Abstract
Retrospective study on the results of microendoscopic decompression surgery for the treatment of cervical myelopathy. The purpose of this study was to describe the microendoscopic laminoplasty (MEL) technique as the surgical method in the treatment of cervical myelopathy, and to document the clinical outcomes for MEL surgery. Endoscopic surgery poses several challenges for the aspiring endoscopic surgeons, the most critical of which is mastering hand–eye coordination. With training in live animal and cadaver surgery, the technical progress has reduced the problem of morbidity following surgery. The authors have performed microendoscopic decompression surgery on more than 2,000 patients for lumbar spinal canal stenosis. Fifty-one patients underwent the posterior decompression surgery using microendoscopy for cervical myelopathy at authors’ institute. The average age was 62.9 years. The criteria for exclusion were cervical myelopathy with tumor, trauma, severe ossification of posterior longitudinal ligament, rheumatoid arthritis, pyogenic spondylitises, destructive spondylo-arthropathies, and other combined spinal lesions. The items evaluated were neurological evaluation, recovery rates; these were calculated following examination using the Hirabayashi’s method with the criteria proposed by the Japanese Orthopaedic Association scoring system (JOA score). The mean follow-up period was 20.3 months. The average of JOA score was 10.1 points at the initial examination and 13.6 points at the final follow-up. The average recovery rate was 52.5%. The recovery rate according to surgical levels was, respectively, 56.5% in one level, 46.3% in two levels and 54.1% in more than three levels. The complications were as follows: one patient sustained a pin-hole-like dura mater injury inflicted by a high-speed air-drill during surgery, one patient developed an epidural hematoma 3 days after surgery, and two patients had the C5 nerve root palsy after surgery. The epidural hematoma was removed by the microendoscopy. All two C5 palsy improved with conservative therapy, such as a neck collar. These four patients on complications have returned to work at the final follow-up. This observation suggests that the clinical outcomes of microendoscopic surgery for cervical myelopathy were excellent or showed good results. This minimally invasive technique would be helpful in choosing a surgical method for cervical myelopathy.
Keywords: Cervical spine, Clinical outcome, Endoscopic surgery, Laminoplasty, Myelopathy
Introduction
Minimally invasive spinal procedures are currently being used effectively to treat a variety of commonly encountered degenerative conditions of the lumbar [7, 9, 13, 16], thoracic [10] and cervical spine [1, 4, 21]. These developments have led to surgical approaches to the spine that can result in less tissue trauma and reduce a patient’s postoperative pain and discomfort, shorten hospital stays, and allow a quicker return to activities of daily living.
Endoscopic surgery poses several challenges for the aspiring endoscopic surgeons, the most critical of which is mastering hand–eye coordination. With training in live animal and cadaver surgery, the technical progress has reduced some problems of morbidity following surgery, such as dural tear, neural deficit, and so on [5]. Recently, microendoscopic decompressive techniques have been developed and applied to various spine pathologies including lumbar spinal stenosis and cervical radiculopathy and myelopathy [1, 4, 7, 9, 13, 16, 21]. Over 2,000 patients of lumbar spinal canal stenosis have undergone the microendoscopic decompression surgery in authors’ institution. The authors have been applying microendoscopic laminoplasty (MEL) as a minimally invasive strategy to cervical posterior decompression surgery. The purpose of this study was to describe the MEL technique (used in 51 patients) as the surgical method in the treatment of cervical myelopathy, and to document the clinical outcomes for MEL surgery.
Materials and methods
Surgical technique of cervical microendoscopic laminoplasty (C-MEL)
General endotracheal anesthesia was induced with adequate intravenous access and an intra-arterial monitoring catheter. The patient was secured in a Mayfield headholder, and was turned to the prone position. The neck was fixed in a neutral position. The fluoroscopic C-arm was brought into the surgical field so that real-time lateral fluoroscopic images could be obtained. The operator generally stood on the side of the approach and video monitors placed opposite to him. Under fluoroscopic guidance held laterally to the patient, the targeting level was marked on the side of the approach. A skin incision approximately 17 mm in length was made at the spinal level to be decompressed. The microendoscopic discectomy (MED) and instrumentation developed by Smith and Foley [16] for lumbar disc disease were used for this procedure. After splitting into paravertebral muscles, the set of serial dilators of the METRx endoscopic system (Medtronic Sofamor Danek, Memphis, TN, USA) was passed to gently dilate the cervical musculature. The 16-mm final working channel was then passed over the dilators and secured to the flexible-arm of the METRx retractor mounted to the table side rail. Final fluoroscopic confirmation of the working channel position was obtained, and the serial dilators were removed. The tubular retractor lay on the lamina and facet joints (Fig. 1), and was tilted parallel to the intervertebral disc (Fig. 2). The endoscope was then attached to the tubular retractor. Bipolar cautery was used to remove any residual muscular and soft tissues overlying the lamina and facet joint in the tubular retractor. With the bony edges well visualized, a small angled endoscopic curette was used to confirm the inter-lamina space and the medial edge of the fact complex. To begin the partial laminectomy (Fig. 3a), a high-speed drill with a long curved endoscopic bit (e.g., Midas-Rex) was used to thin the lamina near the attachment of ligamentum flavum. After adequate drilling, endoscopic Kerrison’s rongeurs were used to continue the removal of the lamina. The superior attachment of ligamentum flavum was exposed, and the procedure was then continued to the superior portion of the inferior lamina. The inferior attachment of ligamentum flavum was exposed. The endoscope was then swung medially and downward to obtain a contralateral view. After the basement of spinal process was drilled, the laminotomy was performed by a long curved high-speed drill with an endoscopic bit (Fig. 3b). The scope was rotated to a lateral position to make use of its 25° viewing angle. As a result of these maneuvers, an excellent viewing angle of nearly 60 to 75° was usually obtained with good contralateral visualization. It was important to continue the contralateral procedure without removing the ligamentum flavum to protect the spinal cord. When the spinal cord was completely decompressed, the floated ligamentum flavum was observed. Attention was directed to removing the ligamentum flavum. A small angled curette, nerve hook, or endoscopic Kerrison’s rongeurs was used to gently dissect the ligament and to identify the plane between the ligamentum flavum and the underlying dura. When the ligamentum flavum was completely removed, the dural pulsation was observed. The C-MEL was completed (Fig. 3c). When the decompression surgery was needed for an adjacent level, the tubular retractor was inclined cranially or caudally. Then, the procedure as the above was added to the adjacent level. For more than three levels another skin incision was added. For example, in case of C3–C7 C-MEL, the skin incisions were made on C4 and C6 levels. A drain was placed at each level to prevent epidural hematoma after surgery. The tubular retractor and endoscope were removed, and the fascia and skin were closed using standard techniques. The drain was placed at the operative level(s) for 48 h to prevent the epidural hematoma after surgery.
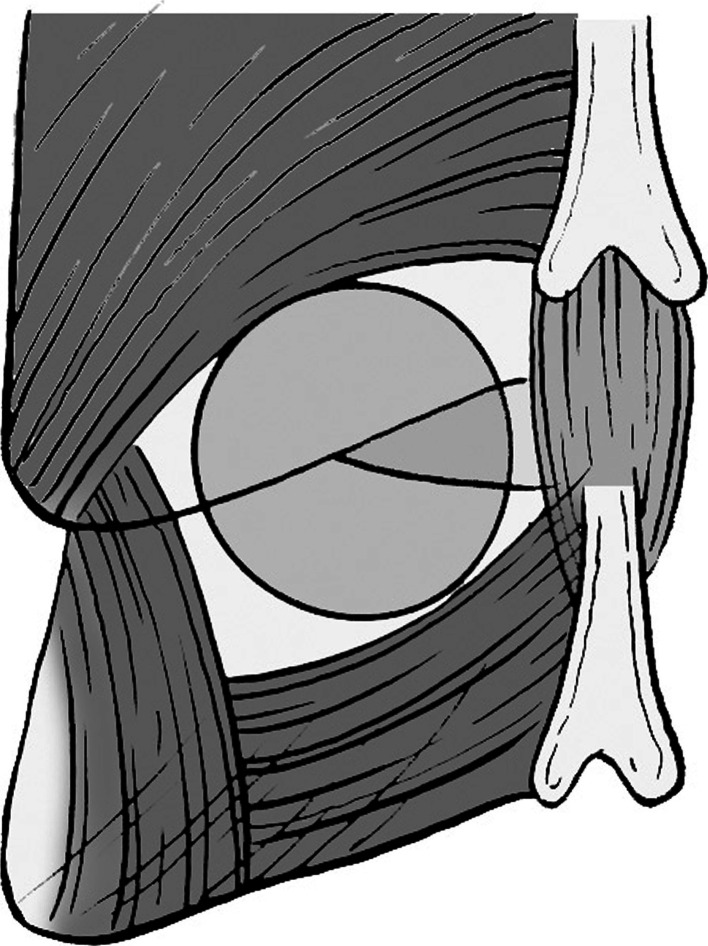
Fig. 1.
The tubular retractor of microendoscopic system lies on the lamina and facet joint
Fig. 2.
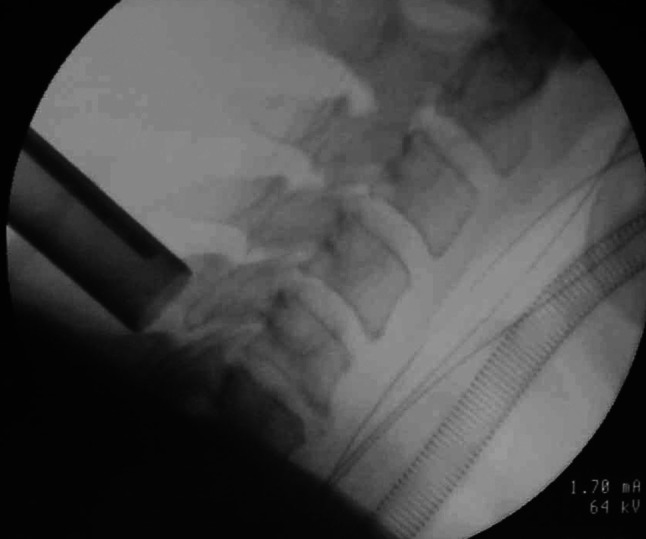
The tubular retractor of microendoscopic system is tilted to the direction of the intervertebral disc. The setting position is confirmed by X-ray
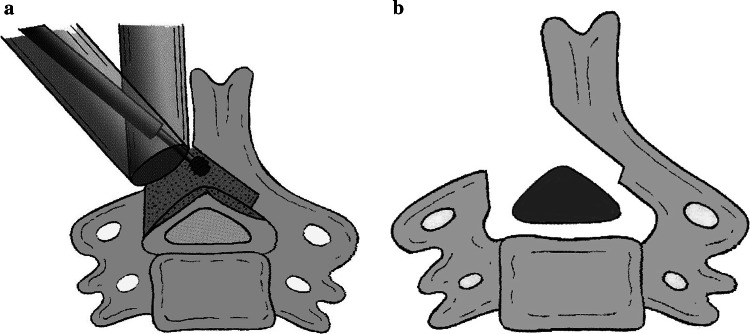
Fig. 3.
The decompression surgery is preformed using a high-speed air-drill. a The hemilaminectomy is performed on the approaching side, and then the laminotomy on the contralateral side is done. b Finally, the laminoplasty is completed to enlarge the spinal canal
Patient population
Between 2003 and 2008, a total of 51 patients with cervical myelopathy underwent this C-MEL at authors’ institution. The criteria for exclusion were cervical myelopathy with tumor, trauma, severe ossification of posterior longitudinal ligament (OPLL), rheumatoid arthritis, pyogenic spondylitises, destructive spondylo-arthropathies, and other combined spinal lesion. There were 34 males and 17 females. The average age at the time of surgery was 62.9 years (range 24–85 years). All patients presented with symptoms of cervical myelopathy, such as clumsiness, numbness of upper and lower extremities, gait disturbance, and urinary disturbance. There were 44 patients with cervical spondylotic myelopathy (CSM), 4 patients with cervical myelopathy due to the calcification of ligamentum flavum (CLF), and 3 patients with cervical intervertebral disc herniation. The spinal cord compression was confirmed by magnetic resonance imaging (MRI), myelography and post-myelography computed tomography (CTM). The following items were assessed both pre- and post-operatively. A contributed lesion was reviewed by a symptom, neurological deficiency and imaging. As much as 26 patients suffered cervical myelopathy on one level, 17 patients on two levels, and 8 patients on more than three levels. These items evaluated were clinical outcomes including complications, neurological evaluation, and recovery rates, which were calculated following examination using Hirabayashi’s method with the criteria proposed by the Japanese Orthopaedic Association scoring system (JOA score) [6, 22], blood loss during surgery, change of C reactive protein (CRP), visual analog scale (VAS) for assessment of treatment of axial pain, Short Form 36 (SF-36). The recovery rate was calculated as follows: recovery rate = 100 × (postoperative JOA score − preoperative JOA score)/(17 − preoperative JOA score).
Statistical analysis
All parameters were analyzed statistically. Either nonparametric Kruskal–Wallis test or Spearman’s correlation coefficient by rank test was used to test differences in the recovery rate of JOA score between groups. The Mann–Whitney U test was used to test differences in the SF-36 subscales between preoperative and the final follow-up. A probability level of <0.05 was considered significant.
Results
The mean follow-up period was 20.3 months (6–56 months). The average of JOA score was 10.1 ± 2.7 points before surgery and 13.6 ± 2.3 points at the final follow-up. The average recovery rate was 52.5 ± 20.3% (Table 1). The duration of symptoms before surgery was an average of 20.1 months (range 1–78 months), and there was no correlation between the duration of symptoms and recovery rates (Spearman’s correlation coefficient by rank test; p = 0.6). As for the contributed levels, the recovery rate was 56.5% on one level, 46.3% on two levels, and 54.1% on more than 3 levels. There were no significant differences between the contributed levels and recovery rates (Kruskal–Wallis test; p = 0.065). Four patients had some perioperative complications. One patient sustained a pin-hole-like dura mater injury inflicted by a high-speed air-drill during surgery. There was little leakage of the cerebrospinal fluid. So, fibrin paste was wound up on dura mater. In another patient who had undergone the C-MEL on one level, although the postoperative drain was retained on the epidural space for 48 h, the spinal cord was compressed by the epidural hematoma 3 days after surgery. He had the progressive quadriplegia, and underwent the emergency surgery to remove the epidural hematoma using microendoscopy. His quadriplegia improved immediately after surgery. After the C-MEL surgery on two or three levels, there were two patients who had temporal C5 nerve root palsy. These two patients were not able to evaluate their upper extremity on the corresponded side to the opened lamina (hemilaminectomy). Their neurological symptoms improved with conservative treatment on a Philadelphia collar. Both patients were able to evaluate their upper extremity a few months later. One of the two patients improved to a full score in manual muscle testing (MMT) for both deltoid and biceps muscles.
Table 1.
JOA score and cervical alignment after C-MEL surgery
| Pre-operation | Final follow-up | |
|---|---|---|
| JOA score (points) | 10.1 ± 2.7 | 13.6 ± 2.3 |
| Recovery rate (%) (JOA score) | 52.5 ± 20.3 | |
| Lordotic angle (degree) (C2–C7) | 13.1 ± 14.0 | 13.5 ± 14.9 |
The mean amount of blood loss in surgery was 35.4 ml on one level, 22.9 ml on two levels, and 44.4 ml on more than three levels. The surgical time was an average of 106 min on one level, 146.4 min on two levels, and 178.2 min on more than three levels. The mean value of CRP 3 days after surgery was 1.1 ± 1.4 mg/dl (normal: <0.3). All patients could get up after awakening from anesthesia immediately and participated in rehabilitation from an early postoperative period. The average period of hospital stay was 8.6 days (range 4–21 days). There was no correlation between the JOA score before surgery and hospital stay (Spearman’s correlation coefficient by rank test; p = 0.28). The VAS of axial symptoms was 46 ± 45 mm before surgery and 15 ± 22 mm at the final follow-up. There was none of the patients whose axial symptoms worsened postoperatively. As for the SF-36, all subscales at the final follow-up have been improved. The scores of role physical, bodily pain, social functioning, and role emotional subscales at the final follow-up were significantly higher than that before surgery (Mann–Whitney U test; p < 0.05, Fig. 4). On the alignment of cervical spine after surgery, no patient changed to kyphosis postoperatively. The lordotic angle of cervical spine (C2–C7) was 13.1 ± 14.0° before surgery and 13.5 ± 14.9° at the final follow-up (Table 1). The local angle of the surgical level was 6.7 ± 10.4° before surgery and 6.7 ± 9.1° at the final follow-up.
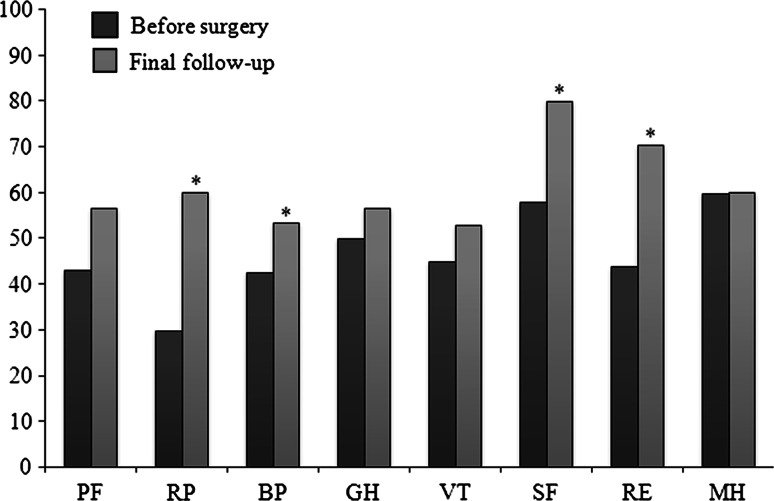
Fig. 4.
All subscales at the final follow-up have been improved. The scores of RP, BP, SF, and RE subscales at the final follow-up were significantly higher than that before surgery. (PF physical functioning, RP role physical, BP bodily pain, SF social functioning, GH general health perceptions, VT vitality, RE role emotional, MH mental health) Significantly different from before surgery (Mann–Whitney U test, *p < 0.05)
Case presentation
Case 1
A 59-year-old woman noticed clumsiness in her finger motions and numbness in both her hands. She attended the authors’ clinic, where she presented in a wheelchair and revealed urinary disturbance. An MRI and CTM showed severe spinal compression caused by the calcification of ligamentum flavum (CLF) at C6–C7. She underwent the C-MEL surgery at C6–C7, and the spinal cord was decompressed. Three years after surgery, her preoperative JOA score improved from 8 to 14 points. The recovery rate of JOA score was 66.7%. This case is presented in Fig. 5.
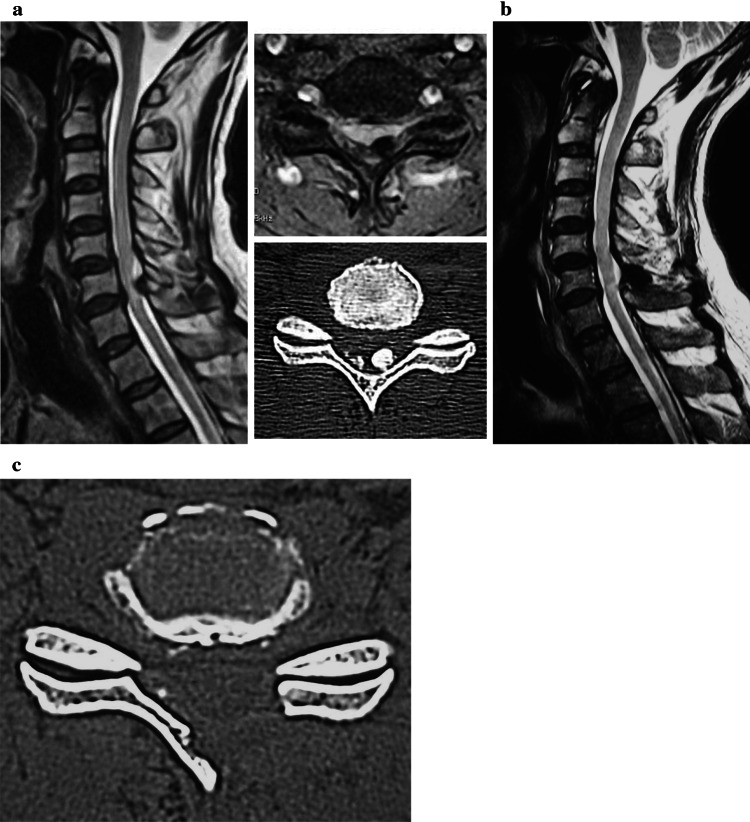
Fig. 5.
a The 67-year-old patient had cervical myelopathy due to the calcification of ligamentum flavum at C6–C7. The JOA score was 8 points at the initial examination. b, c He underwent the microendoscopic laminoplasty at C6–C7, and the spinal cord was decompressed. The JOA score was improving to 14 points at the final follow-up
Case 2
A 64-year-old woman had noticed numbness in both her arms and hands. Gait and urinary disturbance developed gradually, and she visited the authors’ outpatient clinic. The patient presented with spastic quadriparesis, and an MRI showed severe multilevel spinal cord compression by cervical spondylotic myelopathy. She underwent the C-MEL surgery from C4 to C7. Although the spinal cord showed the presence of T2 high signal intensity on MR imaging, the posterior spinal decompression was successful as revealed on MRI after surgery. Two years after surgery, her preoperative JOA score of 9 points improved to 13 points. The recovery rate of JOA score was 50%. This case is presented in Fig. 6.
Fig. 6.
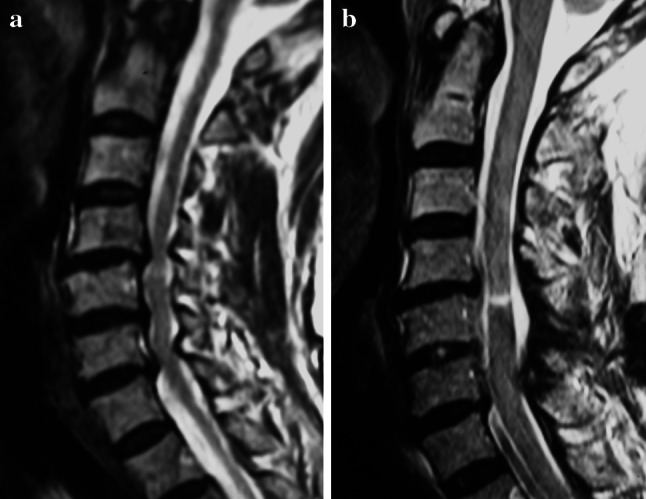
a A 64-year-old patient had cervical spondylotic myelopathy at the multiple levels. The JOA score was 9 points at the initial examination. b She underwent the microendoscopic laminoplasty from C4 to C7. Although the spinal cord was the presence of T2 high signal intensity on MR imaging, the posterior spinal decompression was successful. The JOA score was improving to 13 points at 24 months after surgery
Discussion
Numerous studies in endoscopic surgical visualization have been made for the past decade [1, 4, 7, 9, 10, 13, 16, 21]. Endoscopic assisted procedures have become increasingly popular for the treatment of a wide range of pathologic conditions of the spine, including hyperhydrosis, herniated discs, and fractures. Microendoscopic-assisted decompressive surgeries have been developed in herniated discs and spinal canal stenosis in the lumbar spine [7, 9, 13, 16]. This procedure is attractive because of its small skin incision, gentle tissue dissection, excellent visualization, and ability to achieve results equivalent to those of the open techniques. Visualization of the canal, ligamentum flavum, and existing nerve root interface is facilitated by the endoscope’s 25° viewing angle. Successive angulations of the working channel into a more lateral and horizontal positions allow access to the contralateral dorsal spinal canal because of the endoscope’s 25° angle. This visualization is superior to that of the unilateral open technique, wherein such an extreme contralateral angle of visualization is impossible to obtain with the operating microscope. The endoscopy provides wide visualization through the oblique lens, and the microendoscopic-assisted procedure also allows bilateral decompression via a unilateral approach, the so-called ‘‘unilateral approach and bilateral decompression’’ [11, 15, 19, 20]. In this study, the microendoscopic procedure is the hemilaminectomy on the approaching side and the laminotomy on the contralateral side. Finally, the laminoplasty is completed to enlarge the spinal canal. Exposure of the contralateral attachment of ligamentum flavum is critical to ensuring adequate bilateral decompression. Repositioning the working channel enables the base of the spinous process to be drilled away. This can then be extended to the ventral surface of the contralateral lamina. It is important to keep the surgical procedure, such as drilling without removing the ligamentum flavum to protect the spinal cord. After removal of the ligamentum flavum, the operation is deemed complete when both the posterior protrusion and beat of dural sac are visualized.
This study demonstrates the feasibility of decompressing the spinal canal using a unilateral endoscopic technique [11, 15, 19, 20]. Considerable experience is required to decompress the neural structures adequately. Microendoscopic techniques do involve a very steep learning curve that must be diligently overcome [7, 14]. The field of view through the endoscope is limited, making it difficult to appreciate the amount of bony work that has been performed. The two-dimensional view and hand–eye spatial separation of the endoscopic view can also be extremely disorienting. Ensuring a satisfactory canal decompression, while respecting the integrity of neural elements clearly, requires training and experience. Although the adjustment is being expanded from lumbar spine to cervical spine, authors’ institution makes a standard in the case of the expansion. The standard is that each surgeon needs cadaver training and much experience for lumbar spine diseases, such as herniated intervertebral disc and spinal canal stenosis.
The clinical outcomes of this microendoscopic decompression surgery for cervical myelopathy were excellent or showed good results equal to the reported conventional cervical expansive laminoplasty [2, 6, 8, 23]. The privilege of this surgical procedure was less invasive in comparison with the reported conventional cervical laminoplasty. In this study, the bleeding loss, CRP after surgery, VAS of axial pain, and hospital stay were less than those of conventional techniques in authors’ institution as follows (unpublished data): 30 patients underwent the conventional cervical expansive laminoplasty from 2004 to 2006, (1) the recovery rate was 51.5 ± 24.5%, (2) the mean bleeding loss was 178 ± 198 ml, (3) the mean value of CRP 3 days after surgery was 2.8 ± 1.7 mg/dl, (4) the VAS of axial pain at the final follow-up was 32 ± 32 mm, and (5) the mean hospital stay was 16 ± 3.9 days. As for the perioperative complication of this study, the incidence of C5 nerve root palsy was 2/51 patients (3.9%), and was similar to that of the conventional cervical laminoplasty, which has been reported to average 4.7% (range 0–30%) [17]. The developmental nerve root palsy appeared several days following surgery. The tethering effect on the nerve root induced by excessive posterior shift of the spinal cord after decompression is another hypothesized cause supported by a number of investigators [3, 12, 17, 18], but whether it is the main cause is still controversial.
The indication of this procedure was focused on a cervical myelopathy at one or two levels based on posterior factors, such as calcification or ossification of ligamentum flavum, and spondylotic pincers mechanism. In the multiple levels, the indication was limited on the spondylotic myelopathy excluded the developmental canal stenosis and OPLL. The enlarged space of spinal canal of this procedure was limited in comparison with that of the conventional laminoplasty because of laminotomy technique. For the developmental canal stenosis and continuous or mixed type OPLL, other cervical decompressive surgeries, such as the conventional expansive laminoplasty or anterior corpectomy would be alternative.
Conclusion
This observation suggests that the clinical outcomes of microendoscopic decompression surgery for cervical myelopathy were excellent or showed good results although the number of cases was limited. This minimally invasive technique would be helpful in choosing a surgical method for cervical myelopathy. In future, the minimally invasive spinal surgery will have developed to do it more safely with transducing navigation system and high vision imaging system.
References
- 1.Adamason TE. Microendoscopic posterior cervical laminoforaminotomy for unilateral radiculopathy: results of a new technique in 100 cases. J Neurosurg. 2001;95(Suppl):51–57. doi: 10.3171/spi.2001.95.1.0051. [DOI] [PubMed] [Google Scholar]
- 2.Chiba K, Ogawa Y, Ishii K, Takaishi H, Nakamura M, Maruiwa H, Matsumoto M, Toyama Y. Long-term results of expansive open-door laminoplasty for cervical myelopathy—average 14-year follow-up study. Spine. 2006;31:2998–3005. doi: 10.1097/01.brs.0000250307.78987.6b. [DOI] [PubMed] [Google Scholar]
- 3.Chiba K, Toyama Y, Matsumoto M, Maruiwa H, Watanabe M, Hirabayashi K. Segmental motor paralysis after expansive open-door laminoplasty. Spine. 2002;27:2108–2115. doi: 10.1097/00007632-200210010-00006. [DOI] [PubMed] [Google Scholar]
- 4.Fessler RG, Khoo LT. Minimally invasive cervical microendoscopic foraminotomy: an initial clinical experience. Neurosurgery. 2002;51:S37–S45. [PubMed] [Google Scholar]
- 5.Guiot BH, Khoo LT, Fessler RG. A minimally invasive technique for decompression of the lumbar spine. Spine. 2002;27:432–438. doi: 10.1097/00007632-200202150-00021. [DOI] [PubMed] [Google Scholar]
- 6.Hirabayashi K, Watanabe K, Wakano K, Suzuki N, Satomi K, Ishii Y. Expansive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine. 1983;8:693–699. doi: 10.1097/00007632-198310000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Ikuta K, Arima J, Tanaka T, Oga M, Nakano S, Sasaki K, Goshi K, Yo M, Fukagawa S. Short-term results of microendoscopic posterior decompression for lumbar spinal stenosis. Technical note. J Neurosurg Spine. 2005;2:624–633. doi: 10.3171/spi.2005.2.5.0624. [DOI] [PubMed] [Google Scholar]
- 8.Kawaguchi Y, Kanamori M, Ishihara H, Ohmori K, Abe Y, Kimura T. Pathomechanism of myelopathy and surgical results of laminoplasty in elderly patients with cervical spondylosis. Spine. 2003;28:2209–2214. doi: 10.1097/01.BRS.0000085029.65713.B0. [DOI] [PubMed] [Google Scholar]
- 9.Khoo LT, Fessler RG. Microendoscopic decompressive laminotomy for the treatment of lumbar stenosis. Neurosurgery. 2002;51:S146–S154. [PubMed] [Google Scholar]
- 10.McAfee PC, Regan JR, Zdeblick T, Zuckerman J, Picetti GD, Heim S, Geis WP, Fedder IL. The incidence of complications in endoscopic anterior thoracolumbar spinal reconstructive surgery. A prospective multicenter study comprising the first 100 consecutive cases. Spine. 1995;20:1623–1632. doi: 10.1097/00007632-199507150-00012. [DOI] [PubMed] [Google Scholar]
- 11.McCulloch JA. Microsurgical spinal laminotomies. In: Frymoyer JW, editor. The adult spine: principles and practice. New York: Raven Press; 1991. [Google Scholar]
- 12.Minoda Y, Nakamura H, Konishi S, Nagayama R, Suzuki E, Yamano Y, Takaoka K. Palsy of the C5 nerve root after midsagittal-splitting laminoplasty of the cervical spine. Spine. 2003;28:1123–1127. doi: 10.1097/00007632-200306010-00008. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa Y, Yoshida M, Kawakami M, Ando M, Hashizume H, Minamide A, Maio K, Enyo Y, Okada T. Posterior endoscopic surgery for lumbar disc herniation with contralateral symptoms—a report of two cases. Minim Invasive Neurosurg. 2006;49:282–285. doi: 10.1055/s-2006-950392. [DOI] [PubMed] [Google Scholar]
- 14.Nowitzke AM. Assessment of the learning curve for lumbar microendoscopic discectomy. Neurosurgery. 2005;56:755–762. doi: 10.1227/01.NEU.0000156470.79032.7B. [DOI] [PubMed] [Google Scholar]
- 15.Palmer S, Turner R, Palmer R. Bilateral decompression of lumbar spinal stenosis involving a unilateral approach with microscope and tubular retractor system. J Neurosurg (Spine 2) 2002;97:213–217. doi: 10.3171/spi.2002.97.2.0213. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Cruet MJ, Foley KT, Isaacs RE, Rice-Wyllie L, Wellington R, Smith MM, Fessler RG. Microendoscopic lumbar discectomy: technical note. Neurosurgery. 2002;51:S129–S136. [PubMed] [Google Scholar]
- 17.Sakaura H, Hosono N, Mukai Y, Ishii T, Yoshikawa H. C5 palsy after decompression surgery for cervical myelopathy: review of the literature. Spine. 2003;28:2447–2451. doi: 10.1097/01.BRS.0000090833.96168.3F. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka N, Nakanishi K, Fujiwara Y, Kamei N, Ochi M. Postoperative segmental C5 palsy after cervical laminoplasty may occur without intraoperative nerve injury: a prospective study with transcranial electric motor-evoked potentials. Spine. 2006;31:3013–3017. doi: 10.1097/01.brs.0000250303.17840.96. [DOI] [PubMed] [Google Scholar]
- 19.Tsai RY, Yang RS, Bray RS., Jr Microscopic laminotomies for degenerative lumbar spinal stenosis. J Spin Disord. 1998;11:389–394. [PubMed] [Google Scholar]
- 20.Weiner BK, Walker M, Brower RS, McCulloch JA. Microsurgery for lumbar spinal canal stenosis. Spine. 1999;24:2268–2272. doi: 10.1097/00007632-199911010-00016. [DOI] [PubMed] [Google Scholar]
- 21.Yabuki S, Kikuchi S. Endoscopic partial laminectomy for cervical myelopathy. J Neurosurg Spine. 2005;2:170–174. doi: 10.3171/spi.2005.2.2.0170. [DOI] [PubMed] [Google Scholar]
- 22.Yonenobu K, Abumi K, Nagata K, Taketomi E, Ueyama K. Interobserver and intraobserver reliability of the Japanese orthopaedic association scoring system for evaluation of cervical compression myelopathy. Spine. 2001;26:1890–1894. doi: 10.1097/00007632-200109010-00014. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida M, Otani K, Shibasaki K, Ueda S. Expansive laminoplasty with reattachment of spinous process and extensor musculature for cervical myelopathy. Spine. 1992;17:491–497. doi: 10.1097/00007632-199205000-00004. [DOI] [PubMed] [Google Scholar]