Abstract
The ubiquitin ligase APC/CCdh1 coordinates degradation of key cell cycle regulators. We report here that a nuclear-localized portion of the stress-activated kinase JNK is degraded by the APC/CCdh1 during exit from mitosis and G1 phase of the cell cycle. Expression of a non-degradable JNK induces prometaphase-like arrest and aberrant mitotic spindle dynamics. Moreover, JNK directly phosphorylates Cdh1, during G2 and early mitosis, changing its subcellular localization and attenuating its ability to activate the APC/C during G2/M. The newly identified regulatory mechanism between JNK and Cdh1 reveals an important function for JNK during the cell cycle.
Keywords: JNK, APC/C, Cdh1/fzr, CDK, cell cycle, ubiquitin-proteasome system
Introduction
The capacity of a cell to normally progress through the cell cycle is controlled by complex signaling pathways primarily driven by phosphorylation and ubiquitin-mediated degradation events.
Among the key factors orchestrating cell cycle progression are cyclin-dependent kinases or CDKs, which modulate activity and stability of proteins important for cell cycle progression1. Complementing the activity of CDKs is the anaphase-promoting complex or cyclosome (APC/C), a ubiquitin ligase complex responsible for timely- and spatially-coordinated degradation of cell cycle regulators, conferring directionality and irreversibility to cell cycle transitions2, 3.
APC/C activity requires Cdc20/fzy or Cdh1/fzr adaptor proteins, which recognize specific motifs in protein substrates such as D- and KEN-boxes4–6. A timely switch between APC/CCdc20, which mostly acts during the metaphase-anaphase transition, to APC/CCdh1, which is activated during exit from mitosis and G1, enables use of the APC/C complex to target different substrates at distinct phases of the cell cycle7. This switch is controlled by (i) CDK-mediated phosphorylation of APC/C components, including the activating adaptor subunits Cdc20 and Cdh18–11; (ii) degradation of Cdc20 and Cdh1 through the cell cycle12, 13; and (iii) temporal expression of several APC/C inhibitors, such as Emi1 or Acm1, during the cell cycle14, 15.
Cdh1 phosphorylation by CDKs negatively regulates its ability to activate APC/C during S-phase, G2, and mitosis, when CDKs activity is elevated16–18. Although it is clear that CDKs target several S/TP motifs in Cdh1, detailed mapping of these phosphoacceptor sites and assessment of their relative importance are lacking19.
Here we demonstrate that JNK is activated during G2 and beginning of mitosis. JNK directly phosphorylates human Cdh1 at residues 32, 36, and 151, which inhibit its ability to activate the APC/C during G2, before Cdk1 is readily activated. We further reveal that APC/CCdh1 regulates the stability of nuclear-localized JNK during late mitosis and G1. The significance of this regulation is illustrated by inhibition of JNK degradation during the cell cycle, which results in impaired entry into mitosis and abnormal spindle and chromosomal dynamics.
Results
JNK protein levels are regulated by proteolysis in a cell cycle-dependent manner
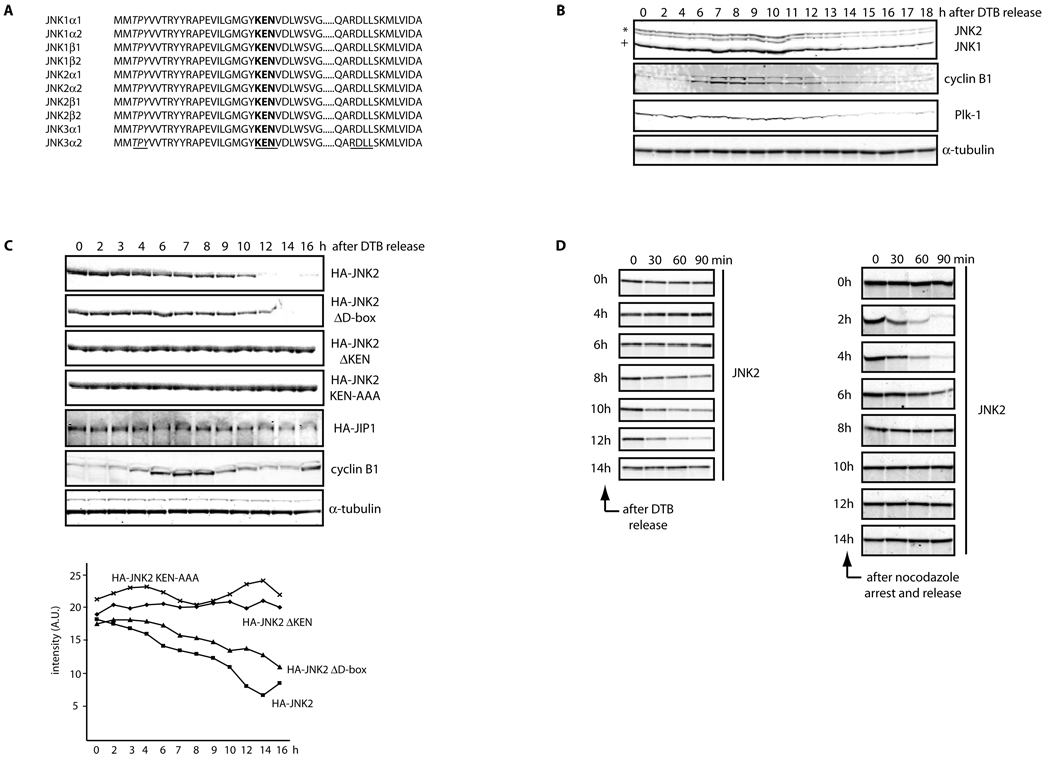
We recently reported the presence of a KEN box, a motif found in APC/C substrates, in all JNK isoforms described thus far in mammals20 (Figure 1A), prompting us to analyze JNK stability during the cell cycle. Analysis of JNK expression in HeLa cells synchronized by a double-thymidine block (which arrests cells at the beginning of S phase) revealed that JNK protein levels are indeed reduced during exit from mitosis and G0/G1 phase (Figures 1B and S1A–B). Similar changes in JNK expression levels through the cell cycle were also observed in cell cycle-synchronized T98G, U2OS, IMR90, HFF-1, and MEF cells (Figure S1C). Cell cycle synchronization in HeLa cells was biochemically confirmed by analysis of cyclin B1 and Plk-1 levels, which are mainly targeted for proteolysis by APC/CCdc20 and APC/CCdh1, respectively (Figure 1B). Cells expressing low levels of ectopic JNK also display cell cycle-dependent fluctuations in JNK levels (Figures 1C and S1D–E), suggesting that changes in JNK levels during the cell cycle are primarily post-translational. Indeed, JNK mRNA levels during the cell cycle were largely unchanged (Figure S1F).
Figure 1. JNK is degraded in vivo and in vitro in a cell cycle- and KEN box-dependent manner.
(A) Multi-alignment of a selected protein sequence from the ten members of the human JNK family. Highlighted in italics and underlined is the activation loop (amino acids 183–185, in JNK2α2); in bold and underlined is the KEN box (amino acids 203–205, in JNK2α2) and underlined a putative Destruction-box (D-box) (amino acids 295–298, in JNK2α2). (B) Extracts prepared from HeLa cells were synchronized by a double-thymidine block (DTB) and analyzed over a period of 18 h by immunoblotting using the indicated antibodies. JNK2 displays as a 54kDa band (*) while JNK1 displays as a 46kDa band (+). FACS analysis confirming the synchronization is shown in Figure S1A. (C) Synchronized HeLa cells overexpressing either HA-tagged JNK2 (wild-type or mutants) or HA-tagged JIP1 (JNK Interacting Protein 1) or control-transfected cells were analyzed by immunoblotting with antibodies against HA-tag, cyclin B1, or tubulin. Quantification of JNK2 (wild-type or mutants) levels for each synchronization is shown in the graph. (D) JNK2 in vitro degradation assays in concentrated extracts prepared from HeLa cells released after being synchronized by either DTB (left panels) or nocodazole arrest (right panels). FACS data is included in Figure S1G. (E) JNK2 in vitro degradation assays in Xenopus laevis egg extracts. Uncropped images for key results of this figure are shown in Figure S7.
To directly assess cell cycle-related changes in JNK stability, we first used in vitro extracts prepared from HeLa cells synchronized either by a double-thymidine block or by nocodazole-arrest. Only extracts prepared from cells exiting from mitosis or in G0/G1 phase could induce degradation of exogenous JNK (Figures 1D, S1G, and S2A). Consistent with these findings, we also observed that the half-life of endogenous JNK is regulated in a cell cycle-dependent manner in both synchronized HeLa and HFF-1 cells (Figures S3A–D).
Interestingly, we noted that timing of JNK degradation in different experimental settings coincides with APC/CCdh1 activation during the mammalian cell cycle13, 21. To fathom cell cycle-associated Cdh1-controlled JNK degradation, we used Xenopus laevis egg extracts, which recapitulate cell cycle transitions in vitro22. JNK was stable in (i) mitotic (CSF, CytoStatic Factor) extracts, (ii) extracts undergoing metaphase-anaphase transition (calcium-treated CSF extracts, which activate the APC/CCdc20), and (iii) interphase extracts (Inter; Figure 1E). Nevertheless, addition of Cdh1 to interphase extracts (which activates APC/CCdh1) was sufficient to cause JNK disappearance. Furthermore, treatment with the proteasome inhibitor MG-132 blocked Cdh1-induced JNK degradation in interphase extracts (Figure 1E). These data indicate cell cycle-regulated degradation of JNK by Cdh1 likely in a KEN-box-dependent manner.
Fine tuning of JNK protein levels by Cdh1
To corroborate that the JNK KEN box acts as a key molecular determinant responsible for JNK degradation20, we analyzed stability of a JNK mutant whose KEN box had been either deleted (JNKΔKEN) or mutated (JNKAAA). In vitro kinase assays showed that JNK kinase activity is unaffected upon deletion or mutation of the KEN box (see Figure S2B). Importantly, expression of either JNKΔKEN or JNKAAA revealed that both are refractory to degradation in vitro (Figures 1E and S2C) and in vivo (Figures 1C and S1E). In contrast, deletion of a putative D-box (JNKΔD-box mutant) only had a mild effect in JNK stabilization (Figures 1C, 1E, and S1E). Altogether, these results indicate that APC/CCdh1 mediates cell cycle-dependent degradation of JNK through the KEN box.
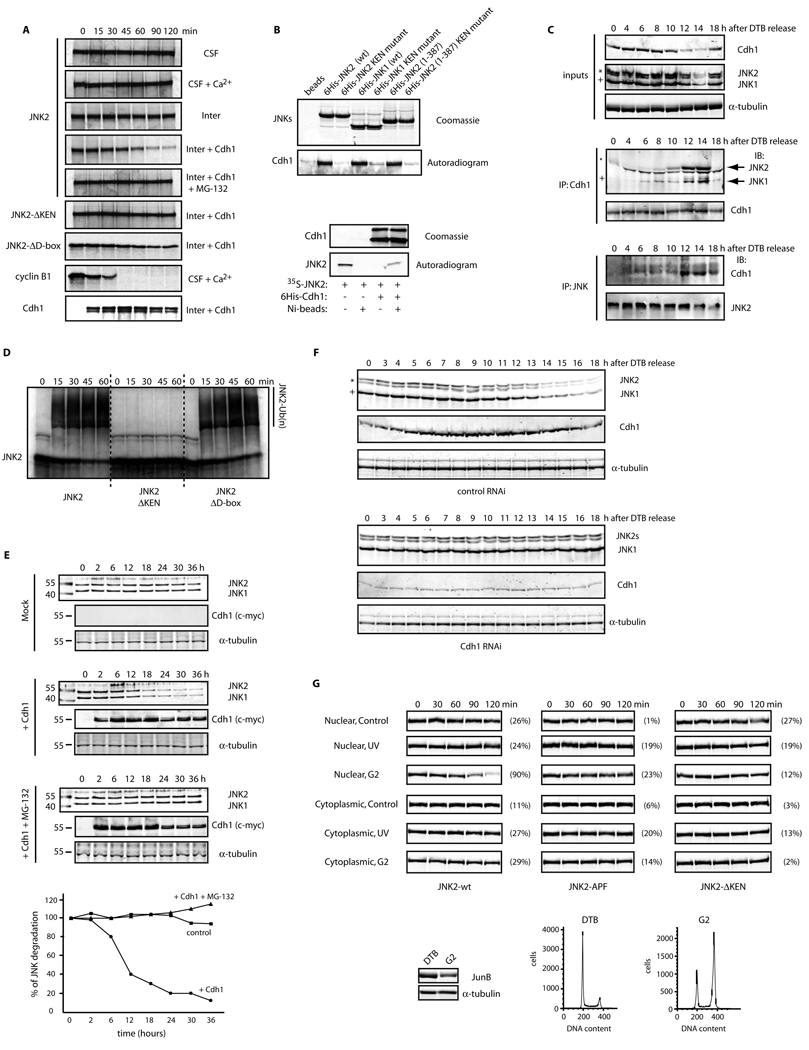
Consistent with the role of Cdh1 in JNK degradation, pull-down assays using recombinant, bacterially-produced, tagged JNK and radiolabeled Cdh1 produced in rabbit reticulocyte lysates revealed that JNK interacts in vitro with Cdh1 (Figures 2A and S2D). Conversely, recombinant Cdh1 (produced and purified from insect cells) was able to pull-down radiolabeled JNK produced in reticulocyte lysates (Figure 2A, lower panels). Further, co-immunoprecipitation assays using either overexpressed or endogenous components confirmed JNK’s association with Cdh1 in vivo (Figures S2E–F). Importantly, robust interaction between endogenous Cdh1 and JNK proteins was cell cycle-dependent and specifically apparent during exit from mitosis and G1 phase of the cell cycle (Figure 2B), when the APC/CCdh1 is known to be activated. Finally, in vitro assays revealed that APC/CCdh1 could ubiquitinate JNK (Figures 2C and S2G). These data suggest that JNK levels are regulated by APC/CCdh1-mediated ubiquitination and subsequent proteasomal degradation.
Figure 2. JNK levels are directly regulated by APC/CCdh1-mediated protein degradation during the cell cycle.
(A) Top panels: in vitro binding assay using recombinant 6×His-tagged JNKs and Cdh1/fzr translated in reticulocyte lysates and radiolabeled with 35S-methionine. Bottom panels: in vitro binding assay using recombinant 6×His-tagged Cdh1 and JNK2 translated in reticulocyte lysates and radiolabeled. Autoradiograms and Coomassie-stained gels are shown. (B) In vivo binding between endogenous JNKs and endogenous Cdh1 immunoprecipitated from synchronized HeLa cells released from a double-thymidine block (DTB). JNK2 displays as a 54kDa band (*) while JNK1 displays as a 46kDa band (+). (C) In vitro ubiquitination assay using JNK2 (wild-type or mutants) and immunoprecipitated APC/C complex from exponentially growing HeLa cells supplemented with Cdh1. (D) Overexpression of myc-tagged Cdh1 induces JNK degradation in HeLa cells. Time-course refers to hours after transfection of cells with Cdh1. Graph shows a quantification of the JNK signal. (E) Cell-cycle-synchronized Cdh1 RNAi’d HeLa cells were analyzed by immunoblotting for expression levels of Cdh1 and JNK. Uncropped images for key results of this figure are shown in Figure S7.
Our experiments in Xenopus egg extracts suggested that Cdh1 is the limiting factor required for cell cycle-dependent degradation of JNK. To test this possibility in mammalian cells, we monitored JNK levels upon exogenous expression of Cdh1. Transient overexpression of Cdh1 resulted in efficient degradation of JNK, which was blocked upon addition of the proteasomal inhibitor MG-132 (Figure 2D). Conversely, depletion of Cdh1 from cells by transfection of shRNA directed against Cdh123 abolished the oscillation of JNK levels seen during the cell cycle (Figures 2E and S2H). These findings strongly suggest that Cdh1 is required to regulate JNK degradation during the cell cycle.
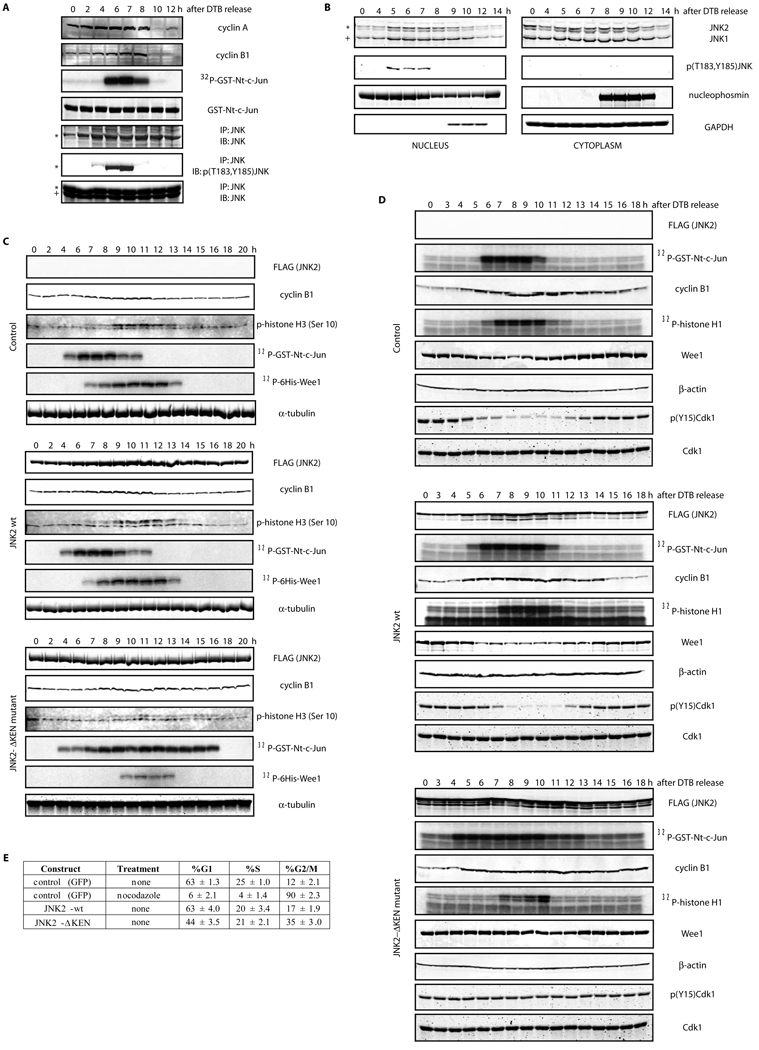
Finally, in order to obtain a clearer understanding of the signaling pathway leading to JNK degradation, we assessed whether JNK isolated from either nucleus or cytoplasm may exhibit different levels of stability in degradation assays in vitro. Our analyses revealed that nuclear-localized JNK is more susceptible to Cdh1-induced degradation (Figure 3A). Indeed, a JNK protein isolated from the nuclear compartment of cells synchronized before entry into mitosis, exhibited the shortest half-life (Figure 3A). Of note, JNK degradation was not detectable in cells subjected to UV-irradiation, suggesting that such degradation occurs in normally cycling cells but not following a genotoxic insult (data not shown). Interestingly, the kinase-deficient JNK mutant (JNKAPF) exhibited a similar pattern seen for the non-degradable version of JNK (JNKΔKEN), indicating that JNK phosphorylation (and activation) may be a prerequisite for its degradation (Figure 3A). These observations reveal that degradation of JNK requires: (i) an intact KEN box, (ii) its prior activation (phosphorylation at amino acids Thr183 and Tyr185 referred to as the TPY motif), (iii) nuclear localization, and (iv) specific G2/M-dependent modification(s).
Figure 3. JNK activation during the cell cycle regulates its subcellular localization and degradation.
(A) In vitro degradation assays, performed in Xenopus laevis interphase egg extracts supplemented with recombinant Cdh1, of FLAG-tagged JNK2 proteins (either wild-type or mutants) isolated from HeLa cells transfected for 48 h. Cells were either untreated (Control), UV-irradiated (45 J/m2) and harvested after 45 min (UV) or synchronized by DTB and harvested before mitosis (6 h after release) (G2). Extracts from these cells were separated into nuclear or cytosolic fractions when indicated. The different extracts were subjected to anti-FLAG immunoprecipitation. The bound material was eluted with the help of a saturating concentration of FLAG peptide, quantified and volume-adjusted before being used in the degradation assays. Signal observed was detected by FLAG immunoblotting and quantified with the help of the Odyssey software (LiCOR Biosciences). Numbers on the right depict the percentage of JNK2 protein degradation for each assay at the 120 min time point. Levels of JunB (whose reduction is a marker of G2-phase) were assessed in the double-thymidine blocked (DTB) versus 6 hours-released G2 extracts. FACS analyses are also included. (B) JNK activity (as detected, by either p-JNK blot or in vitro kinase assay using immunoprecipitated JNK) during the cell cycle in synchronized HeLa cells after release from a double-thymidine block (DTB). (C) HeLa cells synchronized by DTB at early S-phase, were harvested at the indicated times after release and biochemically separated into nuclear and cytosolic fractions. Each fraction was then analyzed with the indicated antibodies by immunoblotting. B23/nucleophosmin and GAPDH serve as nuclear and cytosolic markers, respectively. In this figure, JNK2 displays as a 54kDa band (*) while JNK1 displays as a 46kDa band (+).Uncropped images for key results of this figure are shown in Figure S7.
JNK activation and its role during the unperturbed cell cycle
Timely degradation of JNK, during exit from mitosis and the G1 phase of the cell cycle, implies that its instability is required for cell cycle progression. Since JNK is a kinase, it is plausible that JNK mediates timely phosphorylation of cell cycle regulatory proteins. To assess these possibilities, JNK activity was measured during the cell cycle. Interestingly, JNK activity per se (as monitored by use of a phospho-JNK antibody and in vitro kinase assays) was cell-cycle-regulated and restricted to G2 phase and early mitosis (Figure 3B). Furthermore, we found that a fraction of JNK accumulates in the nucleus during G2 and early M-phase and that this accumulation correlates with JNK activation in the nuclear compartment (Figure 3C).
Given that JNK activation is limited to G2 and early M-phase20, we hypothesized that (i) down-regulation of JNK activity during exit from mitosis is, in part, due to JNK degradation and (ii) JNK activation during G2-M might be required for unperturbed cell cycle progression. To test these possibilities, we employed the non-degradable mutant of JNK (JNKΔKEN). As noted above, we confirmed that this mutant displays kinase activity in vitro (Figure S2B) and is cell cycle-activatable in vivo (Figures 4A and S4A; HFF-1 cells and 4B; HeLa cells). Notably, biochemical analysis of synchronized cultured cells expressing JNKΔKEN revealed prolonged JNK activity during the cell cycle, accompanied by attenuated Cdk1 activity, despite elevated levels of cyclin B1, as compared to either synchronized control cells or cells transfected with wild-type JNK (Figures 4A, S4A, and 4B). Significantly, cells expressing JNKΔKEN also failed to induce Cdk1 dephosphorylation at Tyrosine 15 (an event required for Cdk1 activation) and exhibited deficient degradation of Wee1 during entry into mitosis (Figure 4B). Moreover, JNKΔKEN expression provoked delayed cyclin B1 degradation kinetics during exit from mitosis (Figures 4-B and S4B) and an abnormally higher population of cells in G2- and M-phases, as detected by fluorescence-activated cell sorting (FACS) analysis (Figures 4C, HFF-1; and S4C, HeLa cells). Of note, the degree of G2/M arrest induced by JNKΔKEN expression varied depending on the cell type used despite similar biochemical responses, with non-transformed (normal diploid) cells being affected to a greater degree (compare Figures 4C; HFF-1 cells and S4C; HeLa cells).
Figure 4. Unrestricted activation of JNK during cell cycle progression regulates Wee1’s levels, Cdk1 activity, and entry into mitosis.
(A) Biochemical cell cycle analyses of HFF-1 cells transfected with empty plasmid (Control), JNK2 wild-type (wt) or the JNK2ΔKEN mutant after DTB and release. Levels of overexpressed FLAG-tagged JNKs were analyzed by immunoblot together with levels of cyclin B1, phosphorylated-histone H3 at Serine 10 (p-histone H3) and tubulin (as loading control). Immunokinase assays of JNK (using GST-Nt-c-Jun as substrate) and Cdk1 (using a 6×His-tagged kinase-dead Wee1 as substrate) were performed. FACS data is included in Figure S4A. (B) Similar experiments as described in (A) but using HeLa cells. Levels of overexpressed FLAG-tagged JNKs were analyzed by immunoblot together with levels of cyclin B1, Wee1 and actin (as loading control). Immunokinase assays of JNK (using GST-Nt-c-Jun as substrate) and Cdk1 (using histone H1 as substrate) were performed. (A and B) Degradation pattern of JNK2-wt along the cell cycle is not obvious due to the efficient expression of JNK2 when using the pEF-FLAG plasmid (see Figures S1D–E for details). (C) Flow cytometry cell cycle analyses performed in HFF-1 cells overexpressing the indicated constructs under the stated treatments (noc: nocodazole treatment −18 hours). The percentage of cells in G1, S, and G2/M (a mixed population of cells in G2 and mitosis) phases of the cell cycle are included for one representative experiment. Experiments were repeated at least three times. Uncropped images for key results of this figure are shown in Figure S7.
Elevated levels of Wee1 have been correlated with low levels of Cdk1 activity independently of cyclin levels24. Thus, JNK may directly regulate Wee1 stability. Indeed, we found that JNK interacts with Wee1 in vitro and in vivo using either overexpressed or endogenous components. However, in vitro phosphorylation assays using bacterially purified and active Wee1 and JNK revealed that neither kinase is a substrate for the other (Figures S5A–G). These findings suggest that the JNK effect on Wee1 is likely indirect and could be mediated by members of the Cdc25 family20.
JNK controls microtubules and mitotic spindle dynamics
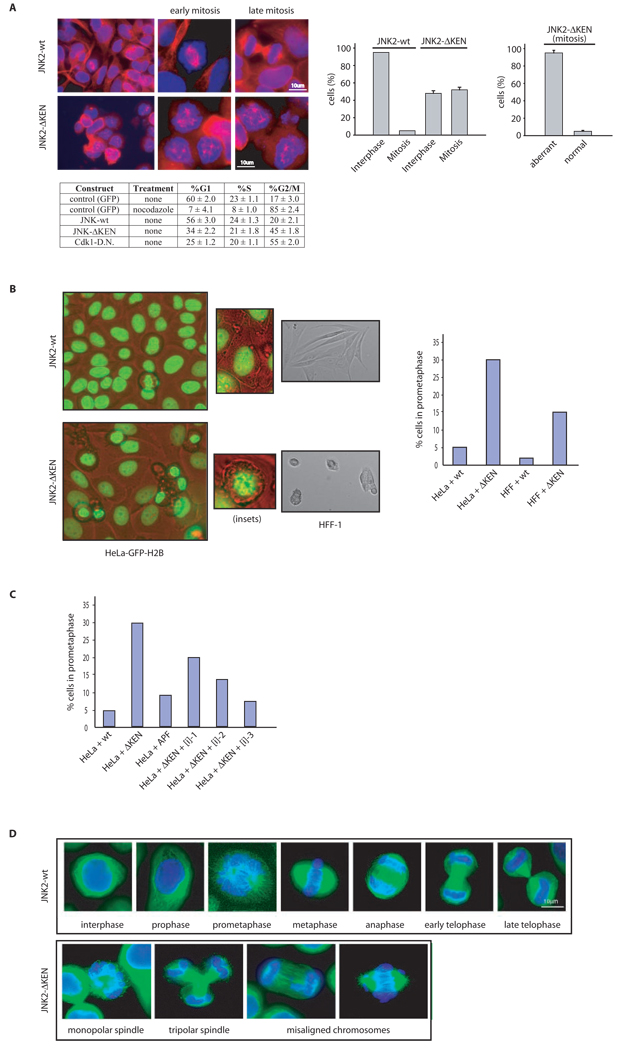
Given the increase in total mitotic index observed in both HFF-1 and HeLa cells expressing the JNKΔKEN mutant (Figures 4C and S4C, respectively), we used immunofluorescence to analyze their mitotic spindle and chromosomal dynamics. HFF-1 cells that showed the most significant G2/M arrest after expression of the JNKΔKEN mutant also displayed aberrant microtubular structures reminiscent of collapsed mitotic spindles (Figure 5A). Furthermore, to determine whether JNKΔKEN-expressing cells were impaired in entry into or exit from mitosis, or both (Figure 5B), we performed live cell imaging using wild-type JNK- and mutant JNKΔKEN-expressing HFF-1 or HeLa cells. Analyses of movies recorded using these cultured cell lines (Figure 5C and Supplemental Movies) revealed that JNKΔKEN-expressing cells exhibit delayed entry into mitosis and instead display a clear prometaphase-like arrest, characterized by highly condensed DNA that failed to align into a metaphase plate (a prerequisite for its proper segregation during progression through mitosis). Furthermore, we confirmed that prometaphase-like arrest induced by JNKΔKEN is mostly due to kinase activity generated by this mutant protein in cells, since arrest is rescued by low doses of a peptidic JNK inhibitor (Figure 5D). Finally, a significant increase in aberrant mitotic figures, including monopolar and multipolar spindles and misaligned and metaphasic lagging chromosomes were noted in HeLa cells, which were more resistant to JNKΔKEN-induced G2/M arrest (Figure 5E). These data establish that inhibition of JNK degradation, coupled with its unrestrained activity throughout the cell cycle, affects entry into mitosis, which is accompanied by abnormal mitotic microtubular and chromosomal structures.
Figure 5. Hyperactivation of JNK during unperturbed cell cycle induces aberrant microtubular and chromosomal structures and a prometaphase-like arrest in cells.
(A) Immunofluorescence microscopy performed in HFF-1 cells. Upper left panels depicts normal spindles in cells expressing JNK2 wild-type. Bottom left panels shows cells arrested in early mitosis and abnormal microtubular structures seen upon expression of JNK2ΔKEN. Tubulin is visualized in red and DNA in blue. Graphs on the right panels correspond to the G2/M (mitosis) arrest quantification observed by microscopy in HFF-1 cells expressing JNK2ΔKEN (n = 900 cells counted) versus JNK2 wild-type (n = 1200 cells counted) and the penetrance of the aberrant microtubular structures found in the cells arrested in the mitosis-like state. (B) Flow cytometry cell cycle analyses performed in HFF-1 cells overexpressing the indicated constructs under the stated treatments (noc: nocodazole −18 hours), for a representative experiment used to perform the microscopy depicted in (A). The percentage of cells in G1, S, and G2/M (a mixed population of G2 and mitosis) phases of the cell cycle are included. Experiments were repeated at least three times. (C) Top panels, captions taken from live imaging movies using either HeLa cells stably transfected with GFP-H2B or HFF-1 cells after overexpression of the indicated JNKs (for 24 h). Bottom panel, quantification of the prometaphase-like arrest induced by JNK2ΔKEN expression (for 24 h) in HeLa and HFF-1 cells. (D) Quantification of the percentage of HFF-1 cells in prometaphase-like arrest (as detected by immunofluorescence analysis after 24 h) under the conditions indicated. APF refers to a kinase-dead version of JNK2 (Thr183Ala, Tyr185Phe mutant)32. [i]-n corresponds to three different concentrations (n=1–3) of JNK inhibitor VII (100 nM, 0.5 µM, and 1 µM) used for 16 h. (E) Immunofluorescence analysis of mitotic spindles in HeLa transfected (for 48 h) with JNK2 wild-type (wt) or KEN-deleted mutant (JNK2-ΔKEN). Tubulin is shown in green and DNA (chromosomal) staining with DAPI in blue.
JNK directly phosphorylates and regulates Cdh1
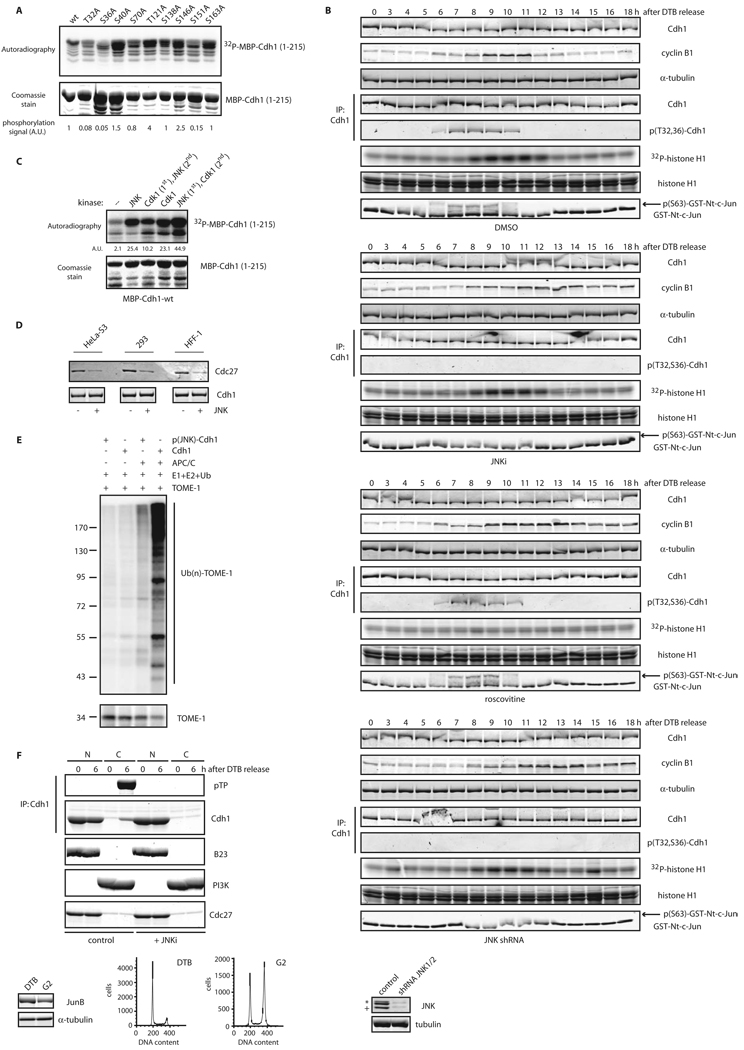
We observed a significant delay in the kinetics of cyclin B1 degradation in synchronized HFF-1 and HeLa cells expressing the JNKΔKEN mutant, despite only a modest G2/M arrest (Figures 4A–C and S4A–C), suggesting that JNK hyperactivation might directly affect APC/C. In addition, in vitro and in vivo assays revealed interaction between JNK and Cdh1 (Figures 2A–B and S2D–F). We therefore asked whether JNK contributes to Cdh1 regulation. Indeed, in vitro kinase analysis revealed that JNKs can phosphorylate Cdh1 within its N-terminal regulatory domain (Figure S6A). Detailed mutagenesis analysis including all putative S/TP sites located in the N-terminus of Cdh1 (amino acids 1–215) identified threonine 32 and serines 36 and 151 as JNK phosphoacceptor sites on Cdh1 in vitro (Figure 6A). To test for potential crosstalk between JNK- and Cdk-mediated Cdh1 phosphorylation, we analyzed the precise kinetics of activation of JNK, Cdk1, and Cdk2 during the cell cycle. We found that initial activation of JNK during the cell cycle preceded Cdk1 and was concomitant with Cdk2 (Figure S6B). Significantly, in vitro analyses revealed that JNK and Cdk2 phosphorylate different residues at the Cdh1 N-terminus (Figure S6C), while Cdk1 was able to phosphorylate all S/TP sites at the Cdh1 N-terminus in vitro (Figures S6D–E). Notably, Cdk1 phosphorylation of Cdh1 in vitro was enhanced when Cdh1 was initially phosphorylated by JNK, indicating that JNK phosphorylation of Cdh1 may prime its subsequent phosphorylation by Cdk1 (Figure 6B).
Figure 6. JNK-mediated phosphorylation of Cdh1 regulates its function.
(A) In vitro kinase assays using active JNK and MBP-Cdh1 wild-type (wt) or single phosphorylation sites mutants. (B) In vitro kinase assays using active JNK and Cdk1 either alone or sequentially (first reaction –priming– was performed using cold ATP) and recombinant MBP-Cdh1 as substrate. (C) Unphosphorylated or in vitro phosphorylated 6×His-Cdh1 by JNK, were used to pull-down Cdc27 from extracts produced from exponentially growing cell lines. (D) In vitro ubiquitination assay using APC/C complex immunoprecipitated from HeLa cells and either unphosphorylated or JNK-phosphorylated Cdh1 and 6×His-TOME-1 as substrate. Ubiquitination reactions were performed in the presence of 32P-labeled ubiquitin (previously phosphorylated by PKA in vitro) and were analyzed by SDS-PAGE and PhosphorImaging. Membrane was probed with TOME-1 antibodies to detect levels of unmodified substrate. (E) Nuclear (N) and Cytosolic (C) fractions produced from HeLa cells synchronized by a DTB and released (0, early S-phase arrest; and 6 h, G2 phase) into control or JNK VII inhibitor-containing media (JNKi; 10 µM added at 4 hours release time-point). Extracts prepared from these fractions were analyzed by Cdc27 immunoblotting and subjected to Cdh1 immunoprecipitation followed by Cdh1 and phospho-ThrPro (pTP) immunoblotting. B23/nucleophosmin and PI3K serve as nuclear and cytoplasmic markers, respectively. Levels of JunB (whose reduction is a marker of G2-phase) were assessed in the 0 h (double-thymidine blocked) versus 6 h-released (G2) extracts, only for the control conditions. FACS analyses are also included. Uncropped images for key results of this figure are shown in Figure S7.
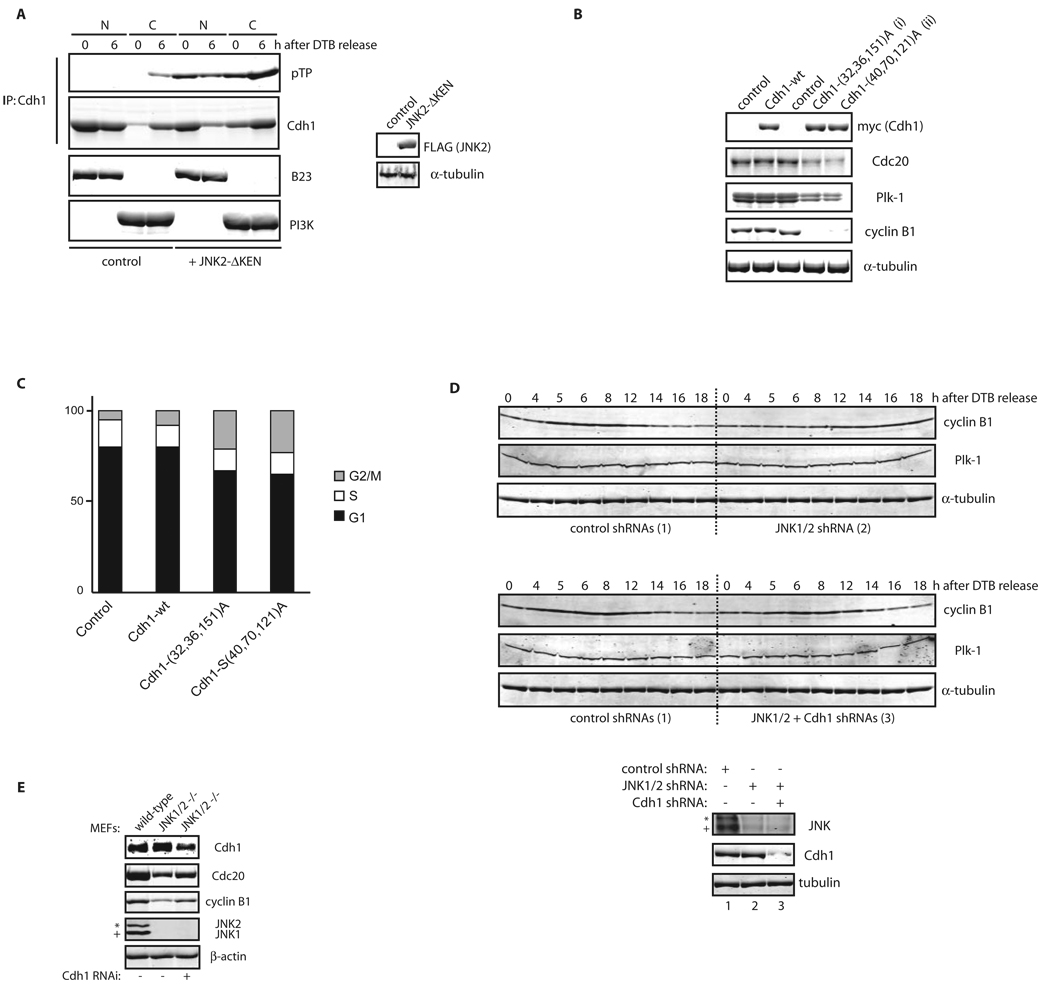
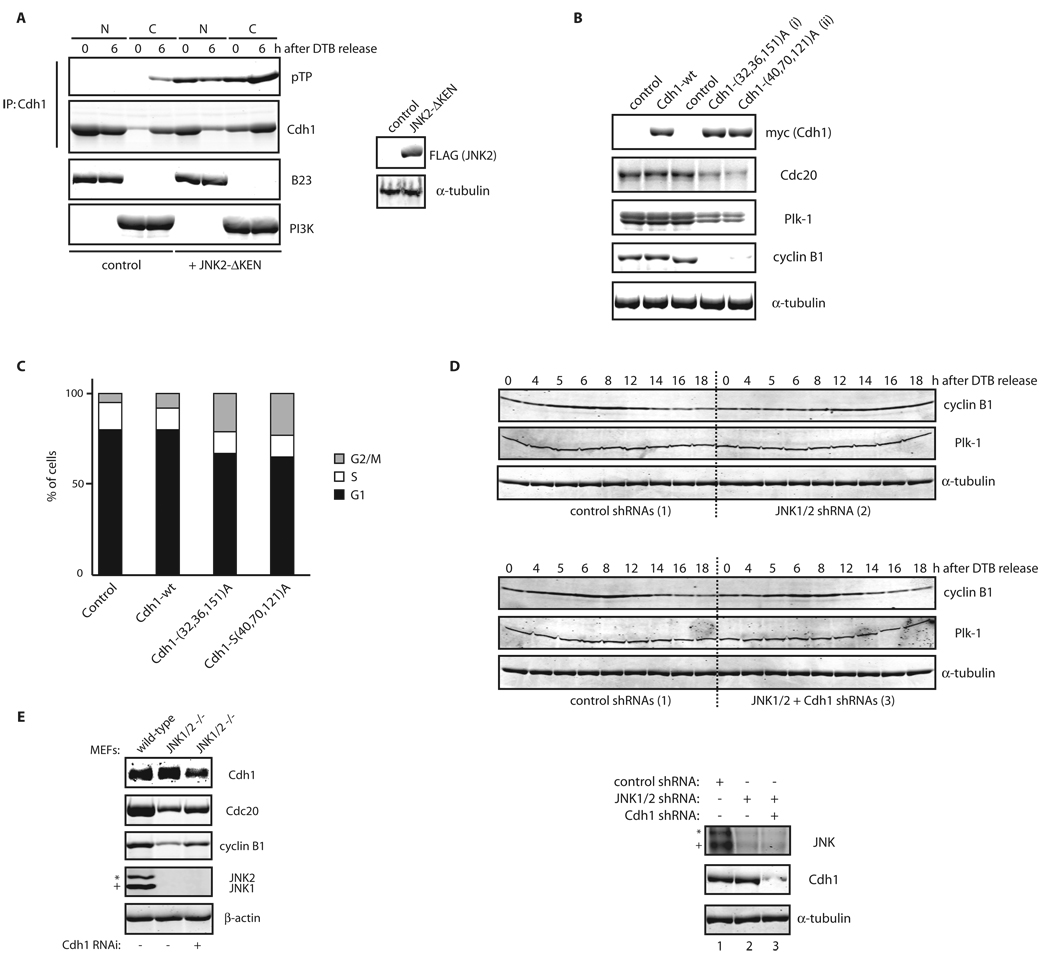
To assess the effect of Cdh1 phosphorylation by JNK, we monitored possible changes in Cdh1’s ability to activate APC/C. A pre-requisite for Cdh1 contribution to APC/C activity is its interaction with the APC/C core complex6. Significantly, when we monitored interaction between recombinant Cdh1 (either unphosphorylated or phosphorylated by JNK in vitro) and Cdc27 (a core subunit of the APC/C), we found that JNK-phosphorylated Cdh1 displayed significantly reduced affinity for Cdc27, in three different cell lines (Figure 6C), which is expected to limit Cdh1-dependent APC/C activity. Moreover, ubiquitination assays indicated that JNK-phosphorylated Cdh1 is less capable of activating APC/C in vitro compared to its unphosphorylated counterpart (Figure 6D). Consistent with these observations, we found that a fraction of Cdh1 relocates in the cytoplasm before mitosis in a JNK-dependent manner (Figure 6E). These observations reveal a mechanism for Cdh1 exclusion from APC/C core components in the presence of active JNK, thereby pointing to the role of JNK in the regulation of APC/C activity.
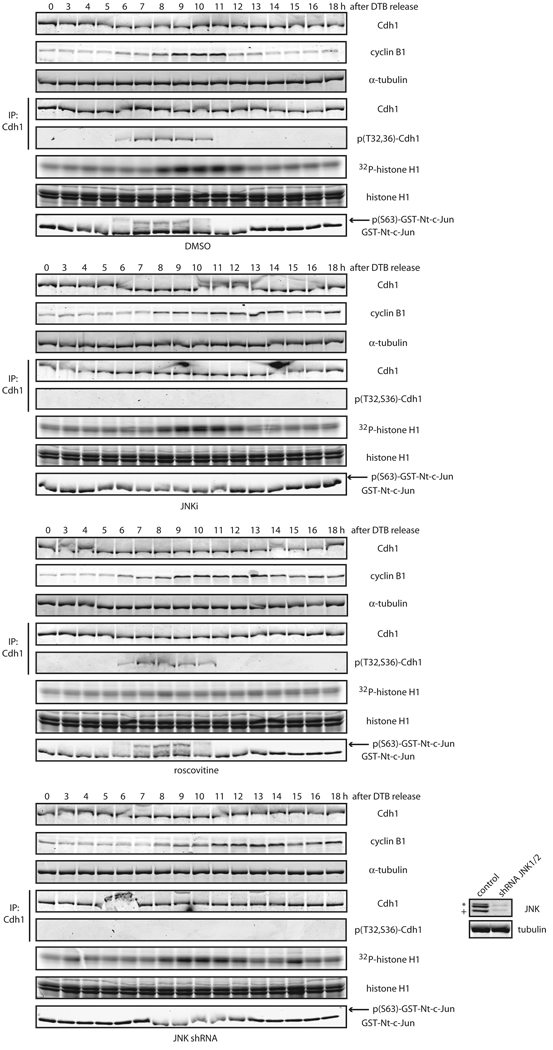
Finally, to test whether activation of JNK during the cell cycle (which occurs mainly in G2) also induces Cdh1 phosphorylation in cells, we synchronized HeLa cells and used a phospho-specific antibody (Figure S6F), raised against a phosphorylated-Thr32/Ser36-containing Cdh1 peptide. We found that Cdh1 phosphorylation at Thr32 and Ser36 likely occurred during G2 and early entry into mitosis, shortly before cyclin B1 readily accumulated in cells (Figure 7). Treatment of cells after release from S-phase arrest with either a peptidic JNK inhibitor or JNK1/2 shRNA abolished Cdh1 phosphorylation at Thr32 and Ser36 (Figure 7). Furthermore, inhibition of Cdk1/2 activation during the cell cycle by use of roscovitine, a specific pan Cdk inhibitor, did not alter Cdh1 phosphorylation at Thr32 and Ser 36 (Figure 7), suggesting that JNK is a bona fide kinase for Cdh1 during the cell cycle that acts independently of Cdks.
Figure 7. JNK phosphorylates Cdh1 in cells independently of CDKs activation.
Phosphorylation status of endogenous Cdh1 after immunoprecipitation at residues Threonine 32 (T32) and Serine 36 (S36) in cell cycle-synchronized HeLa cells after a double-thymidine block (DTB). JNKi (JNK VII inhibitor) was used at 10 µM at the 4 hours release time-point. Roscovitine was utilized at 100 µM at the 6 hours release time-point. Down-regulation of JNK1 and JNK2 achieved by means of shRNA, is shown in the inset panels (bottom right). Western-blot corresponds to the 0 h time-point; JNK2 displays as a 54kDa band (*) while JNK1 displays as a 46kDa band (+). CDKs assays were performed in vitro –using total extracts– by assessing 32P-γ-ATP incorporation in histone H1 as substrate. JNK activity was assessed in vitro, using total extracts incubated with cold ATP and recombinant GST-N-terminus-tagged c-Jun, and revealed by immunoblotting using phospho-Ser63-c-Jun antibodies following a GSH-pull down. Uncropped images for key results of this figure are shown in Figure S7.
JNK limits Cdh1 activation during cell cycle progression
We next assessed whether Cdh1 phosphorylation by JNK regulates cell cycle progression. Expression of the JNKΔKEN mutant in cells correlated with enhanced phosphorylation and cytoplasmic localization of Cdh1 (Figure 8A). This result confirms that JNK-mediated Cdh1 phosphorylation affects its localization and therefore regulates Cdh1’s ability to activate APC/C and recruit its bona-fide substrates. Furthermore, expression of non-phosphorylatable forms of Cdh1, which are mutated in either the JNK or the Cdk2 phosphoacceptor sites characterized here, decreased steady-state levels of Cdc20, Plk-1, and cyclin B1, typical substrates of APC/CCdh1 (Figure 8B), confirming that Cdh1’s phosphorylation by JNK (and Cdk2) negatively regulates its contribution to APC/C function. Moreover, ectopic expression of these non-phosphorylatable Cdh1 mutants was sufficient to block cell cycle progression, as evidenced by induction of G2/M arrest as detected by FACS (Figure 8C). Further, inhibition of JNK activity induced marked reduction and delayed accumulation of cyclin B1 in HFF-1 and HeLa cells, respectively, phenotypes rescued by Cdh1 down-regulation by shRNA (Figures 8D, for HeLa and S6G, for HFF-1). Finally, we found that in MEFs obtained from JNK1/2 DKO animals, expression levels of cyclin B1 and Cdc20 were partially repressed, which could be restored upon inhibition of Cdh1 activity (Figure 8E). These findings suggest that unrestrained activation of Cdh1 occurs during cell cycle progression in the absence of JNK activation.
Figure 8. JNK-mediated phosphorylation of Cdh1 affects cell cycle progression.
(A) Nuclear (N) and Cytosolic (C) fractions produced from HeLa cells, expressing either control or pEF-FLAG-JNK2α2-ΔKEN plasmids, were synchronized by a DTB and release (0; early S-phase arrest and 6 h; G2 phase) and analyzed by Cdh1 immunoprecipitation followed by Cdh1 and phospho-ThrPro (pTP) immunoblotting. B23/nucleophosmin and PI3K serve as nuclear and cytoplasmic markers, respectively. (B) Immunoblot analysis of Cdc20, Plk-1, and cyclin B1 protein levels in cells expressing for 36 h either Cdh1 wild-type (wt) or non-phosphorylatable triple mutants: (i) Threonine 32, Serines 36 and 151 to Alanine (a protein that cannot be phosphorylated by JNK) or (ii) Serines 40 and 70 and Threonine 121 to Alanine (a protein that is significantly less phosphorylated in vitro by Cdk2, see Figure S21). (C) Flow cytometry cell cycle analysis performed in HeLa cells overexpressing the indicated constructs. The percentage of cells in G1, S, and G2/M (a mixed population of G2 and mitosis) phases of the cell cycle are included. (D) HeLa cells infected, with either control or JNK1/2 shRNAs for 24 h, were cell cycle-synchronized by a double-thymidine block (DTB). Cells were also transfected after the first thymidine treatment with either control or Cdh1 shRNAs. Levels of JNK, Cdh1, and tubulin were assessed at time zero before release (bottom panels) and changes in cyclin B1 and Plk-1 levels and tubulin (as loading control) were analyzed during a cell cycle kinetic of 18 h. (E) Extracts from MEFs isolated from either wild-type or JNK1/2 DKO animals were analyzed by immunoblotting using antibodies directed against major cell cycle regulators (as shown), under downregulation of Cdh1 for 36 h by means of shRNA where indicated. In this figure, JNK2 displays as a 54kDa band (*) while JNK1 displays as a 46kDa band (+).Uncropped images for key results of this figure are shown in Figure S7.
Discussion
Our study uncovers an unexpected link between JNK (a major stress kinase) and Cdh1 (an important cell cycle regulator) in the control of APC/C activity and cell cycle progression, through direct phosphorylation and inhibition of Cdh1 function.
The observation that activation of endogenous JNK occurs during G2- and early M-phase20, 25, 26 suggests that JNK degradation is one of the mechanisms responsible for kinase inactivation after mitosis. Consistent with this possibility is the observation that activated JNK is preferentially targeted by APC/CCdh1-mediated degradation. However, initial inactivation of JNK seems to start prior to its ubiquitination and degradation by the APC/CCdh1. The latter suggests the existence of a JNK-specific phosphatase responsible for its inactivation during mitosis, thereby derepressing the APC/CCdh1 complex in conjunction with Cdh1 dephosphorylation mediated by the Cdc14 phosphatases27–29.
It is important to emphasize that the newly discovered function of JNK in cell cycle control is likely of physiological relevance under conditions in which JNK degradation is impaired. Such conditions could occur in settings where JNK expression and activity are constitutively high, and would resemble phenotypes seen following expression of the JNKΔKEN mutant20. Elevated JNK expression or activity, as often seen in human tumors, could be due to increased transcription or impaired degradation and mimic the effects of a non-degradable form of JNK, which deregulates cell cycle progression. In agreement, changes in Cdh1 expression or in the activity of the APC/C would result in increased JNK expression during the cell cycle.
Consistent with the notion that JNK activity is important for cell cycle progression are findings that inhibiting JNK activity either by pharmacological inhibitors30 or genetic deletion31 impairs the G2- to M-phase transition or general cell cycle progression, respectively. Finally, histone H3, Aurora B, and Cdc25C were recently suggested to be regulated by the JNK pathway during the cell cycle20, 25, 26, indicating that JNK may contribute to additional cell cycle-regulated processes.
Overall, our data establish that JNK levels and activity are tightly controlled during the cell cycle to ensure seamless entry into mitosis under normal growth conditions.
Methods
Methods and any associated references are available in the online version of the paper at http://www.nature.com/naturecellbiology/
Supplementary Material
Acknowledgements
We are indebted to R. Agami, M. Brandeis, J. Lukas, J. Bartek, J. Kyriakis, T. Hunter, H. Piwnica-Worms, S. Tsai, J. Hsieh, T. Lorca, and O. Coux for essential reagents used in this work. Supports by NCI grant (CA78419) to Z.A.R. and the Sass foundation to G.J.G. are gratefully acknowledged.
Footnotes
Note: Supplementary Information is available on the Nature Cell Biology website.
Author Contributions
Z.A.R. and G.J.G. designed and G.J.G. performed the experiments. T.T., W.J., and G.J.G. performed the microscopy. M.C. and G.J.G. conducted the FACS analyses. G.J.G. and Z.A.R. organized the study and wrote the paper.
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30:630–641. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Gutierrez GJ, Ronai Z. Ubiquitin and SUMO systems in the regulation of mitotic checkpoints. Trends Biochem Sci. 2006;31:324–332. doi: 10.1016/j.tibs.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pines J. Mitosis: a matter of getting rid of the right protein at the right time. Trends Cell Biol. 2006;16:55–63. doi: 10.1016/j.tcb.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Pfleger CM, Kirschner MW. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 2000;14:655–665. [PMC free article] [PubMed] [Google Scholar]
- 5.Pfleger CM, Lee E, Kirschner MW. Substrate recognition by the Cdc20 and Cdh1 components of the anaphase-promoting complex. Genes Dev. 2001;15:2396–2407. doi: 10.1101/gad.918201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 7.Hagting A, et al. Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. J Cell Biol. 2002;157:1125–1137. doi: 10.1083/jcb.200111001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kramer ER, Scheuringer N, Podtelejnikov AV, Mann M, Peters JM. Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol Biol Cell. 2000;11:1555–1569. doi: 10.1091/mbc.11.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaspersen SL, Charles JF, Morgan DO. Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14. Curr Biol. 1999;9:227–236. doi: 10.1016/s0960-9822(99)80111-0. [DOI] [PubMed] [Google Scholar]
- 10.Zachariae W, Schwab M, Nasmyth K, Seufert W. Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science. 1998;282:1721–724. doi: 10.1126/science.282.5394.1721. [DOI] [PubMed] [Google Scholar]
- 11.Blanco MA, Sanchez-Diaz A, de Prada JM, Moreno S. APC(ste9/srw1) promotes degradation of mitotic cyclins in G(1) and is inhibited by cdc2 phosphorylation. EMBO J. 2000;19:3945–3955. doi: 10.1093/emboj/19.15.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reis A, Levasseur M, Chang HY, Elliott DJ, Jones KT. The CRY box: a second APCcdh1-dependent degron in mammalian cdc20. EMBO Rep. 2006;7:1040–1045. doi: 10.1038/sj.embor.7400772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Listovsky T, et al. Mammalian Cdh1/Fzr mediates its own degradation. EMBO J. 2004;23:1619–1626. doi: 10.1038/sj.emboj.7600149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller JJ, et al. Emi1 stably binds and inhibits the anaphase-promoting complex/cyclosome as a pseudosubstrate inhibitor. Genes Dev. 2006;20:2410–2420. doi: 10.1101/gad.1454006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu JY, Reimann JD, Sorensen CS, Lukas J, Jackson PK. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1) Nat Cell Biol. 2002;4:358–366. doi: 10.1038/ncb785. [DOI] [PubMed] [Google Scholar]
- 16.Listovsky T, Zor A, Laronne A, Brandeis M. Cdk1 is essential for mammalian cyclosome/APC regulation. Exp Cell Res. 2000;255:184–191. doi: 10.1006/excr.1999.4788. [DOI] [PubMed] [Google Scholar]
- 17.Lukas C, et al. Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature. 1999;401:815–818. doi: 10.1038/44611. [DOI] [PubMed] [Google Scholar]
- 18.Sorensen CS, et al. A conserved cyclin-binding domain determines functional interplay between anaphase-promoting complex-Cdh1 and cyclin A-Cdk2 during cell cycle progression. Mol Cell Biol. 2001;21:3692–3703. doi: 10.1128/MCB.21.11.3692-3703.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall MC, Warren EN, Borchers CH. Multi-kinase phosphorylation of the APC/C activator Cdh1 revealed by mass spectrometry. Cell Cycle. 2004;3:1278–1284. doi: 10.4161/cc.3.10.1153. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez GJ, et al. JNK-mediated phosphorylation of Cdc25C regulates cell cycle entry and G(2)/M DNA damage checkpoint. J Biol Chem. 2010;285:14217–14228. doi: 10.1074/jbc.M110.121848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zur A, Brandeis M. Timing of APC/C substrate degradation is determined by fzy/fzr specificity of destruction boxes. EMBO J. 2002;21:4500–4510. doi: 10.1093/emboj/cdf452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutierrez GJ, et al. Meiotic regulation of the CDK activator RINGO/Speedy by ubiquitin-proteasome-mediated processing and degradation. Nat Cell Biol. 2006;8:1084–1094. doi: 10.1038/ncb1472. [DOI] [PubMed] [Google Scholar]
- 23.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 24.Pomerening JR, Kim SY, Ferrell JE., Jr Systems-level dissection of the cell-cycle oscillator: bypassing positive feedback produces damped oscillations. Cell. 2005;122:565–578. doi: 10.1016/j.cell.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Lee K, Song K. Basal c-Jun N-terminal kinases promote mitotic progression through histone H3 phosphorylation. Cell Cycle. 2008;7:216–221. doi: 10.4161/cc.7.2.5155. [DOI] [PubMed] [Google Scholar]
- 26.Oktay K, Buyuk E, Oktem O, Oktay M, Giancotti FG. The c-Jun N-terminal kinase JNK functions upstream of Aurora B to promote entry into mitosis. Cell Cycle. 2008;7:533–541. doi: 10.4161/cc.7.4.5660. [DOI] [PubMed] [Google Scholar]
- 27.Visintin R, et al. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol Cell. 1998;2:709–718. doi: 10.1016/s1097-2765(00)80286-5. [DOI] [PubMed] [Google Scholar]
- 28.Bembenek J, Yu H. Regulation of the anaphase-promoting complex by the dual specificity phosphatase human Cdc14a. J Biol Chem. 2001;276:48237–48242. doi: 10.1074/jbc.M108126200. [DOI] [PubMed] [Google Scholar]
- 29.Bassermann F, et al. The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell. 2008;134:256–267. doi: 10.1016/j.cell.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexaki VI, Javelaud D, Mauviel A. JNK supports survival in melanoma cells by controlling cell cycle arrest and apoptosis. Pigment Cell Melanoma Res. 2008 doi: 10.1111/j.1755-148X.2008.00466.x. [DOI] [PubMed] [Google Scholar]
- 31.Jaeschke A, et al. JNK2 is a positive regulator of the cJun transcription factor. Mol Cell. 2006;23:899–911. doi: 10.1016/j.molcel.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 32.Chen YR, Wang X, Templeton D, Davis RJ, Tan TH. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J Biol Chem. 1996;271:31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.