Abstract
The thoracolumbar junction is the section of the truncal spine most often affected by injuries. Acute instability with structural damage to the anterior load bearing spinal column and post-traumatic deformity represent the most frequent indications for surgery. In the past few years, endoscopic techniques for these indications have partially superseded the open procedures, which are associated with high access morbidity. The particular position of this section of the spine, which lies in the border area between the thoracic and abdominal cavities, makes it necessary in most cases to partially detach the diaphragm endoscopically in order to expose the operation site, and this also provides access to the retroperitoneal section of the thoracolumbar junction. A now standardised operating technique and instruments and implants specially developed for the endoscopic procedure, from angle stable plate and screw implants to endoscopically implantable vertebral body replacements, have gradually opened up the entire spectrum of anterior spine surgery to endoscopic techniques.
Keywords: Thoracolumbar junction, Endoscopic spine surgery, Spinal canal decompression, Spinal lesions, Diaphragm detachment
Introduction
The approach to the anterior column of the thoracolumbar junction of the spine was first described by Hodgson in 1960[16] and cited by Louis in 1977[22]. It represents an extension of deep lateral thoracotomy into the retroperitoneal space and involves almost complete detachment of the diaphragm and in most cases resection of the 10th or 11th rib [7]. This extended approach led to excellent anterolateral exposure of the thoracolumbar junction, making it possible to perform all spine surgery procedures on this section. Until the mid 1990s, we also used this standard approach routinely for all resection and reconstructive interventions on the anterior spine. In view of the access morbidity associated with the open technique, the indications were generally restrictive and reserved for injuries and post-traumatic conditions or illnesses involving extensive structural damage and necessitating reconstruction of the anterior spine. Overall however, there was a clear increase in the number of these operations, resulting on the one hand from a rise in the number of serious spine injuries involving high energy trauma and on the other from a growing number of corrective operations following the failure of inadequate fracture treatment of spinal injuries.
Simultaneously with this development, endoscopic techniques were making their way into spine surgery as into other disciplines. The first publications on this subject [23, 27, 28] describe operations to treat spinal disorders and to remove intervertebral disc material. The first endoscopic spine surgery atlas, now in its second revised edition, was published in 1995 by John Regan and also contains the first references to the use of endoscopy in spine trauma. In the Berufsgenossenschaftliche Unfallklinik in Murnau, we performed our first endoscopic operation on the spine in May 1996 and subsequently developed the endoscopic approach to the thoracolumbar junction described below [6, 18]. The following overview of the endoscopic approach and surgical spectrum is based on the experience of more than 1,300 endoscopic operations on the spine performed in our hospital in Murnau and now in Munich over the past 10 years.
Technical requirements
For this approach we use four reusable flexible threaded trocars with a diameter of 11 mm. Flexible trocars are used in order to reduce the pressure on the intercostal nerve and vascular bundle as much as possible. Light reflections and their interference with the regulation of light intensity can be avoided by using black trocars and instruments with matt surfaces.
A high intensity xenon light source is required to illuminate the thoracic cavity. For image transmission we use a 30° camera which enables us to position the camera far away from the working portal, thus facilitating undisturbed working as well as variable adjustment of the angle of vision. The intraoperative situs is transmitted onto two flat screens that form part of an endoscopy tower containing a digital image recorder and the generator for the ultrasonic knife (Figs. 1, 2). Air insufflation is not required.
Fig. 1.
Standard set up for endoscopic spine surgery. The patient is placed in a true lateral position; four portals are positioned
Fig. 2.
OR setup and instruments
These days various manufacturers offer a set of instruments for soft tissue and bone preparation. Here it is important to make sure that there is a depth scale on both sides and that the instruments are ergonomically designed for good control and handling.
The endoscopic approach to the thoracolumbar junction
The operation is performed under one lung respiration using a double lumen tube. The patient is positioned on his or her side and stabilised in this position with a vacuum mattress or suitable supports at the symphysis and sacrum and at the level of the shoulder blades. The approach side is decided first and foremost by the location of the major vessels, which can be identified from the preoperative CT scan. In most cases the best approach to the thoracolumbar junction is from the left.
As a first step, we draw the affected section of the spine onto the skin of the lateral abdominal and thoracic wall under image intensifier control. Here we pay very careful attention to correct projection of the vertebrae, whose endplates and anterior and posterior margins should be displayed in the central beam, in sharp focus with no double contour. This marking is used as the sole reference for subsequent placement of the portals.
Placing the portals
Opinions on portal placement in the literature are divided and should be seen as depending on the surgical school and specialist area from which the authors originate. Thus, the neurosurgeon and orthopaedic surgeon in the American school around Curtis Dickman [10] and John Regan [28] usually stands in front of the patient, facing and working in a diagonal dorsal direction towards the spinal canal. By contrast, surgeons orientated towards orthopaedics and traumatology are accustomed from open spine surgery to stand behind the patient and to look and operate on the spine from the side.
Independent of this, experience shows that the placement of the trocars with reference to the planned operation and the surgical target area is of fundamental importance for the entire course of the operation. The arrangement we normally use for endoscopic access to the thoracolumbar approach is described below.
The working portal is situated directly above the lesion, the portal for the endoscope is placed over the spine two to three intercostal spaces away from the working portal in a cranial direction. The portals for the retractor and the suction/irrigation instrument are situated ventrally from this point (Fig. 3).
Fig. 3.
Placement of the trocars at the thoracolumbar junction
Approach to the retroperitoneal section of the thoracolumbar junction
Anatomy of the diaphragm
The dome-like diaphragm is firmly connected at its margins with the sternum, ribs and spine, and arches up into the thoracic cavity. The attachment site on the spine and on the directly adjacent ribs is different from one side to the other and has a right (dexter) and left (sinister) crus. The former springs from the lateral surfaces of the L1–L3 vertebrae, the left crus springs from the lateral vertebral wall of the first and second lumbar vertebrae. In a lateral direction, the diaphragm spans the upper portion of the psoas muscle with the medial arcuate ligament, which originates medially from the lateral surface of the first lumbar vertebra and attaches at the transverse process of the same vertebra (Fig. 4). The lateral arcuate ligament in turn arises here at the tip of the transverse process, arches across the lumbar quadrate muscle, which stretches in a cranial direction, and attaches to the inferior margin of the 12th rib. Viewed topographically, the attachments of the diaphragm to the spine lie at the level of the first lumbar vertebra, whereas the lowest point of the thoracic cavity projects with the phrenicocostal sinus at the level of the baseplate of the second lumbar vertebra. This makes it possible to place a trocar intrathoracically in the phrenicocostal sinus, which, after incision of the diaphragm attachment to the spine, provides access to the retroperitoneal section of the thoracolumbar junction right down to the base plate of the second lumbar vertebra. This needs a 4- to 5-cm-long incision; access to the L1–L2 vertebrae is achievable with a shorter incision of 2–3 cm [3, 5, 6, 18] (Fig. 5).
Fig. 4.
View at the diaphragm from below, showing the diaphragm and the anatomical conditions at the thoracolumbar junction. The course of the incision (black interrupted line) runs parallel to the attachment of the diaphragm
Fig. 5.
Endoscopic view at the thoracolumbar junction from above. The course of the incision at the attachment of the diaphragm is marked
Given the anatomical conditions, we consider the nature of the diaphragmatic attachment incision important in order to prevent the occurrence of a postoperative diaphragmatic hernia. Of the two alternatives, radial incision of the diaphragmatic attachment in extension of the spinal axis or semicircular incision running parallel to the diaphragmatic attachment to the spine and the ribs, we prefer the latter for the following reason. Since architecture of the diaphragm is the dome-like, an increase in intraabdominal pressure from a semicircular incision parallel to the attachment causes the resected margins to come together and adhere spontaneously, whereas a radial incision in direct proximity to the orifices of the aorta and the oesophagus weakens the diaphragm fixation and causes the resected margins to gape. In addition, we recommend that every incision in the attachment longer than 2 cm should be sutured endoscopically to be sure of avoiding a hernia.
Potential and technical implementation of endoscopic procedures on the thoracolumbar junction
The past 10 years of endoscopic spine surgery have been characterised by continuous further development of the operating technique accompanied by a considerable expansion of the indication spectrum. Today this covers not only the indications for reconstruction of the anterior spine, including anterior decompression of the spinal canal, vertebral body replacement and ventral instrumentation using angle stable implants, but also the treatment of post-traumatic malpositions and unsuccessful osteosyntheses. It is also possible to treat infections and to remove tumours and metastases endoscopically, although because of the substantial vascularisation in some cases it has to be remembered that the bleeding tendency significantly increases the difficulty of these operations.
Treatment of fractures
Based on our own experience, the results of the multi-centre study of the German Society for Traumatology (DGU) conducted from 1994 to 1996[19] and other publications on the outcomes of surgical treatment of spinal fractures, we must expect a post-traumatic loss of correction to a greater or lesser extent depending on the nature of the spinal injury and the type of surgical treatment. Injuries where we have the major loss of correction are injuries with an anterior column damage combined with a disruption of the posterior tension banding system [2]. As long as the posterior tension banding system is intact, usually at least in younger patients, there is no significant progression of the vertebral body damage. The vertebral body damage reaches immediately after trauma the definitive amount of compression and remains as such as long as the tension banding system is intact.
Operating technique
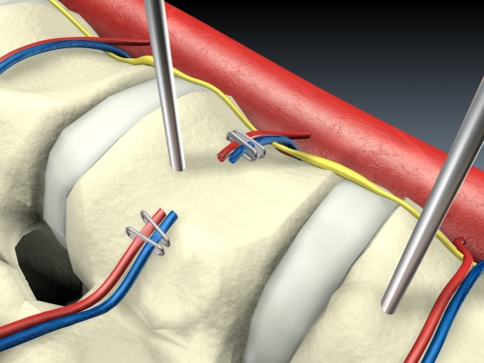
The operating technique described below is used in all reconstructive procedures on the anterior spine and is supplemented with further surgical steps according to indication. After placement of the trocars, the situs is first explored with a blunt probe and the course of the spine and aorta identified. For fracture treatments below the 12th thoracic vertebra, we first incise the diaphragmatic attachment above the spine and push the retroperitoneal fatty tissue away from the psoas muscle (Fig. 5). After mobilising this muscle from ventrally to dorsally, we place the first implants or K-wires under image intensifier control, and these already define the entry point for the screw implants. Under endoscopic conditions it has proved valuable to work with landmarks such as these very early in the course of the operation, since they greatly facilitate orientation in the operating site.
The reconstruction of the anterior spine then proceeds in standardised steps: the segmental vessels are exposed with an elevator, pulled down and mobilised using a 90° angled Overholt, ligated with titanium clips and resected. The lateral vertebral body wall is exposed (Fig. 6).
Fig. 6.
K-wire insertion, exposure and dissection of the segmental vessels
The intervertebral disc(s) is (are) incised. The ventral and dorsal extent of the partial corporectomy is marked using an osteotome. In monosegmental treatment, the caudal limit of the resection is defined using the image intensifier in the AP projection, and osteotomy of the residual vertebral body is performed. We remove the fragments and intervertebral disc material with rongeurs. Particular importance is placed on removing the disc material and freshening up the end plates of the adjacent vertebrae.
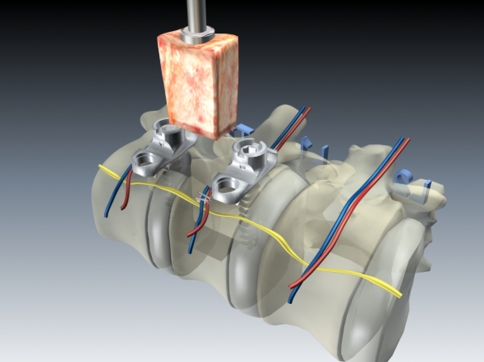
After the length of the defect has been measured a vertebral body replacement of the corresponding length is implanted. In monosegmental treatment we prefer to use an autologous tricortical bone graft, and for bisegmental bridging an expandable titanium cage that we surround with spongiosa harvested from the partial corporectomy (Fig. 7).
Fig. 7.
Insertion of the tricortical bone graft into the partial corporectomy defect Dissection of the retropulsed fragment under direct visualization of the dura. Removal of the fragment
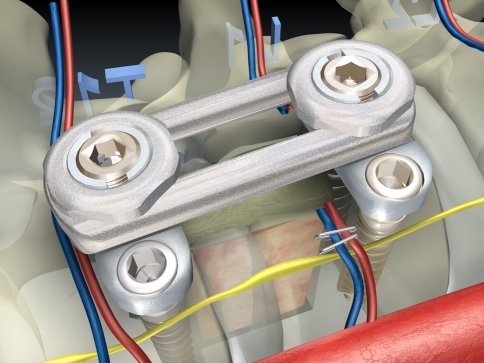
Building on the K-wires or screws inserted in the first step of the operation, we routinely perform a ventral instrumentation. For this we use an angle stable plate and screw implant with the intention of achieving higher primary stability and hence a better initial situation for bone ingrowth in the fusion area (Fig. 8).
Fig. 8.
The anterior reconstruction including bone graft insertion and instrumentation is completed
The operation then concludes with manual endoscopic suturing of the diaphragm incision and the placement of thoracic drainage. Re-inflation of the lung is monitored endoscopically and the four skin incisions are sutured layer by layer after the trocars have been removed.
Decompression of the spinal canal
Depending on the level of stenosis, compression of the spinal canal can lead to a neurological deficit. The spectrum of injuries to the spinal canal, medullary cone and cauda equina ranges from simple contusion to complete tearing of the neural structures. As long as the structures have not been severed, recovery of function and sensory deficits may be possible in principle. This has been demonstrated in both animal investigations [9] and clinical studies [1, 14, 17, 25]. The statements in the literature concerning the effectiveness of spinal canal decompression with regard to influencing progress and final outcome after spinal canal injury are however extremely controversial [8, 11, 14, 15, 21].
We consider the indications for anterior decompression as given when significant narrowing and a neurological deficit remain after primary dorsal reduction and stabilisation. If it is clear from a myelogram that adequate indirect decompression was not achieved through reduction in the primary operation, we then perform a hemilaminectomy as an emergency measure to relieve the compression dorsally and then in a second elective step we attempt to remove the anterior compressing bony fragments and disc material endoscopically.
Operating technique
In our experience it is important to use fixed landmarks such as K-wires or screws to maintain proper alignment and surgical trajectories. Therefore, we recommend placing K-wires or screws under fluoroscopic control into the vertebral bodies above and below the level selected for corporectomy. After this, the adjacent intervertebral discs are incised and removed, and the central part of the vertebral body is resected with osteotomes and rongeurs. Initially, the posterior vertebral body wall is preserved to avoid further canal compromise during the partial corporectomy. In addition, removal of the retropulsed fragment can cause significant bleeding from the epidural venous plexus. Therefore, we recommend completing the partial corporectomy and adjacent diskectomies before the canal decompression. The next step is to identify the pedicle of the fractured vertebral body. In traumatic burst fracture, the pedicles are nearly always preserved and the retropulsed fragment is usually located medial to the pedicle. Thus, the retropulsed fragment is trapped between the two pedicles and is difficult to remove or to reduce. Therefore, we recommend resecting the ipsilateral pedicle with a punch [9] prior to attempting to remove the retropulsed fragment. For this reason the resection of the ipsilateral pedicle has a dual importance: it exposes the spinal canal and at the same time it frees the retropulsed fragment from the pincer grip of the pedicles.
Therefore, after completing the partial corporectomy and resecting the rib heads if necessary, we use a Cobb raspatory to expose the ipsilateral pedicle subperiosteally and to push away the nerve root dorsally. The inferior margin of the pedicle is identified with a nerve hook and the pedicle is transected with a punch (Fig. 9). Removing the dorsocranial section of the vertebral body together with the base of the pedicle exposes the posterior margin fragment and will bring the dura into view (Fig. 10). The compressing fragment can now be lifted off the dura under direct view, mobilised in the direction of the partial corporectomy and resected. A nerve hook is used under image intensifier control to document the completeness of the posterior margin fragment resection in both planes (Fig. 11).
Fig. 9.
Resection of the pedicle in order to expose the lateral dural sac
Fig. 10.
Resection of parts of the postero-lateral aspect of the vertebral body under endoscopic view at the dura
Fig. 11.
The decompression of the spinal canal is completed
We use an expandable titanium cage as a vertebral body replacement because of its higher stability and the smaller risk of dislocation. The operation concludes with the ventral instrumentation and suturing of the diaphragm attachment.
Results
Fracture treatment
In a collective study by the Berufsgenossenschaftliche Unfallklinik Murnau and Stanford University, California, data on 220 patients with unstable injuries of the thoracolumbar junction in the region of the 12th thoracic and first lumbar vertebra were recorded between May 1996 and June 2002 [18]: 186 patients were from the clientele of the BG-Klinik Murnau and 34 from the Stanford University patient base. The average age was 36. The follow-up period was between 4 months and 6 years, with a mean of 2 years. A neurological deficit was present in 43% of the patients, categorised as follows according to the Frankel Scale [13]:16% D, 6% C, 5% C and 15% A with complete neurological deficit symptoms. Eighty-nine patients (40.5%) presented with a fracture of the 12th thoracic vertebra, and 131 patients (59.5%) had a fracture of the first lumbar vertebra.
The injuries were categorised according to the AO classification [24]. All the patients with B and C injuries were primarily dorsally repositioned and stabilised. Thus, of the 220 patients 64.5% (142) were stabilised dorsoventrally and 35.5% of the patients with preoperatively diagnosed type A compression injuries were exclusively ventrally stabilised. Between 1996 and 1999 we used the Z Plate (Sofamor-Danek) for ventral instrumentation and since November 1999 we have used the angle stable MACS TL System (Aesculap) specially designed for the endoscopic technique. At this point in time we predominantly used a tricortical bone graft from the iliac crest as a vertebral body replacement (84%) (Figs. 17, 18, 19, 20) and much less frequently (16%) an extendable titanium cage (Synex®, Synthes). At 1-year follow-up complete fusion could be identified in 85% of the exclusively ventrally treated fractures and in 90% of the dorsoventrally treated fractures. Reconstruction of the spine profile was successful in over 90% of the cases.
Fig. 17.
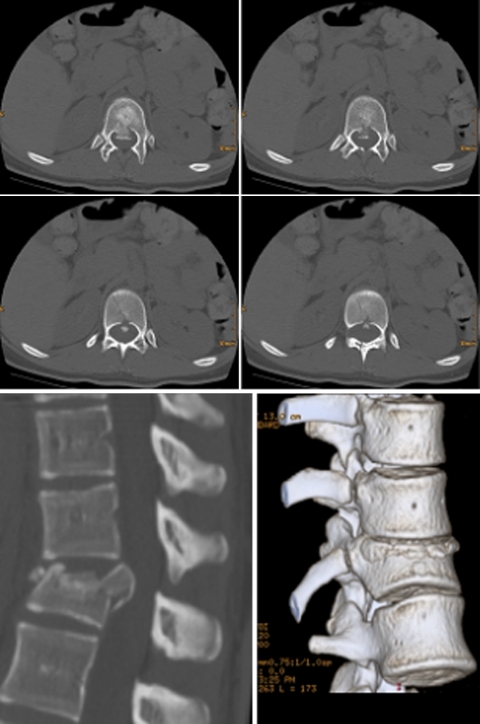
Case example of a burst fracture L1 (type A 3.3) with incomplete neurological deficit (ASIA C) and severe canal compromise. Preoperative X-rays and CT scans
Fig. 18.
Postoperative CT scans for the assessment of spinal canal clearance after having performed dorsal reduction and fixation
Fig. 19.
Postoperative X-rays and CT reconstructions after thoracoscopic anterior decompression, vertebral body replacement (X-Tenz® cage) and anterior instrumentation (MACS TL®)
Fig. 20.

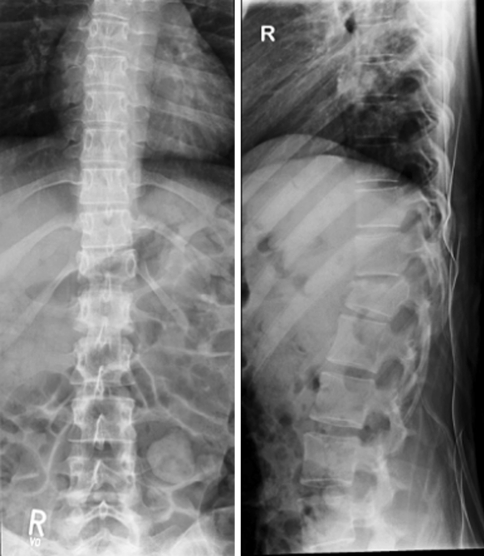
Lateral X-ray view showing the relation of a bisegmental anterior instrumentation T12–L2 to the diaphragm
Case report 1
An 18-year-old woman injured in a car accident suffering from a T12 fracture type B 1.2 according to the AO classification, Frankel E. The anterior column presents an incomplete burst fracture of the T12 vertebra with a split at the lower half of the vertebra. There is a significant narrowing of the spinal canal due to a big fragment of the posterior wall (Figs. 12, 13,14, 15, 16).
Fig. 12.
Case report: 18year-old female after car accident, compression/rotation fissured fracture type C 1.3.2 (AO classification)
Fig. 13.
Transversal CT layer and 2- and 3-dimensional reconstruction of the injury
Fig. 14.
Postoperative X-ray check after initial dorsal bisegmental stabilisation and subsequent thoracoscopic monosegmental ventral fusion with the MACS TL system T11–12
Fig. 15.
Transversal CT layers showing the bicortical screw fixation of the caudal section of Th12, position check of tricortical iliac crest graft and 2D reconstruction to check position after monosegmental stabilisation T11–12 (MACS TL®)
Fig. 16.
Clinical and cosmetic result after suture removal 10 days post-op
Operative planning
Step Indirect decompression of the spinal canal by dorsal reduction and fixation using an internal fixator.
Step Anterior monosegmental fusion with interposition of a tricortical bone graft and ventral instrumentation using MACS TL®, lag screw fixation of the split fracture of the lower half of the T12 vertebra.
The operating time was between 70 min and 9 h, with an average operating time of 3.5 h. The times included in this total for the approach to the retroperitoneal section were between 10 and 20 min including the suture. Passing the learning curve and introducing implants specially developed for the endoscopic technique made it possible to shorten the operating time significantly.
Complications and conversion rates
In 3 of the 220 patients we converted to the open surgery technique, corresponding to a conversion rate of 1.3%. Of these, two occurred in the first five operations. The respective reasons for these were inadequate control of bleeding from the bone and jamming of a screw. Both cases would have been resolvable endoscopically given the present state of technology. During a revision operation following implant failure of a Z Plate a small tear occurred in the aorta as the plate was being exposed, and this was treated by direct suturing of the aorta in open surgery.
Altogether we recorded five cases of implant loosening, of which four involved the Z Plate with loosening of one of the anterior, non-angle stable screws. In one case the entire MACS implant became loose due to an excessively broad indication setting in a case of severe osteoporosis. Of these five cases, three underwent revision surgery because of the accompanying loss of correction.
Approach-related complications such as pleural effusions, persistent pneumothorax or intercostal neuralgia occurred in 5.4% of the cases. In one case, the L1 root was irritated by the application of monopolar current using the dissection hook during endoscopic detachment of the diaphragm. In the follow-up there was no occurrence of diaphragmatic hernia in any of the cases.
Anterior decompression [4]
The results are reported of a study on a consecutive series of 30 patients of the Berufsgenossenschaftliche Unfallklinik Murnau, on whom endoscopic anterior decompression was performed.
Case report 2
A 28-year-old man, injured in skiing accident suffering from a L1 fracture type A 3.3, Frankel D with significant narrowing of the spinal canal (Figs. 17, 18, 19, 20).
Operative strategy
Step Indirect decompression of the spinal canal by dorsal reduction and fixation using an internal fixator.
Step Anterior decompression of the spinal canal, interposition of a distractable cage (X-Tenz®) and anterior bisegmental instrumentation (MACS TL®).
The average preoperative narrowing of the spinal canal, defined according to the Bradford and McBride method, was 55%. The average postoperative clearance of the spinal canal was 110% due to the postoperative larger extension of the spinal canal in comparison to the normal diameter, documented by CT and determined planimetrically. In no case was there a deterioration of the pre-existing neurological situation. In one of the four patients with complete paraplegia there was an improvement in neurological status of one grade on the Frankel Scale. Among the patients with incomplete paraplegia, we observed a significant improvement in neurostatus in 13 out of 20 patients.
The average duration of surgery, including complex correction operations, was 5 h 42 min. The estimated blood loss with routine use of a Cell Saver system was 870 ml. The average stay in intensive care was 1.4 days.
We recorded a complication in 36.7% (11 of 30) of the patients. Of these, five were associated with the harvest of the bone graft from the iliac crest, and essentially concerned postoperative pain in this region. The remaining six complications involved:
postoperative pulmonary insufficiency requiring prolonged artificial respiration in intensive care;
two pleural effusions requiring surgical intervention;
one pulmonary embolism with deep vein thrombosis in the leg and pre-existing total paraplegia;
one transient irritation of the brachial plexus caused by the patient’s position;
one case of persistent damage to the sympathetic nerve plexus.
Discussion
In the past 10 years, endoscopic operations on the spine have developed from an alternative technique to the standard spine surgery procedure, usefully combining the familiar proven techniques from the open procedures of bone and disc resection, vertebral body replacement and ventral instrumentation with the video-assisted operating technique of thoracic surgery (VATS). The subsequent development of instruments and implants as well as the standardisation of the operating procedures meant increasingly that even complex operations could be performed endoscopically and that the initially longer operating times required by the endoscopic procedures could be decreased to a level known from conventional open surgery.
However, the approach to the thoracolumbar junction of the spine, that at the same time represents the border region between the thoracic and abdominal cavity, was for a long time considered difficult to access endoscopically. The lower limit of the thoracoscopically accessible was generally claimed to be the first lumbar vertrebra [10]. With partial detachment of the diaphragm running parallel to the diaphragmatic attachment, as described by us and published in 1998 [6], it is also possible to reach the region of the second lumbar vertebra near to the baseplate under thoracoscopic conditions. This ensures that the first lumbar vertebra, the one most frequently affected by injuries, can also be treated endoscopically using the bisegmental technique.
As shown by the collective study on 220 patients by the BG Unfallklinik Murnau and Stanford University, California, in 2004[18], the approach to the thoracolumbar junction described above has a low complication rate. Crucial for successful implementation of this technique is an incision parallel to the attachment of the diaphragm onto the spine and the ribs. For the approach to the first lumbar vertebra, this incision is about 2 cm long. For the approach to the second lumbar vertebra, an opening of about 4–6 cm is made in the diaphragm. The incision should be closed by suturing to avoid a diaphragmatic hernia. The occurrence of a diaphragmatic hernia must be considered as probably the most serious potential complication, and this has not so far occurred in any case in which the technique we recommend has been used [5, 6, 18]. However, two cases of diaphragmatic hernia are known to us personally following endoscopic diaphragm detachment performed elsewhere. In these cases a radial incision was made over the spine and left unsutured. In both cases, the incarceration of abdominal contents in the hernia gap led to serious complications.
Another approach to the thoracolumbar junction is described by Dickman and Rosenthal [10] in which the patient is placed in a reverse Trendelenburg position in order to shift the intraabdominal organs in a caudal direction and relax the diaphragm. Separation of the pulmonary ligament and mobilisation of the pleura is recommended to give access to the 12th thoracic and first lumbar vertebrae. For lower lying regions the authors recommend the use of retroperitoneally positioned portals.
Our preferred concept of reconstruction of the load bearing anterior spine as a fusion procedure in fracture treatment contains resection of the injured intervertebral discs and the burst sections of the fractured vertebra in the form of a partial corporectomy by preserving the anterior ligament and a small rim of the vertebral body implantation of a vertebral body replacement in the form of an autologous bone graft from the iliac crest or an extendable titanium cage filled and surrounded with spongiosa which will be distracted against the anterior ligament which significantly increases the stability of the construct ventral instrumentation as a purely ventral stabilisation measure or to provide additional stabilisation for a dorsal implant.
The discussion on the sense or necessity of ventral instrumentation in cases where a dorsal stabilisation system has already been implanted is not yet concluded. In biomechanical studies, Knop et al. [20] were able to demonstrate adequate stability using a fixateur interne and an extendable titanium cage (Synex®) that is “braced” against the fixateur. Other current biomechanical studies have shown that ventral instrumentation has a substantial reinforcing influence on the primary stability of the spine section treated with this method [29, 30]. However, the complete resection of the vertebral body and the anterior ligament is not only unnecessary, but it also diminishes the stability given. The crucial question of how much stability a fusion requires for bone ingrowth remains open.
The indication for anterior decompression is the subject of similarly controversial discussion. From animal experiments it is known that both the magnitude of the initial force exerted on the spinal cord and the duration of the compression of neural structures influence not only the severity of the neurological deficits, but also the ability of the spinal cord to recover [9, 25]. Clinical studies were able to demonstrate similar effects in humans [1, 14, 17], although according to Gaebler et al. optimum recovery can be expected when decompression occurs within the first 6 h after the trauma. Therefore, our concept foresees immediate dorsal reduction and stabilisation for indirect reposition of the posterior margin fragment in every case of an unstable injury to the thoracolumbar junction combined with a neurological deficit, and myelographic documentation has proved this to be sufficient to relieve the neural structures in around 80% of the cases. However, if the intraoperative myelogram shows a further contrast medium stop, dorsal decompression is immediately performed. Endoscopic anterior decompression as an elective operation then serves to remove the ventrally compressing fragments with a view to avoiding long term consequences [26].
The special features of the endoscopic technique come into effect particularly in anterior decompression. Among the advantages is the excellent image of the situs obtained with the 30° scope which we routinely use, and which, depending on the rotation of the scope and the nearness to the object, can be compared to the image obtained with the surgical microscope. One of the disadvantages is the two dimensional nature of the image, which makes it difficult or impossible for the surgeon to correctly assess the working angle and the penetration depths of the instruments and screws from an orthograde view. Starting from these premises and based on the analysis of the typical fracture form associated with traumatic spinal canal narrowing, we have developed a procedure adapted to the endoscopic technique, the effectiveness of which we were able to demonstrate in the study of 30 consecutively recorded patients described above [4]. The key site is the pedicle, the resection of which on the one hand provides an unobstructed view onto the dura and on the other exposes the posterior margin fragment, which in most cases is trapped between the intact pedicles, for reposition and resection. The compressing fragments can thus be gently lifted away from the dura with a dissector under direct vision, moved ventrally into the partial corporectomy defect and removed. The study showed an equivalent effectiveness in spinal canal clearance of approximately 100% compared to the open ventral technique. The average operating times have since been reduced from over 5 h to between 3 and 3.5 h.
Complications and complication management
The typology of complications introduced by Aebi [1] also applies in principle for endoscopic spine surgery. These are the complications caused by
Reduction
Approach
Preparation, placement of screws and implants
The implant, lack of fusion, infection.
The reposition of larger deformities via the ventral spine alone is limited for biomechanical reasons. Complications arise here through the inadequate application of force on implants or endplates during attempts at repositioning, and these can lead to primary or premature implant loosening.
However, of greater importance for the endoscopic procedure on the thoracolumbar junction are the approach-related complications. The proximity of the diaphragm and the organs immediately beneath it demands great care in placing the trocars. We recommend that the portal for the endoscope, the one situated furthest in a cranial direction in the thoracic cavity, should be placed first and the thorax opened via a small thoracotomy 2-cm long, allowing the situs to be palpated with the fingers to exclude the possibility of adhesions of the lung, pleura and diaphragm. The other portals can then be placed under endoscopic control. The operation should begin and end with an inspection all around the thoracic cavity. Before the endoscope is removed, it is absolutely essential to monitor the complete re-inflation of the lung endoscopically in order to prevent atelectases and the formation of effusions. To prevent intercostal neuralgias we recommend the use of flexible trocars that reduce the pressure on the intercostal nerve and vascular bundle to an unavoidable minimum. With the minimally invasive approach and the consistent implementation of these recommendations, it was possible to reduce substantially the rate of approach-related complications during surgery. According to an analysis of our first 371 patients this stood at 5.4%. The equivalent rate for open interventions in a multi-centre study by Faciszewski [12] was just over 14%.
With regard to the direct proximity of vital structures, the complication potential of surgical operations on the spine must be regarded as high. While the risk of neurological complications at the level of the thoracolumbar junction is to be regarded as less in comparison with the thoracic spine because of the tapering of the spinal cord and the greater width of the spinal canal, more attention must be paid to the ventrally situated vessels. Injury to these represents a life-threatening complication which has to be brought under control by immediate thoracophenotomy with extensive exposure, mobilisation and tying of the vessels. Dangerous malpositioning of screws and implants can be avoided by using the image intensifier when inserting the vertebral body replacement and screwing in the screws. In our patient series of 371 patients from the first 5 years after introduction of the endoscopic technique the rate of vascular complications (leakage from the aorta) was 0.3%. The equivalent value from the multi-centre study on open procedures is 0.08% [12]. In the same time period we had one spleen injury and temporary damage to the L1 root caused by the application of monopolar current, corresponding to a rate of organ complications of 0.3% for each.
From the group of complications caused by implants, infection or lack of bone fusion, similar results have to be reported for endoscopic and open techniques. The fusion rates are given as between 85 und 90%. The advantages of the smaller approaches with regard to possible contamination of the situs and the development of infection are partly offset by the longer operating times for the endoscopic procedure, at least in the initial phase.
Conclusion
In the last 10 years, endoscopic procedures on the spine have become an alternative standardised spine surgery. The standardisation of procedures and the development of instruments and implants for the minimally invasive operating technique have made a substantial contribution to this. Through the trans-diaphragmatic approach it has been possible to open up the thoracolumbar junction, including the retroperitoneal segments of the spine, to the endoscopic technique. With the extension of the technique to the retroperitoneal sections of the thoracolumbar junction it was possible at the same time to increase the indication spectrum of the endoscopic technique substantially, so that it today includes complete fracture treatment with vertebral body replacement and ventral instrumentation as well as anterior decompression of the spinal canal and the correction of post-traumatic deformities in terms of anterior release and the reconstruction of the anterior spine. Special indications include the endoscopic treatment of infections and tumours, the feasibility of which has been demonstrated in principle in individual studies. The complication rate of the endoscopic procedure is of the same scale as that known from the open procedures, with clear advantages in terms of the reduced access morbidity associated with the minimally invasive technique.
Conflict of interest statement
The author receives royalties from Aesculap, Tuttlingen, Germany and was involved in the technical development of MACS-TL® (constraint plate and screw system for endoscopic anterior instrumentation) and Hydrolift® (Vertebral Body Replacement Device) as a consultant to Aesculap.
References
- 1.Aebi M, Mohler J, Zach G, Morscher E. Analysis of 75 operated thoracolumbar fractures and fracture dislocations with and without neurological deficit. Arch Orthop Trauma Surg. 1986;105:100–112. doi: 10.1007/BF00455844. [DOI] [PubMed] [Google Scholar]
- 2.Beisse R. Endoscopic surgery on the thoracolumbar junction of the spine. Eur Spine J. 2006;15:687–704. doi: 10.1007/s00586-005-0994-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beisse R. Video-assisted techniques in the management of thoracolumbar fractures. Orthop Clin N Am. 2007;38:419–429. doi: 10.1016/j.ocl.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Beisse R, Muckley T, Schmidt MH, Hauschild M, Buhren V. Surgical technique and results of endoscopic anterior spinal canal decompression. J Neurosurg Spine. 2005;2:128–136. doi: 10.3171/spi.2005.2.2.0128. [DOI] [PubMed] [Google Scholar]
- 5.Beisse R, Potulski M, Bühren V. Endoscopic techniques for the management of spinal trauma. Eur J Trauma. 2001;27:275–291. [Google Scholar]
- 6.Beisse R, Potulski M, Temme C, Buhren V. Endoscopically controlled division of the diaphragm. A minimally invasive approach to ventral management of thoracolumbar fractures of the spine. Unfallchirurg. 1998;101:619–627. doi: 10.1007/s001130050315. [DOI] [PubMed] [Google Scholar]
- 7.Blauth M, Knop C, Bastian L. Brust- und Lendenwirbelsäule. Berlin: Springer; 1998. [Google Scholar]
- 8.Bradford DS, McBride GG (1987) Surgical management of thoracolumbar spine fractures with incomplete neurologic deficits. Clin Orthop Relat Res pp May:201–216 [PubMed]
- 9.Cusick JF, Myklebust J, Zyvoloski M, Sances A, Jr, Houterman C, Larson SJ. Effects of vertebral column distraction in the monkey. J Neurosurg. 1982;57:651–659. doi: 10.3171/jns.1982.57.5.0651. [DOI] [PubMed] [Google Scholar]
- 10.Dickman CA, Rosenthal DJ, Perin NI (1999) Thoracoscopic spine surgery. Thieme, New York
- 11.Esses SI, Botsford DJ, Kostuik JP. Evaluation of surgical treatment for burst fractures. Spine. 1990;15:667–673. doi: 10.1097/00007632-199007000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Faciszewski T, Winter RB, Lonstein JE, Francis D, Johnson L. The surgical and medical perioperative complications of anterior spinal fusion surgery in the thoracic and lumbar spine in adults. Spine. 1995;20:1592–1599. doi: 10.1097/00007632-199507150-00007. [DOI] [PubMed] [Google Scholar]
- 13.Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH, Vernon JD, Walsh JJ. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7:179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 14.Gaebler C, Maier R, Kutscha-Lissberg F, Mrkonjic L, Vecsei V. Results of spinal cord decompression and thoracolumbar pedicle stabilisation in relation to the time of operation. Spinal Cord. 1999;37:33–39. doi: 10.1038/sj.sc.3100765. [DOI] [PubMed] [Google Scholar]
- 15.Gertzbein SD, Court-Brown CM, Marks P, Martin C, Fazl M, Schwartz M, Jacobs RR. The neurological outcome following surgery for spinal fractures. Spine. 1988;13:641–644. [PubMed] [Google Scholar]
- 16.Hodgson AR, Stock FE. Anterior spinal fusion a preliminary communication on the radical treatment of Pott’s disease and Pott’s paraplegia. Br J Surg. 1956;44:266–275. doi: 10.1002/bjs.18004418508. [DOI] [PubMed] [Google Scholar]
- 17.Kaneda K, Taneichi H, Abumi K, Hashimoto T, Satoh S, Fujiya M. Anterior decompression and stabilization with the Kaneda device for thoracolumbar burst fractures associated with neurological deficits. J Bone Jt Surg Am. 1997;79:69–83. doi: 10.2106/00004623-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Kim DH, Jahng TA, Balabhadra RS, Potulski M, Beisse R. Thoracoscopic transdiaphragmatic approach to thoracolumbar junction fractures. Spine J. 2004;4:317–328. doi: 10.1016/j.spinee.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Knop C, Blauth M, Bühren V, Hax P-M, Kinzl L, Mutschler W, Pommer A, Ulrich C, Wagner S, Weckbach A, Wetzensen A, Wörsdörfer O. Operative treatment of thoracolumbar fractures-part 3: follow-up. Results of prospective multicenter study by the working group “spine” of the German Society of Trauma surgery. Unfallchirurg. 2001;104:583–600. doi: 10.1007/s001130170089. [DOI] [PubMed] [Google Scholar]
- 20.Knop C, Lange U, Bastian L, Oeser M, Blauth M. Biomechanical compression tests with a new implant for thoracolumbar vertebral body replacement. Eur Spine J. 2001;10:30–37. doi: 10.1007/s005860000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korovessis P, Piperos G, Sidiropoulos P, Karagiannis A, Dimas T. Spinal canal restoration by posterior distraction or anterior decompression in thoracolumbar spinal fractures and its influence on neurological outcome. Eur Spine J. 1994;3:318–324. doi: 10.1007/BF02200144. [DOI] [PubMed] [Google Scholar]
- 22.Louis R. Les theories de l’instabilite’. Rev Chir Orthop. 1977;63:423–425. [PubMed] [Google Scholar]
- 23.Mack MJ, Regan J, Bobechko WP, Acuff TE. Applications of thoracoscopy for diseases of spine. Ann Thorac Surg. 1993;56:736–738. doi: 10.1016/0003-4975(93)90966-L. [DOI] [PubMed] [Google Scholar]
- 24.Magerl F, Aebi S, Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184–201. doi: 10.1007/BF02221591. [DOI] [PubMed] [Google Scholar]
- 25.Pedowitz RA, Garfin SR, Massie JB, Hargens AR, Swenson MR, Myers RR, Rydevik BL. Effects of magnitude and duration of compression on spinal nerve root conduction. Spine. 1992;17:194–199. doi: 10.1097/00007632-199202000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Perrouin-Verbe B, Lenne-Aurier K, Robert R, Auffray-Calvier E, Richard I, Mauduyt de la Greve I, Mathe JF. Post-traumatic syringomyelia and post-traumatic spinal canal stenosis: a direct relationship: review of 75 patients with a spinal cord injury. Spinal Cord. 1998;36:137–143. doi: 10.1038/sj.sc.3100625. [DOI] [PubMed] [Google Scholar]
- 27.Regan JJ, Mack MJ, Oicetti GD. A technical report on video-assisted thoracoscopy in thoracic spinal surgery. Preliminary description. Spine. 1995;20:831–837. doi: 10.1097/00007632-199504000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Rosenthal D, Rosenthal R, Simone A. Removal of a protruded disc using microsurgery endoscopy. Spine. 1994;19:1087–1091. doi: 10.1097/00007632-199405000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Schultheiss M, Hartwig E, Kinzl L, Claes L, Wilke HJ. Thoracolumbar fracture stabilization: comparative biomechanical evaluation of a new video-assisted implantable system. Eur Spine J. 2004;13:93–100. doi: 10.1007/s00586-003-0640-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultheiss M, Hartwig E, Sarkar M, Kinzl L, Claes L, Wilke HJ. Biomechanical in vitro comparison of different mono- and bisegmental anterior procedures with regard to the strategy for fracture stabilisation using minimally invasive techniques. Eur Spine J. 2005;15:82–89. doi: 10.1007/s00586-004-0837-7. [DOI] [PMC free article] [PubMed] [Google Scholar]