Abstract
The immature disc nucleus pulposus (NP) consists of notochordal cells (NCs). With maturation NCs disappear in humans, to be replaced by chondrocyte-like mature NP cells (MNPCs); this change in cell phenotype coincidences with early signs of disc degeneration. The reasons for NC disappearance are important to understand disc degeneration, but remain unknown, yet. This study investigated, whether loading induced a change from a notochordal nucleus phenotype to a chondrocyte-like one. An in vivo disc compression model with fixateur externe was used in 36 mature rabbits. Discs were compressed for different time periods (1, 28, 56 days), and compared with uncompressed control discs (56 days without treatment), and discs with sham compression (28 days). Nucleus cell phenotype was determined by histology and immunohistochemistry. NCs, but not MNPCs highly expressed bone-morphogenetic-protein 2 and cytokeratin 8, thus NC and MNPC numbers could be determined. A histologic score was used to detect structural endplate changes after compression (28 days). Control and sham compressed discs contained around 70% NCs and 30% MNPCs, to be decreased to <10% NCs after 28–56 days of loading. NC density fell sharply by >50% after 28–56 days of compression (P < 0.05 vs. controls). Signs of decreased endplate cellularity and increased endplate sclerosis and fibrosis were found after loading. These experiments show that NCs were less resistant to mechanical stress than MNPCs suggesting that increased intradiscal pressures after loading, and limited nutrition through structurally altered endplates could instigate the disappearance of NCs.
Keywords: Notochordal disc cells, Mechanical stress, Disc compression, Animal model
Introduction
The intervertebral disc (IVD) consists of the central nucleus pulposus (NP), and the surrounding annulus fibrosus (AF). Cells of the disc vary with the anatomical site within the disc. In the outer AF, cells are predominantly fibroblast like and towards the inner annulus the cell phenotype is more chondrocytic [12]. The NP contains at least two different cell types; their distribution depends on the developmental stage of the human disc. In immature human discs, the NP consists of notochordal cells (NCs), which are remnants from the embryonic notochord [5, 9, 34, 39, 44]. NCs are important for the disc development, and it is acknowledged that NCs are the original cell population of the NP. In humans, NCs disappear with disc maturation, to be replaced by chondrocyte-like NP cells; the mature NP cells (MNPCs). Therefore the mature human disc is largely devoid of NCs.
Cell morphology of NCs and MNPCs is different [21]; NCs are larger and contain intracellular vacuole-like structures of yet unknown content [22]. As these vacuoles vary in size and numbers per cell, NCs are heterogeneous [18]. NCs are important to maintain the nucleus hydration and its fluid-like consistency [30], and stimulate surrounding MNPCs to increased extracellular matrix proteoglycan (ECM) production [1, 6]. They also appear to produce higher amounts of ECM themselves [27]. A stimulatory effect of NCs has been determined recently in several in vitro cell culture studies; Oegema et al. [1] hypothesised that NCs secrete a soluble factor, and recently identified the connective tissue growth factor to stimulate surrounding MNPCs [10, 11].
In humans, NCs are believed to disappear around the age of 10, when the disc becomes fibrous, and first signs of disc degeneration occur [29]. NCs persist throughout life in rodents, and non-chondrodystrophoid dogs. These dogs rarely develop spinal disorders; conversely chondrodystrophoid dog species lose NCs during disc maturation, and these species often develop spinal disorders [4]; such observations support the idea that NCs defend the disc from degeneration, in agreement with the findings that first signs of human disc degeneration develop around the time, when the change in nucleus cell phenotype from notochordal to chondrocyte-like occurs [30].
Therefore, the reasons for the disappearance of NCs are important to understand disc degeneration, but are not fully investigated, yet. Some observations support the idea that low nutrient levels could be involved in the disappearance of NCs. The avascular disc is connected by two endplates to the adjacent vertebral bodies, and the nutrition of the disc cells arises predominantly from the endplate vasculature [3]. Thus, a gradient of nutrient availability builds up from endplate to nucleus with lowest levels towards the nucleus centre [15]. In humans, NCs disappear when structural changes in the endplate occur, leading to lower endplate vascular supply [7, 37, 45]. This suggests that a consequent fall in nutrient supply might be involved in the NC disappearance from human discs. Recently, this was supported by in vitro cell culture experiments showing that NCs indeed have higher nutritional demands and less resistance to nutritional stress than MNPCs [18].
Nutritional stress is probably not the only reason for the disappearance of NCs from human discs. As IVDs, and the human IVD in particular, are exposed to mechanical forces during daily activities with high intradiscal pressure values [28, 46], mechanical forces could influence the cellular composition of the NP [2, 38]. Thus, the current study investigated whether mechanical disc loading influenced the distribution of NCs in mature rabbit discs rich of notochordal tissue. This study could help in understanding whether mechanical forces affect the cellular composition of the NP in vivo, and whether mechanical loading could instigate the disappearance of nucleus NCs.
These experiments were performed using an established animal model of disc compression; an external compression device was used to produce a mono-segmental compression in NC-rich discs, and cell densities of NCs and MNPCs were determined in untreated control discs, discs with temporary disc loading, and discs with temporary sham disc loading. NCs were detected by immunohistochemistry that took advantage of the abundant protein expression of cytokeratin 8 [14] and bone-morphogenetic proteins (BMPs) [26, 47] in NCs but not in MNPCs. The results of this study found a decrease in cell densities of NCs, whereas an increase in MNPCs was detected after temporary disc loading, supporting that mechanical stress indeed contributes to the disappearance of NCs.
Methods
Study population
For this in vivo study, 36 female New Zealand white rabbits (skeletally mature, at least 6 months old, weight 3.5–4.2 kg) were approved by the Institutional Review Board of the Animal Experimentation Committee (Regierungspräsidium Karlsruhe, Germany). The animals were randomly assigned to the following groups [31]:
control group: no treatment for 56 days; non-degenerate lumbar discs (n = 6),
1-day compression group (n = 8),
28-day compression group (n = 8),
56-day compression group (n = 8),
28-day sham compression group (n = 6).
As a study limitation, it should be noted that no sham compression of 1 day and 56 days was done.
Surgical procedure
The animals underwent surgery as initially described by Kroeber et al. and adopted by others recently [16, 24, 31]. Briefly, under general anaesthesia, a dorsal approach to the lumbar spine was done in supine animal position. A custom made external loading device (Fig. 1a) was attached to vertical pins and horizontal K-wires, which were inserted into the upper and lower adjacent vertebral body. A spring within the loading device produced a high-disc compression force (disc L3/L4; ~2.4 MPa, or 200 N external loading) during daily activity of the animal (Fig. 1b). In the control group, healthy discs were obtained after 56 days without treatment; the sham compression group underwent surgery with attached, but unloaded compression device, resulting in a temporary segmental immobilization without disc compression [16].
Fig. 1.
Animal model of temporary disc compression. a Model of external disc compression; fixateur externe device. b New Zealand White rabbit with unrestricted activity after surgery with attached and loaded external compression device
Standard histology
After reaching the endpoint, animals were killed, and discs were dissected out for histological evaluation. Discs were fixed in formalin 4%, serially dehydrated in ethanol and embedded in paraffin; 5 μm mid-transverse sections were obtained for appropriate nucleus analysis [16, 31]; In addition, to determine disc height and the structure of the nucleus and endplates, 10-μm vertical sections of the disc segment including adjacent vertebral bodies were obtained (see “Immunohistochemistry for BMP-2 and cytokeratin 8”). Sections were stained with haematoxilin and eosin (H&E); Although in H&E staining, it was possible to differentiate between notochordal and non-notochordal nucleus tissue, it was difficult to identify single cell borders, and thus, impossible to count cells.
Immunohistochemistry for BMP-2 and cytokeratin 8
Additional immunohistochemistric analysis was done for quantitative cell counts. As there is no definitely accepted marker for NCs, a panel of proteins that are highly expressed in human NCs (including several cytokeratin 8, 18, 19, BMP-2, and galektin-3) [26, 47] were extensively pre-tested in rabbit tissue. Finally, immunohistochemistry for BMP-2 (BMP-2 CTS007, R&D Systems) and cytokeratin 8 (Zytomed, Berlin, Germany) was found appropriate to determine the phenotype of rabbit nucleus cells, i.e. whether nucleus cells were rather notochordal or chondrocyte like. The analysis was done stepwise: First H&E staining and BMP-2 immunohistochemistry was used for a semi-quantitative analysis of the total nucleus structure. This analysis evaluated the presence of nucleus areas with abundant BMP-2-positive NCs in tissue:
areas with abundant NCs in nucleus tissue (++NC),
areas with both abundant NCs and areas devoid of NCs in nucleus tissue (+NC),
no areas with abundant NCs in nucleus tissue (−NC).
This analysis was done by two blinded examiners (intraobserver accuracy 100%).
Secondly, the cytokeratin 8 immunohistochemistry (including cell nucleus counter stain) was used to determine cell densities (cells/nucleus square) of cytokeratin 8-positive NCs, cytokeratin 8-negative MNPCs, and total nucleus cell density (NCs + MNPCs). This method was adopted from a recent report in porcine discs [32], and here modified and established for rabbit disc tissue. For quantitative analysis, several representative nucleus areas were chosen, where cells were not aggregated to cell clusters. MNPCs were identified by cell nucleus counter stain in the absence of positive cytokeratin 8 staining. Cells were counted, and the ratio of NCs to MNPCs was determined together with absolute cell numbers. Cytokeratin 8-positive cells were also positive for COL-2 (not shown) and BMP-2. A few cells with weak, diffuse staining were not clearly to identify, and therefore not included in the analysis.
Endplate morphology
Recently, endplate changes after temporary loading have been reported in radiographs [40] and MRIs [31]; such structural endplate alterations after temporary compression could limit nutrient flow to the nucleus cells. Consequently, a low nutrient availability could affect nucleus cell morphology and survival in addition to the increase in hydrostatic pressure during compression. To gain further information about endplate changes, after temporary compression, 10-μm vertical sections of discs with endplate and vertebral bodies were obtained. Sections were stained with H&E, and cartilage endplate morphology was determined in control discs and discs after temporary disc compression (28 days). As a limitation, we were unable to perform additional endplate analysis after 56 days of compression. A semi-quantitative score of endplate degeneration was adopted from a recent report, and included sclerosis, fibrosis and cellularity of endplates (0–2 points; 0 indicating no sclerosis/fibrosis/cellularity, 2 indicating abundant sclerosis/fibrosis/cellularity) [32].
Statistics
Typically, numbers were given as mean with standard error of the means (SEM). A statistical power analysis was adopted from recent studies using this animal model; a group size of n = 6 was found appropriate. Where appropriate statistical tests were done to show differences between the treatment groups; no normal distribution of data was found in the different groups; thus, a non-parametric statistical test was done (Mann–Whitney U test). Excel and SPSS 11.0 were used for statistical analysis.
Results
Animal population
Thirty-four out of 36 animals completed the study without complications. As major surgical complication, one animal developed postoperative incomplete back hint paralysis, and another animal showed auto-aggressive behaviour. Both animals of the 56 days compression group had to be euthanized prior to completion of the study, resulting in n = 6 in this group.
Histology and immunohistochemistry of the disc
Histology
At low magnifications, H&E sections of the discs showed that both control and sham control nucleus consisted of highly vacuolated notochordal tissue, including areas without considerable cellular or tubular structures, and areas with densely clustered cells (Fig. 2a). Following temporary compression of 28 and 56 days, the vacuolated tissue tended to disappear, and the nucleus became more fibrous; similarly the disc height was notably reduced after temporary disc compression (Fig. 2b).
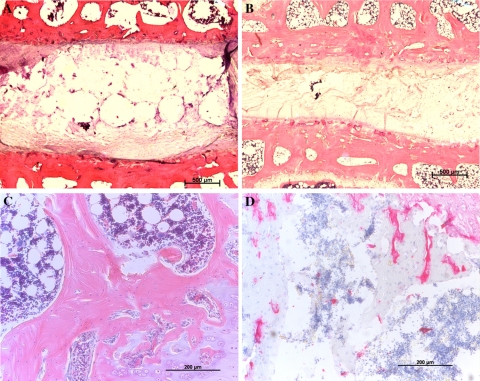
Fig. 2.
Low magnification (a, b) and higher magnification (c, d) vertical sections of intervertebral discs; H&E staining. a An untreated control disc with the typical vacuolated notochordal nucleus (bar 500 μm). b An intervertebral disc after temporary disc compression of 56 days and illustrates differences to control discs, with a reduction in disc height and a more consolidated nucleus pulposus devoid of the typical notochordal nucleus structure (bar 500 μm). c Control endplates and d structurally altered endplates after 28 days of compression
Immunohistochemistry for BMP-2 (Fig. 3e, f)
Fig. 3.
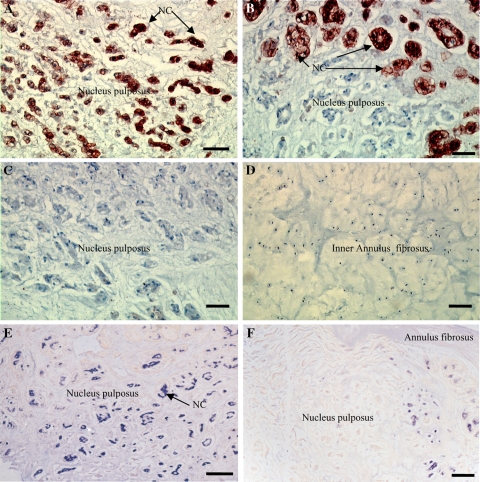
Intervertebral discs with immunohistochemistry for cytokeratin 8 (a–d) and BMP-2 (e, f). a NP area with abundant cytokeratin 8-positive NCs (dark staining) typically found in untreated controls disc (bar 25 μm). b NP area with cytokeratin 8-positive NCs (upper part of the picture) and cytokeratin 8-negative MNPCs (lower part of the picture) typically found after temporary disc compression of 28 days (bar 25 μm). c NP area with no cytokeratin 8-positive NCs and abundant cytokeratin 8-negative MNPCs typically found after disc compression of 56 days (bar 25 μm). d Area of the inner AF with abundant cytokeratin 8-negative AF cells typically found irrespective of the treatment group (bar 25 μm). e NP area with abundant BMP-2-positive NCs (dark staining) typically found in untreated controls disc (bar 25 μm). f NP area with very few BMP-2-positive NCs (dark staining) typically found in disc after 56 days of compression (bar 25 μm)
BMP-2-positive cells were arranged in cell clusters, typical for NCs. The semi-quantitative analysis of the total nucleus structure showed that both control and sham control nucleus consisted largely of areas with abundant NCs (n = 6 each group; 6 ++NC; 0 +NC; 0 −NC). After temporary compression of 1 day, no obvious change in nucleus cell morphology occurred (n = 8; 8 ++NC; 0 +NC; 0 −NC). After temporary compression of 28 days, the nucleus areas with abundant NCs were notably diminished (n = 8; 1 ++NC; 7 +NC; 0 −NC), to be further reduced after temporary disc compression of 56 days; here 50% of animals were devoid of areas with NCs (n = 6; 1 ++NC; 2 +NC; 3 −NC).
Immunohistochemistry for cytokeratin 8 (Figs. 3a–d, 4)
Fig. 4.
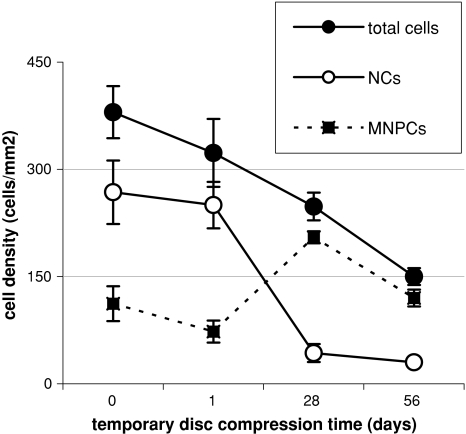
Quantitative analysis of cell densities in the rabbit NP. Cytokeratin 8-positive NCs and cytokeratin 8-negative MNPCs, and total cell numbers (NCs + MNPCs) after different periods of temporary disc compression
In controls, 70 ± 6.8% of cells was cytokeratin 8-positive NCs (Fig. 3a). Almost similar numbers were found in the sham compression group (64 ± 3.7% NCs, P > 0.05 vs. controls) and after disc compression of 1 day (73 ± 1.6% NCs, P > 0.05 vs. controls). However, after disc compression of 28 and 56 days, the percentage ratio of NCs fell considerably (28 days: 13 ± 3.6%, P < 0.01 vs. controls; 56 days: 12 ± 5.4% NCs, P < 0.01 vs. controls; Fig. 3b, c). The cell density analysis is shown in Fig. 4. It should be noted that after temporary disc compression, total nucleus cell numbers considerably decreased; after 56 days the cell density fell to <50% initial numbers, indicating significant cell death after compression. Interestingly, cell numbers of cytokeratin 8-negative MNPCs increased significantly after disc compression of 28 days (P < 0.05 vs. controls), and after 56 days (P > 0.05 vs. controls). However, the cell density of cytokeratin 8-positive NCs fell to <20% of control levels after disc compression of 28 and 56 days (P < 0.01 vs. controls). No cytokeratin 8-positive cells were found in the AF, irrespective of the treatment group (Fig. 3d).
Endplate analysis (Fig. 5)
Fig. 5.
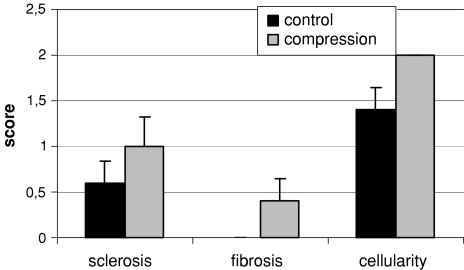
Quantitative results of endplate cellularity, sclerosis and fibrosis
This histological analysis examined n = 5 endplates in the control group, and n = 5 endplates after temporary compression of 28 days. In control endplates, the chondrocyte cell layers were well organised with a high cellularity; only minor endplate sclerosis and no endplate fibrosis was found (Fig. 2c). However, the endplates were more structurally disorganised after temporary compression (Fig. 2d), and the semi-quantitative endplate analysis found a trend to decreased endplate cellularity and increased sclerosis and fibrosis. In details, fibrosis increased from 0 (controls) to 0.4 ± 0.25 (compression; P > 0.05), sclerosis increased from 0.6 ± 0.25 to 1.0 ± 0.32 (P > 0.05), and cellularity decreased from 2 to 1.4 ± 0.25 (P > 0.05; Fig. 5).
Discussion
To gain information on why NCs are not maintained in the NP of human and some animal discs, it was investigated whether mechanical disc loading contributes to the disappearance of NCs from mature, NC-rich rabbit discs. The effects of mechanical loading were analysed by adopting an established in vivo rabbit model of disc compression [16, 17, 24, 31]. With the standard histology and immunohistochemistry, the nucleus cell phenotype was determined, and cell numbers for NCs and MNPCs were analysed after temporary disc compression. The results showed that the nucleus of mature rabbits consisted of approximately 70% cells with notochord phenotype and 30% MNPCs without notochord phenotype, similar to mature porcine discs [32]. After temporary disc compression, <10% of the cells continued to express notochordal phenotype, demonstrating a notochord regression after mechanical loading. This was supported by a previous report showing a nucleus notochord regression after abnormal spinal loading, induced in a bipedal mice model with abnormal upright animal standing [19].
Notochord regression: is increased hydrostatic pressure one key factor?
During temporary disc compression in this model, the intradiscal pressure increased from physiological levels of 0.3–0.9 MPa [17]. Compression of a notochordal-rich nucleus is known to result in stress redistribution, with a compacted peripheral nucleus area leading to a notochordal replacement by fibrocartilage tissue in response to the loading [25, 33]. The current results demonstrate that disc compression of 1 day did not significantly influence cell densities of NCs, indicating that NCs could survive at ~0.9 MPa for a short time period, although regulative processes began after 1 day of compression, as previously indicated by gene expression analysis [32]. In humans, intradiscal pressure experiments found that mechanical stress by upright body position increased intradiscal pressure values. Studies by Nachemson et al. [28] and Wilke et al. [46] showed a wide variation of lumbar intradiscal pressures in vivo during different body positions from 0.1 MPa during lying prone, to 0.5 MPa during standing, and to 2.3 MPa during lifting a 20-kg weight. This indicates that intradiscal pressure varies both after experimental disc compression in rabbits and in response to different body positions in humans. Although the current experimental results show that experimental mechanical stress led to a loss of NCs, it should not necessarily be concluded that human upright body position with an associated increase in intradiscal pressure is ultimately involved in the regression of NCs from human discs. Similarly, spinal disorders were also observed in quadrupeds [4] such as chondrodystrophoid dogs [4], and the variation in intradiscal pressure may also lead to increased nutrition of the disc by means of active pumping mechanisms [42].
Mechanisms of notochord regression: is cell death or (de)differentiation the key factor?
To determine whether NCs die by necrosis or apoptosis, or (de)differentiate into cells not showing a notochordal phenotype anymore, we determined total cell densities and adopted apoptosis data from previous studies showing an approximately fourfold increase in apoptotic cells after 28 days of compression [24]. However, in those studies, it remained unclear whether NCs and/or MNPCs underwent apoptosis. The current results show that NC numbers, as well as total cell numbers, fell significantly after temporary loading (Fig. 4). Conversely, MNPC numbers tended to increase after compression showing that NCs were less resistant against mechanical stress than MNPCs. Moreover, this could also indicate that mechanical stress induced a transformation of cells with typical notochordal phenotype to cells that resemble more chondrocytes without typical notochord protein expression and morphology. The speculation that the fate of NCs involves a phenotype change to MNPCs, as indicated by others recently [8, 21], is supported by a chondrogenic differentiation found in chordoma, a NC tumour [13]. Thus, supporting the current cell density measurements, mechanical stress could indeed induce a morphologic change from NCs to MNPCs, in agreement to a recent in vivo study showing that all cell types in the adult mouse NP, including morphologically MNPCs, were derived from the notochord [8]. More experimental work is required considering this important topic and the current study cannot give definite answers to these question; more studies are needed, including the search for definite notochordal disc markers.
Mechanisms of notochord regression: is malnutrition the key factor?
After temporary disc loading, NCs could disappear due to the continuous increase in intradiscal pressure during compression (from 0.3 to 0.9 MPa [17]). The current 1-day results showed that NC densities were unaffected indicating that NCs could survive under such conditions for at least 1 day. Despite the fact that 56 days of compression probably affects the NC viability, this indicates that other factors may influence the disappearance of NCs, too. A recent study showed that limited nutrient supply affects NC survival in vitro cell cultures, and that NCs were more vulnerable to nutritional stress than MNPCs [18]. In addition, this study found higher endplate porosity in notochordal discs than in non-notochordal discs, indicating that discs with higher endplate porosity tended to maintain NCs. As a decreased endplate porosity hinders the nutrient transport from the endplate vasculature to the nucleus cells [20, 36, 41, 43], the current study explored endplate structures after temporary disc compression, and indeed histological results found moderate structural changes such as endplate fibrosis and sclerosis (Fig. 2). This is in agreement with the previously reported endplate sclerosis in X-rays [40] and MRIs [17] after temporary disc compression. In addition, as a speculation, the endplate vasculature itself could be compressed by the external fixateur, which would further limit the blood and nutrient flow. Thus, both the demonstrated structural endplate changes and the fixateur-related endplate vasculature compression could limit the nutrient supply for NCs, and therefore instigate the disappearance of NCs. Therefore, a systematic investigation into endplate structure in species with discs of different NC content is warranted.
Effect of spinal immobilisation on nucleus cell phenotype
The results after temporary sham compression were remarkable, as this partial immobilisation of the spinal segment [16] did not affect cell densities of NCs, which is in agreement with the other results after temporary sham compression [16, 24, 31]. However, both mechanical overload and immobilisation are believed to cause disc degeneration. Immobilisation of the spine could cause tissue degradation by reduced cellular activity [35], or altered metabolite transport [42]. In vivo experiments with a Ilisarow-type fixateur further supported that both immobilisation and compression produced similar biomechanical changes though after immobilisation the magnitude of changes was lower, and changes occurred slower [23], indicating that longer temporary immobilisation also could have affected NC densities.
Study limitations
Previously, changes after disc compression have been described including a reduction in disc height [24], disc hydration [16], intradiscal pressure [17], and changes in the protein and gene expression of disc constituents, and regulative factors [31]. As these experiments and other animal studies were performed in discs that were anatomically different from mature human discs, i.e. still contain NCs [2, 16], a limitation again should be noted that those changes after disc compression were at least partially influenced by the loss of NCs, as shown in the current study. The disc dehydration with decreased intradiscal pressures [17] and MRI signal intensity [16] was probably related to the regression of NCs with consequent fibrocartilage replacement.
Further limitations of this study should be noted. Owing to methodical reasons, we were unable to perform an additional endplate analysis after temporary compression of 56 days. In consideration of the moderate changes of endplates after 28 days, possibly more severe endplate changes would be present after longer compression. Further, the study power would have been higher if a 7-day compression interval could have been included.
Conclusions
The disappearance of NCs from the NP is important, as NCs protect the disc from degeneration. The few available data and the current study results all support the notion that the regression of NCs is complex and multifactorial. The current results demonstrate that both mechanical stress and structural endplate changes could instigate NC disappearance. This gain in understanding of notochord regression, and further studies addressing the mechanisms and the fate of NCs, will improve the pathophysiologic knowledge of disc degeneration, and might lead to new strategies to prevent and treat disc degeneration.
Acknowledgments
We thank Dr. Jill Urban (Oxford University) for advice in data analysis. Dr. Helga Lorenz did the animal care, and K. Goetzke helped with histological analysis.
Contributor Information
Thorsten Guehring, Phone: +49-621-68100, FAX: +49-621-68103427, Email: tguehrin@ix.urz.uni-heidelberg.de.
Georg W. Omlor, Email: georgomlor@gmx.de
References
- 1.Aguiar DJ, Johnson SL, Oegema TR. Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp Cell Res. 1999;246:129–137. doi: 10.1006/excr.1998.4287. [DOI] [PubMed] [Google Scholar]
- 2.Alini M, Eisenstein SM, Ito K, Little C, Kettler AA, Masuda K, Melrose J, Ralphs J, Stokes I, Wilke HJ. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17:2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benneker LM, Heini PF, Alini M, Anderson SE, Ito K. 2004 Young Investigator Award Winner: vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine. 2005;30:167–173. doi: 10.1097/01.brs.0000150833.93248.09. [DOI] [PubMed] [Google Scholar]
- 4.Bray JP, Burbidge HM. The canine intervertebral disk. Part 2: Degenerative changes—nonchondrodystrophoid versus chondrodystrophoid disks. J Am Anim Hosp Assoc. 1998;34:135–144. doi: 10.5326/15473317-34-2-135. [DOI] [PubMed] [Google Scholar]
- 5.Butler W. Comparative anatomy and development of the mammalian disc. In: Ghosh P, editor. The biology of the intervertebral disc. Boca Raton: CRC Press; 1989. pp. 84–108. [Google Scholar]
- 6.Cappello R, Bird JL, Pfeiffer D, Bayliss MT, Dudhia J. Notochordal cell produce and assemble extracellular matrix in a distinct manner, which may be responsible for the maintenance of healthy nucleus pulposus. Spine. 2006;31:873–882. doi: 10.1097/01.brs.0000209302.00820.fd. [DOI] [PubMed] [Google Scholar]
- 7.Chandraraj S, Briggs CA, Opeskin K. Disc herniations in the young and end-plate vascularity. Clin Anat. 1998;11:171–176. doi: 10.1002/(SICI)1098-2353(1998)11:3<171::AID-CA4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 8.Choi KS, Cohn MJ, Harfe BD. Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Dev Dyn. 2008;237:3953–3958. doi: 10.1002/dvdy.21805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coventry MB. Anatomy of the intervertebral disk. Clin Orthop Relat Res. 1969;67:9–15. doi: 10.1097/00003086-196911000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Erwin WM. The notochord, notochordal cell and CTGF/CCN-2: ongoing activity from development through maturation. J Cell Commun Signal. 2008;2:59–65. doi: 10.1007/s12079-008-0031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erwin WM, Ashman K, O’Donnel P, Inman RD. Nucleus pulposus notochord cells secrete connective tissue growth factor and up-regulate proteoglycan expression by intervertebral disc chondrocytes. Arthritis Rheum. 2006;54:3859–3867. doi: 10.1002/art.22258. [DOI] [PubMed] [Google Scholar]
- 12.Freemont AJ, Watkins A, Le Maitre C, Jeziorska M, Hoyland JA. Current understanding of cellular and molecular events in intervertebral disc degeneration: implications for therapy. J Pathol. 2002;196:374–379. doi: 10.1002/path.1050. [DOI] [PubMed] [Google Scholar]
- 13.Gottschalk D, Fehn M, Patt S, Saeger W, Kirchner T, Aigner T. Matrix gene expression analysis and cellular phenotyping in chordoma reveals focal differentiation pattern of neoplastic cells mimicking nucleus pulposus development. Am J Pathol. 2001;158:1571–1578. doi: 10.1016/S0002-9440(10)64111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gotz W, Osmers R, Herken R. Localisation of extracellular matrix components in the embryonic human notochord and axial mesenchyme. J Anat. 1995;186(Pt 1):111–121. [PMC free article] [PubMed] [Google Scholar]
- 15.Grunhagen T, Wilde G, Soukane DM, Shirazi-Adl SA, Urban JP. Nutrient supply and intervertebral disc metabolism. J Bone Joint Surg Am. 2006;88(Suppl 2):30–35. doi: 10.2106/JBJS.E.01290. [DOI] [PubMed] [Google Scholar]
- 16.Guehring T, Omlor GW, Lorenz H, Bertram H, Steck E, Richter W, Carstens C, Kroeber M. Stimulation of gene expression and loss of anular architecture caused by experimental disc degeneration—an in vivo animal study. Spine. 2005;30:2510–2515. doi: 10.1097/01.brs.0000186591.17114.e9. [DOI] [PubMed] [Google Scholar]
- 17.Guehring T, Unglaub F, Lorenz H, Omlor G, Wilke HJ, Kroeber MW. Intradiscal pressure measurements in normal discs, compressed discs and compressed discs treated with axial posterior disc distraction: an experimental study on the rabbit lumbar spine model. Eur Spine J. 2006;15:597–604. doi: 10.1007/s00586-005-0953-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guehring T, Wilde G, Sumner M, Grunhagen T, Karney G, Tirlapur U, Urban JP. Notochordal intervertebral disc cells—sensitivity to nutrient deprivation. Arthritis Rheum. 2009;60(4):1026–1034. doi: 10.1002/art.24407. [DOI] [PubMed] [Google Scholar]
- 19.Higuchi M, Abe K, Kaneda K (1983) Changes in the nucleus pulposus of the intervertebral disc in bipedal mice. A light and electron microscopic study. Clin Orthop Relat Res, pp 251–257 [PubMed]
- 20.Holm S, Maroudas A, Urban JP, Selstam G, Nachemson A. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8:101–119. doi: 10.3109/03008208109152130. [DOI] [PubMed] [Google Scholar]
- 21.Hunter CJ, Matyas JR, Duncan NA. The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng. 2003;9:667–677. doi: 10.1089/107632703768247368. [DOI] [PubMed] [Google Scholar]
- 22.Hunter CJ, Matyas JR, Duncan NA. The functional significance of cell clusters in the notochordal nucleus pulposus: survival and signaling in the canine intervertebral disc. Spine. 2004;29:1099–1104. doi: 10.1097/00007632-200405150-00010. [DOI] [PubMed] [Google Scholar]
- 23.Iatridis JC, Mente PL, Stokes IA, Aronsson DD, Alini M. Compression-induced changes in intervertebral disc properties in a rat tail model. Spine. 1999;24:996–1002. doi: 10.1097/00007632-199905150-00013. [DOI] [PubMed] [Google Scholar]
- 24.Kroeber MW, Unglaub F, Wang H, Schmid C, Thomsen M, Nerlich A, Richter W. New in vivo animal model to create intervertebral disc degeneration and to investigate the effects of therapeutic strategies to stimulate disc regeneration. Spine. 2002;27:2684–2690. doi: 10.1097/00007632-200212010-00007. [DOI] [PubMed] [Google Scholar]
- 25.Lotz JC, Colliou OK, Chin JR, Duncan NA, Liebenberg E. Compression-induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine. 1998;23:2493–2506. doi: 10.1097/00007632-199812010-00004. [DOI] [PubMed] [Google Scholar]
- 26.McAlinden A, Zhu Y, Sandell LJ. Expression of type II procollagens during development of the human intervertebral disc. Biochem Soc Trans. 2002;30:831–838. doi: 10.1042/BST0300831. [DOI] [PubMed] [Google Scholar]
- 27.Miyazaki T, Kobayashi S, Takeno K, Meir A, Urban J, Baba H (2009) A Phenotypic comparison of proteoglycan production of intervertebral disc cells isolated from rats, rabbits, and bovine tails; which animal model is most suitable to study tissue engineering and biological repair of human disc disorders? Tissue Eng Part A, 15 Aug 2009 [Epub ahead of print] [DOI] [PubMed]
- 28.Nachemson A. Towards a better understanding of low-back pain: a review of the mechanics of the lumbar disc. Rheumatol Rehabil. 1975;14:129–143. doi: 10.1093/rheumatology/14.3.129. [DOI] [PubMed] [Google Scholar]
- 29.Nerlich AG, Schleicher ED, Boos N. 1997 Volvo Award winner in basic science studies. Immunohistologic markers for age-related changes of human lumbar intervertebral discs. Spine. 1997;22:2781–2795. doi: 10.1097/00007632-199712150-00001. [DOI] [PubMed] [Google Scholar]
- 30.Oegema TR., Jr The role of disc cell heterogeneity in determining disc biochemistry: a speculation. Biochem Soc Trans. 2002;30:839–844. doi: 10.1042/BST0300839. [DOI] [PubMed] [Google Scholar]
- 31.Omlor GW, Lorenz H, Engelleiter K, Richter W, Carstens C, Kroeber MW, Guehring T. Changes in gene expression and protein distribution at different stages of mechanically induced disc degeneration–an in vivo study on the New Zealand white rabbit. J Orthop Res. 2006;24:385–392. doi: 10.1002/jor.20055. [DOI] [PubMed] [Google Scholar]
- 32.Omlor GW, Nerlich A, Wilke HJ, Pfeiffer M, Lorenz H, Schaaf-Keim M, Bertram H, Richter W, Carstens C, Guehring T (2009) A new porcine in vivo model of disc degeneration-response of annulus fibrosus cells, chondrocyte-like nucleus pulposus cells and notochordal nucleus cells to partial nuceleotomy. Spine (in press) [DOI] [PubMed]
- 33.Palmer EI, Lotz JC. The compressive creep properties of normal and degenerated murine intervertebral discs. J Orthop Res. 2004;22:164–169. doi: 10.1016/S0736-0266(03)00161-X. [DOI] [PubMed] [Google Scholar]
- 34.Peacock A. Observations on the postnatal structure of the intervertebral disc in man. J Anat. 1952;86:162–179. [PMC free article] [PubMed] [Google Scholar]
- 35.Setton LA, Chen J. Cell mechanics and mechanobiology in the intervertebral disc. Spine. 2004;29:2710–2723. doi: 10.1097/01.brs.0000146050.57722.2a. [DOI] [PubMed] [Google Scholar]
- 36.Soukane DM, Shirazi-Adl A, Urban JP. Computation of coupled diffusion of oxygen, glucose and lactic acid in an intervertebral disc. J Biomech. 2007;40:2645–2654. doi: 10.1016/j.jbiomech.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Taylor T, Twomey L. The development of the human intervertebral disc, chap 2. In: Ghosh P, editor. The biology of the intervertebral disc. Boca Ratoon: CRC Press; 1988. pp. 39–82. [Google Scholar]
- 38.Taylor TK, Melrose J, Burkhardt D, Ghosh P, Claes LE, Kettler A, Wilke HJ. Spinal biomechanics and aging are major determinants of the proteoglycan metabolism of intervertebral disc cells. Spine. 2000;25:3014–3020. doi: 10.1097/00007632-200012010-00008. [DOI] [PubMed] [Google Scholar]
- 39.Trout JJ, Buckwalter JA, Moore KC, Landas SK. Ultrastructure of the human intervertebral disc. I. Changes in notochordal cells with age. Tissue Cell. 1982;14:359–369. doi: 10.1016/0040-8166(82)90033-7. [DOI] [PubMed] [Google Scholar]
- 40.Unglaub F, Guehring T, Lorenz H, Carstens C, Kroeber MW. Effects of unisegmental disc compression on adjacent segments: an in vivo animal model. Eur Spine J. 2005;14:949–955. doi: 10.1007/s00586-004-0800-7. [DOI] [PubMed] [Google Scholar]
- 41.Urban JP, Holm S, Maroudas A, Nachemson A (1977) Nutrition of the intervertebral disk: an in vivo study of solute transport. Clin Orthop Relat Res, pp 101–114 [PubMed]
- 42.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29:2700–2709. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 43.Urban MR, Fairbank JC, Etherington PJ, Loh FL, Winlove CP, Urban JP. Electrochemical measurement of transport into scoliotic intervertebral discs in vivo using nitrous oxide as a tracer. Spine. 2001;26:984–990. doi: 10.1097/00007632-200104150-00028. [DOI] [PubMed] [Google Scholar]
- 44.Walmsley R. The development and growth of the intervertebral disc. Edinb Med J. 1953;60:341–364. [PMC free article] [PubMed] [Google Scholar]
- 45.Whalen JL, Parke WW, Mazur JM, Stauffer ES. The intrinsic vasculature of developing vertebral end plates and its nutritive significance to the intervertebral discs. J Pediatr Orthop. 1985;5:403–410. doi: 10.1097/01241398-198507000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Wilke HJ, Neef P, Caimi M, Hoogland T, Claes LE. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine. 1999;24:755–762. doi: 10.1097/00007632-199904150-00005. [DOI] [PubMed] [Google Scholar]
- 47.Zhu Y, McAlinden A, Sandell LJ. Type IIA procollagen in development of the human intervertebral disc: regulated expression of the NH(2)-propeptide by enzymic processing reveals a unique developmental pathway. Dev Dyn. 2001;220:350–362. doi: 10.1002/dvdy.1115. [DOI] [PubMed] [Google Scholar]