Abstract
The Escherichia coli D-galactose and D-glucose receptor possesses a Ca(II)-binding site closely related in structure and metal-binding characteristics to the eukaryotic EF-hand sites. Only the structure of the Ca(II)-occupied site is known. To investigate the structural change triggered by Ca(II) and Sr(II) binding, we have used 19F NMR to probe five 5-fluorotryptophan (5F-Trp) and seven 3-fluorophenylalanine (3F-Phe) positions in the structure, extending the approach described in the preceding article. Of particular interest were two 5F-Trp residues near the N terminus of the Ca(II) site at positions 127 and 133. Substitution of the larger Sr(II) for Ca(II) triggered 19F NMR frequency shifts of the 5F-Trp127 and -133 resonances, indicating a detectable structural change in the Ca(II) site. In contrast, the three 5F-Trp resonances from distant regions of the structure exhibited no detectable frequency shifts. When the metal was removed from the Ca(II) site, the 5F-Trp127 and -133 frequencies shifted to a new value similar to that observed for free 5F-Trp in aqueous solvent, and this new frequency was a function of the H2O to D2O ratio, indicating that the residues had become solvent exposed. Metal removal yielded small or undetectable frequency shifts for the three distant 5F-Trp resonances and for four of the five resolved 3F-Phe resonances. The allosteric coupling of the metal and sugar binding sites was observed to be slight: depletion of metal ions was observed to reduce the D-galactose affinity of the receptor by 2-fold. Together the results indicate that the structural changes in the Ca(II) site are primarily localized in the region of the site. Removal of the metal ion from the site exposes the nearby 5F-Trp127 and -133 residues to the solvent, suggesting that the empty site has a more open structure. Evidence for a similar opening of eukaryotic EF-hand sites to solvent upon removal of metal is discussed. Such a structural change could play an important role in facilitating substrate binding and dissociation.
The Escherichia coli D-galactose and D-glucose receptor possesses a single Ca(II) binding site as well as its sugar binding cleft (Vyas et al., 1987). One function of this Ca(II) site is to stabilize the folded structure of the receptor (Vyas et al., 1987; Luck, Careaga, and Falke, unpublished results), although additional functions have not been ruled out. The structure and the metal-binding properties of the Ca(II) site are remarkably similar to those of the EF-hand class of Ca(II) sites found in eukaryotic proteins involved in Ca(II) signaling and other functions (Vyas et al., 1987, 1989; Snyder et al., 1990; Strynadka James, 1989). However, the eukaryotic EF-hand sites occur in cooperative pairs that yield complex substrate-binding properties; in contrast the D-galactose and D-glucose receptor possesses only one Ca(II) site. Due to this singularity the bacterial site has become a useful model system for the investigation of the molecular mechanisms underlying selective Ca(II) binding (Snyder et al., 1990) and is used in the present study to investigate the structural change triggered by metal binding.
Extensive information is available regarding the structures of the Ca(II)-occupied conformers of the bacterial site and of a number of EF-hand sites (Vyas et al., 1987; Strynadka & James, 1989). Like the EF-hand sites, the bacterial site uses seven oxygens in a pentagonal-bipyramidal geometry to coordinate bound Ca(II). In addition the identities and conformations of the coordinating amino acids in the bacterial site fall within the ranges typically observed in EF-hand sites. The bacterial site contains one unique structural feature: its backbone structure consists of two stretches of polypeptide, nine and two amino acids in length, which are distant in the primary sequence but adjacent in the folded structure. In contrast, the consensus eukaryotic EF-hand site is composed of a continuous polypeptide loop, 12 amino acids in length. However, the backbone coordinates of the first nine residues in the bacterial and eukaryotic loops are similar or identical, as are those of the last residue. Further evidence for structural similarity is provided by observed parallels in metal binding affinities and selectivity (Vyas et al., 1987, 1989; Snyder et al., 1990).
The structural changes that occur when Ca(II) binds to EF-hand and other Ca(II) sites remain largely uncharacterized. Currently there exists no EF-hand site whose structure has been determined in both the Ca(II)-occupied and empty states. For convenience the structural changes triggered by Ca(II) binding can be separated into (1) local structural changes involving the residues of the metal-binding loop and its immediate vicinity and (2) allosteric structural changes that occur at distant sites in the protein structure. A preliminary model for the local structural changes triggered by metal binding to an EF-hand site is suggested by a comparison of the empty sites I and II of troponin C with the Ca(II)-occupied sites III and IV in the same structure (Herzberg & James, 1988; Satyshur et al., 1988; Strynadka & James, 1989). The empty sites I and II exhibit a remarkably open structure in which the distance between coordinating oxygens ranges up to 12 Å, while Ca(II)-occupied sites typically exhibit maximum oxygen–oxygen internuclear distances of 5 Å. One driving force that could contribute to the open structure of an empty Ca(II) site is the electrostatic repulsion between the negatively charged coordinating side chains. This picture suggests that side chains in the site and others nearby may be more exposed to the aqueous solvent when the metal is removed—a prediction that is testable by NMR.
The present study uses the 19F NMR approach outlined in the preceding article (Luck Falke, 1991) to investigate the structural changes triggered in the receptor by Ca(II) and other metal ions. Again the receptor is labeled at its five tryptophan positions with 5-fluorotryptophan (5F-Trp) or at its seven phenylalanine positions with 3-fluorophenylalanine (3F-Phe). Figure 1 of the preceding article illustrates the locations of these labeling sites in the receptor structure (Vyas et al., 1987) and reveals that Trp positions 127 and 133 lie near the N terminus of the main Ca(II) loop, which begins with the side-chain ligand Asp134. Phe143 lies near the C terminus of this same loop, which ends at Gln142. These three labeling sites provide a means to probe local structural changes in the vicinity of the Ca(II) site, while the other nine Trp and Phe sites enable distant structural changes to be monitored. The results indicate that Ca(II) binding triggers a local structural change involving primarily the Ca(II) site itself, such that residues in the vicinity of the Ca(II) site become exposed to the aqueous solvent upon removal of the substrate metal.
Experimental Procedures
Isolation of the Fluorine-Labeled Receptor
The 5F-Trp- or 3F-Phe-labeled receptor was produced by biosynthetic incorporation of the appropriate fluorinated amino acid in E. coli auxotroph strains, according to the procedures given in the preceding article for expression of wild-type receptor (Luck & Falke, 1991).
The present study required the apo-Ca(II) site. In order to fully remove metal ions from the site, the protocol used to isolate the labeled receptor was modified as follows. Periplasmic components including the receptor were isolated and concentrated as previously described and then subjected to a series of dialysis steps, each for 6–12 h at 4 °C. Unfolding buffer (two changes of 3.0 M guanidine HCl, 100 mM KCl, 20 mM EDTA, 10 mM Tris, pH 7.1 with HCl, and 0.5 mM phenylmethanesulfonylfluoride) denatured the protein and released the bound metal and sugar substrates (modified from Miller et al., 1983). Refolding buffer (four changes of 100 mM KCl, 20 mM EDTA, and 10 mM Tris, pH 7. with HCl) renatured the protein in the absence of Ca(II). The water used was deionized and then glass-distilled, and the KCl used was ultra-Ca(II)-free for Ca(II) electrode work (Orion Instruments Inc.). Dialysis tubing and glassware were treated with EDTA to remove contaminating metal ions. Dialyzed samples were further concentrated by ultrafiltration (Amicon apparatus, YM10 membranes) to yield a final concentration of 200–500 μM for NMR. It was found that when metals were removed from the 3F-Phe receptor, it was necessary to add 1 mM D-glucose to all buffers following the unfolding buffer to prevent precipitation.
Addition of Substrates to the Fluorine-Labeled Receptor
D-Glucose was simply added to obtain a final concentration of 1 mM as indicated in the figures. Addition of Ca(II) or Sr(II) began with dialysis to remove EDTA so that protons would not be released by the metal–EDTA equilibrium; the dialysis buffer contained 100 mM KCl, 10 mM Tris, pH 7.1 with HCl, and 0.25–2.0 mM of the appropriate metal as the dichloride salt.
Quantitation of D-Galactose Binding by Equilibrium Dialysis
[4,5-3H] D-Galactose (Du Pont NEN Research Products) was purified by thin-layer chromatography on silica gel plate in 7:4:2 1-butanol/ethanol/H2O (v/v/v) and diluted with nonradioactive D-galactose to a known specific activity. Equilibrium dialysis was carried out in pairs of microdialysis chambers separated by a 12000–14000 MW cutoff filter treated with EDTA (Spectrapore, Inc.). The receptor [10 μM, sugar- and Ca(II)-free] was added to one chamber, and radiolabeled D-galactose (varying concentration) was added to the other chamber in each pair. The dialysis buffer in both chambers contained 100 mM KCl, 10 mM Tris, pH 7.1 with HCl, and either 0.5 mM EDTA or 0.5 mM CaCl2. Equilibration was reached after 8–12 h at 5 °C. Binding data were converted to plots of receptor-bound sugar vs free sugar; then the dissociation constant and its standard error were determined by nonlinear least-squares best-fit analysis.
NMR Measurements
19F NMR spectra were obtained at 470 MHz on a Varian VXR 500 spectrometer as described in the preceding article (Luck & Falke, 1991). The standard sample contained 10% D2O, 100 mM KCl, 10 mM Tris, pH 7.1 with HCl, either 20 mM EDTA or a metal salt, and 75 μM of either 3F-Phe (for 5F-Trp-labeled receptor) or 5F-Trp (for 3F-Phe-labeled receptor) as a frequency reference and integration standard. All chemical shifts are reported relative to trifluoroacetic acid at 0 ppm. Standard spectral parameters were 12000-Hz spectral width, 16K data points, 60° pulse width, 0.68-s acquisition time, 1.0-s relaxation delay, 25-Hz line broadening, at 25 °C with no decoupling.
Solvent-induced isotope-shift (SIIS) experiments were carried out by dialyzing receptor samples against different ratios of D2O to H2O containing 100 mM KCl, 20 mM EDTA, 10 mM Tris, pH 7.1 with HCl. All spectra were acquired locked on D2O (frequency shifts measured from locked and unlocked spectra differed by <10%). Solvent-induced frequency shifts were measured as follows. The frequency of any given resonance was a linear function of the D2O to H2O ratio, which enabled the frequency in 100% H2O to be measured by extrapolation of the best-fit line. This extrapolated frequency was then subtracted from the measured frequencies in other D2O to H2O ratios to yield the frequency shift relative to 100% H2O. Standard spectral parameters were the same as above.
Protein Graphics and Distance Measurements
Receptor coordinates were analyzed on an Evans and Sutherland PS-300 graphics system driven by a VAXstation 3500 with Biosym Technologies Insight graphics software.
Error Estimates
Errors were determined as described in the accompanying article. 19F NMR frequency shifts exhibited absolute errors of ±0.1 ppm, while ratios of integrated resonances yielded relative errors of ±20%.
Results
Preparation of 5F-Trp- and 3F-Phe-Labeled Receptors and Acquisition of 19F NMR Spectra
5F-Trp or 3F-Phe was biosynthetically incorporated into the E. coli D-galactose and D-glucose receptor; then the labeled receptor was isolated to ≥90% purity. The preceding article (Luck & Falke, 1991) quantitated the extent of fluorine incorporation (65 ± 10% for 5F-Trp, 20 ± 10% for 3F-Phe), assigned the 5F-Trp 19F NMR resonances, and examined the effects of the fluorine label on Tb(III) dissociation from the Ca(II) site. It was found that a nearby 5F-Trp residue decreased the residence time of the metal in the site by 1.6- to 2.5-fold while 3F-Phe had no significant effect. These results indicate that the fluorine labeling leaves the Ca(II) site structure fully intact.
Much of the present study focuses on the SF-Trp residues near the N terminus of the Ca(II)–binding loop: 5F-Trp127 and -133. Examination of the 19F NMR resonances of 5F-Trp127 and -133 reveals that each residue gives rise to a pair of resonances (preceding article, also below). These double resonances stem from two distinct structures of the Ca(II) site that interconvert slowly on the NMR time scale. The molecular basis of these two structures has not yet been determined, although a plausible explanation is that cis and trans isomers of Pro231 yield two conformers of the contacting residue, Trp133, which in turn contacts Trp127. Irregardless of the source of this conformational duality, the present results demonstrate that 5F-Trp127 and -133 can be used to probe the structural effects of metal substitution and to investigate the structure of the empty site.
Structural Effects of Substitution of Sr(Il) for Ca(Il)
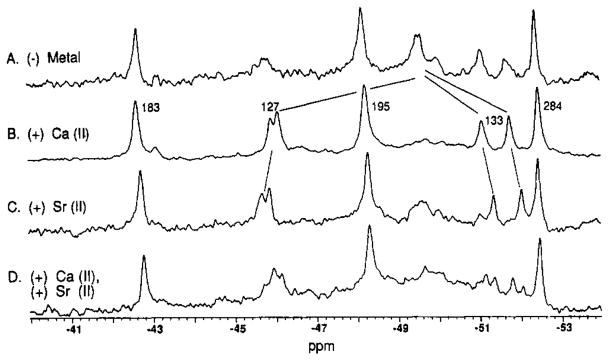
The group IIa metals Ca(II) and Sr(II) differ in diameter by 0.3 Å [their effective ionic radii are 1.06 and 1.21 Å, respectively, Shannon (1976)], and both are substrates of the Ca(II) site (Snyder et al., 1990). In order to determine the effect of an identically charged but larger metal on receptor structure, Sr(II) was substituted for Ca(II) in the receptor containing bound D-glucose in the sugar binding cleft. The structural environments of nearby residues are indeed altered by this substitution: the indole rings of Trp127 and -133 lie within 4.5 and 9.5 Å of the bound Ca(II), respectively, and Figure 1 indicates that their 5F-Trp resonances undergo significant resonance frequency shifts of +0.3 ± 0.1 and −0.3 ± 0.1 ppm, respectively, when Ca(II) is replaced by Sr(II). Similar frequency shifts are observed when the Sr(II) substitution is carried out in receptor fully depleted of bound sugar, as illustrated in Figure 2. In contrast the more distant 5F-Trp indoles 183, 195, and 284, which lie 26, 16, and 41 Å away from the bound metal, respectively, yield 5F-Trp resonances that are not detectably shifted by the Sr(II) substitution when the sugar cleft either is occupied by D-glucose or is empty (Figures 1 and 2). This result is most conclusive in the spectrum of a mixture of Ca(II)- and Sr(II)-loaded receptors, in which the 5F-Trp183, -195, and -284 resonances show no evidence of broadening due to heterogeneity (Figure 1D). Together the results indicate that substitution of a larger substrate metal ion generates a local structural change at the Ca(II) site that does not detectably perturb the sampled distant sites, suggesting that the Ca(II) site is not strongly coupled to other regions of the receptor.
FIGURE 1.
Binding of Ca(II) and Sr(II) to the D-glucose-occupied, F5-Trp-labeled receptor: 19F NMR spectra. In each case the 5- fluorotryptophan (5F-Trp) incorporation was 65 ± 10% (Luck & Falke, 1991). Samples contained 200–500 μM 5F-Trp-labeled receptor depleted of metal ions, 100 mM KCl, 10 mM Tris, pH 7.1 with HCl,1mM D-glucose, 10% D2O, and (A) 20 mM EDTA, (B) 0.25 mM CaCl2, (C) 2 mM Srcl2, or (D) 0.1 mM CaCl2 and 2.0 mM SrCl2. Spectra were recorded at 470 MHz, 25 °C. Frequencies are relative to 3F-Phe as an internal standard, and the indicated assignments are from the preceding article.
FIGURE 2.
Binding of Ca(II) and Sr(II) to the sugar-empty, 5F- Trp-labeled receptor: 19F NMR spectra. Samples contained 200–500 μM 5F-Trp-labeled receptor depleted of metal ions and sugar, with 100 mM KCl, 10 mM Tris, pH 7.1 with HCl, 10% D2O, and (A) 20 mM EDTA, (B) 0.5 mM CaCl2, or (C) 2.0 mM SrCl2. Other sample and spectral parameters at 470 MHz were as in Figure 1.
Structural Effects of Metal Removal
The effects of metal removal from the Ca(II) site on 5F-Trp residues were determined first for the receptor containing bound D-glucose, as illustrated in Figure 1A,B. When the metal is removed from the receptor, 60 ± 10% of the integrated intensity is lost from the 5F-Trp127 resonance and 20 ± 10% is lost from the 5F-Trp133 resonance. A new resonance at −49.6 ppm is simultaneously observed with the same integrated intensity (within error) as that lost from 5F-Trp127 and -133 resonances. When Ca(II) is added back to the metal-depleted site, the intensity at −49.6 ppm disappears and the 5F-Trp127 and -133 resonances are restored to their initial full intensities (data not shown). These reversible frequency shifts are even more dramatic when the receptor possesses an empty sugar cleft, as illustrated in Figure 2A,B. In this case when metal is removed, 100 ± 20% of the detectable 5F-Trp127 and 60 ± 10% of the 5F-Trp133 integrated intensities are reversibly shifted to −49.6 ppm. The simplest explanation for these results is that removal of the metal places the 5F-Trp127 and -133 residues in new but similar environments. In contrast to the 5F-Trp127 and -133 resonances, the 5F-Trp183, -195, and -284 resonances exhibit small or undetectable frequency shifts (≤0.1 ppm) upon removal of the metal from either the D-glucose-occupied or the sugar-empty receptor (Figures 1 and 2). These results are fully consistent with the interpretation that the Ca(II) site is local and does not trigger large structural changes in the sampled distant regions of the receptor.
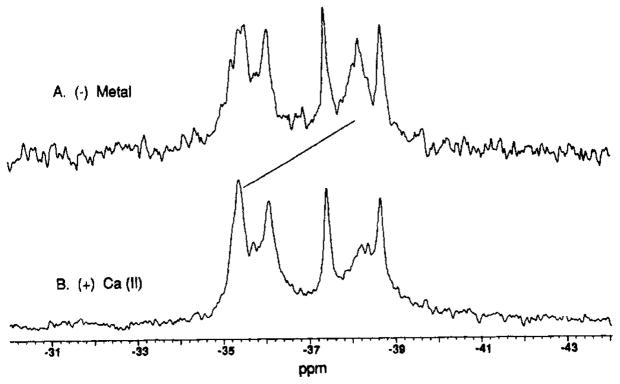
The 3F-Phe-labeled receptor containing bound D-glucose was used as an independent system in which to test the hypothesis that the Ca(II)-triggered structural change is localized. Although the 3F-Phe resonances have not been assigned, five of the seven resonances are sufficiently well resolved to be useful as probes. As illustrated in Figure 3, when the metal is removed from the site, four of the five resolved 3F-Phe resonances are not detectably shifted while one shifts dramatically to a new resonance frequency of −38.4 ppm. Only one of the 3F-Phe residues lies near the Ca(II) site: 3F-Phe143 is at the base of the Ca(II) loop. Thus at least three of the four resonances observed to be unaffected by metal removal are distant from the Ca(II) site. Together the 5F-Trp and 3F-Phe results again provide strong evidence that the Ca(II) site generates only local structural changes. [In principle the failure to detect frequency changes is a negative result; however, the possibility that Ca(II) binding or Sr(II) substitution triggers a global structural change that spuriously does not shift 19F NMR frequencies at multiple sites is unlikely because the 19F nucleus is so environmentally sensitive (Luck & Falke, 1991).]
FIGURE 3.
Binding of Ca(II) to the D-glucose-occupied, 3F-Phe-labeled receptor: 19F NMR spectra. 3F-Phe incorporation was 20 ± 10%. Samples contained 200–500 μM labeled receptor, 100 mM KCl, 10 mM Tris, pH 7.1 with HCl, 1 mM D-glucose, 10% D2O, and (A) 20 mM EDTA or (B) 0.5 mM CaCl2. Spectral parameters at 470 MHz were as in Figure 1, except that 5F-Trp was used as an internal frequency standard.
The nature of the structural changes triggered by Ca(II) binding was further tested by examining the effect of Ca(II)-site occupancy on the sugar binding equilibrium at the sugar binding cleft, 27 Å distant from the bound metal ion. The binding of [3H] D-galactose to the sugar cleft was measured by equilibrium dialysis for the Ca(II)-occupied and metal-depleted receptors; the resulting dissociation constants are 230 ± 90 nM and 500 ± 100 nM, respectively. This 2-fold change in sugar affinity corresponds to a +0.5 kcal/mol change in the D-galactose binding free energy of −9.2 kcal/mol. The observation of a small but detectable interaction between the Ca(II) and sugar sites is consistent with the small frequency shift observed for the Ca(II)-site 5F-Trpl33 when sugar binds (preceding article, Figure 5). However, the small magnitude of these changes suggests that the biological function of the Ca(II) is not simply to allosterically switch the sugar site between high- and low-affinity states.
Nature of the New Resonances That Appear When the Metal Is Removed
The observation that the 5F-Trp127 and -133 resonances are shifted to −49.6 ppm by metal removal suggests that these residues become exposed to the aqueous solvent when the Ca(II) site is empty, since free 5F-Trp in H2O also exhibits a resonance frequency of −49.6 ppm (Figure 4). Unfolding the receptor by chemical denaturation (3 M guanidine HCl) or by heat (67.5 °C) shifts all the receptor 5F-Trp resonances to frequencies near this water-exposed value of −49.6 ppm (Figure 4). Together these results support the conclusion that metal removal causes local unfolding of the Ca(II) site.
FIGURE 4.
19F NMR spectra of folded and unfolded 5F-Trplabeled receptor and of aqueous 5F-Trp. All samples contained 100 mM KCl, 10 mM Tris, pH 7.1 with HCl, 1 mM D-glucose, and 10% D2O; samples B–E also contained 200–500 μM 5F-Trp-labeled receptor. Individual samples contained (A) 10 mM 5F-Trp, free amino acid, (B) 3 M guanidine HCl, (C) heat treatment at 67.5 °C for 1 h prior to and during spectral accumulation, (D) 20 mM EDTA, and (E) 0.5 mM CaCl2. Other sample and spectral parameters at 470 MHz were as in Figure 1.
As an independent test of solvent exposure, the resonance at −49.6 ppm generated by metal removal was examined in a solvent-induced isotope-shift (SIIS) experiment. Generally, when the solvent surrounding an exposed fluorine is changed from H2O to D2O, the 19F NMR resonance undergoes a large frequency shift proposed to stem from the different hydrogen-bond strengths of F···H–O and F···D–O (Hagen et al., 1978). For a solvent-exposed 5F-Trp resonance, this shift is 100 Hz. The shift observed for the −49.6 ppm resonance of the metal-depleted receptor is 96 Hz, over 2-fold larger than that of any other receptor resonance; plotted in Figure 5 are the SIIS data for the −49.6 ppm resonance, the 5F-Trp195 resonance, and the free 5F-Trp resonance. The results confirm that 5F-Trp127 and -133 become exposed to solvent when the Ca(II) site is empty. It is interesting to note that the unassigned 3F-Phe resonance a −38.4 ppm, which is generated by metal removal, is near the frequency of −38.0 ppm observed for free 3F-Phe in H2O, indicating that the local unfolding of the Ca(II) site may also expose a 3F-Phe residue to the solvent.
FIGURE 5.
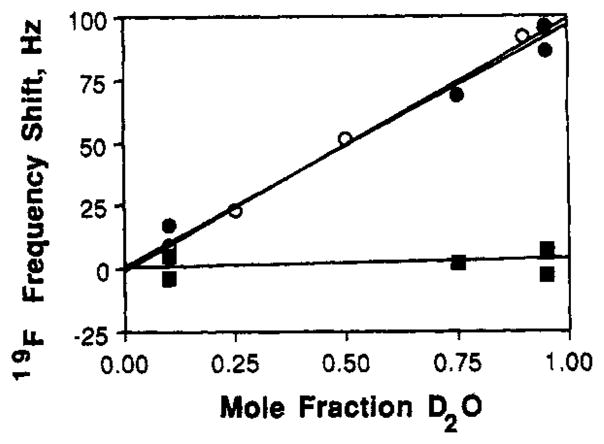
Solvent-induced isotope shifts for free 5F-Trp and receptor SF-Trp resonances. Measured for each resonance was the frequency shift caused by increasing the mole fraction of solvent D2O; this frequency shift is a measure of solvent exposure. Samples contained either 5 mM free 5F-Trp (open symbols, free amino acid) or 200–500 μM 5F-Trplabeled receptor depleted of metal and sugar (filled symbols); all samples contained 100 mM KCl, 10 mM Tris, pH 7.1 with HCl, 20 mM EDTA, and the indicated mole fraction D2O. The 5F-Trpl27 and -133 resonances in the metal-empty Ca(II) site were monitored at −49.6 ppm (closed circles), while the buried 5F-Trp195 resonance was monitored at −48. ppm (closed squares). Spectra at 470 MHz were analogous to that in Figure 2A.
The observation that metal removal shifts 5F-Trp integrated intensity to −49.6 ppm and shifts 3F-Phe intensity to −38.4 ppm raises the possibility that these new solvent-exposed intensities are due to denatured receptor molecules rather than to specific residues in the vicinity of the Ca(II) site. In this picture the disappearance of the 5F-Trp127 and -133 intensities would be ascribed to their involvement in local structural fluctuations with intermediate kinetics on the NMR time scale (preceding article, eq 1). Such a picture is disfavored by the following evidence: (1) the integrated solvent-exposed intensity is reproducible in different samples, whereas protein denaturation is generally not reproducible; (2) the appearance of the solvent-exposed intensity is reversed by Ca(II) binding, while denaturation of the receptor at NM concentrations is generally not reversible; (3) denaturation of the receptor at NMR concentrations would generally yield a precipitate, but none is observed; and (4) the integrated intensities of the solvent- exposed residues are within error (±20%) the same as those lost from specific 5F-Trp or 3F-Phe resonances. In short, the available evidence indicates that the removal of the metal from the Ca(II) site primarily impacts residues in the vicinity of the site and exposes these residues to the solvent.
Implications for the Kinetics of Metal Binding and Dissociation
The metal binding and dissociation reactions of the Ca(II) site have been proposed to be slow, exhibiting half-times of τ½ > 1 min for Tb(III) binding and dissociation (Vyas et al., 1989; Snyder et al., 1990). The present study provides limits on the kinetics of (a) the interconversion of the empty and the metal-occupied site and (b) and Ca(II)-Sr(II) exchange reaction.
For the Ca(II) on-off reaction, the relevant NMR time scale is 1 ms, as calculated from the frequency shift of the upfield 5F-Trp133 resonance to −49.6 ppm upon removal of Ca(II) (Figures 1 and 2, and preceding article, eq 1). Distinct resonances are observed in the same spectra for the 5F-Trp127 and -133 residues and for the −49.6 ppm resonance (Figure 1A), indicating a sum of distinct spectra from the Ca(II)- occupied site and the empty site. It follows that the interconversion time of these structures must satisfy τic ≫ 1 ms, verifying the slow binding and dissociation of the Ca(II) ion.
For the Ca(II)-Sr(II) exchange reaction, the relevant NMR time scale is 7 ms, based on the frequency shift of the 5F- Trpl27 and -133 residues upon Sr(II) substitution (Figures 1 and 2, and preceding article, eq 1). To probe the exchange kinetics, a saturating mixture of Ca(II) and Sr(II) was added to the metal-depleted receptor containing bound D-glucose in the sugar cleft. The resulting 19F NMR resonances of 5F- Trp127 and -133, displayed in Figure 1D, are the sums of distinct resonances from the Ca(II)-occupied and Sr(II)-occupied structures. The interconversion of these structure is thus in the slow interconversion limit and must satisfy τic ≫ 7 ms (preceding article, eq l), verifying the slowness of the Ca(II)-Sr(II) exchange.
Discussion
The present observations indicate that the fluorine atoms of residues 5F-Trp127 and -133 become exposed to solvent when the metal ion is removed from the Ca(II) site. Such a result is straightforward to explain by using the available crystal structure of the Ca(II)-occupied receptor (Vyas et al., 1987). The structure shows that the nine-residue loop (positions 34–142) providing five of the seven coordinating oxygens forms a turn connecting two nearly antiparallel secondary structure elements: an α-helix and a β-strand. A plausible model for the structural change is that removal of Ca(II) from the site enables the Ca(II)-binding turn to open by a small angle, thereby lessening the steric constraints on the 5F-Trp127 and -133 indole rotations. The observed solvent exposure of the associated 19F nuclei could represent either a static or dynamic structural change that places the fluorinated rings in contact with surface water molecules.
The proposed opening of the site upon removal of the metal may also occur in eukaryotic EF-hand sites, which exhibit similarities to the bacterial site extending to specific features of the aromatic residues located near the bound metal ion. In the bacterial structure, Trp133 occupies the n − 1 position relative to n − 1 the position of the first coordinating side chain, Asp 134. Similarly in EF-hand structures, the n − 1 position is typically occupied by an aromatic residue and the first coordinating side chain is Asp with few exceptions. In both the bacterial and eukaryotic sites, the aromatic ring at n − 1 is oriented away from the site such that it approaches the maximal distance from the bound metal ion (Vyas et al., 1987; Strynadka & James, 1989). Evidence that EF-hand sites may also open when metal is removed is provided by a comparison of EF-hand sites I and III in the troponin C structure, which both possess Phe at their n − 1 positions (Satyshur et al., 1988; Herzberg James, 1988; Strydnadka James, 1989). Site I in the troponin C structure is empty, and its n − 1 aromatic ring (Phe 29) is oriented outward to the solvent where it is largely solvent exposed. In contrast, site III is Ca(II)-occupied, and its n − 1 ring (Phe 105) is an interface residue but largely buried. This picture suggests that the structures of the bacterial and EF-hand sites are similar not only when occupied by Ca(II) but when empty as well. Further structural details are needed: a complete molecular understanding of Ca(II) binding and dissociation will be possible only when the empty and Ca(II)-occupied structures of the same Ca(II) site have been determined.
The local nature of the structural changes at the bacterial Ca(II) site is not surprising, since the site has no known allosteric function. The small (2-fold) effect of Ca(II) binding on the sugar binding affinity at the distant sugar cleft is consistent with this picture. The local nature of the structural change at the Ca(II) site is in sharp contrast to the global structural changes triggered by sugar binding to the D-galactose and D-glucose receptor (preceding paper). Together these studies illustrate the usefulness of solution 19F NMR in the analysis of both local and global structural changes in biomolecules. Future applications of the 19F NMR approach to the bacterial chemosensory system, and to other sensory and signaling systems, will be designed to further elucidate the molecular events and mechanisms of these pathways and their protein components.
Acknowledgments
We are indebted to our co-workers Louise Ingalls and Stephanie Boehme for carrying out the D-galactose binding measurements. We thank Dr. Florante A. Quiocho and his co-workers for providing crystallographic coordinates. We also thank Dr. Daniel Celander for commenting on the manuscript.
Footnotes
This investigation was supported by the National Institutes of Health Grant R01-GM40731 and by a University of Colorado CRCW Junior Faculty Development Award (J.J.F.).
Registry No. Ca, 7440-70-2; D-galactose, 59-23-4; D-glucose, 50-99-7.
References
- Hagen DS, Weiner JH, Sykes BD. Biochemistry. 1978;17:3860–3866. doi: 10.1021/bi00611a028. [DOI] [PubMed] [Google Scholar]
- Herzberg O, James MN. J Mol Biol. 1988;203:761–779. doi: 10.1016/0022-2836(88)90208-2. [DOI] [PubMed] [Google Scholar]
- Luck LA, Falke JJ. Biochemistry. 1991 (preceding paper in this issue) [Google Scholar]
- Miller DM, Olson JS, Pflugrath JW, Quiocho FA. J Biol Chem. 1983;258:13665–13672. [PubMed] [Google Scholar]
- Satyshur KA, Rao RT, Pyzalska D, Drendel W, Greaser M, Sundaralingham M. J Biol Chem. 1988;263:1628–1647. [PubMed] [Google Scholar]
- Shannon RD. Acta Crystalfogr. 1976;A32:751–767. [Google Scholar]
- Snyder EE, Buoscio BW, Falke JJ. Biochemistry. 1990;29:3937–3943. doi: 10.1021/bi00468a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strynadka NC, James MN. Annu Rev Biochem. 1989;58:951–998. doi: 10.1146/annurev.bi.58.070189.004511. [DOI] [PubMed] [Google Scholar]
- Vyas MN, Jacobsen BL, Quiocho FA. J Biol Chem. 1989;264:20817–20821. [PubMed] [Google Scholar]
- Vyas NK, Vyas MN, Quiocho FA. Nature. 1987;327:635–638. doi: 10.1038/327635a0. [DOI] [PubMed] [Google Scholar]
- Vyas NK, Vyas MN, Quiocho FA. Science. 1988;242:1290–1295. doi: 10.1126/science.3057628. [DOI] [PubMed] [Google Scholar]