Abstract
Traumatic injuries of the spine and spinal cord are common and potentially devastating lesions. We present a comprehensive overview of the classification of vertebral fractures, based on morphology (e.g., wedge, (bi)concave, or crush fractures) or on the mechanism of injury (flexion-compression, axial compression, flexion-distraction, or rotational fracture-dislocation lesions). The merits and limitations of different imaging techniques are discussed, including plain X-ray films, multi-detector computed tomography (MDCT), and magnetic resonance imaging (MRI) for the detection. There is growing evidence that state-of-the-art imaging techniques provide answers to some of the key questions in the management of patients with spine and spinal cord trauma: is the fracture stable or unstable? Is the fracture recent or old? Is the fracture benign or malignant? In summary, we show that high-quality radiological investigations are essential in the diagnosis and management of patients with spinal trauma.
Keywords: Spine, trauma; Spine, fractures; Spine, injuries; Spine, MR; Spine, CT
Introduction
Trauma to the spine and spinal cord is a potentially devastating injury [1]. It can be accompanied by significant neurological damage, including paraplegia, quadriplegia, or even death. Patients who present with complete spinal cord injuries, without discernable motor or sensory preservation on neurological examination, have a very poor prognosis. On the other hand, patients who present with an incomplete injury may regain a large amount of useful function, or be spared the progression to complete injury with rapid diagnosis and treatment of fracture fragments, hematomas, or other lesions which compress the spinal cord. Imaging studies are essential to confirm the exact location of the injury, to assess the stability of the spine, and to define the repercussion of the trauma on the diameters of the spinal canal and neural foramina, as well as on the spinal cord and nerve roots.
Vertebral fractures predominantly affect young men (traumatic injuries) and elderly women (osteoporotic fractures) [2]. At the time of injury, the average age of patients with traumatic spine lesions is 32 years and 55% of those injured are aged 16–30 years. Approximately, half of spinal injuries occur in the cervical spine, the other half involves the thoracic, lumbar, and sacral areas. Motor vehicle accidents (MVA) are the principal cause of spine trauma and account for approximately 40% of reported cases. Other injuries are typically the result of a fall or sporting activities [3]. In the United States, violence (gunshot, stabbing, etc.,) accounts for up to 25% of cases. The incidence of spinal injuries due to violence is increasing, whereas the incidence of injuries due to MVA is declining.
Classification of vertebral fractures
Vertebral morphology
Several classification systems for spine trauma are in use. Most classifications are based on the mechanism of injury or anatomical changes, but their clinical usefulness is limited by the lack of quantifiable management parameters. Ideally, vertebral fractures should be graded on the basis of clinically relevant and measurable parameters such as: neurological function impairment (modified Frankel grading method [4]), spinal canal deformity, and biomechanical stability [5].
When a patient with spine trauma is referred for imaging, the exact mechanism of trauma is unknown in many cases. Therefore, most radiologists use a pragmatic approach to the classification and description of vertebral fractures which is based on vertebral morphology [6]. This classification system takes into account the loss of height of the vertebral body and the location of the fracture. Osteoporotic fractures can be classified into three major types, depending on the location of the fracture lines [6]:
wedge fracture, usually involving the anterior (or less commonly the posterior) edge of the vertebral body;
concave or biconcave fracture, involving the central part of the vertebra (Fig. 1);
crush fracture, involving a combination of anterior, posterior and central elements.
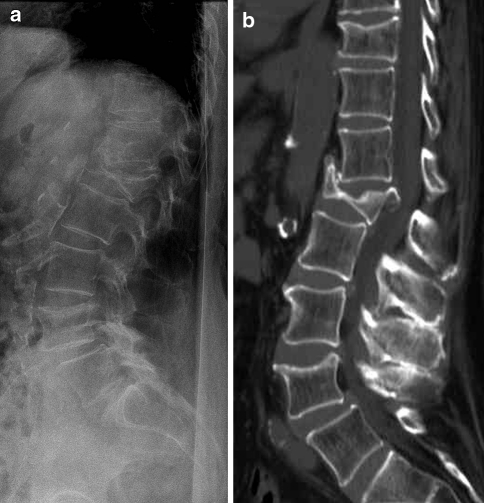
Fig. 1.
Biconcave fracture of L1 in an 86-year-old woman with severe osteoporosis. Plain X-rays (a) show the biconcave fracture, with vertebral body height <50% of normal. The spinal canal is difficult to assess. A non-contrast CT scan with sagittal reformatted images (b) clearly shows retropulsion of a bone fragment into the spinal canal with compression of the conus medullaris. The bone fragment is, even in retrospect, difficult to see on the plain films
Within each group, the deformity can be graded semiquantitatively according to the loss of vertebral body height [7]:
Grade I: vertebral body height is >75% of normal value
Grade II: vertebral body height is between 50 and 75% of normal value
Grade III: vertebral body height is <50% of normal value
Even more important is to assess the deformity of the spinal canal and neural foramina. In spine fractures, the spinal canal is often narrowed from translation and intrusion of vertebral body fragments. On sagittal views, which can be obtained through multiplanar reformation of volumetric CT data sets or from sagittal MR images, the anterior–posterior canal diameter can be measured. Some authors have suggested measuring the cross-sectional area compromise, especially in thoracic and lumbar spine injuries [5].
Mechanism of injury
With reference to Denis’ three-column theory of spinal stability [8], fractures of the spine can be classified based on the pattern of injury and the forces involved [9]. The mechanism of injury reflects the mechanical mode of failure of the vertebral bodies.
Flexion-compression mechanism (wedge or compression fracture)
The combination of flexion and compression forces typically causes an anterior wedge compression fracture. The anterior column is compressed, with variable involvement of the middle and posterior column. Three subtypes can be defined [10, 11]. In the first pattern, only the anterior column is implicated (stable fracture). This results in anterior wedging of the vertebral body. The loss of anterior vertebral body height is usually <50%. In the second pattern, there is an anterior column involvement and posterior column ligamentous failure (potentially unstable fracture). Imaging studies reveal anterior wedging and increased interspinous distance. The loss of vertebral body height is usually >50%. In the third pattern, there is failure of all three columns (unstable fracture). Imaging studies demonstrate anterior wedging and posterior vertebral body disruption. Dislodged bone fragments in the spinal canal may cause compression of the spinal cord or nerve roots.
Axial-compression mechanism (burst fracture)
A burst fracture (also known as crush fracture) is caused by axial compression forces. This injury is associated with high energy trauma (e.g., fall from a great height, MVA, and sports-related trauma). Burst fractures are most commonly found at the thoracolumbar junction and between levels T5 and T8 [12].
A burst fracture is characterized by a loss of height of the vertebral body. The fracture implicates the anterior and middle columns; the state of the posterior column determines whether the fracture is stable or unstable [13]. Posterior element displacement and/or vertebral body or facet dislocation or subluxation is found in unstable fractures. Displacement of bone fragments into the spinal canal may cause compression of the spinal cord or nerve roots, as well as vascular injury.
Flexion-distraction mechanism (Chance fractures)
The combination of flexion and distraction forces can cause a Chance (or seatbelt) fracture. This is a type of thoracolumbar injury in which the posterior column is involved with injury to ligamentous components, bony components, or both. Chance fractures are often associated with intra abdominal injuries [14]. The pathophysiology depends on the axis of flexion. Several subtypes exist. In the most common type of Chance fracture, the axis of flexion is anterior to the anterior longitudinal ligament (ALL). This results in a horizontal fracture of the bony elements along with disruption of the supraspinous ligament. Imaging studies display an increase in the interspinous distance and may show horizontal fracture lines through the pedicles, transverse processes, and pars interarticularis. On axial CT scans, the pedicular fracture lines are seen as a gradual loss of definition of the pedicles; this appearance has been called the “dissolving pedicle sign” [14]. With more severe flexion-distraction forces, the axis of flexion lies behind the ALL. These Chance fractures can be accompanied by a burst-type vertebral fracture with posterior cortex buckling or retropulsion. This is an unstable injury. Moreover, if the pars interarticularis is disrupted, the instability of the injury is increased, and this can lead to significant subluxation. Neurological sequels, when present, are related to the degree of compression of the neural elements.
Rotational fracture-dislocation mechanism
The precise mechanism of this fracture is a combination of lateral flexion and rotation with or without a component of posterior-anteriorly directed force. The resultant injury pattern is failure of both the posterior and middle columns with varying degrees of anterior column insult. The rotational force is responsible for disruption of the posterior ligaments and facet joint. With sufficient rotational force, the upper vertebral body rotates and carries the superior portion of the lower vertebral body along with it. This causes the radiographic “slice” appearance sometimes seen with these types of injuries.
Imaging studies
Technique
The main objectives of the radiological examination in the clinical setting of spinal trauma are to depict the spinal axis rapidly and accurately, and to guide potential surgical decompression. Several imaging modalities can be used, but nowadays multi-detector computer tomography (MDCT) and magnetic resonance (MR) imaging are the most important imaging modalities [15].
Plain X-ray films
Plain X-ray films are a ‘quick & dirty’ way to assess the spine, and are readily available in most hospitals and trauma centers. Plain radiographs may be helpful in fracture screening, and are mainly used to detect a spinal deformity. Indications for obtaining “surveillance” radiographs of the thoracic and lumbar spine in patients with blunt injuries include: back pain, fall from a height of 10 feet or more, ejection from a motorcycle/motor vehicle crash at 50 mph or more, Glasgow coma scale (GCS) score of ≤8, and neurological deficit [16]. In the United States and Canada, two decision rules have been developed to permit selective ordering of cervical spine radiographs, with the purpose of rapidly ruling out injury to the cervical spine in low-risk trauma patients and to reduce health care expenditures. The “National Emergency X-Radiography Utilization Study (NEXUS) Low-Risk Criteria (NLC)” were first described in 1992 [17], and subsequently validated in a study involving 34,069 patients [18, 19]. More recently, the “Canadian C-Spine Rule (CCR)” was developed for use with alert (GCS = 15) and stable cervical spinal trauma patients by evaluating 8,924 cases [20]. For alert trauma patients who are in stable condition, the CCR was found to be superior to the NLC with respect to sensitivity and specificity for cervical spine injury [21]. All patients with blunt spinal trauma who do not meet the clinical low-risk criteria should be referred for spinal imaging [1].
Plain X-ray films, even with the best possible technique, underestimate the amount of traumatic spine injury, and lesion(s) may be missed (Fig. 2). The difficulty in “clearing” the cervical spine (i.e., excluding a fracture) in trauma patients is well known to most radiologists. Hairline fractures or non-displaced fractures are difficult to detect on conventional radiographs. In patients with osteoporosis, a wrong diagnosis of latent vertebral fracture is often made when it is based on plain X-ray imaging [22]. In the cervical spine, plain X-ray films detect only 60–80% of fractures; a significant number of fractures are not visible, even when three views of the spine are obtained [23]. In a series of 216 consecutive patients with cervical injuries, using a combination of three X-ray views (anteroposterior, cross-table lateral, and open-mouth odontoid), 61% of all fractures were missed, 36% of (sub-) luxations were missed, and 23% of patients were falsely identified having normal spines, of whom half had in fact unstable cervical injuries [24]. Despite swimmers views, repeated attempts at open-mouth odontoid views, and other permutations of imaging, it is very often difficult to depict the entirety of the cervical spine to a satisfactory extent.
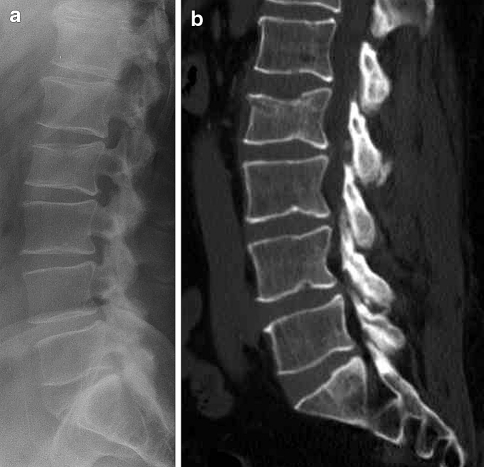
Fig. 2.
A 54-year-old man developed back pain after a mild trauma. Plain X-rays of the lumbar spine (a) show a slight loss of height with anterior wedging of L2. Non-contrast CT scan with sagittal reformatted images (b) reveals a fracture of the upper endplate of L2, as well a subchondral fracture extending to the anterior wall of the vertebral body
Therefore, with these limitations in mind, and given the speed and precision provided by modern MD CT units, it has become the policy of many major trauma centers to use MD CT as the primary imaging modality in high risk patients with blunt cervical spine injury [25].
(Multidetector) computed tomography
Computed tomography (CT), and in particular MDCT, plays a critical role in the rapid assessment of the (poly-)traumatized patient [26]. Early on, many trauma centers adopted the technique of thin-section CT with reformation in sagittal or coronal planes to evaluate the spine. The widespread availability of spiral CT and subsequently MDCT, refined the technique and allowed the rapid acquisition of data sets which provided confidence in diagnosis and increased utilization.
CT screening has a higher sensitivity and specificity for evaluating cervical spine injury compared with plain film radiographs [25, 27, 28]. In the cervical spine, CT detects 97–100% of fractures, but its accuracy in detection of purely ligamentous injuries has not been documented [23]. A recent study assessed that CT was the most efficient imaging tool with a sensitivity of 100%, whereas a single cross-table lateral view had a sensitivity of only 63% in detecting skeletal injuries of the cervical spine [29].
An additional advantage is that CT allows more rapid radiological clearance of the cervical spine than radiography [27, 28]. For these reasons, many major trauma centers nowadays have replaced plain film radiographs with spiral CT or MDCT as the standard of care in the initial evaluation of the cervical spine in moderate to severe trauma patients [27]. Although CT, and especially MDCT, is more costly than plain radiographs, it has been shown that it can actually decrease institutional costs (when settlement costs are taken into account) due to the reduction of the incidence of paralysis resulting from false-negative imaging studies [30].
The most important limitation of this technique is the inability to provide screening for ligamentous injury and spinal cord lesions. Furthermore, the interpretation of (MD) CT data is more complicated in patients with severe degenerative disease. CT provides overall superior depiction of the bony anatomy of the spinal canal in the trauma patient. It can also depict significant soft tissue abnormalities, such as traumatic disk herniations, significant epidural hemorrhage, and other injuries (though not of the spinal cord; Fig. 3). It is clear that MR imaging is superior in this regard, but the review of spine CT in a trauma patient should include careful review of the soft tissue windows.
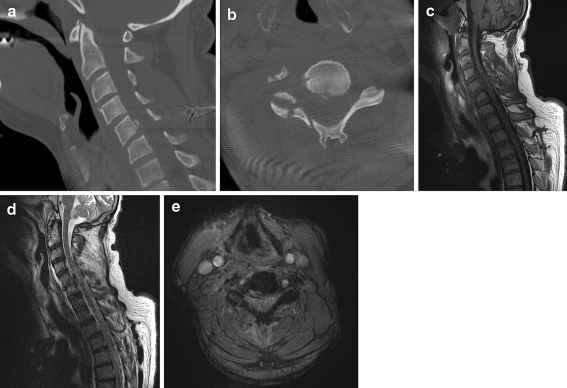
Fig. 3.
A 55-year-old man suffered a severe neck trauma in a motor vehicle accident. A non-contrast CT scan with sagittal (a) and axial (b) reformatted images shows a posterior–inferior avulsion fracture of the C5 vertebral body, fracture-luxation of the right C4-C5 facetjoint and there is also a fracture of the right lamina. The subsequent MRI-examination, with sagittal T1-weighted (c), T2-weighted (d), and axial T2*-weighted images (e) reveals a traumatic disk herniation with compression of the spinal cord and intramedullary areas of hyperintensity, indicating spinal cord edema
Traditionally, CT of the thoracic and lumbar spine is commonly performed to evaluate suspicious levels on plain film studies, or to evaluate the patient with a known level of injury. Recent literature data indicate that MDCT diagnoses thoracolumbar spine fractures more accurately than plain X-ray films [31, 32]. CT screening shortens the time to removal of spine precautions. Moreover, a CT scan-based diagnosis does not appear to result in greater radiation exposure and improves resource use. As with the cervical spine, reformatted sagittal and coronal images are also helpful to demonstrate abnormalities in alignment, and to clarify the nature of fractures which are seen on the axial images.
MR imaging
Thanks to its increased availability for the emergency room physician, MR imaging is starting to play an increasingly important role in the assessment of spine trauma patients [15]. Thanks to its inherently superior contrast resolution, MR imaging is the preferred technique for the detection of soft tissue injuries [33]. It is mainly used to exclude occult injuries and to identify spinal cord lesions [34]. MR imaging is the modality of choice for assessing traumatic lesions involving the intervertebral disks and spinal ligaments [3]. It has been recommended that cervical spine trauma patients with negative standard radiographs and suspected occult cervical injury should be investigated by MR imaging to detect ligamentous injuries that were not seen on plain X-ray studies [35]. The typical exam protocol for detecting spinal ligamentous injury includes sagittal T1, sagittal gradient recalled T2*, and sagittal STIR images, as well as axial imaging (Fig. 4). Edema in the interspinous or supraspinous ligaments is particularly conspicuous on STIR images. Some observers prefer fat-suppressed T2 images, which provide similar conspicuity of the changes seen in ligamentous injury. In a consecutive retrospective series of 89 patients, ligamentous injury was found in seven patients, of whom two underwent surgery because of the findings on the MR imaging study [35]. A more recent study showed that, in cervical spine trauma, MR imaging was highly sensitive for injury to the intervertebral disk (93%), posterior longitudinal ligament (93%), and interspinous soft tissues (100%), but was less sensitive in assessing injury to the anterior longitudinal (71%) and flavum (67%) ligaments [36].
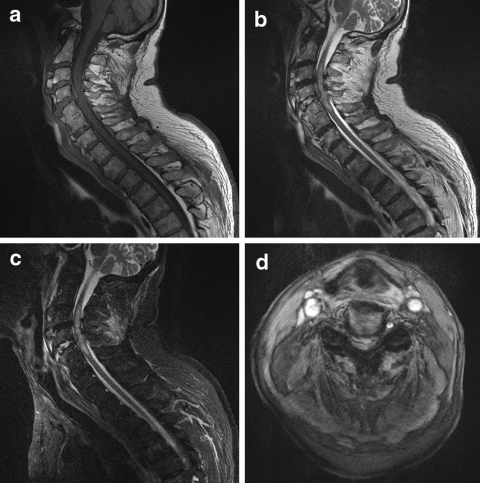
Fig. 4.
A 82-year-old man became tetraplegic after falling down some stairs. He was known to suffer from diffuse idiopathic skeletal hyperostosis (DISH). An MRI-examination of the cervical spine was performed with sagittal T1-weighted images (a), sagittal T2- weighted images (b), sagittal STIR images (c), and axial T2*- weighted images (d). There is a traumatic dislocation of C4-C5, with a massive disk extrusion, narrowing of the spinal canal and extrinsic cord compression. There is extensive intramedullary edema, indicating cord contusion. The STIR images, with fat suppression, show bone marrow edema
Any patient with presumed spinal cord injury should undergo an MR imaging examination as soon as possible. In patients with spinal cord injury, MR imaging is able to reveal the location and severity of the lesion and, at the same time, to indicate the cause of spinal cord compression [37]. This is especially useful in the management of patients with incomplete spinal cord injury, for whom surgical intervention may prevent further deterioration [37]. Several types of traumatic spinal cord lesions can be found: intramedullary hemorrhage, spinal cord contusion/edema, extrinsic compression by a bone fragment or a traumatic disk herniation, and even complete transsection of the cord [38]. MR imaging helps in predicting neurological recovery [39]. Neurological recovery is usually insignificant in patients with intramedullary hemorrhage or cord transsection, whereas patients with cord edema or contusion may significantly recover from neurological dysfunction [39]. One should bear in mind that plain radiographs, and even MDCT, do not rule out injury to the spinal cord [40]. The concept of spinal cord injury without radiological abnormality is known by the acronym SCIWORA. New imaging techniques such as diffusion-weighted MR imaging (DWI), may provide important information complimentary to conventional MRI to allow a better prognostic evaluation of recovery from SCIWORA [41].
Finally, MR imaging is not only useful in the soft tissue injuries associated with spine trauma, but also demonstrates changes within the bone marrow of traumatized vertebrae which are unapparent on plain film studies, such as bone contusions [42]. For the detection of bone marrow edema, sagittal T2-weighted sequences with spectral fat saturation or STIR images with fat suppression, are most useful. It is not uncommon to find multiple levels of involvement, and some trauma centers mandate evaluation of the other spinal segments to exclude additional injury.
Questions to be answered
Stable versus unstable fracture?
Successful management of traumatic spine injuries requires understanding of the concepts of spinal stability and instability [9]. Determination of spinal stability is important because treatment strategies rely heavily on this assessment [43]. There are several classification systems which correlate the pattern of injury with the fracture type and the probable forces involved. White and Panjabi proposed to use a checklist point system to assess spinal stability for each of the cervical, thoracic, and lumbar spine segments [44]. Another well-known and widely used classification is the three-column model of the spine, which was introduced by Denis in 1983 [8]. This system describes both the functional units that contribute to the stability of the spine and the destabilizing effect of injuries to the various columns.
The anterior column is defined as containing the anterior longitudinal ligament, the anterior half of the vertebral body, and the related portion of the intervertebral disk and its annulus fibrous.
The middle column contains the posterior longitudinal ligament, the posterior half of the vertebral body, and the intervertebral disk and its annulus.
The posterior column contains the bony elements of the posterior neural arch and the ligamental elements, which include the flavum, interspinous, and supraspinous ligaments. The joint capsule of the intervertebral articulations is also part of the posterior column.
Disruption of two or more columns results in an unstable arrangement. Examples of stable lesions include: avulsion fracture, spinous process fracture, osteophyte fracture, transverse process fracture, and injury to trabecular bone. In the evaluation of unstable injuries such as, for example, crush fractures, MDCT is useful for assessing the bone fragments, whereas MR imaging is superior for demonstrating spinal cord injury and paraspinal hematoma (Fig. 5).
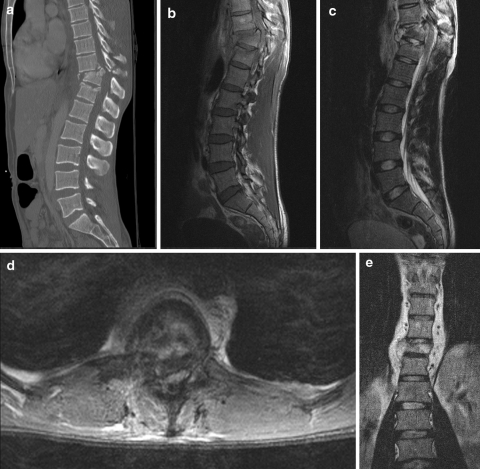
Fig. 5.
A 28-year-old man was injured in a motor vehicle accident. CT scan of the thoracolumbar spine was performed with sagittal mutiplanar reformations (a). There is an unstable burst fracture of Th11 with retropulsion of bone fragments into the spinal canal, and kyphotic angulation. In order to assess the spinal cord, MRI of the thoracolumbar spine was performed with sagittal T1-weighted images (b), sagittal T2-weighted images (c), axial T2-weighted images (d). The spinal canal is narrowed with extrinsic compression of the spinal cord and intramedullary focal areas of hyperintensity, indicating spinal cord oedema. The coronal T1-weighted image (e) shows the paravertebral hematoma
Recent versus old fracture?
This question is difficult to answer, particularly on conventional X-ray studies [6]. Sometimes it is impossible to answer with certainty on the basis of a single imaging study. On imaging studies, recent fractures tend to be associated with one or more of the following characteristics: impaction of bone trabeculae (plain X-ray), bone marrow edema (MR), pre-and paravertebral hemorrhage (MR or CT), epidural hemorrhage (MR or CT), and spinal cord edema (MR). Bone marrow edema on fat-saturated or fat-suppressed MR images is a good indicator of a recent fracture; it decreases gradually over time, but its disappearance is unrelated to relief of pain [42].
Benign versus malignant fracture?
Benign, atraumatic vertebral compression fractures of the thoracic or lumbar spine are commonly found on spine imaging studies, especially in elderly patients. Osteoporosis is the most common cause of benign vertebral compression fractures. The prevalence of osteoporotic fractures increases steadily with age and women are at greatest risk. Malignant (pathologic) vertebral fractures are most commonly the result of metastatic disease of primary cancers affecting the lung, prostate, and breast. Occasionally, a malignancy affects the spine itself or is the result of meningeal neoplasia.
Unfortunately, it is not possible to reliably distinguish between benign versus malignant vertebral fractures using conventional X-rays or CT. MR imaging is more useful because it shows the anatomic distribution and intensity of signal changes of bone and adjacent tissues, contrast enhancement characteristics, and changes over time [45]. MR imaging findings indicative of metastatic compression fractures include: convex posterior border of the vertebral body (expansion of the vertebral body due to the underlying tumor), abnormal signal intensity involving pedicles or posterior elements, epidural mass, encasing epidural mass, focal paraspinal mass, and other spinal metastases [46]. MR imaging findings evocative of acute osteoporotic compression fractures are: low-signal-intensity band on T1- and T2-weighted images, spared normal bone marrow signal intensity of the vertebral body, retropulsion of a posterior bone fragment, and multiple compression fractures [Jung et al. 46]. However, on conventional MR imaging sequences, benign and malignant fractures of the spine may present similar signal intensity characteristics; for example, acute healing compression fractures may mimic the findings of metastatic lesions [45].
With advanced MR imaging techniques, it may become possible to distinguish between benign versus malignant fractures. Several investigators have suggested that diffusion-weighted MR imaging is useful to distinguish between pathologic fractures from benign vertebral compression fractures (with bone marrow edema) [47–49]. Pathologic fractures with metastatic tumor infiltration tend to be associated with restricted diffusion (high signal intensity on diffusion-weighted scans and low signal on apparent diffusion coefficient maps), as opposed to vertebral bone marrow edema from benign fracture. However, other authors have argued that diffusion-weighted MR imaging of the spine compared to non-contrast T1-weighted imaging showed no advantage in the detection and characterization of vertebral metastases, but diffusion-weighted MR imaging was considered superior to T2-weighted imaging [50]. On in-phase/opposed-phase chemical shift imaging, a significant difference in signal intensity was found between benign compression fractures and malignancy [51, 52]. MR spectroscopy has been applied in the study of osteoporotic fractures, and demonstrated an increase in saturated fats [53]. This finding presumably reflects the increase in the intertrabecular spaces (which are filled with fat), associated with the trabecular thinning in osteoporosis.
Conclusion
Radiological investigation is of paramount importance in the diagnosis and management of patients with spinal trauma. The main objectives of imaging patients with spinal trauma are: rapid and accurate depiction of the spinal axis, identification of (potentially) unstable injuries, and indication of signs for surgical decompression. For the investigation of low-risk patients (who are alert and in stable condition) with cervical spine trauma, clinical guidelines (NLC and CCR) have been developed to determine which individuals do not require spinal imaging. Plain X-rays of the spine play a limited role in the detection of vertebral fractures. In spine trauma patients with moderate or high risk, CT, and especially MDCT, is the modality of choice for assessing the degree of vertebral collapse and for measuring the diameter of the bony spinal canal. MDCT is superior to all other imaging modalities in the detection of vertebral fractures and unstable injuries. However, CT is of limited value for assessing the spinal cord. Therefore, MR imaging should be used whenever a spinal cord lesion or an occult injury is suspected (Fig. 6). MR imaging is the method of choice for assessing spinal cord lesions, ligamentous injury, and vertebral bone marrow edema. Advanced MR techniques, including diffusion-weighted imaging, in-phase/opposed-phase chemical shift imaging, and MR spectroscopy hold promise in distinguishing benign versus malignant fractures.
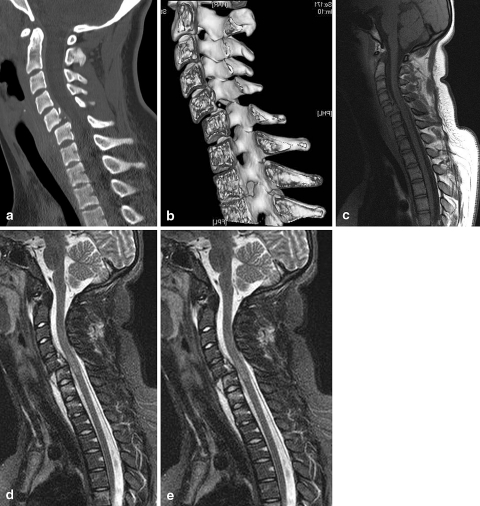
Fig. 6.
A 14-year-old girl was bicycling to school when she was hit by a car; according to witnesses of the accident, she was catapulted into the air and fell on her head. Upon admission, the patient could only flex her right arm; there was no movement in the other limbs. A CT scan of the cervical spine was ordered. Sagittal multiplanar reformatted image (a) and volume rendering image with cut-away (b) reveal a traumatic fracture-luxation at C5-C6, with anterolisthesis of C5. An MR examination of the cervical spine was performed with sagittal T1-weighted images (a), sagittal T2- weighted images (b), and sagittal STIR images (c). This examination confirms the deformity of the spinal canal, and also intramedullary edema (best seen T2-weighted images) and bone marrow edema (best seen on the fat-suppressed STIR images)
Conflict of interest statement
None of the authors has any potential conflict of interest.
References
- 1.Goethem JW, Maes M, Ozsarlak O, Hauwe L, Parizel PM. Imaging in spinal trauma. Eur Radiol. 2005;15:582–590. doi: 10.1007/s00330-004-2625-5. [DOI] [PubMed] [Google Scholar]
- 2.Hu R, Mustard CA, Burns C. Epidemiology of incident spinal fracture in a complete population. Spine. 1996;21:492–499. doi: 10.1097/00007632-199602150-00016. [DOI] [PubMed] [Google Scholar]
- 3.Parizel PM, Gielen JL, Vanhoenacker FM (2007) The spine in sports injuries: the cervical spine. In: Vanhoenacker FM, Maes M, Gielen JL (eds) Imaging of orthopedic sports injuries. Springer, Berlin, pp 377–390
- 4.Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH, Vernon JD, Walsh JJ. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I Paraplegia. 1969;7:179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 5.Tsou PM, Wang J, Khoo L, Shamie AN, Holly L. A thoracic and lumbar spine injury severity classification based on neurologic function grade, spinal canal deformity, and spinal biomechanical stability. Spine J. 2006;6:636–647. doi: 10.1016/j.spinee.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Lenchik L, Rogers LF, Delmas PD, Genant HK. Diagnosis of vertebral osteoporotic fractures: importance of recognition and description by radiologists. AJR Am J Roentgenol. 2004;183:949–958. doi: 10.2214/ajr.183.4.1830949. [DOI] [PubMed] [Google Scholar]
- 7.Genant HK, Wu CY, Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 8.Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8:817–831. doi: 10.1097/00007632-198311000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Vollmer DG, Gegg C. Classification and acute management of thoracolumbar fractures. Neurosurg Clin N Am. 1997;8:499–507. [PubMed] [Google Scholar]
- 10.Ferguson RL, Allen BL., Jr A mechanistic classification of thoracolumbar spine fractures. Clin Orthop Relat Res. 1984;189:77–88. [PubMed] [Google Scholar]
- 11.Singh K, Vaccaro AR, Eichenbaum MD, Fitzhenry LN. The surgical management of thoracolumbar injuries. J Spinal Cord Med. 2004;27:95–101. doi: 10.1080/10790268.2004.11753737. [DOI] [PubMed] [Google Scholar]
- 12.Bensch FV, Koivikko MP, Kiuru MJ, Koskinen SK. The incidence and distribution of burst fractures. Emerg Radiol. 2006;12:124–129. doi: 10.1007/s0010140-005-0457-5. [DOI] [PubMed] [Google Scholar]
- 13.McAfee PC, Yuan HA, Fredrickson BE, Lubicky JP. The value of computed tomography in thoracolumbar fractures. An analysis of one hundred consecutive cases and a new classification. J Bone Joint Surg Am. 1983;65:461–473. [PubMed] [Google Scholar]
- 14.Bernstein MP, Mirvis SE, Shanmuganathan K. Chance-type fractures of the thoracolumbar spine: imaging analysis in 53 patients. AJR Am J Roentgenol. 2006;187:859–868. doi: 10.2214/AJR.05.0145. [DOI] [PubMed] [Google Scholar]
- 15.Bagley LJ. Imaging of spinal trauma. Radiol Clin North Am. 2006;44:1–12. doi: 10.1016/j.rcl.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Frankel HL, Rozycki GS, Ochsner MG, Harviel JD, Champion HR. Indications for obtaining surveillance thoracic and lumbar spine radiographs. J Trauma. 1994;37:673–676. doi: 10.1097/00005373-199410000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman JR, Schriger DL, Mower W, Luo JS, Zucker M. Low-risk criteria for cervical-spine radiography in blunt trauma: a prospective study. Ann Emerg Med. 1992;21:1454–1460. doi: 10.1016/S0196-0644(05)80059-9. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman JR, Mower WR, Wolfson AB, Todd KH, Zucker MI. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. National Emergency X-Radiography Utilization Study Group. N Engl J Med. 2000;343:94–99. doi: 10.1056/NEJM200007133430203. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman JR, Wolfson AB, Todd K, Mower WR. Selective cervical spine radiography in blunt trauma: methodology of the National Emergency X-Radiography Utilization Study (NEXUS) Ann Emerg Med. 1998;32:461–469. doi: 10.1016/S0196-0644(98)70176-3. [DOI] [PubMed] [Google Scholar]
- 20.Stiell IG, Wells GA, Vandemheen KL, Clement CM, Lesiuk H, Maio VJ, Laupacis A, Schull M, McKnight RD, Verbeek R, Brison R, Cass D, Dreyer J, Eisenhauer MA, Greenberg GH, MacPhail I, Morrison L, Reardon M, Worthington J. The Canadian C-spine rule for radiography in alert and stable trauma patients. JAMA. 2001;17(286):1841–1848. doi: 10.1001/jama.286.15.1841. [DOI] [PubMed] [Google Scholar]
- 21.Stiell IG, Clement CM, McKnight RD, Brison R, Schull MJ, Rowe BH, Worthington JR, Eisenhauer MA, Cass D, Greenberg G, MacPhail I, Dreyer J, Lee JS, Bandiera G, Reardon M, Holroyd B, Lesiuk H, Wells GA. The Canadian C-spine rule versus the NEXUS low-risk criteria in patients with trauma. N Engl J Med. 2003;349:2510–2518. doi: 10.1056/NEJMoa031375. [DOI] [PubMed] [Google Scholar]
- 22.Ito Z, Harada A, Matsui Y, Takemura M, Wakao N, Suzuki T, Nihashi T, Kawatsu S, Shimokata H, Ishiguro N. Can you diagnose for vertebral fracture correctly by plain X-ray? Osteoporos Int. 2006;17:1584–1591. doi: 10.1007/s00198-006-0123-0. [DOI] [PubMed] [Google Scholar]
- 23.Crim JR, Moore K, Brodke D. Clearance of the cervical spine in multitrauma patients: the role of advanced imaging. Semin Ultrasound CT MR. 2001;22:283–305. doi: 10.1016/S0887-2171(01)90023-X. [DOI] [PubMed] [Google Scholar]
- 24.Woodring JH, Lee C. Limitations of cervical radiography in the evaluation of acute cervical trauma. J Trauma. 1993;34:32–39. doi: 10.1097/00005373-199301000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Griffen MM, Frykberg ER, Kerwin AJ, Schinco MA, Tepas JJ, Rowe K, Abboud J. Radiographic clearance of blunt cervical spine injury: plain radiograph or computed tomography scan? J Trauma. 2003;55:222–226. doi: 10.1097/01.TA.0000083332.93868.E2. [DOI] [PubMed] [Google Scholar]
- 26.Linsenmaier U, Krotz M, Hauser H, Rock C, Rieger J, Bohndorf K, Pfeifer KJ, Reiser M. Whole-body computed tomography in polytrauma: techniques and management. Eur Radiol. 2002;12:1728–1740. doi: 10.1007/s00330-001-1225-x. [DOI] [PubMed] [Google Scholar]
- 27.Antevil JL, Sise MJ, Sack DI, Kidder B, Hopper A, Brown CV. Spiral computed tomography for the initial evaluation of spine trauma: a new standard of care? J Trauma. 2006;61:382–387. doi: 10.1097/01.ta.0000226154.38852.e6. [DOI] [PubMed] [Google Scholar]
- 28.Blackmore CC, Mann FA, Wilson AJ. Helical CT in the primary trauma evaluation of the cervical spine: an evidence-based approach. Skeletal Radiol. 2000;29:632–639. doi: 10.1007/s002560000270. [DOI] [PubMed] [Google Scholar]
- 29.Platzer P, Jaindl M, Thalhammer G, Dittrich S, Wieland T, Vecsei V, Gaebler C. Clearing the cervical spine in critically injured patients: a comprehensive C-spine protocol to avoid unnecessary delays in diagnosis. Eur Spine J. 2006;15:1801–1810. doi: 10.1007/s00586-006-0084-1. [DOI] [PubMed] [Google Scholar]
- 30.Grogan EL, Morris JA, Jr, Dittus RS, Moore DE, Poulose BK, Diaz JJ, Speroff T. Cervical spine evaluation in urban trauma centers: lowering institutional costs and complications through helical CT scan. J Am Coll Surg. 2005;200:160–165. doi: 10.1016/j.jamcollsurg.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 31.Hauser CJ, Visvikis G, Hinrichs C, Eber CD, Cho K, Lavery RF, Livingston DH. Prospective validation of computed tomographic screening of the thoracolumbar spine in trauma. J Trauma. 2003;55:228–234. doi: 10.1097/01.TA.0000076622.19246.CF. [DOI] [PubMed] [Google Scholar]
- 32.Wintermark M, Mouhsine E, Theumann N, Mordasini P, Melle G, Leyvraz PF, Schnyder P. Thoracolumbar spine fractures in patients who have sustained severe trauma: depiction with multi-detector row CT. Radiology. 2003;227:681–689. doi: 10.1148/radiol.2273020592. [DOI] [PubMed] [Google Scholar]
- 33.Wilmink JT. MR imaging of the spine: trauma and degenerative disease. Eur Radiol. 1999;9:1259–1266. doi: 10.1007/s003300050832. [DOI] [PubMed] [Google Scholar]
- 34.Adams JM, Cockburn MI, Difazio LT, Garcia FA, Siegel BK, Bilaniuk JW. Spinal clearance in the difficult trauma patient: a role for screening MRI of the spine. Am Surg. 2006;72:101–105. [PubMed] [Google Scholar]
- 35.Geck MJ, Yoo S, Wang JC. Assessment of cervical ligamentous injury in trauma patients using MRI. J Spinal Disord. 2001;14:371–377. doi: 10.1097/00002517-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Goradia D, Linnau KF, Cohen WA, Mirza S, Hallam DK, Blackmore CC. Correlation of MR imaging findings with intraoperative findings after cervical spine trauma. AJNR Am J Neuroradiol. 2007;28:209–215. [PMC free article] [PubMed] [Google Scholar]
- 37.Forster BB, Koopmans RA. Magnetic resonance imaging of acute trauma of the cervical spine: spectrum of findings. Can Assoc Radiol J. 1995;46:168–173. [PubMed] [Google Scholar]
- 38.Goethem JW, Ozsarlak O, Parizel PM. Cervical spine fractures and soft tissue injuries. JBR-BTR. 2003;86:230–234. [PubMed] [Google Scholar]
- 39.Kulkarni MV, McArdle CB, Kopanicky D, Miner M, Cotler HB, Lee KF, Harris JH. Acute spinal cord injury: MR imaging at 1.5 T. Radiol. 1987;164:837–843. doi: 10.1148/radiology.164.3.3615885. [DOI] [PubMed] [Google Scholar]
- 40.Kalra V, Gulati S, Kamate M, Garg A. SCIWORA-Spinal Cord Injury Without Radiological Abnormality. Indian J Pediatr. 2006;73:829–831. doi: 10.1007/BF02790395. [DOI] [PubMed] [Google Scholar]
- 41.Shen H, Tang Y, Huang L, Yang R, Wu Y, Wang P, Shi Y, He X, Liu H, Ye J. Applications of diffusion-weighted MRI in thoracic spinal cord injury without radiographic abnormality. Int Orthop. 2007;31:375–383. doi: 10.1007/s00264-006-0175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voormolen MH, Rooij WJ, Graaf Y, Lohle PN, Lampmann LE, Juttmann JR, Sluzewski M. Bone marrow edema in osteoporotic vertebral compression fractures after percutaneous vertebroplasty and relation with clinical outcome. AJNR Am J Neuroradiol. 2006;27:983–988. [PMC free article] [PubMed] [Google Scholar]
- 43.Kim CW, Perry A, Garfin SR. Spinal instability: the orthopedic approach. Semin Musculoskelet Radiol. 2005;9:77–87. doi: 10.1055/s-2005-867098. [DOI] [PubMed] [Google Scholar]
- 44.White AA, Panjabi MM (1990) Clinical Biomechanics of the Spine, 2nd edn). Lippincott Williams & Wilkins, Philadelphia. ISBN 0-397-50720-8
- 45.An HS, Andreshak TG, Nguyen C, Williams A, Daniels D. Can we distinguish between benign versus malignant compression fractures of the spine by magnetic resonance imaging? Spine. 1995;20:1776–1782. doi: 10.1097/00007632-199508150-00005. [DOI] [PubMed] [Google Scholar]
- 46.Jung HS, Jee WH, McCauley TR, Ha KY, Choi KH. Discrimination of metastatic from acute osteoporotic compression spinal fractures with MR imaging. Radiographics. 2003;23:179–187. doi: 10.1148/rg.231025043. [DOI] [PubMed] [Google Scholar]
- 47.Baur A, Stabler A, Bruning R, Bartl R, Krodel A, Reiser M, Deimling M. Diffusion-weighted MR imaging of bone marrow: differentiation of benign versus pathologic compression fractures. Radiology. 1998;207:349–356. doi: 10.1148/radiology.207.2.9577479. [DOI] [PubMed] [Google Scholar]
- 48.Park SW, Lee JH, Ehara S, Park YB, Sung SO, Choi JA, Joo YE. Single shot fast spin echo diffusion-weighted MR imaging of the spine: is it useful in differentiating malignant metastatic tumor infiltration from benign fracture edema? Clin Imaging. 2004;28:102–108. doi: 10.1016/S0899-7071(03)00247-X. [DOI] [PubMed] [Google Scholar]
- 49.Thurnher MM, Bammer R. Diffusion-weighted magnetic resonance imaging of the spine and spinal cord. Semin Roentgenol. 2006;41:294–311. doi: 10.1053/j.ro.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Castillo M, Arbelaez A, Smith JK, Fisher LL. Diffusion-weighted MR imaging offers no advantage over routine noncontrast MR imaging in the detection of vertebral metastases. AJNR Am J Neuroradiol. 2000;21:948–953. [PMC free article] [PubMed] [Google Scholar]
- 51.Erly WK, Oh ES, Outwater EK. The utility of in-phase/opposed-phase imaging in differentiating malignancy from acute benign compression fractures of the spine. AJNR Am J Neuroradiol. 2006;27(6):1183–1188. [PMC free article] [PubMed] [Google Scholar]
- 52.Zajick DC, Jr, Morrison WB, Schweitzer ME, Parellada JA, Carrino JA. Benign and malignant processes: normal values and differentiation with chemical shift MR imaging in vertebral marrow. Radiology. 2005;237:590–596. doi: 10.1148/radiol.2372040990. [DOI] [PubMed] [Google Scholar]
- 53.Fanucci E, Manenti G, Masala S, Laviani F, Di Costanzo G, Ludovici A, et al. Multiparameter characterisation of vertebral osteoporosis with 3-Tesla MR. Radiol Med (Torino) 2007;112(2):208–223. doi: 10.1007/s11547-007-0136-6. [DOI] [PubMed] [Google Scholar]