Abstract
Objectives
To determine if sensory and motor nerve function was associated cross-sectionally with quadriceps strength or ankle dorsiflexion strength in an older community-based population.
Design, Setting, Participants
Health, Aging, and Body Composition Study participants (N=2059; 50% men; 37% black, aged 73-82 years) in 2000-2001.
Intervention
n/a
Measurements
Quadriceps and ankle strength were measured with an isokinetic dynamometer. Sensory and motor peripheral nerve function in the legs/feet was assessed by 10-g and 1.4-g monofilaments, vibration threshold, and peroneal motor nerve conduction amplitude and velocity.
Results
Monofilament insensitivity, poorest vibration threshold quartile (>60 μ), and poorest motor nerve conduction amplitude quartile (<1.7 mV) were associated with 11%, 7%, and 8% lower quadriceps strength (all p<0.01), respectively, compared to the best peripheral nerve function categories in adjusted linear regression models. Monofilament insensitivity and lowest amplitude quartile were both associated with 17% lower ankle strength (p<0.01). In multivariate analyses, with monofilament insensitivity (β=-7.19), vibration threshold (β=-0.097), and motor nerve conduction amplitude (β=2.01) in the same model, each contributed independently to lower quadriceps strength (all p<0.01). Monofilament insensitivity (β=-5.29) and amplitude (β=1.17) each contributed independently to lower ankle strength (all p<0.01). Lean mass did not explain the associations of peripheral nerve function with strength.
Conclusions
Reduced sensory and motor peripheral nerve function is related to poorer lower-extremity strength in older adults, suggesting a mechanism for the relationship with lower-extremity disability.
Keywords: quadriceps strength, peripheral nerves, performance
Introduction
Loss of lean muscle mass, or sarcopenia, is thought to account for much of the loss of strength and function in older adults (1-3). However maintenance or gain of muscle mass may not prevent age-related strength decline (3). In addition to lower lean mass, aging muscle is characterized by loss of muscle fibers, predominantly type 2 fast twitch fibers, and an increase in grouping or “clustering” of type 1 fibers (4). Despite the known atrophy and denervation contributing to the pathophysiology of muscle aging, little is known about the role of poor peripheral nerve function in age-related strength loss. Potentially peripheral nerve function in the lower leg is more strongly related to distal strength. However, an association with proximal strength is also plausible if systemic factors are damaging the peripheral nerves throughout the lower extremities.
The incidence (5) and prevalence of poor peripheral nerve function is higher in older adults, even those without diabetes (6-11). In the U.S. for 1999-2000, 28% of adults aged 70-79 years and 35% of adults aged >80 years had loss of protective sensation at the foot, with a higher prevalence in diabetic adults (11). Diabetes was associated with greater loss of strength (12-13) in the Health, Aging and Body Composition (Health ABC) Study and is associated with poorer self-reported and objective physical performance measures U.S. adults (14-16) and the Health ABC cohort (17). Poor peripheral nerve function may be related to reduced physical performance in older diabetic adults (18-21).
Reduced sensory and motor peripheral nerve function was related to poorer Health ABC physical performance battery scores and its individual components (usual and narrow walking speed, standing balance, chair stands), independent of diabetes (22). Lower extremity strength may be a pathway through which poor peripheral nerve function is related to reduced physical function and greater disability in old age (22). We examined if reduced sensory and motor nerve function is associated cross-sectionally with lower quadriceps strength or ankle dorsiflexion strength in an older community-based population.
Methods
Study participants
The Health, Aging, and Body Composition Study (Health ABC) is an ongoing prospective cohort study investigating changes in body composition as a common pathway by which multiple diseases contribute to disability. Participants were well-functioning adults aged 70-79 years at baseline examination in 1997-1998 (N=3075; 48.4% male; 41.6% black). Participants were a volunteer group from a large mass mailing to 1) a random sample of white Medicare beneficiaries and 2) all age-eligible black community residents, in Pittsburgh, PA and Memphis, TN. Eligible participants reported no difficulty walking a quarter of a mile (400 m), climbing 10 steps, or performing activities of daily living; were free of life-threatening cancers with no active treatment within the past 3 years; and planned to remain within the study area for >3 years. Participants provided informed consent prior to exams, approved by the institutional review boards at the University of Pittsburgh and the University of Tennessee Health Science Center. Of 3075 participants at baseline, 2096/2493 (84.0%) with a clinic/home 2000-2001 exam had a quadriceps strength assessment. The remaining cohort had an in-person physical performance battery though not quadriceps strength (N=383), an in-person exam without physical function testing (N=14), telephone follow-up only (N=282), were deceased (N=187), withdrew (N=11) or missed the exam (N=102). We excluded participants missing fasting blood glucose (n=22) or with diabetes onset of < 20 years old (n=5). We included 2059 participants (686 white men, 334 black men, 617 white women, 422 black women) with cross-sectional measures of quadriceps strength and peripheral nerve function from 2000-2001, representing 67.0% of baseline participants and 83.0% with a 2000-2001 exam. Of these, 1744 (607 white men, 260 black men, 534 white women, 343 black women) had data on ankle strength a year later at a 2001-2002 exam.
Sensory and motor peripheral nerve function
Peripheral nerve function measurements included monofilament sensitivity, average vibration detection threshold on the bottom of the great toe in μ (VSA-3000 Vibratory Sensory Analyzer, Medoc), and peroneal motor nerve conduction amplitude in mV and velocity in m/s from the popliteal fossa to ankle (NeuroMax 8, XLTEK). These measures represent standard peripheral nerve function assessments that have been previously validated. Monofilament insensitivity was defined as inability to feel 3 of 4 touches at the dorsum of the great toe for the 10-g standard monofilament (lack of standard sensation) or as inability to feel 3 of 4 touches for the 1.4-g monofilament (lack of light touch sensation). Clinic examiners with training and certification in the nerve function measures performed the tests on the right leg unless it was contraindicated due to knee replacement, amputation, ulcer, trauma, or surgery. Feet were warmed to 30°C prior to testing.
Quadriceps and ankle strength
Knee extension strength was measured concentrically from 90° to 30° at 60° per second on an isokinetic dynamometer (Kin-Com 125 AP Dynamometer) in 3-6 trials after a warm-up trial at sub-maximal effort (23, 24). The right leg was tested unless a participant had knee pain or a knee replacement. Quadriceps strength was calculated as the mean maximal torque (Nm) from the three best trials. Participants were excluded for a systolic blood pressure greater than 199 mmHg, diastolic blood pressure greater than 109 mmHg, history of brain aneurysm or stroke, bilateral knee replacement, or severe bilateral knee pain. The observed coefficient of variation was 11.8% in our inter-examiner reliability study. Ankle dorsiflexion strength was assessed a year after the peripheral nerve measures and measured concentrically at 60 degrees per second on an isokinetic dynamometer (Kin-Com 125 AP Dynamometer) in 3-6 trials. The right leg was tested on all participants unless contraindicated. Ankle dorsiflexion strength was calculated as the mean maximal torque produced (Nm) between 72° and 30° of ankle extension.
Other measures
Height was measured using a stadiometer. Weight was measured with a calibrated balance beam scale. Total whole body bone mineral-free lean mass and total whole body fat mass were assessed by dual-energy X-ray absorptiometry (DXA; Hologic 4500A, Hologic, Inc.). Diabetes was defined as self-reported physician diagnosis (not only during pregnancy), hypoglycemic medication use, or fasting glucose >126 mg/dl (>7.0 mmol/l) after a >8 hour fast (25). Health histories included smoking (1999-2000), alcohol consumption frequency at baseline, osteoarthritis (1999-2000), prevalent disease at baseline (peripheral arterial disease; cerebrovascular disease – transient ischemic attack/stroke; cardiovascular disease -bypass/coronary artery bypass graft, carotid endarterectomy, myocardial infarction, angina, congestive heart failure); and eye diseases (1999-2000; retinopathy/retinal disease, cataracts, glaucoma). Weekly physical activity from walking and stair climbing (kcal/kg/week), knee pain on most days/past month, and depressive symptoms on the Center for Epidemiologic Studies Depression (CES-D) scale (26) were determined by an interviewer-administered questionnaire.
Cognitive function was measured with the Modified Mini-Mental State Examination (3MSE) and attention, psychomotor speed, and executive function were measured with the Digit Symbol Substitution (DSS) test (27). Cystatin-C (>1 mg/dl) and serum creatinine >1.5 mg/dl for men and >1.3 mg/dl for women defined renal insufficiency at baseline (28). Hypertension was defined through self-reported physician diagnosis, medication use, and/or a blood pressure at the clinic exam. Ankle-brachial index (AAI; continuous or with a cutpoint of <0.9) assessed subclinical cardiovascular disease.
Statistical analyses
Univariate associations were tested separately for sex differences (men vs. women) and race differences within sex (white men vs. black men and white women vs. black women) using Pearson chi square methods and Fishers exact test when appropriate. Nonparametric one way Mann-Whitney tests were performed for non-normal distributions. Outcomes of average quadriceps strength and ankle strength were calculated using ANCOVA, adjusted for age, sex, race, clinic site, diabetes, height, lean mass, fat mass, and peripheral nerve function (monofilament sensitivity, average vibration threshold, amplitude, velocity). Statistical significance was reported at p<0.01 due to multiple comparisons.
Strength (either quadriceps or ankle) was the outcome in stepwise multiple linear regression. Peripheral nerve function measures were the independent variables of interest and adjustments included age, sex, race, clinic site, diabetes, height, fat mass, lean mass and variables detailed in “Other Measures”. Linear regression models met underlying assumptions and were built progressively by entering variables stepwise as follows: peripheral nerve function, demographic factors, diabetes, body composition variables, lifestyle factors and finally chronic health conditions. Diabetes, demographic factors, and body composition variables were retained in all models and other variables were removed in a stepwise manner at p>0.10. Vibration threshold was also analyzed by quartiles due to its skewed distribution to very low or very high threshold values, though results did not change. Interactions with each of the peripheral nerve function measures and sex, race and diabetes were entered in the final models. Stratified models by race were performed for the quadriceps strength outcome since the interaction term with amplitude was significant. Analyses on non-diabetic participants, excluding diabetic participants, were also performed to show that these associations with peripheral nerve function were not limited to diabetic adults. Multicollinearity for independent variables was assessed using the variance inflation factor (VIF), the inverse of the proportion of variance not accounted for by other independent variables; no VIF was >10 and the mean VIF for each regression model was < 2 (29). Data were analyzed using SPSS (SPSS, Inc.) statistical software.
Results
Descriptives by race and sex subgroup are given in Table 1, with significant differences indicated by sex and by race within sex (white men vs. black men and white women vs. black women). The participants were 49.5% men and 36.7% black. Table 2 illustrates the differences in peripheral nerve function measures by sex and by race within sex. Men had reduced sensory and motor nerve function compared to women for all measures (Table 2). White men had worse vibration threshold than black men. White women had worse amplitude than black women.
Table 1.
Descriptive characteristics by sex and race.*
| Men | Women | |||
|---|---|---|---|---|
| White N=686 |
Black N=334 |
White N=617 |
Black N=422 |
|
| Age (years)† | 76.7 + 2.9 | 76.4 + 2.8 | 76.4 + 2.8 | 76.1 + 2.9 |
| Diabetes (%)† | 20.3‡ | 27.2 | 10.2‡ | 25.7 |
| Body composition & physical ability | ||||
| Height (m)† | 1.7 + 0.06 | 1.7 + 0.07 | 1.6 + 0.06 | 1.6 + 0.06 |
| BMI (kg/m2) | 27.1 + 3.7 | 27.2 + 4.4 | 26.0 + 4.4‡ | 29.3 + 5.9 |
| Bone-free total lean mass (kg)† | 54.2 + 6.6‡ | 55.5 + 8.0 | 37.6 + 5.0‡ | 42.2 + 6.3 |
| Total fat mass (kg)† | 24.3 + 7.0‡ | 23.2 + 7.7 | 26.4 + 7.9‡ | 30.5 + 9.9 |
| Quadriceps strength (Nm)† | 116.6 +29.2 | 118.7 +36.2 | 70.6 +19.1‡ | 77.3 + 23.3 |
| Ankle strength (Nm)† | 54.8 + 26.1 | 52.1 + 18.4 | 32.7 +10.0‡ | 35.4 + 12.3 |
| Lifestyle characteristics | ||||
| Current smoker (%) | 4.2‡ | 14.6 | 5.5‡ | 9.4 |
| Drinking frequency >1/week (%)† | 46.3‡ | 25.3 | 30.5‡ | 11.9 |
| Physical activity (kcal/kg/week) † | 7.2+10.1 | 5.7+18.8 | 5.5+18.2‡ | 3.7+7.4 |
| Chronic health conditions | ||||
| Cardiovascular disease history (%)† | 24.2‡ | 18.2 | 9.2‡ | 12.9 |
| Cerebrovascular disease history (%) | 5.0 | 6.4 | 6.6 | 5.3 |
| Peripheral arterial disease history (%)† | 5.8 | 3.8 | 1.8‡ | 4.6 |
| Ankle-arm index <0.9 (%) | 10.9‡ | 24.9 | 10.0‡ | 23.0 |
| Hypertension (%) | 67.0‡ | 77.1 | 67.3‡ | 82.4 |
| Systolic blood pressure (mmHg) † | 136.7+19.4‡ | 141.2 + 20.6 | 138.8+18.0‡ | 142.9+ 20.7 |
| Diastolic blood pressure (mmHg) | 73.2 + 10.0‡ | 76.4 + 10.5 | 71.8 + 10.3‡ | 75.3 + 10.6 |
| Retinal disease/Retinopathy (%) | 8.3‡ | 3.9 | 7.1 | 5.2 |
| Glaucoma (%) | 9.1‡ | 19.5 | 7.4‡ | 17.9 |
| Cataracts (%)† | 42.5‡ | 35.0 | 53.6 | 55.1 |
| Cystatin-C (mg/L)† | 1.06 + 0.3‡ | 1.02 + 0.2 | 0.99 + 0.2‡ | 0.95 + 0.2 |
| Creatinine >1.5 men/1.3 women (%)† | 4.4‡ | 13.9 | 2.1‡ | 6.0 |
| CES-D depression index† | 4.8 + 4.8‡ | 6.4 + 6.5 | 6.6 + 6.5‡ | 7.4 + 6.8 |
| Knee pain, most days/month (%)† | 15.8 | 17.7 | 18.7‡ | 24.2 |
| Osteoarthritis (%)† | 6.4 | 7.5 | 9.1‡ | 12.8 |
Continuous value shown as mean + standard deviation, except for those with wide ranges.
p<0.05 for men vs. women
p<0.05 white vs. black within sex
Table 2.
Descriptive characteristics of sensory and motor peripheral nerve function by sex and race.*
| Men | Women | |||
|---|---|---|---|---|
| White men N=686 |
Black men N=334 |
White women N=617 |
Black women N=422 |
|
| Sensory peripheral nerve function | ||||
| No 10-g monofilament detection (%)† | 11.6 | 13.3 | 4.9 | 6.2 |
| No 1.4-g monofilament detection (%)† | 49.9 | 55.4 | 37.2 | 40.6 |
| Vibration threshold (μ)† | 59.7 + 35.7‡ | 55.6 + 36.8 | 44.7 + 32.6 | 40.5 + 29.3 |
| Motor peripheral nerve function | ||||
| Amplitude (mV)† | 2.9 + 1.9 | 3.2 + 2.0 | 3.4 + 1.9‡ | 3.8 + 2.1 |
| Velocity (m/s)† | 41.9 + 4.8 | 41.9 + 4.9 | 44.2 + 5.4 | 44.7 + 5.8 |
Continuous value shown as mean + standard deviation.
p<0.05 for men vs. women
p<0.05 white vs. black within sex
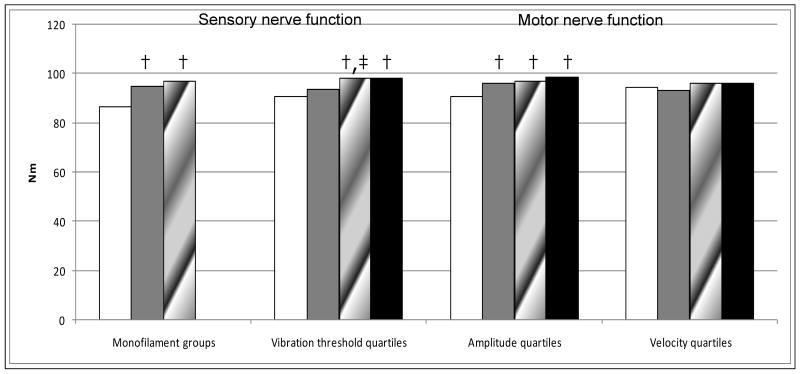
Poor sensory (monofilament and vibration threshold) and motor (amplitude but not velocity) nerve function were associated with reduced quadriceps strength. In adjusted analyses, the worst nerve function categories for monofilament sensitivity, vibration threshold and amplitude were associated with 11%, 7%, and 8% lower mean quadriceps strength respectively, compared to the best nerve function (Figure 1). Poor sensory and motor nerve function were associated with reduced ankle strength as well. Monofilament insensitivity and the lowest amplitude quartile were both associated with 17% lower ankle strength (p<0.01), compared to the best respective nerve function group or quartile. Any monofilament sensitivity (either to the 1.4-g or 10-g) was related to higher ankle strength compared to insensitivity (44.9 Nm and 43.8 Nm vs. 37.3 Nm; p<0.01). Amplitude showed a trend across the two poorest quartiles, with the lowest ankle strength in the worst quartile compared to each of the other quartiles (39.3 Nm and. 44.0 Nm vs. 45.5 Nm and 47.2 Nm; p<0.01 for each comparison) and the 2nd quartile having lower ankle strength than the best quartile (44.0 Nm vs. 47.2 Nm; p<0.01). The lowest quartile of velocity had lower ankle strength compared to the third quartile (42.0 Nm vs. 46.6 Nm; p<0.01).
Figure 1.
Difference in quadriceps strength* by peripheral nerve function measures§.
*Adjusted for age, sex, race, clinic site, diabetes, height, total lean and fat mass
† p<0.01 pairwise comparison with 1st group
‡ p<0.01 pairwise comparison with 2nd group
§ Monofilament groups (left to right): None, 10-g, 1.4-g;
Vibration threshold quartiles (left to right): 60-120μ, 30-50 μ, 10-20μ, 0μ;
Amplitude quartiles (left to right): <1.7mV, 1.7-3.0 mV, 3.1-4.5 mV, >4.5 mV;
Velocity quartiles (left to right): <39.3 m/s, 39.3-43.0 m/s, 43.1-46.8m/s, >46.8 m/s
Peripheral nerve function measures had a modest correlation between measures (r=0.03-0.22), adjusted for age, sex and race, and each measures was entered into the model as an independent contributor to the strength outcomes. When all nerve function measures were included in the model simultaneously, 10-g monofilament insensitivity (β=-7.19), vibration threshold (β=-0.097), and motor nerve conduction amplitude (β=2.01) each contributed independently to lower quadriceps strength in the fully adjusted models (Table 3a, Model 1; all p<0.01), with no relationship with velocity. Adjustment for lifestyle factors and chronic health conditions had little effect on β estimates for the peripheral nerve function measures (Table 3a; Model 2). Interactions of peripheral nerve function measures with sex and diabetes were not significant in the final model. The interaction term of amplitude with race was significant, with a stronger relationship of quadriceps strength and amplitude for black participants (Table 3 a; Model 3) The beta of the interaction term reflects the strength of the relationship in black compared with white participants and the beta for the white participants is obtained by subtracting the beta of the interaction term from the beta of the main effect. In separate subgroup analyses by race, the direction of the associations with peripheral nerve function were consistent in each group, however significant for 10-g monofilament insensitivity in the white participants (β=-7.70, p=0.003), but not the black participants (β=-6.61, p=0.09), and significant for vibration threshold (β=-0.10, p=0.003) and amplitude (β=2.13, p<0.001) for the black participants, but not the white participants (β=-0.03, p=0.21 and β=0.59, p=0.09, respectively). Associations with quadriceps strength were the similar for non-diabetic participants (Table 3a; Model 4).
Table 3.
Table 3a. Multivariate linear regression model* for quadriceps strength (Nm) with all nerve function measures were included as independent contributors. [Variables with p>0.1 or excluded from the model were listed as not applicable (n/a).]
| Model 1: Minimally adjusted (N=1474) | Model 2: Fully adjusted (N=1745) | Model 3: Fully adjusted with interaction terms (N=1745) | Model 4: Excluding diabetic participants, fully adjusted with interaction terms (N=1427) | |||||
|---|---|---|---|---|---|---|---|---|
| β | p-value | β | p-value | β | p-value | B | p-value | |
| Monofilament detection: none | -7.71 | 0.005 | -6.91 | 0.002 | -7.19 | 0.001 | -9.27 | <0.001 |
| 10-g only | -1.62 | 0.20 | -1.44 | 0.22 | -1.39 | 0.22 | -0.53 | 0.67 |
| Vibration threshold (μ) | -0.063 | 0.002 | -0.051 | 0.004 | -0.097 | 0.001 | -0.098 | 0.005 |
| Amplitude (mV) | 1.23 | <0.001 | 1.14 | <0.001 | 2.01 | <0.001 | 2.18 | <0.001 |
| Velocity (m/s) | -0.001 | 0.997 | n/a | >0.1 | n/a | >0.1 | n/a | >0.1 |
| Diabetes | -4.69 | 0.004 | -5.28 | <0.001 | -5.24 | <0.001 | n/a | n/a |
| Black race | -2.14 | 0.10 | 0.67 | 0.62 | -1.36 | 0.66 | -2.51 | 0.47 |
| Vibration threshold*Black race | n/a | n/a | n/a | n/a | -0.065 | 0.06 | -0.070 | 0.08 |
| Amplitude*Black race | n/a | n/a | n/a | n/a | 1.46 | 0.01 | 1.77 | 0.004 |
| Table 3b. Multivariate linear regression model* for ankle dorsiflexion strength (Nm) with all nerve function measures were included as independent contributors. [Variables with p>0.1 or excluded from the model were listed as not applicable (n/a).] | ||||||
|---|---|---|---|---|---|---|
| Model 1: Minimally adjusted (N=1273) | Model 2: Fully adjusted (N=1563) | Model 3: Excluding diabetic participants, fully adjusted (N=1334) | ||||
| β | p-value | β | p-value | β | p-value | |
| Monofilament detection: none | -4.93 | 0.06 | -5.29 | 0.004 | n/a | >0.1 |
| 10-g only | 0.074 | 0.95 | -0.502 | 0.60 | n/a | >0.1 |
| Vibration threshold (μ) | -0.017 | 0.33 | n/a | >0.1 | n/a | >0.1 |
| Amplitude (mV) | 0.849 | 0.004 | 1.17 | <0.001 | 1.18 | <0.001 |
| Velocity (m/s) | 0.117 | 0.28 | n/a | >0.1 | n/a | >0.1 |
| Diabetes | -2.22 | 0.12 | -1.72 | 0.15 | n/a | n/a |
| Black race | -3.90 | 0.001 | -1.91 | 0.09 | -1.70 | 0.17 |
Model 1: Adjusted for age, sex, race, clinic site, diabetes, height, total lean mass and fat mass.
Model 2: Model 1 + drinking frequency, knee pain, cerebrovascular disease, cystatin-C, systolic and diastolic blood pressure, AAI, CES-D score, DSS score
Model 3: Model 2 + vibration*Black race interaction and amplitude*Black race interaction
Model 4: Model 3 excluding diabetic participants and diabetes variable
Model 1: Adjusted for age, sex, race, clinic site, diabetes, height, total lean mass and fat mass.
Model 2: Model 1 + glaucoma, diastolic blood pressure, CES-D score, DSS score
Model 3: Model 2 + excluding diabetic participants and diabetes variable
When all nerve function measures were included in the model simultaneously, 10-g monofilament insensitivity (β=-5.29) and motor nerve conduction amplitude (β=1.17) each contributed independently to lower ankle strength (Table 3b, Model 1; p<0.01). No relationship between vibration threshold or velocity and ankle strength was found. Interactions of peripheral nerve function measures with sex, race and diabetes were not significant for ankle strength. Excluding diabetic participants eliminated associations of 10-g monofilament insensitivity and ankle strength though other associations remained similar (Table 3b; Model 3).
Discussion
In these community-dwelling older adults, poor sensory and motor nerve function was associated cross-sectionally with lower quadriceps and ankle strength, independent of diabetes and lean mass. This finding may have important implications for lower-extremity disability in older adults and suggest that the peripheral nerves are related to muscle function in older adults. To our knowledge, the association of sensory and motor nerve function with lower-extremity strength has not been investigated in ambulatory, community-dwelling older adults. The lower strength that we noted for the poorest nerve function was on the magnitude of double or triple the percent strength loss per year that was previously reported in our study (3). Our results suggest that poor peripheral nerve function is associated with some of the lower strength observed in aged populations.
In diabetes, severe peripheral neuropathy and pre-clinical declines in peripheral nerve function, with both sensory and motor nerve involvement, are quite clearly related to muscle atrophy (30-32) and decline in strength (33-34). This muscle atrophy has been shown to be greater distally in the leg compared to proximally (31) and strength loss also occurs distally as well as proximally (33). In older adults, it is possible that early loss of peripheral nerve function may first affect strength with atrophy occurring as a very late phenomenon. We have previously reported that the loss of quadriceps strength occurs in older adults even with the maintenance or gain of muscle mass (3). Both the 10-g insensitivity and the more sensitive test of sensory nerve function, higher vibration threshold, were related to reduced quadriceps strength while only the 10-g insensitivity was related to reduced ankle strength. Insensitivity to the 10-g monofilament is a clinical assessment found to be predictive of future foot ulcers (35). We are uncertain as to why nerve conduction velocity showed a weaker association with strength than amplitude. Lower amplitude may be related to axonal damage of the nerve and lower velocity may be related to demyelination of the nerve (36). Interestingly, the InCHIANTI Study found that amplitude, but not velocity, was independently related to calf muscle density in older adults (37). Additional evidence from the InCHIANTI Study and our previous work in the Health ABC Study indicates that amplitude and velocity may each be related to different aspects of physical function (22, 38).
Studies of older diabetic adults support that reduced peripheral nerve function may play a role in their poorer physical performance (18-20, 22, 39). In our analyses, the relationship between sensory and motor nerve function and quadriceps strength remained significant after diabetic participants were excluded from analyses. The relationship between motor nerve function and ankle strength also remained significant after excluding those with diabetes. Additionally, we have previously reported in this population that peripheral nerve function is associated with physical performance independently of diabetes status and found that it attenuated the relationship of quadriceps strength to performance by 13% using the Health ABC performance battery, a supplemented version of the lower-extremity battery from the Established Populations for the Epidemiologic Studies of the Elderly (22,40-41). More support of an independent association of peripheral nerve function and motor performance in older adults, was found in a cohort of disabled women aged >65 years in which poor balance and gait speed were associated with reduced sensory nerve function - but not with self-reported diabetes status after adjustment for the nerve function measures (21). However in contrast to our results, this study did not find a relationship of sensory nerve function and quadriceps strength (21) in disabled older women. In older Italian adults, clinically-assessed distal symmetrical neuropathy was associated with a twofold increased risk of prospective physical performance decline independent of diabetes (42).
Our study is unique in its inclusion of standardized sensory and motor peripheral nerve function assessments across the spectrum of function in older adults, instead of using clinical cutpoints. These peripheral nerve function measures were chosen due to their established use in epidemiologic studies, though were limited to large fiber nerves, rather than small fiber nerves. Ideally nerve conduction studies test multiple nerves to confirm polyneuropathy, though we were not attempting to define clinical disease. The peroneal nerve, rather than the sural, was selected since it is a motor nerve and more likely to have a response in older adults (6, 9). We also had distal and proximal measures of strength and showed that both are related to peripheral nerve function. However, quadriceps strength was measured cross-sectionally with the peripheral nerve function whereas the ankle strength was measured a year later. These results are consistent with our previous report of both distal and proximal bone mineral density sites showing a relationship with peripheral nerve function (43). We adjusted for total lean mass, although lean mass did not explain the associations of peripheral nerve function with strength. Peripheral nerve function was likely reduced in non-study participants and Health ABC Study participants without a follow-up; therefore results may apply only to ambulatory community-dwelling older adults. Future studies should address associations in a wider spectrum of peripheral nerves and in older adults with greater age ranges and physical abilities.
Poor sensory and motor peripheral nerve function was related to lower-extremity strength, both distally and proximally and independent of diabetes and lean mass, in this biethnic study of older men and women. Considering the high prevalence of poor peripheral nerve function among older adults in our study and in the U.S. population (11) peripheral nerve deficits are an unappreciated problem in the elderly. Our cross-sectional work provides a first reported association of peripheral nerve function and strength in older adults and serves as a basis for future research to determine the impact of peripheral nerve function decline on disability. Longitudinal studies are needed to confirm the importance of peripheral nerve function decline in the loss of strength with aging, as this may be a mechanism for lower-extremity disability.
Acknowledgments
This work was funded by the National Institutes on Aging (NIA) contract numbers N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; 1-R01-AG 028050 (to ESS) and supported in part by the Intramural Research Program of the NIH, National Institute on Aging; University of Pittsburgh Claude D. Pepper Older Americans Independence Center (P30-AG024827) Pilot Grant (to ESS) and the American Diabetes Association (1-04-JF-46 to ESS). We thank the participants and staff for their efforts, Dr. Robert Boudreau for statistical insights, Ms. Mei Yang for performing analyses, and Ms. Michelle Utz-Kiley for assistance with manuscript preparation.
Footnotes
Conflict of Interest Disclosure: The authors report no conflict of interest.
Author Contributions: ESS: Participated in study concept and design, data analysis and interpretation, and preparation of the manuscript.
ND: Participated in data analysis and interpretation, and preparation of the manuscript.
AVS: Participated data analysis and interpretation and preparation of manuscript.
HER: Participated in study concept and design, data analysis and interpretation and preparation of the manuscript.
BHG: Participated in data analysis and interpretation and preparation of the manuscript.
KAF: Participated in data analysis and interpretation and preparation of the manuscript.
RIS: Participated in data analysis and interpretation and preparation of the manuscript
AIV: Participated in data analysis and interpretation and preparation of the manuscript
TBH: Participated in study concept and design, acquisition of subjects and data, analysis and interpretation of data, and preparation of the manuscript.
ABN: Participated in study concept and design, acquisition of subjects and data, analysis and interpretation of data, and preparation of the manuscript.
Sponsor's Role: The funding institutes had no role in the design, methods, subject recruitment, data collection, analysis, and preparation of manuscript or in the decision to submit the manuscript for publication.
References
- 1.Evans W. Functional and metabolic consequences of sarcopenia. J Nutr. 1997;127:998S–1003S. doi: 10.1093/jn/127.5.998S. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 3.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2006;61A:M1059–M1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 4.Andersen JL. Muscle fiber type adaptation in the elderly human muscle. Scand J Med Sci Sports. 2003;13:40–47. doi: 10.1034/j.1600-0838.2003.00299.x. [DOI] [PubMed] [Google Scholar]
- 5.Baldereschi M, Inzitari M, Di Carlo A, Farchi G, Scafato E, Inzitari D, The ILSA Working Group Epidemiology of distal symmetrical neuropathies in the Italian elderly. Neurology. 2007;68:1460–1467. doi: 10.1212/01.wnl.0000260606.36443.29. [DOI] [PubMed] [Google Scholar]
- 6.Rivner MH, Swift TR, Malik K. Influence of age and height on nerve conduction. Muscle Nerve. 2001;24:1134–1141. doi: 10.1002/mus.1124. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs JM, Love S. Qualitative and quantitative morphology of human sural nerve at different ages. Brain. 1985;108:897–924. doi: 10.1093/brain/108.4.897. [DOI] [PubMed] [Google Scholar]
- 8.Franklin GM, Kahn LB, Baxter J, Marshall JA, Hamman RF. Sensory neuropathy in non-insulin-dependent diabetes mellitus: The San Luis Valley Diabetes Study. Am J Epidemiol. 1990;131:633–643. doi: 10.1093/oxfordjournals.aje.a115547. [DOI] [PubMed] [Google Scholar]
- 9.Stetson DS, Albers JW, Silverstein BA, Wolfe RA. Effects of age, sex, and anthropmetric factors on nerve conduction measures. Muscle Nerve. 1992;15:1095–1104. doi: 10.1002/mus.880151007. [DOI] [PubMed] [Google Scholar]
- 10.Resnick HE, Vinik AI, Heimovitz HK, Brancati FL, Guralnik JM. Age 85+ years accelerates large-fiber peripheral nerve dysfunction and diabetes contributes even in the oldest-old: The Women's Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2001;56:M25–M31. doi: 10.1093/gerona/56.1.m25. [DOI] [PubMed] [Google Scholar]
- 11.Gregg EW, Sorlie P, Paulose-Ram R, et al. Prevalence of lower-extremity disease in the U.S. adult population >40 years of age with and without diabetes: 1999-2000 National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:1591–1597. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 12.Park SW, Goodpaster BH, Strotmeyer ES, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: The Health, Aging and Body Composition Study. Diabetes Care. 2007;30:1507–1512. doi: 10.2337/dc06-2537. [DOI] [PubMed] [Google Scholar]
- 13.Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: The Health, Aging and Body Composition Study. Diabetes. 2006;55:1813–1818. doi: 10.2337/db05-1183. [DOI] [PubMed] [Google Scholar]
- 14.Gregg EW, Beckles GLA, Willismason DF, Leveille SG, Langlois JA, Engelgau MM, Narayan KMV. Diabetes and physical disability among older U.S. adults. Diabetes Care. 2000;23:1272–1277. doi: 10.2337/diacare.23.9.1272. [DOI] [PubMed] [Google Scholar]
- 15.Ryerson B, Tierney EF, Thompson TJ, Engelgau MM, Wang J, Gregg EW, Geiss LS. Excess physical limitations among adults with diabetes in the U.S. population, 1997-1999. Diabetes Care. 2003;26:206–210. doi: 10.2337/diacare.26.1.206. [DOI] [PubMed] [Google Scholar]
- 16.Gregg EW, Mangione CM, Cauley JA, Thompson TJ, Schwartz AV, Ensrud KE, Nevitt MC, Study of Osteoporotic Fractures Research Group Diabetes and incidence of functional disability in older women. Diabetes Care. 2002;25:61–67. doi: 10.2337/diacare.25.1.61. [DOI] [PubMed] [Google Scholar]
- 17.de Rekeneire N, Resnick HE, Schwartz AV, et al. Diabetes is associated with subclinical functional limitation in non-disabled older persons: The Health, Aging and Body Composition Study. Diabetes Care. 2003;26:3257–3263. doi: 10.2337/diacare.26.12.3257. [DOI] [PubMed] [Google Scholar]
- 18.Cavanagh PR, Derr JA, Ulbrecht JS, Maser RE, Orchard TJ. Problems with gait and posture in neuropathic patients with insulin-dependent diabetes mellitus. Diabet Med. 1992;9:469–474. doi: 10.1111/j.1464-5491.1992.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 19.Petrofsky J, Lee S, Macnider M, Navarro E. Autonomic, endothelial function and the analysis of gait in patients with type 1 and type 2 diabetes. Acta Diabetol. 2005;42:7–15. doi: 10.1007/s00592-005-0168-0. [DOI] [PubMed] [Google Scholar]
- 20.Resnick HE, Stansberry KB, Harris TB, Tirivedi M, Smith KS, Morgan P, Vinik AI. Diabetes, peripheral neuropathy, and old age disability. Muscle Nerve. 2002;25:43–50. doi: 10.1002/mus.1217. [DOI] [PubMed] [Google Scholar]
- 21.Resnick HE, Vinik AI, Schwartz AV, Leveille SG, Barncati FL, Balfour J, Guralnik JM. Independent effects of peripheral nerve dysfunction on lower-extremity physical function in old age: The Women's Health and Aging Study. Diabetes Care. 2000;23:1642–1647. doi: 10.2337/diacare.23.11.1642. [DOI] [PubMed] [Google Scholar]
- 22.Strotmeyer ES, de Rekeneire N, Schwartz AV, et al. The relationship of reduced peripheral nerve function and diabetes to physical performance in older white and black adults: The Health, Aging and Body Composition (Health ABC) Study. Diabetes Care. 2008;31:1767–1772. doi: 10.2337/dc08-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman AB, Haggerty CL, Goodpaster B, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:323–30. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- 24.Wilhite MR, Cohen ER, Wilhite SC. Reliability of concentric and eccentric measurements of quadriceps performance using KIN-COM dynamometer: the effect of testing order for three different speeds. J Orthop Sports Phys Ther. 1992;15:175–182. doi: 10.2519/jospt.1992.15.4.175. [DOI] [PubMed] [Google Scholar]
- 25.Report of The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2003;26:S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 26.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 27.Mehta KM, Simonsick EM, Rooks R, Newman AB, Pope SK, Rubin SM, Yaffe K, Health, Aging and Body Composition Study Black and white differences in cognitive function test scores: What explains the difference? J Am Geriatr Soc. 2004;52:2120–2127. doi: 10.1111/j.1532-5415.2004.52575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shlipak MG, Wassel Fyr CL, Chertow GM, et al. Cystatin C and mortality risk in the elderly: The Health, Aging, and Body Composition Study. Nephrol. 2006;17:254–261. doi: 10.1681/ASN.2005050545. [DOI] [PubMed] [Google Scholar]
- 29.Chatterjee S, Price B. Regression Analysis by Example. 2nd. John Wiley & Sons; New York, NY, USA: 1991. pp. 191–192. [Google Scholar]
- 30.Bus SA, Yang QX, Wang JH, Smith MB, Wunderkucg R, Cavanagh PR. Intrinsic Muscle atrophy and toe deformity in the diabetic neuropathic foot. Diabetes Care. 2002;25:1444–1450. doi: 10.2337/diacare.25.8.1444. [DOI] [PubMed] [Google Scholar]
- 31.Andersen H, Gadeberg PC, Brock B, Jakobsen J. Muscular atrophy in diabetic neuropathy: a stereological magnetic resonance imaging study. Diabetologia. 1997;40:1062–1069. doi: 10.1007/s001250050788. [DOI] [PubMed] [Google Scholar]
- 32.Greenman RL, Khaodhiar L, Lima C, Dihn T, Giurini JM, Veves A. Foot small muscle atrophy is present before the detection of clinical neuropathy. Diabetes Care. 2005;28:1425–1430. doi: 10.2337/diacare.28.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen H, Nielsen S, Mogensen CE, Jakobsen J. Muscle strength in type 2 diabetes. Diabetes. 2004;53:1543–1548. doi: 10.2337/diabetes.53.6.1543. [DOI] [PubMed] [Google Scholar]
- 34.Andreassen CS, Jakobsen J, Andersen H. Muscle weakness: a progressive late complication in diabetic distal symmetric polyneuropathy. Diabetes. 2006;55:806–812. doi: 10.2337/diabetes.55.03.06.db05-1237. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong DG, Lavery LA, Vela SA, Quebedeaux TL, Fleischli JG. Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Arch Intern Med. 1998;158:289–292. doi: 10.1001/archinte.158.3.289. [DOI] [PubMed] [Google Scholar]
- 36.Arezzo JC, Zotova E. Electrophysiologic measures of diabetic neuropathy: mechanism and meaning. Int Rev Neurobio. 2002;50:229–255. doi: 10.1016/s0074-7742(02)50079-9. [DOI] [PubMed] [Google Scholar]
- 37.Lauretani F, Bandinelli S, Bartali B, et al. Axonal degeneration affects muscle density in older men and women. Neurobiol Aging. 2006;27:1145–1154. doi: 10.1016/j.neurobiolaging.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDermott MM, Guralnik JM, Albay M, Bandinelli S, Miniati B, Ferrucci L. Impairments of muscles and nerves associated with peripheral arterial disease and their relationship with lower extremity functioning: The InCHIANTI Study. J Am Geriatr Soc. 2004;52:405–410. doi: 10.1111/j.1532-5415.2004.52113.x. [DOI] [PubMed] [Google Scholar]
- 39.Volpato S, Blaum C, Resnick H, Ferrucci L, Fried LP, Guralnik JM. Comorbidities and impairments explaining the association between diabetes and lower extremity disability: The Women's Health and Aging Study. Diabetes Care. 2002;25:678–683. doi: 10.2337/diacare.25.4.678. [DOI] [PubMed] [Google Scholar]
- 40.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and predication of mortality and nursing home admission. J Gerontol Med Sci. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 41.Simonsick EM, Newman AB, Nevitt MC, Kritchevsky SB, Ferrucci L, Guralnik JM, Harris T, Health ABC Study Group Measuring higher level physical function in well-functioning older adults: Expanding familiar approaches in the Health ABC Study. J Gerontol Med Sci. 2001;56:644–649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 42.Inzitari M, DiCarlo A, Baldereschi M, et al. Risk and predictors of motor-performance decline in a normally functioning population-based sample of elderly subjects: The Italian Longitudinal Study on Aging. J Am Geriatr Soc. 2006;54:318–324. doi: 10.1111/j.1532-5415.2005.00584.x. [DOI] [PubMed] [Google Scholar]
- 43.Strotmeyer ES, Cauley JA, Schwartz AV, de Rekeneire N, Resnick HE, Zmuda JM, Shorr RI, Tylavsky FA, Vinik AI, Harris TB, Newman AB, Health ABC Study Reduced peripheral nerve function is related to lower hip BMD and calcaneal QUS in older white and black adults: The Health, Aging and Body Composition Study. J Bone Miner Res. 2006;21:1803–1810. doi: 10.1359/jbmr.060725. [DOI] [PubMed] [Google Scholar]