Abstract
Publications relating brainstem radiation toxicity to quantitative dose and dose–volume measures derived from three-dimensional treatment planning were reviewed. Despite the clinical importance of brainstem toxicity, most studies reporting brainstem effects after irradiation have fewer than 100 patients. There is limited evidence relating toxicity to small volumes receiving doses above 60–64 Gy using conventional fractionation and no definitive criteria regarding more subtle dose–volume effects or effects after hypofractionated treatment. On the basis of the available data, the entire brainstem may be treated to 54 Gy using conventional fractionation using photons with limited risk of severe or permanent neurological effects. Smaller volumes of the brainstem (1–10 mL) may be irradiated to maximum doses of 59 Gy for dose fractions ≤2 Gy; however, the risk appears to increase markedly at doses >64 Gy.
Keywords: Brainstem, Radiation, Tolerance, NTCP
1. CLINICAL SIGNIFICANCE
Central nervous system (CNS) tolerance to radiation therapy (RT) is of concern for patients treated for primary or meta-static disease involving the brain and head and neck.
2. ENDPOINTS
The common toxicity criteria of the Cancer Therapy Evaluation Program (CTEP) grades brainstem injury on the basis of symptoms (Grade 1—mild or asymptomatic; Grade 2—moderate, not interfering with activities of daily living (ADLs); Grade 3—severe interference with ADLs, possible intervention; Grade 4—life-threatening or disabling, intervention indicated; and Grade 5—Death) (1). Severe RT-induced CNS injury is typically manifest months to years after treatment. Tumor recurrence and constitutional symptoms from other diseases and treatments may confound the diagnosis. The study of RT-induced CNS injury is challenging because (1) the incidence of injury is generally low, (2) survivals are short for most patients, (3) formal grading of brainstem effects is subjective and is often characterized categorically (yes–no) for cranial neuropathy, and (4) for patients with intracranial tumors, it is often difficult to distinguish between side effects and disease progression. For patients irradiated to head and neck sites, the distinction between brainstem and other neurological complications is often unclear.
3. CHALLENGES OF DEFINING VOLUMES
Defining the brainstem on axial imaging is usually straightforward, although it requires special attention to the superior extent and interfaces at the cerebral and cerebellar peduncles where the brainstem borders are indistinct. The brainstem includes the midbrain, pons, and medulla. The midbrain is inferior to the third ventricle and the optic tracts. The inferior border of brainstem is at the pyramidal decussation found at the level of the foramen magnum where the brainstem becomes the spinal cord. Segmentation or visualization of coronal or sagittal planes may be helpful when defining the brainstem on neuroimaging. The brainstem is a stable structure; however, anatomic shift may occur from tumor and after surgery. By neuroimaging, Luft et al. determined average brainstem volume in 48 healthy volunteers (average age 40 years, range, 20–73) to be 34 (range, 27– 43) mL (2). Merchant et al. reported age-dependent increases in brainstem volume in children diagnosed with infratentorial ependymoma (3). The volume of the brainstem can be affected by surgery and neurodegenerative conditions (4).
4. REVIEW OF DOSE–VOLUME DATA
A literature review was undertaken to extract relevant brainstem tolerance data from studies published in the era of CT-based treatment planning, including recent or active protocols in which brainstem or neurologic toxicity was reported. The review focused on articles that provided quantitative brainstem dose and dose–volume measures related to toxicity (5–24). General features of studies representing the adult population are listed in this section and in Table 1. Because of the marked interstudy variations in reporting dose and outcome, it was not possible to generate a unifying dose–response curve from the available data.
Table 1.
Selected reports of planning constraints used to limit brain stem toxicity
| Photon constraints No. patients | Particle constraints No. patients | ||
|---|---|---|---|
| Jian11* | aV65 <3 mL (BID) | Weber17† | Surface ≤63 CGE |
| 48 | aV60 <5 mL (BID) | 29 | Center ≤54 CGE |
| Hoppe 9‡ | Dmax <50 Gy | Nishimura15§ | Surface <64 CGE |
| 85 | 14 | Center <53 CGE | |
| Daly12‡ | D1% ≤54 Gy | Noel16¶,|| | Surface ≤64 CGE |
| 36 | 45 | Center ≤53 CGE | |
| Schoenfeld5* | aV55 <0.1 cc | Debus6,7¶,||,** | Surface ≤64 CGE |
| 100 | 367 | Center ≤53 CGE | |
| Wenkel14¶,**,†† | Surface ≤64 CGE | ||
| 46 | Center ≤53 CGE | ||
Abbreviation: aVXX = Absolute volume at dose XXGy; BID = twice daily; CGE = Cobalt Gray Equivalent; Dmax = maximum dose.
Either nasopharynx, oropharynx, hypopharynx or larynx.
Chordoma or chondrosarcoma.
Nasal Cavity and/or paranasal sinus.
Olfactory neuroblastoma.
Photons + proton boost.
Base of skull.
Studies that noted complications if limits were exceeded.
Meningioma.
Only five of the reviewed studies had more than 50 patients (5–10), and most did not undertake statistical analysis of toxicity. The reported range of median follow-up times was 9–60 months with death limiting follow-up in some studies (18, 22, 23). Brainstem necrosis or MRI-based evidence of injury were reported in five studies (6, 7, 13, 14, 21). Neurologic toxicities were reported in eight (6–8, 10, 11, 13, 14, 20). Treatment planning limits on the high dose component of the brainstem dose–volume histogram (DVH) in multifraction studies are shown in Table 1.
Five studies used photons only, at conventional (1.2- to 2-Gy) fractionation (5, 9, 11–13). Uy et al. (13) reported brainstem necrosis for 1 of 40 meningioma patients treated with serial tomotherapy. For this patient, the treatment plan maximum dose (Dmax) was 55.6 Gy, and the absolute volume of brainstem that exceeded 54 Gy (aV54) was 4.7 mL. Reporting on 48 patients with nasopharyngeal cancer treated with 1.2 Gy/fraction twice daily to 74.4 Gy and concomitant chemotherapy, Jian (11) noted 3 patients with Grade 1 neurologic deficit.
Five studies used protons only (15, 17) or a mixture of protons and photons (6, 7, 14, 16) using a relative biologic effectiveness (RBE) dose conversion factor of 1.1 from physical dose to cobalt gray equivalent (CGE). A change in proton dose calibration in 1995 affected two series (6, 7, 14). Proton doses were 6.5% greater than originally stated. The doses reported by Wenkel et al. (14) were recalculated to reflect the new calibration whereas those reported by Debus et al. (6, 7) were not. These studies placed separate limits on the maximum dose to the center and surface of the brainstem (Table 1). Debus’s study was the largest, reporting on 367 skull-base tumor patients treated with a combination of photon and proton conformal radiation therapy between 1974 and 1995. There were 19 late brainstem-related toxicities, including three deaths. Significant predictors of toxicity by univariate analysis were as follows: Dmax >64 Gy, aV50 >5.9 mL, aV55 >2.7 mL, aV60 >0.9 mL, two or more skull-based surgeries, diabetes, and high blood pressure. Predictors by multivariate analysis were aV60 >0.9 mL CGE, two or more skull-based surgeries, and diabetes. In Wenkle’s study of 46 patients with recurrent meningioma, the median Dmax brainstem dose was 58.0 (range, 12.1–66.3) CGE. One patient developed brainstem injury with a dose that exceeded an unspecified constraint value by 10%; two others with neurologic toxicities had brainstem doses that exceeded the constraints in Table 1; less restrictive constraints had been used initially. Two small studies demonstrated high brainstem doses without toxicities (15, 17). Median doses of 63.1 (range, 49.6–68.1) CGE and 48.5 (range, 15.8–63.3) CGE to the surface and center of the brainstem, respectively, were safe for the 29 patients in the study by Weber et al. (17). Brainstem DVHs were evaluable in 11 of the 14 patients treated without brainstem complications at 2.5 CGE/fraction in the study by Nishimura et al. (15). Maximum brainstem dose ranged from 50.7 to 66.3 CGE for four patients with the largest values. Dose to center was 63.7 CGE for the patient who received 66.3 CGE to the surface. Dose to center was <35.1 CGE in the others.
Single fraction stereotactic radiosurgery (SRS) was used in five studies (8, 10, 18–20). Each included a wide range of prescription doses and isodose levels, making it difficult to draw conclusions. The largest SRS study, by Foote et al. (10), followed 149 vestibular schwannoma patients treated with LINAC-based SRS between 1988 and 1998; 41 were treated before and 108 after 1994. Large single fractions (10–22.5 Gy) were used. Their analysis revealed a “learning curve” with a 5% and 2% actuarial 2-year rate of facial and trigeminal neuropathies, respectively, for patients treated after 1994 compared with 29% for both neuropathies for the earlier patients. Significant risk factors by univariate analysis for cranial nerve palsy were Dmax ≥17.5 Gy (facial neuropathy), prescribed dose≥12.5 Gy (any cranial neuropathy), prior open resection, age <62 years, pons-petrous tumor diameter >8 mm, tumor volume >1.7 mL; length of irradiated cranial nerve >16 mm, distance from brainstem to end of tumor in petrous bone, and planning without MR imaging (trigeminal neuropathy). Risk factors on multivariate (multiple Cox regression) analysis wereDmax, treatment before or after 1994, previous resection, and distance from brainstem to end of tumor in petrous bone. Substituting prescription dose for Dmax made a small loss in predictive strength. Authors concluded that there was significant increase in nerve complication for peripheral doses ≥15 Gy on the basis of cutpoint analysis.
There was only one multifractionated SRS study (21). Clark et al. found brainstem complications in 4 of 20 patients treated for meningioma with a hypofractionation protocol of six fractions of 7 Gy each normalized to 90 % of the maximum target dose. The brainstem was near enough to receive dose for all patients in this group. Complications were found to correlate with a mean biological effective dose (linear-quadratic model, α/β = 2.5 Gy) >70 Gy.
Pediatric CNS tumors
No toxicity was reported in pediatric patients with brainstem glioma (treated with opposed lateral fields that encompassed the majority of the brainstem) to doses of 54–60 Gy at 2 Gy/fraction, 75.6 Gy at 1.26 Gy twice daily (22) or 78 Gy at 1 Gy twice daily (23). The primary limitation of these studies was the short median survival, ≤12 months. Of 32 patients treated to 72 Gy twice daily, in combination with recombinant beta-interferon, there was at least one treatment-related death (24).
There is no evidence that the tolerance of the pediatric patient differs from the adult. Most pediatric protocols for CNS tumors recommend doses >54 Gy, and separate brainstem dose limits are usually absent.
Merchant et al. studied 68 patients with infratentorial ependymoma treated with surgery and conformal RT (54– 59.4 Gy) (3). With follow-up of 5 years post-RT, partial recovery of tumor/surgery-acquired neurological deficits was more common in patients with fewer surgeries, fewer CSF shunting procedures, smaller tumor sizes, and smaller RT planning target volumes, as well as in female patients. In patients with full or normal recovery, a considerable portion of the brainstem received over 60 Gy (aV60 = 7.8 ± 1.4 mL, V60 = 37% ± 6.3%). There was no difference in brainstem recovery based on absolute (15.4±0.9 mL) or percent (76.4% ± 3%)volume of the brainstem that received more than 54 Gy. Difference in these values for patients without full recovery was not statistically significant. One male patient died with autopsy-confirmed residual tumor and focal areas of brainstem necrosis. His mean brainstem dose was 59 Gy, and he had significant perioperative morbidity after two surgeries, including hemiparesis and unilateral and complete cranial nerve deficits involving the lower cranial nerves.
5. FACTORS AFFECTING RISK
An increased rate of toxicity has been associated with targets that are larger and closer to the brainstem (10, 18), lack of MRI-based planning (10), the number of surgeries, hydro-cephalus, diabetes, and hypertension (3, 6, 10).
6. MATHEMATICAL/BIOLOGICAL MODELS
The 1991 Emami review(25), with supporting data available at that time, specified a 5-year, 5% rate of complications, which they defined as “necrosis/infarct,” would result from 50, 53, and 60 Gy delivered to the whole, two thirds, and one third of the brainstem, respectively (25). The corresponding Lyman- Kutcher-Berman (LKB) parameters for calculation of the normal tissue complication probability (NTCP) were n = 0.16, m = 0.14, with a tolerance dose for 50% probability of having this complication (TD50) equal to 65Gy (26).These parameters may be overly conservative. For example, they estimate a 12% risk of severe complications for 54 Gy to the whole brainstemor 3% risk of complications when the particle constraints (Table 1) are used to characterize the brainstem DVH for partial volume irradiation. The estimated risks are large in comparison to those observed in the studies cited, suggesting the need for further examination of these parameter values. For example, a Lyman model with larger TD50 (±72 Gy) or smaller m (±0.1) would reduce the predicted risks to <5% or <1%, respectively. Larger n (±0.25) would predict smaller risk for exposures that followed constraints such as those in Table 1 but not for irradiation of the whole brainstem. Studies with sufficient dose–volume complication data for quantitative examination of model parameters for conventional fractionation are needed.
7. SPECIAL SITUATIONS
Applicability of the linear quadratic (LQ) model for extreme hypofractionation is controversial. Flickinger et al. (27) attempted to fit the LQ model to a variety of neurologic outcomes, including facial neuropathy (31 events/218 acoustic neuroma patients) for patients receiving stereotactic radio-surgery with ≥2 years of follow-up. Their attempts to fit these complications and other endpoints failed because they required large negative values for α/β. Meeks et al. fit the Lyman plus LQ model to the outcomes (cranial neuropathy) and DVHs of 118 patients treated with LINAC-based radiosurgery for acoustic neuroma (based on patients from a previous study with ≥1 year follow-up) (28). Multivariate analysis showed brainstem Dmax >16 Gy as the most significant risk factor for delayed cranial neuropathy. They found that the original LKB model parameters with α/β = 3.3 Gy resulted in NTCP estimates that underestimated complication probability (26). The first 50 patients were used to derive a better fit and the results were applied to all 118 patients. The best fit was achieved using n = 0.04, m = 0.15, α/β = 2.1 Gy, and TD50 = 15.3 Gy; no confidence intervals were given. They found agreement with their NTCP calculation for these two groups with 33.2 % and 5.7 % before and after 1994, respectively. The average NTCP was 7.2% (range, 0%–80%) vs. 77% (range, 29%–100%) for patients with no permanent vs. permanent cranial neuropathy. Their curve on 3% iso-complication extrapolated to 14.2 Gy for partial volume ≈0 (i.e., Dmax). Using their parameters, we calculate NTCP values equal to 1%, 13%, 61%, and 94% for partial volume irradiation of one third of the brain stem to doses of 12.5, 14.2, 16, and 17.5 Gy, respectively, demonstrating agreement with their fig. 3a. We approximate NTCP results for Dmax by calculating for a small partial volume (1%), finding values of 0.2 %, 3.2%, 26%, and 68% for the same doses. This illustrates the rapid increase in NTCP over the range of doses discussed for SRS.
8. RECOMMENDED DOSE–VOLUME LIMITS
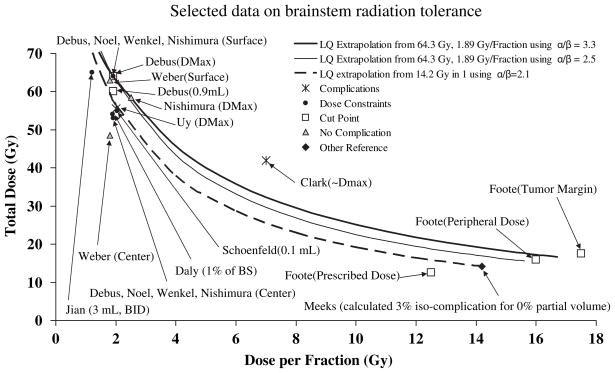
Data and LQ models of isoeffect doses for total dose vs. dose per fraction are presented in Fig. 1. Data are grouped using the categories of reported complication, cut point, dose constraint, no complication, and other reference. Cut points were dose values reported to be statistically significant for increased risk but not necessarily for low vs. high risk. Reported dose constraints used in treatment planning are presumed to be associated with low risk, although authors do not quantify the expected incidence. The “no complication” category includes reports that provided statistical data on brainstem doses >50 Gy. Data not applicable to the other categories are categorized as “other reference,” i.e., the calculated 3% isocomplication, Dmax value extrapolated in the previous section, Special Situations. Because a range of dose fractionation schedules are used, three curves have been calculated using the linear quadratic model using α/β values discussed by authors examined in this study. The solid curves identify schedules isoeffective to 64.3 Gy treated in 1.89 Gy/fraction with α/β = 3.3 (thick) (28) or 2.5 (thin) (21). The dashed curve shows schedules isoeffective to 14.2 Gy treated in a single fraction using α/β = 2.1 (28).
Fig. 1.
Comparison of selected data on brainstem tolerance and dose constrains compared to linear quadratic (LQ) model extrapolations. Data points are marked with the corresponding author and dose parameter considered in parenthesis (e.g., surface or maximum dose). Center, 0.9 mL, 0.1 mL, and 3 mL refer to the minimum dose to that hottest volume. Some data were estimated from the cited articles. Cut points illustrate thresholds determined by authors to correlate with significant increase in incidence of brainstem necrosis or neuropathy. Little quantitative data on brainstem doses is available in the dose range of stereotactic radiosurgery and hypofractionation. BID = twice daily; BS = Brain Stem; Dmax = maximum dosage.
The entire brainstem may be treated to 54 Gy using conventional fractionation with limited risk of severe or permanent neurological effects (3). Smaller volumes of the brainstem (1–10 cc) may be irradiated to maximum doses of 59 Gy for dose fractions ≤2 Gy. The risk appears to increase markedly at doses >64 Gy. However, there is insufficient information to determine whether there is a further volume effect.
Figure 1 highlights the lack of information in the 4 to 8 Gy range. The applicability of the LQ fit curves to this region of hypofractionated regimens is unknown. There is only one reported study in the intermediate hypofractionation range, and its impact is blurred by use of “effective” vs. delivered dose statistics (21). We emphasize that in presenting curves that pass through this middle-fraction-size region, we are not making recommendations for clinical choice of threshold.
For single fraction SRS, maximum brainstem dose of 12.5 Gy is associated with low (<5%) risk. Higher doses (15– 20 Gy) have been used with low reported incidence of complication in patient groups with poor prognosis for long-term survival (e.g., brain stem metastases) (18, 31). However, the apparent safety of these higher doses may be an artifact of the poor survival. Thus, additional longer-term data are needed before recommending these higher doses as relatively safe.
9. FUTURE TOXICITY STUDIES
Obtaining deeper understanding of tolerance thresholds and dose, volume, and fractionation effects is hampered by the lack of clearly defined data in the literature. To provide unambiguous data of the range of doses safely employed by clinicians and improve understanding of dose–volume effects, we make the following suggestions:
We encourage publication of detailed brainstem dose– volume and outcomes data for patients with long-term follow-up even when no toxicity has been observed. These data are especially needed for emerging fractionated SRT regimens.
In the absence of a formal structure for interinstitutional NTCP data sharing, we suggest that future studies be designed with mechanisms for acquiring and reporting detailed dosimetric and outcome information in a form that might be used for NTCP modeling. For example, publication of an “atlas” as described by Jackson, is a technically simple way to provide detailed information from a study in which DVHs are produced as part of the normal planning process (29). For hypofractionated treatments, the method of correcting dose distribution information for fractionation should be clearly described, but the underlying physical dose–volume information should also be made accessible.
Common, clinically practical, formal grading systems should be used to define the toxicities (see the next section, Toxicity Scoring) in future publications to facilitate data pooling and intercomparisons.
At a less detailed level, studies of brainstem toxicity outcomes should report the mean and standard deviation of at least four brainstem dosimetric variables: Dmax, Dmax per fraction, D1mL, and mean dose. For patients experiencing brainstem radiation necrosis or severe neuropathy, the specific values of these points should be noted.
Development of formal, communitywide methods for collection of multi-institutional data from both academic and nonacademic clinics would support the goal of obtaining sufficient data for robust modeling.
10. TOXICITY SCORING
The Common Toxicity Criteria system was replaced by the Common Terminology for Criteria for Adverse Events (CTCAE v4.0) for use in CTEP protocols. The brainstem is one component of the brain for which specific toxicity assessment is possible (1, 30). The baseline history and physical examination is requisite to the longitudinal study of brainstem function. Special attention should be given to the baseline neurologic exam and the assessment of cranial nerve, motor, sensory, and cerebellar function. Heart rate and blood pressure assessments are critical for patients with a prior history of surgery near the brainstem. A history of postoperative seizure, apnea, and neurogenic hypertension should be documented, along with developmental progression for very young children. The same assessments should be repeated at regular intervals, with documentation of improving or worsening of symptoms. Longitudinal studies of brainstem effects should consider T1, T2, and diffusion-tensor imaging to evaluate the brainstem and white matter trajectories for signs of ischemia due to the combined effects of tumor and/or surgery and for structural alterations that might be used to predict late effect (6, 7).
Acknowledgments
We thank Drs. Brian Kavanagh, John Kirkpatrick, and John Flickinger for their helpful suggestions and express special thanks to Dr. Larry Marks for his diligent efforts in editing this article.
Footnotes
Conflicts of interest: none.
References
- 1.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events, Version 4.0. Publish Date: May 27, 2009. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
- 2.Luft AR, Skalej M, Schulz JB, et al. Patterns of age-related shrinkage in cerebellum and brainstem observed in vivo using three-dimensional MRI volumetry. Cereb Cortex. 1999;9:712– 721. doi: 10.1093/cercor/9.7.712. [DOI] [PubMed] [Google Scholar]
- 3.Merchant TE, Chitti RM, Li C, et al. Factors associated with neurological recovery of brainstem function following postoperative conformal radiation therapy in infratentorial ependymoma. Int J Radiat Oncol Biol Phys. 2010;76:496–503. doi: 10.1016/j.ijrobp.2009.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klockgether T, Skalej M, Wedekind D, et al. Autosomal dominant cerebellar ataxia type I. MRI-based volumetry of posterior fossa structures and basal ganglia in spinocerebellar ataxia types 1, 2 and 3. Brain. 1998;121:1687–1693. doi: 10.1093/brain/121.9.1687. [DOI] [PubMed] [Google Scholar]
- 5.Schoenfeld GO, Amdur RJ, Morris CG, et al. Patterns of failure and toxicity after intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;71:377–385. doi: 10.1016/j.ijrobp.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Debus J, Hug EB, Liebsch NJ, et al. Brainstem tolerance to conformal radiotherapy of skull base tumors. Int J Radiat Oncol Biol Phys. 1997;39:967–975. doi: 10.1016/s0360-3016(97)00364-7. [DOI] [PubMed] [Google Scholar]
- 7.Debus J, Hug EB, Liebsch NJ, et al. Dosevolume tolerance of the brainstem after high-dose radiotherapy. Front Radiat Ther Oncol. 1999;33:305–314. doi: 10.1159/000061211. [DOI] [PubMed] [Google Scholar]
- 8.Pollock BE, Gorman DA, Brown PD. Radiosurgery for arteriovenous malformations of the basal ganglia, thalamus, and brainstem. J Neurosurg. 2004;100:210–214. doi: 10.3171/jns.2004.100.2.0210. [DOI] [PubMed] [Google Scholar]
- 9.Hoppe BS, Stegman LD, Zelefsky MJ, et al. Treatment of nasal cavity and paranasal sinus cancer with modern radio-therapy techniques in the postoperative setting—the MSKCC experience. Int J Radiat Oncol Biol Phys. 2007;67:691–702. doi: 10.1016/j.ijrobp.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Foote KD, Friedman WA, Buatti JA, et al. Analysis of risk factors associated with radiosurgery for vestibular schwannoma. J Neurosurg. 2001;95:440–449. doi: 10.3171/jns.2001.95.3.0440. [DOI] [PubMed] [Google Scholar]
- 11.Jian JJ, Cheng SH, Tsai SY, et al. Improvement of local control of T3 and T4 nasopharyngeal carcinoma by hyperfractionated radiotherapy and concomitant chemotherapy. Int J Radiat Oncol Biol Phys. 2002;53:344–352. doi: 10.1016/s0360-3016(02)02709-8. [DOI] [PubMed] [Google Scholar]
- 12.Daly ME, Chen AM, Bucci MK, et al. Intensity-modulated radiation therapy for malignancies of the nasal cavity and paranasal sinuses. Int J Radiat Oncol Biol Phys. 2007;67:151–157. doi: 10.1016/j.ijrobp.2006.07.1389. [DOI] [PubMed] [Google Scholar]
- 13.Uy NW, Woo SY, Teh BS, et al. Intensity-modulated radiation therapy (IMRT) for meningioma. Int J Radiat Oncol Biol Phys. 2002;53:1265–1270. doi: 10.1016/s0360-3016(02)02823-7. [DOI] [PubMed] [Google Scholar]
- 14.Wenkel E, Thornton AF, Finkelstein D, et al. Benign meningioma: partially resected, biopsied, and recurrent intracranial tumors treated with combined proton and photon radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48:1363–1370. doi: 10.1016/s0360-3016(00)01411-5. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura H, Ogino T, Kawashima M, et al. Proton-beam therapy for olfactory neuroblastoma. Int J Radiat Oncol Biol Phys. 2007;68:758–762. doi: 10.1016/j.ijrobp.2006.12.071. [DOI] [PubMed] [Google Scholar]
- 16.Noël G, Habrand JL, Mammar H, et al. Combination of photon and proton radiation therapy for chordomas and chondrosarcomas of the skull base: The Centre de ProtonthŽrapie D’Orsay experience. Int J Radiat Oncol Biol Phys. 2001;51:392–398. doi: 10.1016/s0360-3016(01)01634-0. [DOI] [PubMed] [Google Scholar]
- 17.Weber DC, Rutz HP, Pedroni ES, et al. Results of spot-scanning proton radiation therapy for chordoma and chondrosarcoma of the skull base: The Paul Scherrer Institut experience. Int J Radiat Oncol Biol Phys. 2005;63:401–409. doi: 10.1016/j.ijrobp.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 18.Kased N, Huang K, Nakamura JL, et al. Gamma Knife radiosurgery for brainstem metastases: The UCSF experience. J Neuro-oncol. 2008;86:195–205. doi: 10.1007/s11060-007-9458-4. [DOI] [PubMed] [Google Scholar]
- 19.Fuentes S, Delsanti C, Metellus P, et al. Brainstem metastases: Management using gamma knife radiosurgery. Neurosurgery. 2006;58:37–42. doi: 10.1227/01.neu.0000190655.95669.5c. [DOI] [PubMed] [Google Scholar]
- 20.Maruyama K, Kondziolka D, Niranjan A, et al. Stereotactic radiosurgery for brainstem arteriovenous malformations: Factors affecting outcome. J Neurosurg. 2004;100:407–413. doi: 10.3171/jns.2004.100.3.0407. [DOI] [PubMed] [Google Scholar]
- 21.Clark BG, Souhami L, Pla C, et al. The integral biologically effective dose to predict brainstem toxicity of hypo-fractionated stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 1998;40:667–675. doi: 10.1016/s0360-3016(97)00734-7. [DOI] [PubMed] [Google Scholar]
- 22.Freeman CR, Krischer JP, Sanford RA, et al. Final results of a study of escalating doses of hyperfractionated radiotherapy in brainstem tumors in children: a Pediatric Oncology Group study. Int J Radiat Oncol Biol Phys. 1993;27:197–206. doi: 10.1016/0360-3016(93)90228-n. [DOI] [PubMed] [Google Scholar]
- 23.Packer RJ, Boyett JM, Zimmerman RA, et al. Outcome of children with brainstem gliomas after treatment with 7800 cGy of hyperfractionated radiotherapy. A Children’s Cancer Group Phase I/II trial. Cancer. 1994;74:1827–1834. doi: 10.1002/1097-0142(19940915)74:6<1827::aid-cncr2820740628>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 24.Packer RJ, Prados M, Phillips P, et al. Treatment of children with newly diagnosed brainstem gliomas with intravenous recombinant beta-interferon and hyperfractionated radiation therapy: A Children’s Cancer Group Phase I/II study. Cancer. 1996;77:2150–2156. doi: 10.1002/(SICI)1097-0142(19960515)77:10<2150::AID-CNCR28>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 25.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 26.Burman C, Kutcher GJ, Emami B, et al. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys. 1991;21:123–135. doi: 10.1016/0360-3016(91)90172-z. [DOI] [PubMed] [Google Scholar]
- 27.Flickinger JC, Kondziolka D, Lunsford LD. Radiobiological analysis of tissue responses following radiosurgery. Technol Cancer Res Treat. 2003;2:87–92. doi: 10.1177/153303460300200203. [DOI] [PubMed] [Google Scholar]
- 28.Meeks SL, Buatti JM, Foote KD, et al. Calculation of cranial nerve complication probability for acoustic neuroma radiosurgery. Int J Radiat Oncol Biol Phys. 2000;47:597–602. doi: 10.1016/s0360-3016(00)00493-4. [DOI] [PubMed] [Google Scholar]
- 29.Jackson A, Yorke ED, Rosenzweig KE. The atlas of complications incidence: A proposal for a new standard for reporting the results of radiotherapy protocols. Semin Radiat Oncol. 2006;16:260–268. doi: 10.1016/j.semradonc.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Pollock BE, Flickinger JC. A proposed radiosurgery-based grading system for arteriovenous malformations. J Neurosurg. 2002;96:79–85. doi: 10.3171/jns.2002.96.1.0079. [DOI] [PubMed] [Google Scholar]
- 31.Lorenzoni JG, Devriendt D, Massager N. Brain stem metastases treated with radiosurgery: prognostic factors of survival and life expectancy. Surg Neurol. 2009;71:188–196. doi: 10.1016/j.surneu.2008.01.029. [DOI] [PubMed] [Google Scholar]