Abstract
Rationale
An increasing number of investigators utilize the marble-burying assay despite the paucity of information available regarding what underlies the behavior.
Objectives
We tested the possibility that a genetic component underlies marble burying in mice and if there is a genetic correlation with other anxiety-like traits. Since findings reported in the literature indicate that marble-burying behavior reflects an anxiety-like response, we explored the assumption that the novel nature of a marble induces this anxiety. Finally, we investigated how the natural response of a mouse to dig relates to the marble-burying phenomenon.
Methods
We examined ten different inbred mouse strains to determine if marble-burying behavior is genetically regulated and correlated with anxiety-like traits in two other assays. We employed multiple variants of the “traditional” marble-burying assay to address how issues such as the novelty of marbles and digging behavior contribute to marble burying.
Results
Marble-burying behavior varied across strain and did not correlate with anxiety measures in other assays. Multiple tests conducted to reduce the novelty of marbles failed to alter burying behavior. Additionally, digging behavior correlated with marble burying, and the presence of marbles did not significantly impact the digging response.
Conclusions
Our results indicate that mouse marble burying is genetically regulated, not correlated with other anxiety-like traits, not stimulated by novelty, and is a repetitive behavior that persists/perseveres with little change across multiple exposures. Marble burying is related to digging behavior and may in fact be more appropriately considered as an indicative measure of repetitive digging.
Keywords: Marble burying, Anxiety, Perseverative, Obsessive–compulsive, Digging
Introduction
In response to an aversive stimulus, many animals will freeze, flee, or engage in aggressive behavior (Bolles 1970); in addition, many rodents (including rats and mice) exhibit burying behavior, commonly referred to as “defensive burying” (Pinel and Treit 1978; Treit et al. 1981; Pinel et al. 1989; for review see De Boer and Koolhaas 2003). Burying behavior in rodents often refers to the displacement of bedding material using the snout and forepaws in an effort to cover an object (e.g., Pinel and Treit 1978). Rodents will engage in “defensive burying” in response to aversive stimuli including shock prods, air puffs, noxious food, and scorpions (Pinel and Treit 1978; Terlecki et al. 1979; Wilkie et al. 1979; Poling et al. 1981; Londei et al. 1998). Rodents will also bury nonaversive unconditioned objects, such as food pellets and glass marbles (Terlecki et al. 1979; Poling et al. 1981; Broekkamp et al. 1986); however, it is debatable whether this is also a “defensive” response. The marble-burying assay was developed to take advantage of this inherent burying behavior to evaluate how many novel, but innocuous, glass marbles a rodent would bury.
In recent years, a number of investigators have utilized the marble-burying assay as a tool for assessing either anxiety-like and/or repetitive-like behaviors in mice. Early pharmacological studies helped establish marble burying (MB) as a potential anxiety model due to its sensitivity to anxiolytics such as diazepam (Broekkamp et al. 1986); later studies have continued to illustrate decreases in marble burying with various anxiolytics (i.e., Njung’e and Handley 1991a; Ichimaru et al. 1995; Borsini et al. 2002; Nicolas et al. 2006; Li et al. 2006). In addition, other drugs such as antidepressants, including selective serotonin reuptake inhibitors and tricyclic antidepressants, also decrease marble burying (i.e., Broekkamp et al. 1986; Njung’e and Handley 1991a, b; Ichimaru et al. 1995; Borsini et al. 2002; Takeuchi et al. 2002; Li et al. 2006; Nicolas et al. 2006). However, questions regarding the specificity of marble burying as an indicator of anxiety alone have been raised in part due to studies illustrating a decrease in marble burying in response to drugs such as typical antipsychotics (Broekkamp et al. 1986). It is commonly assumed that mice bury marbles because the novelty of the object elicits the burying response. Yet, several studies suggest that marble burying is not necessarily stimulated by novelty (Broekkamp et al. 1986; Njung’e and Handley 1991a; Gyertyán 1995), but rather it simply associates with digging behavior and reflects a more obsessive/compulsive-like behavior (Njung’e and Handley 1991a; Gyertyán 1995; Londei et al. 1998; Masuda et al. 2000; Deacon and Rawlins 2005).
In order to appropriately interpret behavioral differences between subjects, it is imperative that researchers thoroughly understand the fundamental behavior itself. Therefore, to better understand the nature of marble-burying behavior in mice, we designed several experiments to address the following issues/questions. First, if marble burying is an “anxiety-related” response, then one would hypothesize that there would be a correlation between marble burying and responses on other anxiety-related assays. Second, if mice are burying marbles due to their novelty, then mice familiar with marbles should bury fewer marbles. Finally, marble burying may be simply a byproduct of digging behavior.
Methods and experimental design
Subjects
Experiment 1 consisted of 2- to 4-month-old male mice from the following ten inbred mouse strains (12 mice/strain): 129S1/SvImJ (129S1), A/J, AKR/J (AKR), BALB/cByJ (BALB), C3H/HeJ (C3H), C57BL/6J (B6), CBA/J (CBA), DBA/2J (D2), FVB/NJ (FVB) (Jackson Labs, Bar Harbor, ME, USA), and 129S6/SvEv Tac (129S6; Taconic, Germantown, NY, USA). All remaining experiments utilized 2- to 4-month-old male C57BL/6J mice. All mice were allowed at least 10 days to acclimate after arrival before testing commenced. Mice were group housed on a 12:12-h light–dark (LD) cycle, in type 2 microisolator cages (four mice/cage) with food and water available ad libitum. Mice were housed with corncob bedding. Mice were transferred to the testing room followed by a 30-min acclimation period that preceded each test. All experiments were conducted between 8:00 A.M. and 12:00 P.M. All testing was approved by the Baylor Institutional Animal Care and Use Committee in accordance with National Institutes of Health guidelines.
Apparatus
Marble burying
In experiment 1, the cage preparation involved filling clean cages (27×16.5×12.5 cm) with 4.5-cm corncob bedding, followed by gently overlaying 20 black glass marbles (15 mm diameter) equidistant in a 4×5 arrangement. Testing consisted of a 30-min exploration period. In the remaining experiments, 4.5 cm SANI-CHIP bedding was used, and mice were tested for 20 min. We switched to SANI-CHIP bedding following the observation that marbles are more easily buried in this medium, and since marbles are buried quicker, we can decrease the testing time allowing for greater throughput. The number of marbles buried (>50% marble covered by bedding material) was recorded. White noise (55 dB) was present during testing.
Open-field activity
Animal activity was recorded using the VersaMax Animal Activity Monitoring System (AccuScan Instruments, Columbus, OH, USA), which includes an empty clear Plexiglas (40×40×30 cm) open-field arena. Specific measures chosen for analysis included total distance traveled (cm), center distance ratio [measure of anxiety (Treit and Fundytus 1989) defined as the ratio between distance the subject traveled in the arena center (22.5 cm× 22.5 cm) vs. the total distance traveled], and number of “stereotypy” (operationally defined by the computer when the subject repeatedly breaks the same infrared beam consecutively without breaking an adjacent beam, with each stereotypic event separated by at least 1 s). Data was collected over 2-min intervals across a 30-min testing period, but the analysis incorporated the entire 30 min of data. Overhead bright lights (750 lux) and white noise (55 dB) were utilized during testing.
Light–dark exploration
The test apparatus consisted of a polypropylene box (44× 21×21 cm) unequally divided into two chambers separated by a small opening (8.5×5 cm). Two thirds of the total area was a brightly lit chamber (750 lux) while the remaining one third was a dark chamber. Each subject was placed in the brightly lit side of the box furthest from the dark chamber and allowed 10 min exploration. Transitions between the two chambers were recorded using the Psion Workabout (Psion Teklogix, Hebron, KY, USA). A transition event occurred when the subject and all four of its paws transferred from one chamber to the other. White noise (55 dB) was utilized during testing.
Experiment 1: strain distribution pattern in MB, OFA, and LD
Inbred strain studies provide scientists with a powerful tool to not only gain insights into the genetic regulation of a behavioral trait but also to evaluate the degree to which particular behaviors are associated. There are large differences in anxiety-related responses among inbred strains of mice (Trullas and Skolnick 1993; Crawley et al. 1997; Homanics et al. 1999; Rogers et al. 1999; van Gaalen and Steckler 2000; Bouwknecht and Paylor 2002). Pharmacological experiments suggest that marble-burying behavior can be used to assess anxiety-related and obsessive/compulsive/repetitive behaviors in mice (e.g., Broekkamp et al. 1986; Njung’e and Handley 1991a). Although the literature indicates that various strains of mice have been used in marble-burying studies (e.g., Broekkamp et al. 1986; Njung’e and Handley 1991a; Gyertyán 1995; Ichimaru et al. 1995; Londei et al. 1998; Nicolas et al. 2006), no systematic inbred strain study has been performed. Therefore, experiment 1 determined (a) if there are strain differences in marble burying and (b) if marble-burying behavior is correlated with responses in other assays of anxiety-related behaviors.
Test Paradigm
Each mouse (12 mice/strain) was tested in each test and in one of three orders: MB → open-field activity (OFA) → LD, OFA → LD → MB, or LD → MB → OFA. Mice were tested in the MB for 30 min, the OFA for 30 min, and the LD for 10 min. Data were analyzed using one-way analysis of variance (ANOVA) with post hoc analysis using Fisher’s least significant difference. In addition, data were analyzed for association/correlation using the Pearson correlation test.
Experiment 2a: reducing the novelty of marbles by exposure in home cage
Most laboratory mice have never been exposed to glass marbles, which leads to a logical speculation that the novelty of the marble induces the response of marble burying. If novelty drives burying behavior, then increasing the familiarity of a mouse with marbles should hypothetically decrease marble burying. Experiment 2a was designed to directly address this issue by exposing mice to marbles in their home cage prior to testing. There were three groups in this experiment (eight mice/group): One group had no preexposure to the marbles; the second group had a clean set of ten marbles placed in their home cage each day for 5 days; the last group had a clean set of ten marbles placed in their home cage the day before testing. On the day of testing, all mice were tested for MB as described above. Data were analyzed using one-way ANOVA.
Experiment 2b: reducing the novelty of marbles by repeat testing
In this experiment, we tested the hypothesis that if mice bury marbles because they are novel, then retesting the mice should result in a reduction in marble burying. If, however, marble burying represents a response that is highly repetitive/stereotypic or perseverative, then marble burying would remain consistent across multiple exposures. For this experiment, one group of mice was given five consecutive tests, each separated by 1 h, while the second group was given five tests separated by 24 h (12 mice/group). Data were analyzed using one-way ANOVA with repeated measures. Post hoc analysis was performed using within-subjects contrasts.
Experiment 2c: marbles vs. food
If mice bury marbles due to their novelty, then a reasonable assumption would be for a mouse to display less burying behavior when presented with a more familiar object (i.e., food pellets). For this experiment, mice were tested for burying behavior in the presence of either marbles or food pellets. The food pellets were selected from the home cage of each mouse. Food pellets of approximately the same size as the marbles were used (~15 mm long). Cages were prepared with 4.5 cm of SANI-CHIPS and either 12 marbles or 12 food pellets aligned in a 3×4 arrangement on the bedding surface. Twelve mice were randomly assigned to the marble- or food-prepared cage and allowed 20 min exploration. Two days later, the tests were reversed for each mouse such that all mice were tested in both the marble- and food-prepared cages. Data were analyzed using two-way ANOVA (object buried × test order).
Experiment 3: do mice avoid marbles when given an opportunity?
This experiment was designed to test the hypothesis that marble-burying behavior is elicited in a similar way to that seen in defensive burying, i.e., to avoid a “noxious” (or novel) stimulus. In the standard marble-burying assay, marbles are spread throughout a majority of the cage surface. Therefore, this condition does not provide equal space free from the presence of the marbles. We tested the possibility that mice might avoid marbles when given the opportunity by testing mice in a larger cage where marbles were presented in one of two conditions: across the entire cage surface or only across half the cage. Large rodent cages (43.5×22×15.5 cm) were prepared with 4.5 cm SANI-CHIPS. Marbles were positioned on the bedding surface with either 20 marbles (in a 4×5 arrangement) spread across a majority of the cage or only across half the cage. Each mouse freely explored one of the two cage conditions for 20 min (ten mice/group). The number of marbles buried was recorded. Data were analyzed using one-way ANOVA.
Experiment 4: digging or burying?
Recognizing what mouse behaviors contribute to the process of marble burying is important to the understanding of the marble-burying assay. When mice are placed in cages with new bedding, three prominent behaviors observed include locomotor activity, rearing, and digging. The marble-burying test typically involves the use of a cage with clean bedding, and therefore, it is reasonable to question how these three behaviors might affect marble-burying behavior. Since the burying of any object must involve the displacement of bedding material to cover that object, the most reasonable assumption is that of these three behaviors, digging is the one most likely to influence marble burying. We attempted to address this question through multiple experiments designed to quantify digging behavior during tests for marble burying.
A digging event was considered to be an obvious directed action by any of the four paws or snout toward the displacement of bedding material. During the course of a 20-min experiment, the experimenter observed the behavior of a mouse in the test cage for a 2-s period and recorded whether digging was or was not observed during that sample window. Digging events were recorded every 20 s for a total of 60 observations during a 20-min test.
Experiment 4a: digging in marble vs. nonmarble cage
Since digging appears to be a natural innate behavior in mice, particularly in response to placement in a cage with clean bedding, one obvious question is how digging behavior quantitatively changes in the presence of marbles. In this experiment, 14 mice were placed in a cage with clean bedding (4.5 cm SANI-CHIPS) that either did or did not contain marbles (× 20, arranged as in the MB assay). One day later, the tests were reversed for each mouse such that all mice were tested in both cage setups. Data were analyzed using two-way ANOVA (digging × test order).
Experiment 4b: digging in the presence of marbles vs. food
To extend our understanding of what factors may modulate the manifestation of digging behavior, we explored the possibility that familiar objects (food) and novel objects (marbles) might differentially influence digging. Therefore, digging behavior was recorded during experiment 2c. Data were analyzed using one-way ANOVA.
Experiment 4c: will mice selectively dig in a location where marbles are present or absent?
To further explore the possibility that marbles influence digging behavior, mice were observed for digging events in an experimental setup using the large cage that had marbles placed over only half the cage (cages were created similarly to those created for experiment 3). Mice (N=8) were tested for 20 min and were tested once daily for five consecutive days to determine if the digging and marble burying changed with repeated testing. In addition to counting the number of marbles buried and recording digging behavior, the percentage of digging events on the marble side vs. that on the nonmarble side were calculated: # of digging events observed on side X/# times mouse observed on side X). Furthermore, a ratio was calculated for % marbles buried: % time digging events occurred on the marble side (# digging events on the marble side/# opportunities for digging events for entire cage). Data were analyzed using one-way ANOVA or two-way ANOVA (% digging × side of cage) with repeated measures. Follow-up analyses of significant interaction terms were performed using simple-effects tests. In addition, marble burying and digging activity were analyzed for correlation using the Pearson correlation test.
Experiment 5: reducing the novelty of the bedding
During normal cage changing, mice will quickly explore the new clean cage and often display digging behavior. Therefore, it is possible that marble burying is simply a “reaction” to being placed into a cage with clean bedding. In this experiment, marble-burying behavior was measured using the standard “clean” bedding setup or in their home cage. If mice bury marbles as a reaction to the presence of new bedding, then mice that are tested in their home cage should bury fewer marbles compared to mice tested with new bedding.
Mice (N=12) were single housed in new cages with 3 cm SANI-CHIPS 24 h before testing. The following day, mice were tested for marble burying and digging activity in one of two conditions: home cage or new cage. The home cage condition refers to mice tested in the cage in which they had been single housed. The new cage condition refers to mice tested in the typical “new cage” setup prepared for testing with 3 cm bedding and 20 marbles. Each mouse was briefly (less than 1 min) transferred to a clean cage while appropriate home cages were prepared by leveling the bedding material and overlaying 20 marbles. Mice were then transferred to either their prepared home cage or the clean testing cage. Digging activity was recorded in addition to the number of marbles buried at the termination of the 20-min experiment. In order to determine if there was an interaction between familiarity with the marbles and the test, mice were returned to their original group housing cages for 2 days, and the experiment (starting from the single housing) was repeated with each mouse tested using the condition not previously tested. Data were analyzed using two-way ANOVA (test cage condition × test order). Follow-up analysis of interactions was performed with the simple effects test.
Results
Experiment 1: strain distribution pattern in MB, OFA, and LD
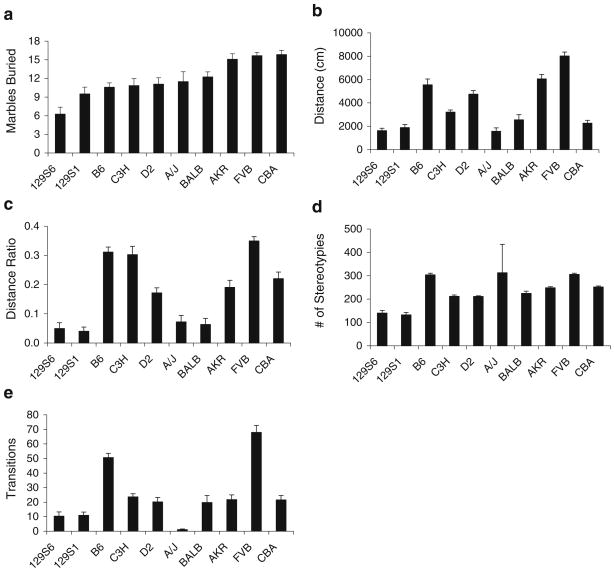
The data in Fig. 1a clearly demonstrate the significant differences in marble burying across the ten inbred strains of mice, with a significant main effect of strain [F(9,110)= 9.13, p<0.001]. Strains AKR/J, FVB/NJ, and CBA/J buried the most marbles while 129S6/SvEv Tac mice buried the least.
Fig. 1.
Behavioral responses of ten inbred strains of mice on the marble-burying, open-field, and light–dark tests. The number of marbles buried among inbred strains of mice (a); open-field exploratory activity as measured by the total distance traveled (b); “anxiety” in the open field as measured by the center distance/total distance ratio (c); number of stereotypies in the open field as measured by the number of repeated photobeam interruptions (d); “anxiety” in the light–dark test as measured by the number of transitions between the light and dark compartments. All values represent means ± standard error of the mean
Figure 1b–e illustrates the strain distribution for the total distance, center ratio, and “stereotypy” measures in the open-field test and the number of light–dark transitions in the LD test. In order to easily compare the strain distribution pattern, the order of strains in all figures for Fig. 1 match the order from the marble-burying test (see Fig. 1a). Significant main effects of strain were found for each of the OFA and LD measures [total distance: F(9,110)=45.49, p<0.001; center ratio: F(9,110)=32.54, p<0.001; “stereotypy”: F(9,110)= 2.72, p=0.007; LD transitions: F(9,110)=39.60, p<0.001]. It is important to note that the OFA and LD strain survey data presented here are consistent with strain survey data in the literature (Bolivar et al. 2000; Bouwknecht and Paylor 2002; Milner and Crabbe 2008).
Pearson’s correlation analysis (Table 1) revealed that the anxiety-like measures for LD (transitions) and OFA (center ratio) significantly correlated with one another (R= 0.826, p=0.003), but neither LD nor OFA measures correlated with the number of marbles buried (R=0.434, p=0.211; R=0.505, p=0.137, respectively). In addition, activity in the OFA (total distance traveled) significantly correlated with LD transitions (R=0.852, p=0.002) and the OFA center ratio (R=0.748, p=0.013), but not with the number of marbles buried (R=0.531, p=0.115). Interestingly, although it failed to reach statistical significance, the measure with the highest correlation value with the number of marbles buried was the “stereotypy” measure from OFA (R=0.620, p=0.056).
Table 1.
Pearson r correlations between measures within three behavioral assays: marble burying (# marbles buried), light–dark exploration (transitions), and open-field activity (total distance traveled, center/total distance traveled ratio, “stereotypy”)
| Marbles buried | Light– dark transitions | Total distance | Center/total distance | “Stereotypy” | |
|---|---|---|---|---|---|
| Marbles buried | 1 | ||||
| Light–dark transitions | 0.434 | 1 | |||
| Total distance | 0.531 | 0.852* | 1 | ||
| Center/total distance | 0.505 | 0.826* | 0.748* | 1 | |
| “Stereotypy” | 0.620 | 0.512 | 0.511 | 0.571 | 1 |
Data includes analysis across ten inbred strains (n=12/strain)
p<0.05
Experiment 2a: reducing the novelty of marbles by exposure in home cage
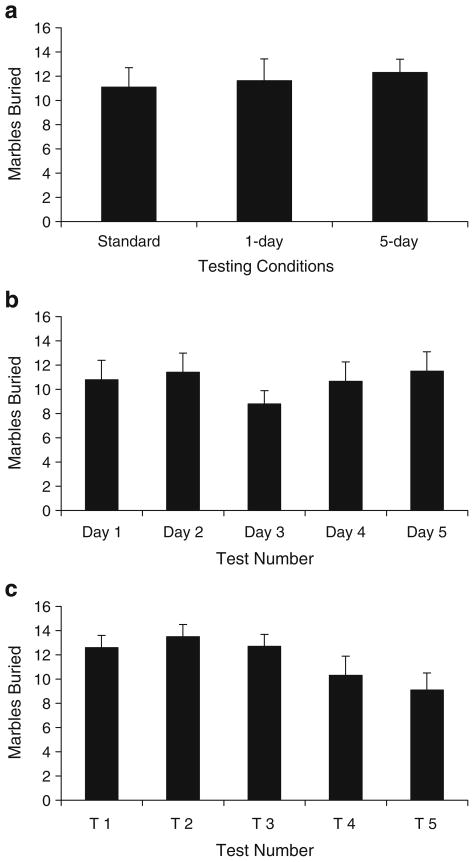
In the home cage habituation paradigm, mice were exposed to marbles in their home cage for either 1 or 5 days, and their performance in the marble-burying experiment was compared to mice not preexposed to marbles. There were no significant differences in the number of marbles buried between the three testing groups (F(2,21)=0.17, p=0.847; Fig. 2a).
Fig. 2.
The number of marbles buried in mice naïve (standard) or familiarized (1 and 5 day) to marbles in the home cage prior to testing (a); the number of marbles buried following repeated testing during five consecutive days (b) or five 1-h intervals on the same day (c). All values represent means ± standard error of the mean
Experiment 2b: reducing the novelty of marbles by repeat testing
In this experiment, mice repeatedly went through the marble-burying test in order to reduce marble novelty. When mice were tested once daily for five consecutive days, we observed no difference in the number of marbles buried over the 5-day testing period (F(4,44)=2.26, p= 0.078; Fig. 2b). However, mice tested five times within the same day did exhibit a significant main effect of test number (F(4,44)=4.15, p=0.006; Fig. 2c). Follow-up analysis using within-subject contrasts revealed a significant decrease in marbles buried during the fifth test compared to the first test [F(1,11)=4.95, p=0.048], while neither the second, third nor fourth test significantly varied from the first test.
Experiment 2c: marbles vs. food
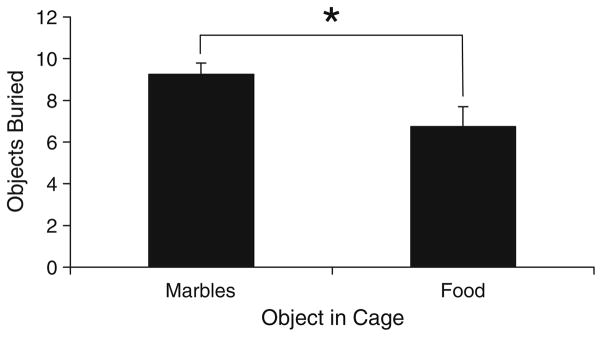
To determine if mice will bury a very familiar object, such as food pellets from their own cage, we compared the burying behavior of mice presented with either marbles or food. It is important to note the difficulty in directly comparing marbles to food since they are different in size and shape. Each mouse was tested once with marbles and once with food pellets using a balanced crossover design. The results illustrate that mice clearly bury familiar food pellets, although the number buried is less than the number of marbles buried (F(1,20)=4.86, p=0.039; Fig. 3). ANOVA revealed no effect of test history [F(1,20)=0.09, p=0.772], indicating that the results are not significantly influenced by which test was performed first.
Fig. 3.
The number of marbles or food pellets buried. All values represent means ± standard error of the mean. *p<0.05
Experiment 3: do mice avoid marbles when given an opportunity?
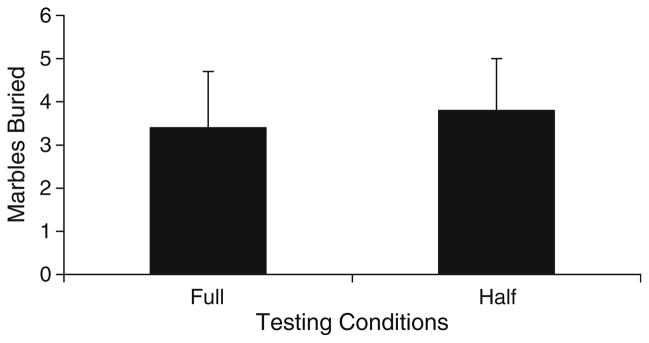
In order to determine if mice will avoid marbles when given the opportunity, mice were tested for marble-burying behavior in one of two conditions: a large cage with marbles spread throughout the entire bedding surface or a large cage with marbles covering only half the surface. No significant difference was found between either of the two cage conditions (F(1,18)=0.05, p=0.831; Fig. 4) indicating that mice bury similar amounts of marbles even when provided sufficient space to “avoid” the marbles.
Fig. 4.
The number of marbles buried in a large arena containing marbles spread across either the entire arena (full) or only half the arena (half). All values represent means ± standard error of the mean
Experiment 4a: digging in marble vs. nonmarble cage
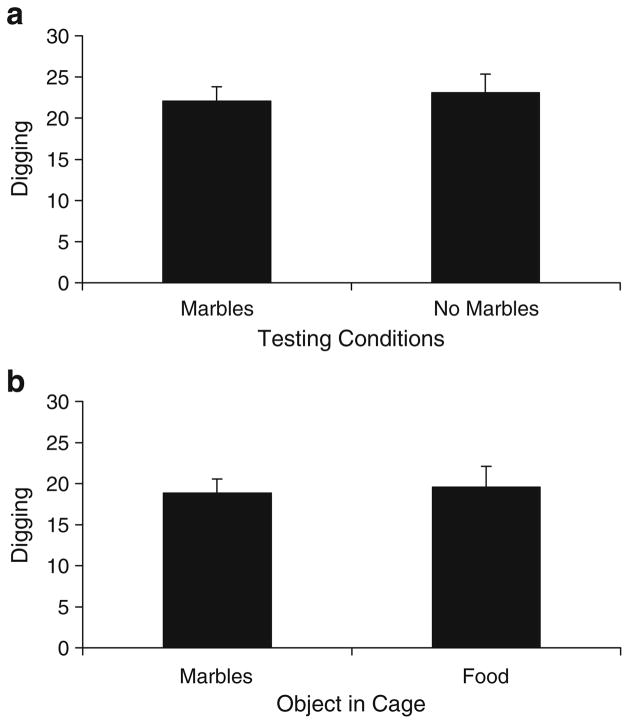
We evaluated mouse-digging behavior in cages with clean bedding in the presence or absence of marbles to determine if marbles affect digging behavior. Figure 5a clearly demonstrates that the presence of marbles did not influence the number of digging observations recorded [F(1,24)= 0.11, p=0.739].
Fig. 5.
Digging behavior in mice. The number of digging observations in either the presence or absence of marbles (a); the number of digging observations in the presence of either marbles or food pellets (b). All values represent means ± standard error of the mean
Experiment 4b: digging in the presence of marbles vs. food
When mice are placed in a cage with either marbles or food pellets present on the bedding surface, we find that the novelty of an object (marble) has no affect on the number of digging observations observed when compared to digging in the presence of a more familiar object (food pellet) (F(1,22)=0.06, p=0.808; Fig. 5b).
Experiment 4c: will mice selectively dig in a location where marbles are present or absent?
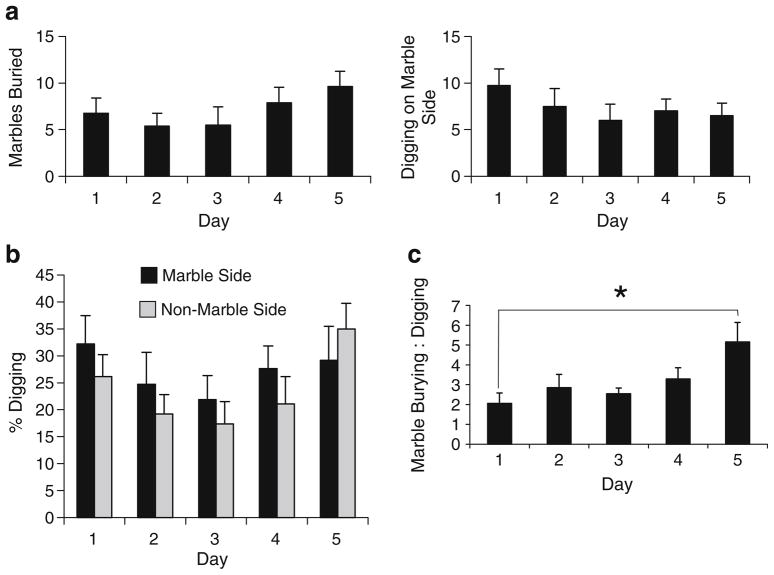
To determine if digging behavior changes across repeated testing, we evaluated burying and digging behavior in a large cage containing marbles on half of the surface of the bedding. Consistent with the data from Fig. 2b, the number of marbles buried did not significantly change across testing days (F(4,28)=1.92, p=0.135; Fig. 6a). Furthermore, digging observation on the marble side also did not significantly change over the multiple testing days (F(4,28)=1.32, p= 0.288; Fig. 6a). On the first day of testing, mice spent approximately 50% of the time on the marble side of the cage, and while repeat testing led to a decrease in the percentage of time spent on the marble side, this was not significant [F(4,28)=2.35, p=0.079; data not shown].
Fig. 6.
Digging activity and marble burying in a large arena half covered with marbles across five testing days. The total number of marbles buried and the number of digging observations on the marble side of the arena (a); the % of time spent digging on each side of the arena (calculated as the number of digging events on that side divided by 60, the total number of events recorded; b); the ratio of the % of marbles buried per the % of recorded digging events on the marble side (c). All values represent means ± standard error of the mean.
*p<0.05
When examining the percentage of digging events observed for each of the two sides, an overall main effect of testing day was observed (F(4,56)=3.25, p=0.018; Fig. 6b); however, no interaction was observed between testing day and cage side [marble vs. nonmarble; F(4,56)= 0.84, p=0.509]. In order to compare the number of marbles buried to digging activity on the marble side, we calculated a ratio for % marbles buried: % digging events observed on the marble side (Fig. 6c). Repeated-measures ANOVA demonstrated a significant main effect of day [F(4,24)= 3.68, p=0.018], and follow-up analysis illustrated a significant increase between test days 1 and 5 [F(1,6)= 9.11, p=0.023] indicating that there is some dissociation between marble burying and digging and that they are not completely coupled responses.
Pearson’s correlation was implemented in order to evaluate how marble burying in this experimental design might correlate with digging and, more specifically, the location of those digging events (marble vs. nonmarble side). For each of the five testing days, the number of marbles buried significantly correlated with both the total number of digging events as well as the digging events observed on the marble side (p<0.05; Table 2). On the other hand, marble burying significantly correlated with digging observed on the nonmarble side only on days 1 and 2.
Table 2.
Pearson r correlations between the total number of marbles buried and total digging events observed, digging events observed on the marble side, or those observed on the nonmarble side
| Testing day | Total digging vs. marble burying | Digging on marble side vs. marble burying | Digging on nonmarble side vs. marble burying |
|---|---|---|---|
| 1 | 0.879* | 0.759* | 0.725* |
| 2 | 0.812* | 0.735* | 0.720* |
| 3 | 0.805* | 0.981* | 0.094 |
| 4 | 0.813* | 0.761* | 0.532 |
| 5 | 0.801* | 0.781* | 0.524 |
p<0.05
Experiment 5: reducing the novelty of the bedding
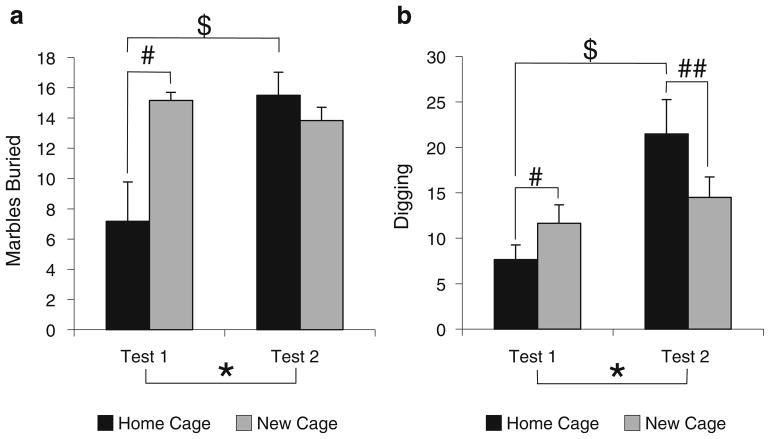
To determine the impact of novel “burying media”, we measured marble-burying and digging behavior in a cage with new bedding (i.e., traditional marble-burying test setup) compared to burying in a cage using “home cage” bedding. Figure 7 displays that test media can impact marble-burying and digging responses in a complex manner that is dependent on familiarity.
Fig. 7.
The number of marbles buried (a) or digging observations (b) in either the home cage or a cage with new bedding across two tests. Asterisk, single number sign, double number sign, and dollar sign indicate significant differences (p<0.05). The asterisk denotes that overall mice buried more marbles (a) and displayed more digging (b) responses during the second test vs. the first test. The single number sign, double number sign, and dollar sign denote significant interactions: (single number sign) mice buried more marbles (a) and dug more (b) in the new cage compared to the home cage, but only when tested in this condition first; (double number sign) mice dug (b) more in the home cage, but only when tested in this condition second; (dollar sign) mice buried more marbles (a) and dug more (b) in the second test, but only when tested in the home cage. All values represent means ± standard error of the mean
For marble burying, there was an overall order effect on marble burying [test 2 > test 1; F(1,20)=9.10, p=0.007], but the main effect of test media was not significantly different [F(1,20)=3.91, p=0.062]. However, the interaction (test media × test order) was significant [F(1,20)=4.77, p= 0.041]. Simple effects analysis of the interaction indicated that there was a significant effect of test order, but only in the home testing condition [test 2 > test 1; F(1,10)=7.54, p= 0.021]. There was no significant order effect in the traditional new cage setup [F(1,10)=1.68, p=0.224]. In addition, the simple effects analysis also indicated that mice in the home cage buried fewer marbles compared to the new cage, but this effect was only observed during test 1 [F (1,10)=5.86, p=0.036]. Thus, the test media can alter marble burying, but only in naive mice.
Similar to that observed for marble burying, there was an overall effect of test order for the digging measure [test 2 > test 1; F(1,20)=4.73, p=0.042], but the main effect of test media was not significantly different [F(1,20)=0.35, p= 0.560]. However, the interaction (test media × test order) was significant [F(1,20)=10.86, p=0.004]. Simple effects analysis of the interaction indicated that there was a significant effect of test order, but only in the home testing condition [F(1,10)=11.56, p=0.007]. There was no significant test order effect in the traditional new cage setup [F (1,10)=0.89, p=0.368]. In contrast to the marble-burying findings, the simple effects analysis also indicated that mice in the home cage had fewer digging events compared to those in the new cage during test 1 [F(1,10)=6.23, p= 0.032], but significantly more during test 2 [F(1,10)=5.34, p=0.043]. Thus, digging behavior is also affected by the familiarity of the test media in opposite directions dependent on prior exposure to the marble test.
Discussion
In order to probe for a possible genetic component in marble-burying behavior, a survey was conducted across multiple inbred strains. The strain survey for marble burying clearly demonstrated a strain-dependent distribution in the marble-burying behavior demonstrating that genetic differences regulate the marble-burying response in mice. The fact that the data are continuously distributed indicates that marble-burying behavior is regulated by multiple genetic factors. Since digging is likely integral to burying behavior, it is interesting to note that these results parallel previous findings that the genetic background affects digging behavior (Webster et al. 1981; Dudek et al. 1983; Kitaoka 1994).
A number of researchers utilize marble burying as a model for anxiety-like traits, yet it is not clear if mice bury marbles because of “anxiety”. Much of the data supporting this assumption come from pharmacological studies where agents shown to be anxiolytic in humans also decrease marble burying in mice (Broekkamp et al. 1986; Njung’e and Handley 1991a; Ichimaru et al. 1995; Li et al. 2006; Nicolas et al. 2006). If mice bury marbles due to “increased anxiety”, one might expect marble burying to correlate with other anxiety indicators such as transitions in the light–dark box or the center/total distance traveled ratio in the OFA assay. Experiment 1 demonstrated that LD and OFA anxiety-like measures correlated well with one another, but neither correlated with marble burying. In addition, marble-burying behavior is not correlated with overall activity demonstrating that this behavior is not simply a reflection of mouse activity. The lack of a significant correlation between marble burying, the center ratio in the open field, and transitions in the light–dark box does not necessarily mean that there is no “anxiety” component to marble burying, but only that this measure does not correlate with indicators of anxiety in other tests. Anxiety in humans can be characterized by a wide number of different classifications including panic disorder, phobias, social anxiety disorder, and obsessive–compulsive disorder. While mice may be considered “simpler” mammals compared with humans, it is not unreasonable to imagine that mice may respond differently on different assays used to assess anxiety-like traits, suggesting that different situations elicit different anxiety-like traits in mice (File 1996). Therefore, if an anxiety component exists in the marble-burying assay, it is clearly different from that displayed in other assays.
Behaviors that one might expect from an animal experiencing anxiety brought on by some external factor may include efforts to avoid the object (run away, freeze) or efforts to eliminate the object (attack, bury). The marble-burying assay likely forces some form of interaction between the subject and the marbles since the marbles are spread throughout the surface of the cage. If marbles represent an anxiety-provoking stimulus, one might expect that given the opportunity, a mouse might choose to avoid the stimulus. Our results illustrate that cages prepared with marbles spread across either half the cage or the entire cage did not affect the average number of marbles buried. In fact, mice did not preferentially spend more time on one side of the test area, supporting previous findings by Njung’e and Handley (1991a). In the experiment by Njung’e and Handley, a two-chambered system was used in which marbles were placed in only one chamber, providing mice the opportunity to choose between chambers. Similar to our findings, mice explored both chambers equally. One might assume that when given a choice between an area with or without marbles, a mouse spending an equal amount of time between these two areas indicates that the marbles are not affecting anxiety-like behaviors. However, mice are very exploratory animals and what we observe could be the result of an exploratory drive that overrides any possible anxiety-related drive to avoid the marbles.
The presence of a novel object (i.e., glass marbles) in a cage may trigger a behavioral response from mice resulting in burying behavior targeted toward that object. We tested this hypothesis by studying burying behavior under various conditions with reduced object novelty. The results from these experiments indicate that marble-burying behavior is a consistent repetitive behavior that can be evaluated over a series of several days without a significant alteration, and increasing the familiarity with marbles has little effect on burying behavior. It is possible that we did not provide sufficient time for the mice to become “familiar” with the marbles. However, the time provided for these mice to become familiar with the marbles should be sufficient, based on experiments such as object preference (Dodart et al. 1997). In addition, mice bury food pellets, which are clearly very familiar. The fact that more marbles were buried compared to food may simply reflect that we could not make the food pellets the exact same size, shape, and weight as the marbles; these subtle physical differences may have influenced the observed burying behavior. Furthermore, the nature of food pellets being important for sustenance may alter how a mouse, for example, interacts with the food. It is possible that even with similar digging behaviors, mice may interact more with the food and conceivably uncover previously buried food in the process. However, we did note during testing that there was less direct manipulation of the food compared to that of the marbles (data not shown). Nevertheless, despite the differences between marbles and food pellets, mice readily bury both items.
Since mice readily dig in cages with fresh bedding, it is not difficult to imagine a scenario where marbles are covered due to bedding flying in the air from the digging action. Therefore, to further understand the nature of marble-burying behavior in mice, we also examined digging behavior. A study by Gyertyán (1995) previously speculated that marble burying is more likely an indicator of digging activity as opposed to a directed action toward burying marbles due to an anxiety-provoked state, and our data is consistent with this hypothesis. Objects such as marbles and food pellets have no apparent effect on digging behavior. On the other hand, the familiarity of the bedding material plays a role not only in decreasing digging but also affects frequency with which marbles are buried. Additionally, the fact that digging activity and the number of marbles buried correlate well with one another further supports some connection between these two phenomena. Considering that digging is the most logical method by which one would expect a mouse to intentionally or unintentionally bury objects in a cage, it therefore makes sense for these two factors to be linked with each other. In fact, the number of marbles buried during the marble assay may serve as a convenient readout for the more generalized highly repetitive digging behavior that mice routinely display. It should be noted that some of the present data do indicate that although marble-burying behavior mirrors digging behavior, some of the data reveal that burying and digging can be dissociated in some situations. For example, in experiment 4c, we observed that the proportion of marble burying to digging changed significantly across days due to the fact that the number of digging events decreased across days while the number of marbles buried increased by the last day of testing. This finding suggests that marble burying may become more efficient with repeated testing. In addition, in experiment 5, we observed that digging behavior in the home cage vs. the new cage was altered in opposite directions during the first and second tests, while the test media impacted marble burying only during test 1. Thus, marble burying does indeed reflect or mirror digging, but these two behaviors are not 100% synonymous and can be dissociated.
Studies addressing the burrowing behaviors in both rats and mice have been published over the last several decades (i.e., Ruffer 1965; Berry 1968; Pinel and Treit 1978; Adams and Boice 1981; Pinel et al. 1989; Schmid-Holmes et al. 2001). Rodents generally engage in digging behavior to build burrows in the wild that serve a variety of purposes including safety from predators, food storage, thermoregulation from harsh climate conditions, and nesting (McNab 1966; Studier and Baca 1968; Fleming and Brown 1975; Boice 1977; Bouchard and Lynch 1989). While most laboratory rodents are kept under very protective conditions, negating any obvious “need” for burrows, studies have shown that even laboratory rodents can and will build burrows when provided the opportunity (Boice 1977; Boice and Adams 1980; Dudek et al. 1983; Bouchard and Lynch 1989). Some animal studies have described what is considered a “behavioral need” regarding a “compulsion” for an animal to display behaviors that result in something that the environment around them already fulfills (Hughes and Duncan 1988). For example, Hughes et al. (1989) showed that laying hens display nest-building behaviors even in the presence of a “functional” nest they previously prepared. Similarly, mice will continue to engage in burrowing behaviors even when in a compartment where they had previously constructed a burrow (Sherwin et al. 2004). In this regard, mice vigorously and repeatedly digging in a clean cage in the presence or absence of marbles may be satisfying this innate “need” for burrowing by engaging in a highly repetitive/perseverative digging behavior.
In conclusion, using an inbred strain survey strategy, our findings indicate that although marble burying differs among inbred strains of mice, it is not correlated with “anxiety-related” responses in the open-field or light–dark tests, nor is it correlated with overall exploratory activity. Furthermore, marble-burying behavior is not altered by increased experience with marbles or the marble-burying test. However, there does appear to be an interaction between marble-burying behavior and familiarity with the burying substrate. Lastly, marble-burying behavior is clearly associated with basic digging behavior. Taken together, we suggest that marble-burying behavior is an accurate reflection of repetitive digging behavior that is perseverative and highly resistant to change based on familiarity. While we may never be able to answer the question as to why a mouse digs or why/how a mouse buries marbles, the current results demonstrate that marble-burying/digging behavior is not correlated with exploratory activity or other measures of anxiety-like traits, is not affected by object novelty, nor does it change significantly with repeated testing. This supports the contention that these responses or traits are highly repetitive and perseverative and as such provide a very useful tool to quantitatively evaluate repetitive/perseverative responses in mice.
Acknowledgments
This work was supported by the Baylor Fragile X Center and the Baylor EKS IDDRC (NICHD, HD24064). A.T. and A.B. received partial support from NIGMS training grants T32 GM08307 and TM GM008507, respectively. We would like to thank Dr. Corinne Spencer, Shannon Hamilton, and Randi-Michelle Cowin for their valuable input and suggestions during the course of this study.
Contributor Information
Alexia Thomas, Department of Neuroscience, Baylor College of Medicine, Houston, TX 77030, USA.
April Burant, Molecular and Human Genetics, Baylor College of Medicine, Houston, TX 77030, USA.
Nghiem Bui, Molecular and Human Genetics, Baylor College of Medicine, Houston, TX 77030, USA.
Deanna Graham, Molecular and Human Genetics, Baylor College of Medicine, Houston, TX 77030, USA.
Lisa A. Yuva-Paylor, Molecular and Human Genetics, Baylor College of Medicine, Houston, TX 77030, USA
Richard Paylor, Email: rpaylor@bcm.edu, Department of Neuroscience, Baylor College of Medicine, Houston, TX 77030, USA, Molecular and Human Genetics, Baylor College of Medicine, Houston, TX 77030, USA.
References
- Adams N, Boice R. Mouse (Mus) burrows: effects of age, strain, and domestication. Anim Learn Behav. 1981;9:140–144. [Google Scholar]
- Berry RJ. The ecology of an island population of the house mouse. J Anim Ecol. 1968;37(2):445–470. [Google Scholar]
- Boice R. Burrows of wild and albino rats: effects of domestication, outdoor raising, age, experience, and maternal state. J Comp Physiol Psychol. 1977;91(3):649–661. doi: 10.1037/h0077338. [DOI] [PubMed] [Google Scholar]
- Boice R, Adams N. Outdoor enclosures for feralizing rats and mice. Behav Res Meth Instrum. 1980;12:577–582. [Google Scholar]
- Bolivar VJ, Caldarone BJ, Reilly AA, Flaherty L. Habituation of activity in an open field: a survey of inbred strains and F1 hybrids. Behav Genet. 2000;20(4):285–293. doi: 10.1023/a:1026545316455. [DOI] [PubMed] [Google Scholar]
- Bolles RC. Species-specific defense reactions and avoidance learning. Psychol Rev. 1970;77(1):32–48. [Google Scholar]
- Borsini F, Podhorna J, Marazziti D. Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology. 2002;163:121–141. doi: 10.1007/s00213-002-1155-6. [DOI] [PubMed] [Google Scholar]
- Bouchard PR, Lynch CB. Burrowing behavior in wild house mice: variation within and between populations. Behav Genet. 1989;19 (3):447–456. doi: 10.1007/BF01066170. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Paylor R. Behavioral and physiological mouse assays for anxiety: a survey in nine mouse strains. Behav Brain Res. 2002;136:489–501. doi: 10.1016/s0166-4328(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Broekkamp CL, Rijk HW, Joly-Gelouin D, Lloyd KL. Major tranquillizers can be distinguished from minor tranquillizers on the basis of effects on marble burying and swim-induced grooming in mice. Eur J Pharmacol. 1986;126:223–229. doi: 10.1016/0014-2999(86)90051-8. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Deacon RMJ, Rawlins JNP. Hippocampal lesions, species-typical behaviours and anxiety in mice. Behav Brain Res. 2005;156:241–249. doi: 10.1016/j.bbr.2004.05.027. [DOI] [PubMed] [Google Scholar]
- DeBoer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur J Pharmacol. 2003;463:145–161. doi: 10.1016/S0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- Dodart JC, Mathis C, Ungerer A. Scopolamine-induced deficits in a two-trial object recognition task in mice. Neuroreport. 1997;8 (5):1173–1178. doi: 10.1097/00001756-199703240-00023. [DOI] [PubMed] [Google Scholar]
- Dudek BC, Adams N, Boice R, Abbott ME. Genetic influences on digging behaviors in mice (Mus musculus) in laboratory and seminatural settings. J Comp Psychol. 1983;97 (3):249–259. [PubMed] [Google Scholar]
- File SE. Recent developments in anxiety, stress, and depression. Pharmacol Biochem Behav. 1996;54(1):3–12. doi: 10.1016/0091-3057(95)02175-2. [DOI] [PubMed] [Google Scholar]
- Fleming TH, Brown GJ. An experimental analysis of seed hoarding and burrowing behavior in two species of Costa Rican heteromyid rodents. J Mammal. 1975;56(2):301–315. [Google Scholar]
- Gyertyán I. Analysis of the marble burying response: marbles serve to measure digging rather than evoke burying. Behav Pharmacol. 1995;6:24–31. [PubMed] [Google Scholar]
- Homanics GE, Quinlan JJ, Firestone LL. Pharmacologic and behavioral responses of inbred C57BL/6J and strain 129/SvJ mouse lines. Pharmacol Biochem Behav. 1999;63(1):21–26. doi: 10.1016/s0091-3057(98)00232-9. [DOI] [PubMed] [Google Scholar]
- Hughes BO, Duncan IJH. The notion of ethological ‘need’, models of motivation and animal welfare. Anim Behav. 1988;36 (6):1696–1707. [Google Scholar]
- Hughes BO, Duncan IJH, Brown MF. The performance of nest building by domestic hens: is it more important than construction of a nest? Anim Behav. 1989;37(2):210–214. [Google Scholar]
- Ichimaru Y, Egawa T, Sawa A. 5-HT1A-receptor subtype mediates the effect of fluvoxamine, a selective serotonin reuptake inhibitor, on marble-burying behavior in mice. Jpn J Pharmacol. 1995;68:65–70. doi: 10.1254/jjp.68.65. [DOI] [PubMed] [Google Scholar]
- Kitaoka A. Defensive aspects of burrowing behavior in rats (Rattus norvegicus): a descriptive and correlational study. Behav Processes. 1994;31:13–28. doi: 10.1016/0376-6357(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Li X, Morrow D, Witkin JM. Decreases in nestlet shredding of mice by serotonin uptake inhibitors: comparison with marble burying. Life Sci. 2006;78(17):1933–1939. doi: 10.1016/j.lfs.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Londei T, Valentini AMV, Leone VG. Investigative burying by laboratory mice may involve non-functional, compulsive, behaviour. Behav Brain Res. 1998;94:249–254. doi: 10.1016/s0166-4328(97)00162-9. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Ishigooka S, Matsuda Y. Digging behavior of ddY mouse. Exp Anim. 2000;49(3):235–237. doi: 10.1538/expanim.49.235. [DOI] [PubMed] [Google Scholar]
- McNab BK. The metabolism of fossorial rodents: a study of convergence. Ecology. 1966;47(5):712–733. [Google Scholar]
- Milner LC, Crabbe JC. Three murine anxiety models: results from multiple inbred strain comparisons. Genes Brain Behav. 2008;7:496–505. doi: 10.1111/j.1601-183X.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- Nicolas LB, Kolb Y, Prinssen EPM. A combined marble burying-locomotor activity test in mice: a practical screening test with sensitivity to different classes of anxiolytics and antidepressants. Eur J Pharmacol. 2006;547:106–115. doi: 10.1016/j.ejphar.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Njung’e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol Biochem Behav. 1991a;38 (1):63–67. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- Njung’e K, Handley SL. Effects of 5-HT uptake inhibitors, agonists and antagonists on the burying of harmless objects by mice; a putative test for anxiolytic agents. Br J Pharmacol. 1991b;104:105–112. doi: 10.1111/j.1476-5381.1991.tb12392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinel JPJ, Treit D. Burying as a defensive response in rats. J Comp Physiol Psychol. 1978;92(4):708–712. [Google Scholar]
- Pinel JPJ, Symons LA, Christensen BK, Tees RC. Development of defensive burying in Rattus norvegicus: experience and defensive responses. J Comp Psychol. 1989;103(4):359–365. doi: 10.1037/0735-7036.103.4.359. [DOI] [PubMed] [Google Scholar]
- Poling A, Cleary J, Monaghan M. Burying by rats in response to aversive and nonaversive stimuli. J Exp Anal Behav. 1981;35:31–44. doi: 10.1901/jeab.1981.35-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DC, Jones DNC, Nelson PR, Jones CM, Quilter CA, Robinson TL, Hagan JJ. Use of SHIRPA and discriminant analysis to characterise marked differences in the behavioural phenotype of six inbred mouse strains. Behav Brain Res. 1999;105:207–217. doi: 10.1016/s0166-4328(99)00072-8. [DOI] [PubMed] [Google Scholar]
- Ruffer DG. Burrows and burrowing behavior of Onychomys leucogaster. J Mammal. 1965;46(2):241–247. [Google Scholar]
- Schmid-Holmes S, Drickamer LC, Robinson AS, Gillie LL. Burrows and burrow-cleaning behavior of house mice (Mus musculus domesticus) Am Midl Nat. 2001;146:53–62. [Google Scholar]
- Sherwin CM, Haug E, Terkelsen N, Vadgama M. Studies on the motivation for burrowing by laboratory mice. Appl Anim Behav Sci. 2004;88:343–358. doi: 10.1016/j.applanim.2004.03.009. [DOI] [Google Scholar]
- Studier EH, Baca TP. Atmospheric conditions in artificial rodent burrows. Southwest Nat. 1968;13(4):401–410. [Google Scholar]
- Takeuchi H, Yatsugi S, Yamaguchi T. Effect of YM992, a novel antidepressant with selective serotonin re-uptake inhibitory and 5-HT2A receptor antagonistic activity, on a marble-burying behavior test as an obsessive-compulsive disorder model. Jpn J Pharmacol. 2002;90:197–200. doi: 10.1254/jjp.90.197. [DOI] [PubMed] [Google Scholar]
- Terlecki LJ, Pinel JPJ, Treit D. Conditioned and unconditioned defensive burying in the rat. Learn Motiv. 1979;10:337–350. [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1989;31:959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Treit D, Pinel JPJ, Fibiger HC. Conditioned defensive burying: a new paradigm for the study of anxiolytic agents. Pharmacol Biochem Behav. 1981;15(4):619–626. doi: 10.1016/0091-3057(81)90219-7. [DOI] [PubMed] [Google Scholar]
- Trullas R, Skolnick P. Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology. 1993;111:323–331. doi: 10.1007/BF02244948. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, Steckler T. Behavioural analysis of four mouse strains in an anxiety test battery. Behav Brain Res. 2000;115:95–106. doi: 10.1016/s0166-4328(00)00240-0. [DOI] [PubMed] [Google Scholar]
- Webster DG, Williams MH, Owens RD, Geiger VB, Dewsbury DA. Digging behavior in 12 taxa of muroid rodents. Anim Learn Behav. 1981;9(2):173–177. [Google Scholar]
- Wilkie DM, MacLennan AJ, Pinel JPJ. Rat defensive behavior: burying noxious food. J Exp Anal Behav. 1979;31 (3):299–306. doi: 10.1901/jeab.1979.31-299. [DOI] [PMC free article] [PubMed] [Google Scholar]