Abstract
Chronic spinal disc disease leads to disorders in postural movement coordination. An incorrect asymmetrical movement pattern for the lower limbs loading impairs proprioception and deteriorates postural stability, particularly when the vision is occluded. The standard surgical treatment improves biomechanical conditions in the lumbar spine, reduces pain, yet does it reduce the stability deficit in the upright position? An answer to the latter question would help work out targeted therapy to improve postural stability. We hypothesized that the standard surgical treatment would improve postural stability reflected by decreased sway variability accounting for better use of proprioceptive inputs postoperatively. Thirty-nine patients with lumbar disc herniation participated in the study. Their postural sway was recorded in anterior/posterior and medial/lateral planes with their eyes open or closed (EC) before and after surgery. The variability, range, mean velocity of the recorded time series and the area of the ellipse enclosed by the statokinesiogram were used as measures of postural stability. Preoperatively, EC condition resulted in an increased variability and mean velocity of postural sway, while postoperatively it caused an increase in sway mean velocity and sway area only with no effect on sway variability and range. The comparison of the balance before and after the surgery in the EC condition showed significant decrease in all parameters. In the early postoperative period, the patients recover the ability to control their postural sway in EC within normal limits, however, at the expense of significantly increased frequency of corrective torques. It is probably a transient short-term strategy needed to compensate for the recovery phase when the normal weighting factors for all afferents are being reestablished. We propose that this transient postoperative period may be the best timing of therapeutic intervention targeted at facilitating and reinforcing the acquisition of correct motor patterns.
Keywords: Postural control, Postural balance, Lumbar disc herniation, Surgical treatment
Introduction
Human postural balance relies on information from somatosensory, vestibular and visual systems [20, 28]. Postural stability depends also on the efficiency of the motor function: joint stability and muscle activity [7]. The performance of the postural balance system is affected by age, neurological dysfunctions [3], cerebrocranial injuries [11], and motor organ diseases [7, 31].
A vital role in maintaining balance is played by the spine. Dysfunctions of the spine influence on control of posture in upright position. Lower back pain is a significant social problem. It has been estimated that 70–85% of all people at some point in their life suffers from low-back pain. In recent years, the percentage of patients undergoing surgery due to herniated disc increased significantly, particularly in the USA. The lumbar disc herniation (LDH) is the third ranking most common cause of surgical procedures [1].
In patients suffering from low-back pain syndrome, significant deficits in postural balance have been reported [19, 22, 24]. The suggested causes of these deficits were pain [24, 25] and impairment of the proprioceptive input in the area of the spine and lower limbs [12, 18, 34]. Asell et al. [2] reported that patients with chronic lower back pain did not differ from controls in terms of the lumbar spine motor performance; other investigations, however, have reported such differences [12, 26, 27]. According to Sipko et al. [33] patients with intervertebral disk disease are characterized by asymmetrical leg loading. This asymmetry, as a combined result of the radiating pain and disturbed proprioceptive input, often leads to postural imbalance and other motor deficits. Based on the study by Morag et al. [23], muscular pareses in patients with LDH cause gait disorders, which is considered one of the indications for the surgery.
In the investigation by Bouche et al. [4] poorer postural balance was found in patients after surgical treatment of herniated disc as compared to the controls. The occurrence of pain in these patients had an influence on postural stability disorders both with open eyes (EO) and with closed eyes (EC). According to Leinonen et al. [18], patients with sciatica had deteriorated postural control and proprioception in the lumbar spine. Surgical treatment improved their proprioception; yet, it did not decrease their postural instability, as was indicated in tests made 3 months after the surgery [18].
It is difficult to explain why the surgical elimination of the reasons for main pathological symptoms such as pain and proprioceptive disorders does not lead to improved postural control in subjects with LDH. After surgery, these subjects promptly acquire favorable condition to make use of the reestablished proprioceptive function. We believe that if it was not for the retention of incorrect compensatory motor patterns and the lack of targeted therapy to aid the consolidation of correct motor patterns, they should present normal postural stability months after surgery. As a first step to verify the latter proposition, the assessment of postural control in these subjects shortly after surgery is required. However, to the best of our knowledge, there are no studies on postural control of patients with LDH in the early postoperative period. Leinonen et al. [18] and Bouche et al. [4] assessed stability of posture 3 months after the surgery, while Dubourg et al. [8] evaluated recovery from paresis due to LDH a month after the surgery, respectively.
The purpose of the study was to compare selected postural sway measures in patients before and shortly after surgery. We hypothesized that postural control would improve postoperatively. As these patients have been reported to significantly rely on vision [4], we expected larger changes in postural stability with eyes closed than with eyes open.
Materials and methods:
Subjects
A group of 39 patients undergoing surgery due to LDH were investigated in the Military Clinic Hospital in Wroclaw. The group consisted of 16 women (44%) and 23 men (56%), aged from 26 to 70 years.
Based on the description of magnetic resonance (MR) results, the following levels of disc hernia were found in 44% subjects—multilevel hernias, in 15% at L4/L5 level, in 29% at L5/S1 level, 3% stenosis of the spinal canal, 3% hernia at L5/S1 level with stenosis of the spinal canal, 3% multilevel hernias and spondylolisthesis, 3% hernia at the L4/L5 level and spondylolisthesis.
The directions of disc hernias were as follows in 40% of subjects: central, 22.5% central right-sided, 15% central left-sided, 20% central bilateral, 2.5% right-sided. All subjects gave informed consent prior to participation, and the procedures were approved by the University Bioethics Committee.
Experimental protocol
Postural stability was assessed in two consecutive 30 s quiet stance periods, first with eyes open (EO) and then with eyes closed (EC) on a pressure plate before and after (3–4 days) surgery. The subjects were asked to stand as still as possible and to preserve the symmetry of stance regarding equal loading of their legs. Further instructions included arms at the sides, feet hip apart and, in the EO stance, fixing the gaze on a small cross-painted on the otherwise blank wall at the distance of 1 m. The sampling frequency was 10 Hz resulting in 300 samples of the centre of force (COF) recorded using a PEL 38 pressure plate manufactured by MEDICAPTEURS (France). This measuring mat consists of 1,024 pressure sensors, recording vertical pressure forces of the plantar side of the feet on the ground. The COF is automatically calculated by the system’s software in the anterior/posterior (AP) and medial/lateral (ML) planes and describes postural sway excursions over time [10]. The recorded COF signals were used to compute the COF variability (standard deviation), range, mean velocity, and area of ellipse enclosed by the statokinesiogram.
Statistical analysis
All data were tested for normal distribution and homogeneity of variances and failed to meet these assumptions. Thus, we used the Wilcoxon’s matched pairs test to compare the postural stability measures between the pre- and postoperative status and between the EO versus EC condition in both planes of movement separately. The results are presented as median values. The level of significance was set at P = 0.05.
Results
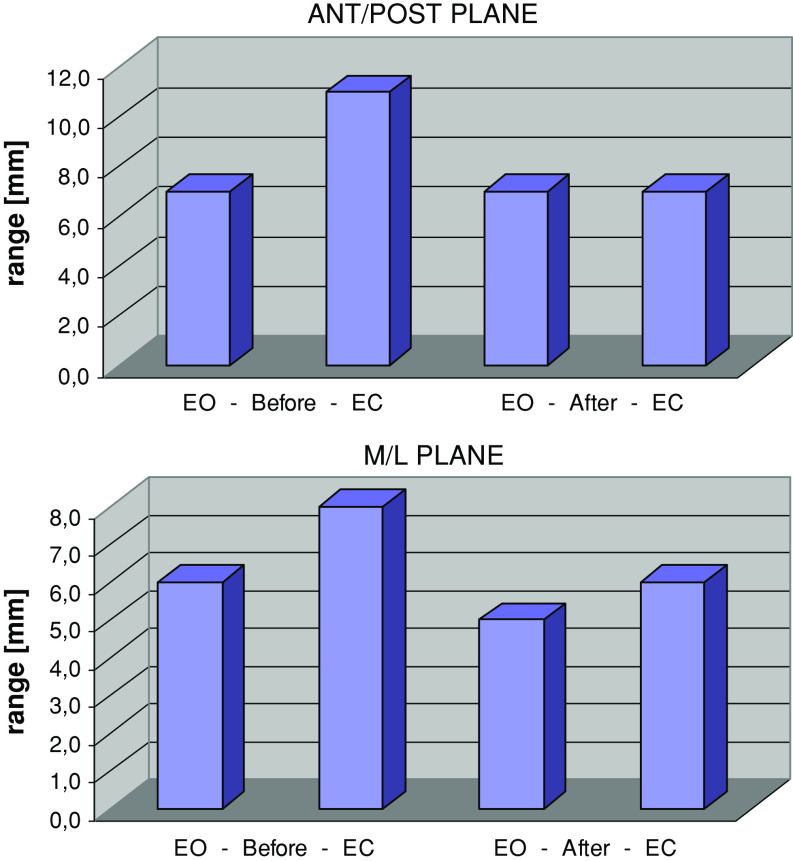
Prior to the surgery, EC resulted in a significant increase in all stabilographic parameters: sway variability (P < 0.0005, P < 0.05), range (P < 0.0003, P = 0.007), and mean velocity (P < 0.0001) in the AP and ML plane, respectively (Figs. 1, 2, 3). Sway area also increased (P < 0.0004) (Fig. 4). These results indicated a large reliance on visual information in subjects with LDH.
Fig. 1.
Median of sway range (mm) in anterior/posterior and medial/lateral plane in tests with eyes open (EO) and eyes closed (EC) before and after surgery
Fig. 2.
Median of sway variability (mm) in anterior/posterior and medial/lateral plane in tests with eyes open (EO) and eyes closed (EC) before and after the surgery
Fig. 3.
Median of sway velocity (mm/s) in anterior/posterior and medial/lateral plane in tests with eyes open (EO) and eyes closed (EC) before and after the surgery
Fig. 4.
Median of area of ellipse enclosed by statokinesiogram (mm2) in tests with eyes open (EO) and eyes closed (EC) before and after surgery
After the surgery, EC caused a significant increase in sway mean velocity (P < 0.0001 in the AP and the ML plane) and sway area (P < 0.01) only, while the sway variability measures remained unaffected. Thus, the reliance on vision disappeared, and was substituted for other sensory modalities.
In EO condition, there were no differences between stability measures before and after the surgery indicating an effective compensatory contribution of vision to balance before the surgery. However, in the EC condition, the surgery resulted in a significant decrease in all stability parameters: sway variability (P < 0.002 in AP plane), range (P < 0.005, P < 0.02), and mean velocity (P < 0.01, P < 0.01) in the AP and ML plane, respectively (Figs. 1, 2, 3). Sway area also decreased (P < 0.02) (Fig. 4). The latter results pointed out the beneficial outcome of the surgery on the non-visual modalities contributing to body balance.
Discussion
In this paper, we investigated stability of the upright stance in LDH patients before the surgery and in the early postoperative period. We hypothesized that this surgery should result in the instantaneous change in postural strategies leading to the improvement in postural control. Our results confirmed this hypothesis because all measures of postural sway variability significantly decreased after surgery in EC condition.
In line with the several other studies [4, 18], the variability of postural sway in our patients before surgery was larger with EC than with EO (by 80–100% in the AP and 40–70% in the ML plane, respectively). Such a strong reliance on vision does not occur in healthy people. It is generally accepted that the cause of such substantial differences in patients with LDH is impairment of the proprioceptive system [12, 18, 34], the significance of which in maintaining stable position is fundamental [29, 32]. Peterka et al. [29] estimated that in typical condition while standing on a firm base of support, healthy persons rely 70% on somatosensory information. Yet, body balance in our patients before surgery in EO condition was similar to that of healthy persons suggesting that the former subjects had learned to effectively use visual information to compensate for deteriorated proprioception. Consequently, postural control in our patients was practically optimal and the subsequent reintegration of proprioception after surgery could not have caused further reduction of the sway variability in the EO condition. It concurs with the results of several authors [9] that certain level of sway variability is indispensable for the continuous monitoring of the body position against stability limits.
Postoperatively, in the EC condition all stability measures decreased indicating improved balance of the subjects. Most likely, this improvement accounted for a better function of their proprioceptive system and similar reasoning was presented in a number of papers [5, 12, 34]. However, we believe that our results provide evidence that may shed more light on neuromotor antecedents of the postoperative balance improvement in our patients, namely on postural strategies. The best way to demonstrate this evidence is to analyze the changes in postural control between the EO and EC, in the pre- and postoperative patients, separately. Before surgery, eyes closure led to almost identical increases in sway variability and mean velocity. The mean velocity of a time series is a product of its variability and frequency [11], thus, the resulting increase in the sway mean velocity was caused solely by a similar increase in the sway variability, without any significant changes in sway frequency. The latter parameter represents the activity of the CNS in the monitoring the body position and velocity with respect to the stability limits, and some authors argued that frequency might be important in identifying changes in postural strategies, particularly in terms of the level of muscle co-contraction and stiffening the posture [35]. In fact, it has been demonstrated in several studies that increased difficulty of task has resulted in higher frequency, always associated with higher postural stiffness [6, 16, 17]. In view of the latter evidence, our results indicate that our chronic patients before surgery, when challenged by occluded vision, were not able to adopt efficient control strategy to counteract significantly increased sway amplitude.
In contrast, our patients after surgery displayed no changes in sway variability and large increase in sway mean velocity during the EC as compared to EO stances. Taking into account, the aforementioned relationship between the mean velocity and the remaining sway parameters, the increase in the mean velocity could have been caused only by substantial increase in the sway frequency. To present in a different way, closing the eyes by patients after surgery prompted their CNS to increase the activity of the body position monitoring and/or the rate of reciprocal muscle co-contraction which accounted for the improved proprioceptive function. It seems interesting to compare the latter results with other studies concerned with healthy adults whose postural stability is fairly robust to occluded vision displaying moderate increase in sway variability and no changes in mean velocity, when comparing EC to EO condition. It suggests that occluded vision is not regarded by healthy persons as a real threat to the equilibrium system and their CNS can easily hold out against this challenge using intact vestibular and proprioceptive pathways. However, the proprioceptive system in our patients after surgery, although its function has been restored, was still in a transient phase from learned compensatory patterns and the accuracy of the spatial orientation information was reduced. The CNS assessed these compromised orientation cues that arose from still incorrect sensory reweighting strategies as a threat to postural stability and employed tighter (higher frequency) control of posture in our patients during EC stances. This reasoning concurs with the transient increases in body sway that have occurred after restoration or alteration of visual and proprioceptive cues [13, 30]. Despite the excessive sway frequency as compared to healthy persons, the optimistic outcome was that the postoperative patients regained their ability to use higher frequency of corrective movements to stabilize the body. We believe that it may be the best timing to start therapeutic intervention targeted at facilitating and reinforcing the acquisition of correct motor patterns. The benefit of very early therapy has been recently demonstrated by Millisdotter and Strömqvist [21].
Besides the improved proprioceptive input, another reason for facilitating these corrections may be the elimination of pain which is in line with the study by Kuczyński and Paluch [15] who found significant negative relationship between the pain level and sway frequency in persons with low-back pain. Similarly, Bouche et al. [4] reported that sciatica patients after lumbar discectomy developed visual compensation mechanisms that were sufficient in case of pain relief only.
The role of pain relief (and its biomechanical consequences) in the recovery of normal balance control in patients with LDH, in view of the presented evidence, is worth additional discussion. Mok et al. [22] reported reduced ability to control a hip strategy for balance control in persons with LBP. During balance tests, subjects are usually instructed to stand motionless. Healthy people during easy tasks fulfill the condition of one-segmental inverted pendulum and use a so-called ankle strategy shifting the body by torques at the ankle [14]. In more difficult tests, such as standing on a short base of support, these subjects tend to use hip strategy that involves generation of horizontal shear forces. If the function of any subsystem of the balance control is deteriorated, hip strategy may be used even in relatively easy trials. Hence, postural control normally involves elements of both strategies, though the emphasis will vary depending on the postural context [22]. Biomechanically, ankle strategy has low frequency of sway due to a large moment of inertia of the body around the ankle joints. In hip strategy, the upper and lower body, each having significantly lower moment of inertia as compared to the whole body, move in a relatively independent way in what allows to generate higher frequencies of sway without producing excessive sway magnitude. It is possible that postural sway in our preoperative patients was based on ankle strategy and indicated no involvement of increased sway frequency, while after surgery these persons were able to use hip strategy that increased sway mean velocity as the result of increased sway frequency.
Conclusion
Postural performance in quiet standing with EO in patients with LDH does not differ from that in healthy persons. This is achieved by the overreliance of the former subjects on visual cues and may contribute to a delay and difficulties in the reweighting of sensory information following a surgery. Occluded vision leads to significantly exacerbated postural stability in these patients who are unable to properly monitor their posture due to incorrect proprioceptive information and/or to counteract excessive sway by means of tighter control. In the early post - operative period the patients recover the ability to control their postural sway with EC within normal limits, however at the expense of significantly increased frequency of corrective torques. It is probably a transient short-term strategy needed to compensate for the recovery phase when the normal weighting factors for all afferents are being reestablished. We propose that this transient postoperative period may be the best timing of therapeutic intervention targeted at facilitating and reinforcing the acquisition of correct motor patterns.
References
- 1.Andersson GBJ. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 2.Asell M, Sjolander P, Kerschbaumer H, Djupsjobacka M. Are lumbar repositioning errors larger among patients with chronic low back pain compared with asymptomatic subjects? Arch Phys Med Rehabil. 2006;87:1170–1176. doi: 10.1016/j.apmr.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 3.Baloh RW, Jacobson KM, Enrietto JA, Corona S, Honrubia V. Balance disorders in older persons: quantification with posturograhy. Otolaryngol Head Neck Surg. 1998;119:89–92. doi: 10.1016/S0194-5998(98)70177-9. [DOI] [PubMed] [Google Scholar]
- 4.Bouche K, Stevens V, Cambier D, Caemaert J, Danneels L. Comparison of postural control in unilateral stance between healthy controls and lumbar discectomy patients with and without pain. Eur Spine J. 2006;15:423–432. doi: 10.1007/s00586-005-1013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brumagne S, Cordo P, Lysens R, et al. The role of paraspinal muscle spindles in lumbosacral position sense in individuals with and without low back pain. Spine. 2000;25:989–994. doi: 10.1097/00007632-200004150-00015. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter MG, Frank JS, Silcher CP, Peysar GW. The influence of postural threat on the control of upright stance. Exp Brain Res. 2001;138:210–218. doi: 10.1007/s002210100681. [DOI] [PubMed] [Google Scholar]
- 7.Davids K, Kingsbury D, George K, O’Connel M, Stock D. Interacting constraints and the emergence of postural behavior in ACL-deficient subjects. J Mot Behav. 1999;31:358–366. doi: 10.1080/00222899909601000. [DOI] [PubMed] [Google Scholar]
- 8.Dubourg G, Rozenberg S, Fautrel B, et al. A pilot study on the recovery from paresis after lumbar disc herniation. Spine. 2002;27:1426–1431. doi: 10.1097/00007632-200207010-00010. [DOI] [PubMed] [Google Scholar]
- 9.Gatev P, Thomas S, Kepple T, Hallett M. Feedforward ankle strategy of balance during quiet stance in adults. J Physiol. 1999;514:915–928. doi: 10.1111/j.1469-7793.1999.915ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerbino PG, Griffin ED, Zurakowski D. Comparison of standing balance between female collegiate dancers and soccer players. Gait Posture. 2007;26:501–507. doi: 10.1016/j.gaitpost.2006.11.205. [DOI] [PubMed] [Google Scholar]
- 11.Geurts ACH, Ribbers GM, Knop JA, Van Limbeck J. Identification of static and dynamic instability following traumatic brain injury. Arch Phys Med Rehabil. 1996;77:639–644. doi: 10.1016/S0003-9993(96)90001-5. [DOI] [PubMed] [Google Scholar]
- 12.Gill KP, Callaghan MJ. The measurement of lumbar proprioception in individuals with and without low back pain. Spine. 1998;23:371–377. doi: 10.1097/00007632-199802010-00017. [DOI] [PubMed] [Google Scholar]
- 13.Hay L, Bard C, Fleury M, Teasdale N. Availability of visual and proprioceptive afferent messages and postural control in elderly adults. Exp Brain Res. 1996;108:129–139. doi: 10.1007/BF00242910. [DOI] [PubMed] [Google Scholar]
- 14.Horak FB, Nashner LM. Central programming of postural movements: adaptation to altered support surface configuration. J Neurophysiol. 1986;55:1369–1381. doi: 10.1152/jn.1986.55.6.1369. [DOI] [PubMed] [Google Scholar]
- 15.Kuczynski M, Paluch P. Postural stability in patients with back pain. Acta Bioeng Biomech. 1999;1(2):19–23. [Google Scholar]
- 16.Kuczyński M. The second order autoregressive model in the evaluation of postural stability. Gait Posture. 1999;9:50–56. doi: 10.1016/S0966-6362(99)00003-X. [DOI] [PubMed] [Google Scholar]
- 17.Kuczyński M, Ostrowska B. Understanding falls in osteoporosis: the viscoelastic modeling perspective. Gait Posture. 2006;23(1):51–58. doi: 10.1016/j.gaitpost.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Leinonen V, Kankaanpaa M, Luukkonen M, Kansanen M, Hanninen O, Airaksinen O, Taimela S. Lumbar paraspinal muscle function, perception of lumbar position and postural control in disc herniation-related back pain. Spine. 2003;28(8):842–848. doi: 10.1097/00007632-200304150-00019. [DOI] [PubMed] [Google Scholar]
- 19.Luoto S, Aalto H, Taimela S, Hurri H, Pyykko I, Alaranta H. One-footed and externally disturbed two-footed postural control in patients with chronic low back pain and healthy control subject. Spine. 1998;23(19):2081–2089. doi: 10.1097/00007632-199810010-00008. [DOI] [PubMed] [Google Scholar]
- 20.Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-C. [DOI] [PubMed] [Google Scholar]
- 21.Millisdotter M, Strömqvist B. Early neuromuscular customized training after surgery for lumbar disc herniation: a prospective controlled study. Eur Spine J. 2007;16:19–26. doi: 10.1007/s00586-005-0044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mok NW, Brauer SG, Hodges PW. Hip strategy for balance control in quiet standing is reduced in people with low back pain. Spine. 2004;29(6):E107–E112. doi: 10.1097/01.BRS.0000115134.97854.C9. [DOI] [PubMed] [Google Scholar]
- 23.Morag E, Hurwitz DE, Andriacchi TP, Hickey M, Andersson J. Abnormalities in muscle function during gait in relation to the level of lumbar disc herniation. Spine. 2000;25(7):829–833. doi: 10.1097/00007632-200004010-00011. [DOI] [PubMed] [Google Scholar]
- 24.Moseley GL, Hodges PW. Are the changes in postural control associated with low back pain caused by pain interference? Clin J Pain. 2005;21:323–329. doi: 10.1097/01.ajp.0000131414.84596.99. [DOI] [PubMed] [Google Scholar]
- 25.Moseley GL, Nicholas MK, Hodges PW. Pain differs from non-painful attention-demanding or stressful tasks in its effect on postural control patterns of trunk muscles. Exp Brain Res. 2004;156:64–71. doi: 10.1007/s00221-003-1766-0. [DOI] [PubMed] [Google Scholar]
- 26.Newcomer KL, Laskowski ER, Bing Yu, Johnson JC, Kai-Nan An. Differences in repositioning error among patients with low back pain compared with control subjects. Spine. 2000;25:2488–2493. doi: 10.1097/00007632-200010010-00011. [DOI] [PubMed] [Google Scholar]
- 27.O’Sullivan PB, Burnet A, Floyd AN, et al. Lumbar repositioning deficit in a specific low back pain population. Spine. 2003;28:1074–1079. doi: 10.1097/00007632-200305150-00022. [DOI] [PubMed] [Google Scholar]
- 28.Paulus WM, Straube A, Brandt T. Visual stabilization of posture: physiological stimulus characteristics and clinical aspects. Brain. 1984;107(Pt 4):1143–1163. doi: 10.1093/brain/107.4.1143. [DOI] [PubMed] [Google Scholar]
- 29.Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 2002;88:1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- 30.Peterka RJ, Loughlin PJ. Dynamic regulation of sensorimotor integration in human postural control. J Neurophysiol. 2004;91:410–423. doi: 10.1152/jn.00516.2003. [DOI] [PubMed] [Google Scholar]
- 31.Rougier P, Belaid D, Cantalloube S, Lamotte D, Deschamps J. Quiet postural control of patients with total hip arthroplasty following joint arthritis. Motor Control. 2008;12:136–150. doi: 10.1123/mcj.12.2.136. [DOI] [PubMed] [Google Scholar]
- 32.Simoneau G, Ulbrecht J, Derr J, Cavanagh P. Role of somatosensory input in the control of human posture. Gait Posture. 1995;3:115–122. doi: 10.1016/0966-6362(95)99061-O. [DOI] [Google Scholar]
- 33.Sipko T, Chantsoulis-Supińska M, Żmuda M, Zwoliński J. Postural balance in the early post-operative period in patients with intervertebral disk disease following surgery. Ortop Traumatol Rehabil. 2008;10(3):226–237. [PubMed] [Google Scholar]
- 34.Taimela S, Kankaanpaa M, Luoto S. The effect of lumbar fatigue on the ability to sense a change in lumbar position: a controlled study. Spine. 1999;24:1322–1327. doi: 10.1097/00007632-199907010-00009. [DOI] [PubMed] [Google Scholar]
- 35.Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K. Stiffness control of balance in quiet standing. J Neurophysiol. 1998;80:1211–1221. doi: 10.1152/jn.1998.80.3.1211. [DOI] [PubMed] [Google Scholar]