOI] [PubMed] [Google Scholar]
- 18.Lecci A, Capriati A, Altamura M, Maggi CA. Tachykinins and tachykinin receptors in the gut with special reference to NK2 receptors in human. Auton Neurosci. 2006;126-127:232–249. doi: 10.1016/j.autneu.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Bornstein JC, Costa M, Grider JR. Enteric motor and interneuronal circuits. Neurogastroenterol Motil. 2004;16 1:34–38. doi: 10.1111/j.1743-3150.2004.00472.x. [DOI] [PubMed] [Google Scholar]
- 20.Heuckeroth RO, Enomoto H, Grider JR, Golden JP, Hanke JA, Jackman A, Molliver DC, Barrdgett ME, Snider WD, Johnson EM, Milbrandt J. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron. 1999;22:253–263. doi: 10.1016/s0896-6273(00)81087-9. [DOI] [PubMed] [Google Scholar]
- 21.Rossi J, Luukko K, Poteryaev D, Laurikainen A, Sun YF, Laakso T, Eerikainen S, Tuominen R, Lakso M, Rauvala H, Arumae U, Pasternack M, Saarma M, Airaksinen MS. Retarded growth and deficits in the enteric and parasympathetic nervous system in mice lacking GFRα2, a functional neurturin receptor. Neuron. 1999;22:243–252. doi: 10.1016/s0896-6273(00)81086-7. [DOI] [PubMed] [Google Scholar]
- 22.Gianino S, Grider JR, Cresswell J, Endomoto H, Heuckeroth RO. GDNF determines enteric neuron numbers by controlling precursor proliferation. Development. 2003;130:2187–2198. doi: 10.1242/dev.00433. [DOI] [PubMed] [Google Scholar]
- 23.McDonagh SC, Lee J, Izzo A, Brubaker PL. Role of glial cell line-derived neurotrophic factor family receptor α2 in the actions of the glucagon-like peptides on the murine intestine. Am J Physiol. 2007;293:G461–G468. doi: 10.1152/ajpgi.00424.2006. [DOI] [PubMed] [Google Scholar]
- 24.Grider JR. Identification of neurotransmitters regulating intestinal peristaltic reflex in humans. Gastroenterology. 1989;97:1414–1419. doi: 10.1016/0016-5085(89)90384-3. [DOI] [PubMed] [Google Scholar]
- 25.Grider JR. Tachykinin as transmitters of ascending contractile component of the peristaltic reflex. Am J Physiol. 1989;257:G709–G714. doi: 10.1152/ajpgi.1989.257.5.G709. [DOI] [PubMed] [Google Scholar]
- 26.Grider JR. CGRP as a transmitter in the sensory pathway mediating peristaltic reflex. Am J Physiol. 1994;266:G1139–G1145. doi: 10.1152/ajpgi.1994.266.6.G1139. [DOI] [PubMed] [Google Scholar]
- 27.Grider JR, Jin JG. Distinct populations of sensory neurons mediate the peristaltic reflex elicited by muscle stretch and mucosal stimulation. J Neurosci. 1994;14:2854–2860. doi: 10.1523/JNEUROSCI.14-05-02854.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckland ME, Cunningham AM. Alterations in expression of the neurotrophic factors glial cell line-derived neurotrophic factor, ciliary neurotrophic factor and brain-derived neurotrophic factor, in the target-deprived olfactory neuroepithelium. Neuroscience. 1999;90:333–347. doi: 10.1016/s0306-4522(98)00270-x. [DOI] [PubMed] [Google Scholar]
- 29.Saland LC, Cunningham LA, Su C, Morales M, Gaddy J. Glial cell line-derived neurotrophic factor in the rat pituitary gland. Brain Res Bull. 2000;52(2):109–113. doi: 10.1016/s0361-9230(00)00242-2. [DOI] [PubMed] [Google Scholar]
- 30.Okragly AJ, Haak-Frendscho M. An acid-treatment method for the enhanced detection of GDNF in biological samples. Exp Neurol. 1997;145:592–6. doi: 10.1006/exnr.1997.6500. [DOI] [PubMed] [Google Scholar]
- 31.Schafer KH, Mestres P. The GDNF-induced neurite outgrowth and neuronal survival in dissociated myenteric plexus cultures of the rat small intestine decreases postnatally. Exp Brain Res. 1999;125:447–452. doi: 10.1007/s002210050702. [DOI] [PubMed] [Google Scholar]
- 32.Acevedo JR, Kuemmerle JF, Grider JR. Regulation of neurite growth in cultured myenteric neurons by endogenous neurotropin-3 and glial cell-derived neurotrophic factor. Gastroenterology. 1996;110:A1055. [Google Scholar]
- 33.Chalazonitis A, Pham TD, Rothman TP, DiStefano PS, Bothwell M, Blair-Flynn J, Tessarollo L, Gershon MD. Neurotrophin-3 is required for the survival-differentiation of subsets of developing enteric neurons. J Neurosci. 2001;21:5620–5636. doi: 10.1523/JNEUROSCI.21-15-05620.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dolatshad NF, Silva AT, Saffrey MJ. Identification of GFR alpha-2 isoforms in myenteric plexus of postnatal and adult rat intestine. Brain Res Mol Brain Res. 2002;107:32–38. doi: 10.1016/s0169-328x(02)00446-1. [DOI] [PubMed] [Google Scholar]
- 35.Anitha M, Chandrasekharan AM, Salgado JR, Grouzmann E, Mwangi S, Sitaraman SV, Srinivasan S. Glial-derived neurotropic factor modulates enteric neuronal survival and proliferation through neuropeptide Y. Gastroenterology. 2006;131:1164–1178. doi: 10.1053/j.gastro.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grider JR, Langdon LE. Physiological role of neuropeptide Y in the regulation of the ascending phase of the peristaltic reflex. Am J Physiol. 2003;285:G1139–G1146. doi: 10.1152/ajpgi.00082.2003. [DOI] [PubMed] [Google Scholar]


