Abstract
Introduction:
Multiple myeloma (MM) is a relatively common and incurable hematological malignancy. Currently, there is no single standard therapy, with choice of treatment dependent on individual patient factors. Lenalidomide is an immunomodulatory drug with potent antitumor, antiangiogenic, immunomodulatory, and proapoptotic activity in MM.
Aims:
To evaluate the evidence for the use of lenalidomide in its current indication in relapsed or refractory MM, and additionally its investigational use for the treatment of newly diagnosed MM.
Evidence review:
In patients with relapsed and refractory MM, adding lenalidomide to high-dose dexamethasone significantly improves response rates and time-to-progression, relative to high-dose dexamethasone alone. This translates into a significant extension of overall survival (with a median extension of 9.1 months in a pivotal phase III study). Outcome is independent of patient age, number of previous therapies, type of previous therapy (including thalidomide or autologous stem cell transplantation), renal impairment, and β2-microglobulin level. Evidence suggests that combining lenalidomide with low-dose dexamethasone improves outcomes in patients with newly diagnosed disease and is superior to lenalidomide combined with high-dose dexamethasone. Myelosuppression is the predominant toxicity observed, although some studies have shown high incidences of venous thromboembolism in the absence of prophylactic antithrombotic anticoagulation therapy. There is currently only limited evidence regarding the health economics of lenalidomide.
Role in therapy:
The encouraging results obtained with lenalidomide alone and in combination with dexamethasone in patients with relapsed or refractory MM have led to its adoption as a recommended therapy in patients who have received at least one prior treatment. Emerging evidence supports the ongoing investigation of lenalidomide in combination with low-dose dexamethasone, and in other combinations including bortezomib, for use both in relapsed, refractory, and newly diagnosed MM.
Keywords: lenalidomide, evidence, multiple myeloma, outcomes, treatment
Core evidence clinical impact summary for lenalidomide in relapsed, refractory multiple myeloma
| Outcome measure | Evidence | Implications |
|---|---|---|
| Patient-oriented evidence | ||
| Prolongation of OS | Substantial | Median OS is significantly longer with lenalidomide plus dexamethasone than with dexamethasone alone |
| Prolongation of TTP | Substantial | Median TTP is significantly longer with lenalidomide plus dexamethasone than with dexamethasone alone |
| Adverse events | Substantial | A significantly higher proportion of patients treated with lenalidomide plus dexamethasone report grade 3 or 4 adverse events, with neutropenia, thrombocytopenia, anemia, and venous thromboembolic events being the most important |
| Improvement in quality of life | No evidence | |
| Disease-oriented evidence | ||
| Improvement in response rate (overall and complete response) | Substantial | A significantly higher proportion of patients treated with lenalidomide plus dexamethasone compared with dexamethasone alone respond to treatment |
| Economic evidence | ||
| Health care resource utilization | Limited | Direct drug costs of the approved lenalidomide regimen appear to be similar to or higher than related novel agent regimens |
| Cost effectiveness | No evidence | |
Abbreviations: OS, overall survival; TTP, time to progression.
Scope, aims, and objectives
Lenalidomide1 in combination with dexamethasone is indicated for the treatment of multiple myeloma (MM) in patients who have received at least one prior therapy.2,3 This review provides a background to MM, summarizes current therapies and unmet needs, and evaluates the current evidence for the use of lenalidomide. Disease-oriented outcomes are evaluated, including response rates, response duration, time-to-progression (TTP), overall survival (OS), and one-year survival, as well as safety and tolerability. A search of the literature to-date did not identify any studies with patient-reported outcomes, such as quality of life, functional status, treatment satisfaction, adherence, or symptom relief. These parameters of clinical benefit are therefore not included in this review.
Methods
The English language medical literature was reviewed to identify abstracts and articles relating to lenalidomide in MM. Relevant databases were searched on April 11th, 2008 using the search terms “lenalidomide OR Revlimid OR CC-5013 AND ‘multiple myeloma’”. Each database was searched from the beginning of the database to the date of the search, unless otherwise specified. The following databases, including proceedings of oncology-based meetings, were searched for relevant abstracts and full text articles:
Cochrane Database of Systematic Reviews (CDSR), http://www.cochrane.org
ClinicalTrials.gov clinical trial register, http://www.clinicaltrials.gov
ClinicalStudyResults.org clinical trial register, http://www.clinicalstudyresults.org
American Society of Hematology (ASH), 2005–2007, http://bloodjournal.hematologylibrary.org/search.dtl
International Myeloma Working Group (IMWG), 2007
European Hematology Association (EHA), 2008, http://www.ehaweb.org/
American Society of Clinical Oncology (ASCO), 2005–2007, http://www.asco.org/portal/site/ASCO
An additional search of the English language literature on PubMed conducted on April 11, 2008 was further refined to provide specificity for the search using the limits “clinical trial”, “meta analysis”, “randomized controlled trial”, and “humans”. The original PubMed search yielded 228 records including abstracts, and was subsequently narrowed to 18 records with these limits imposed. Six records were excluded from these 18 records for reasons of relevance (n = 2), incorrect indication (n = 3), and nonsystematic review (n = 1). A further search of PubMed conducted on September 1, 2008 and time-limited from the previous search but otherwise using the same limits yielded one additional record, which was included. A similar search of the ASCO website on the same day yielded 13 additional abstracts, while hand-searching of the European Hematology Association (EHA; http://www.ehaweb.org/) 2008 meeting abstracts produced 10 new abstracts. Eighteen of these records were included in the clinical evidence. No systematic reviews were identified for the use of lenalidomide in MM. Two papers and 18 abstracts were of level 2 evidence, and another 11 papers and 25 abstracts were of level ≥3 evidence. The levels of evidence identified from the literature searches are summarized in Table 1. Criteria for exclusion were nonsystematic reviews, case studies, case series, phase I clinical trials or interim analyses of phase I/II clinical trials, and duplicate abstracts defined as presentation of similar data in the same calendar year. Substudy analyses were included at the same level of evidence as for the original study. Descriptive and observational studies, including retrospective studies, were included only for evaluation of safety.
Table 1.
Evidence base included in the review
| Category |
Number of records |
|
|---|---|---|
| Full papers | Abstracts | |
| Initial search | 228 | 216 |
| Records excluded | 216 | 191 |
| Records included | 12 | 25 |
| Search update, new records | 1 | 23 |
| Records excluded | 0 | 5 |
| Records included | 1 | 18 |
| Level 1 clinical evidence | 0 | 0 |
| Level 2 clinical evidence | 2 | 18 |
| Level ≥ 3 clinical evidence | 11 | 25 |
| Trials other than RCT | 11 | 25 |
| Case reports | 0 | 0 |
| Economic evidence | 0 | 3 |
Notes: For definition of levels of evidence, see Core Evidence website (http://www.dovepress.com/core-evidence-journal).
Abbreviation: RCT, randomized clinical controlled trial.
Disease overview
MM is a hematological malignancy of plasma cells characterized by clonal expansion, bone marrow infiltration, lytic bone disease, hypercalcemia, renal insufficiency, and the presence, in the vast majority of patients, of immunoglobulin paraproteins (M-protein) in the serum and/or urine.4 The disease arises from a B-cell of the normal germinal center as a result of a chromosomal translocation that places an oncogene under the control of immunoglobulin enhancers.5 Despite recent therapeutic advances, including high-dose chemotherapy and autologous stem cell transplantation (SCT), MM is an incurable disease with a median overall survival (OS) of three to four years and a five-year relative survival of approximately 33% in 2007.6,7 During the past 10 years, survival rates for MM have increased; however, relapse remains inevitable and, until recently, there were few effective salvage therapies.8 Novel treatment options, such as thalidomide, bortezomib, and lenalidomide, are increasingly recognized as important and potent new therapies in overcoming resistant disease and contributing to improved outcome.8,9
Epidemiology
In the US, MM is the second most common hematologic malignancy after non-Hodgkin’s lymphoma, with an estimated 19,920 new cases in 2008.10 This figure represents about 1.4% of all new cancer cases, including 14% of new hematologic malignancies.10 However, with a median survival in the order of three to four years,6 the disease claims a higher proportion of cancer-related deaths, estimated at 10,690 or 2% of all cancer deaths, including 20% of deaths due to hematologic malignancies.10
MM is predominantly a disease of older patients, with a mean age at diagnosis of 70 years.11 During 2000–2005, approximately 64% of diagnoses of MM were in people aged 65 years and older, and around 96% were in people aged 45 years and older. Although age is the most significant risk factor for MM, disease incidence is also higher among men than women (7.0 vs 4.6 per 100,000, respectively) and among African-Americans than Caucasian Americans (men: 14.4 vs 6.6 per 100,000, respectively; females: 9.8 vs 4.1 per 100,000, respectively).11 The economic burden of MM has yet to be well described, but its high mortality and considerable antecedent morbidity is likely to make this substantial.
Clinical features
MM can be classified on the basis of symptoms, with symptomatic disease requiring evidence of related organ- or tissue-impairment, which is typically manifested by increased calcium, renal insufficiency, anemia, and/or bone lesions secondary to the plasma cell proliferative process.12 Other symptoms include bone pain, fatigue, fractures, recurrent infections, and weakness. Although the detection of immunoglobulin M-protein is characteristic, this is patient-specific and is absent in the 1%–2% of patients with nonsecretory MM.4 In asymptomatic or smoldering MM, M-protein and/or bone marrow clonal cells are present, but there is no related organ- or tissue-impairment. Up to 25% of patients may have a smoldering pattern of disease at presentation.13
Diagnosis
A diagnosis of MM is often made incidentally during investigations of other conditions or as part of routine screening, as overt features of the disease may be absent. Assessment of serum and urine samples for M-proteins helps to establish a diagnosis, with immunofixation considered the gold standard when looking to confirm the presence of M-proteins and to distinguish heavy versus light chain types.12 Monoclonal gammopathies need to be excluded from polyclonal gammopathies because only the former are associated with neoplasia or potential neoplastic events. Serum protein electrophoresis is a suitable screening assay for M-protein whenever MM or related disorders are suspected, or in the presence of unexplained weakness, fatigue, anemia, infection, back pain, osteopenia, osteolytic lesions, or spontaneous fractures.12 Elevation of erythrocyte sedimentation rate, increased serum viscosity, hypergammaglobulinemia, hypercalcemia, Bence Jones proteinuria, renal insufficiency, and immunoglobulin deficiency (especially in the context of recurrent infection) may also be indicative and warrant screening for M-protein. Studies should include complete blood count, serum chemistry, bone marrow aspirate, and trephine biopsy for cytogenetic analysis of immunoglobulin translocations, as well as fluorescence in situ hybridization (FISH) and assessment of β2-microglobulin, C-reactive protein, and lactate dehydrogenase.12
A diagnosis of MM requires M-protein levels of ≥30 g/L and/or ≥10% or more plasma cells in the bone marrow.12 When these features are present together with related organ- or tissue-impairment, a diagnosis of symptomatic (versus asymptomatic) MM may be applied. Any patient with a serum M-protein level of <30 g/L and/or <10% clonal plasma cells in the bone marrow in the absence of myeloma-related organ- or tissue-impairment is considered to have monoclonal gammopathy of undetermined significance (MGUS).
Disease staging
Two main staging systems are currently in use in MM, the International Staging System (ISS) and the Durie–Salmon system.6,14 The staging system most widely used since 1975 is the Durie–Salmon system, which is based on four clinical parameters that predict tumor burden: hemoglobin level; serum calcium level; number of bone lesions; and M-protein levels14 (Table 2). Serum creatinine level is additionally used to sub-categorize patients in each of the three stages according to renal function.
Table 2.
| Stage | Durie–Salmon criteria | ISS criteria |
|---|---|---|
| I | All of the following:
|
Serum β2-M < 3.5 mg/L and serum albumin ≥3.5 g/dL |
| II | Neither stage I nor stage III | Neither stage I nor stage III according to the following subcategories:
|
| III | One or more of the following:
|
Serum β2-M ≥ 5.5 mg/L |
Notes:
Durie-Salmon sub-classifications (either A or B): A: serum creatinine<2.0 mg/dL; and B: serum creatinine ≥2.0 mg/dL.
Abbreviations: β2-M, β2-microglobulin; Ig, immunoglobulin; ISS, International Staging System.
Although the Durie–Salmon system remains in widespread use, it is limited by observer dependence on assessments of the number of lytic lesions, by the characterization of new prognostic factors, and some redundancy. With respect to the latter, patients with stage I disease are not separated from those with smoldering myeloma in that neither group requires immediate treatment.15 Similarly, patients with either stage II or III disease typically have active, symptomatic myeloma. Moreover, with the recognition of the prognostic value of serum β2-microglobulin and serum albumin, clinicians are increasingly complementing the Durie–Salmon system with the ISS.6
The ISS has been proposed as a simple, reliable, and more cost-effective predictor of survival in MM.6,15 Based on a collaboration involving investigators from 17 institutions worldwide and data on 11,171 previously untreated symptomatic myeloma patients, the ISS separates patients into three prognostic groups based on serum ß2-microglobulin and albumin levels at the time of starting initial systemic therapy (Table 2). The ISS has been validated by geographic region, by age (<65 years versus ≥65 years), by standard therapy versus autologous SCT, and in comparison with the Durie–Salmon and other staging systems.6,16
Prognosis
There is significant variation in the survival of patients with MM. Based on the ISS, the median survival of patients with stage I, II, or III disease is estimated at 62, 44, and 29 months, respectively.6 Although serum β2-microglobulin and albumin levels combine in the ISS to provide a powerful prognostic tool, a number of independent prognostic markers have been described that may further assist in predicting outcome.17 Many established prognostic markers allowing identification of high-risk patients early in the disease course have been derived from studies of conventional chemotherapy and include age, β2-microglobulin level, World Health Organization (WHO) performance status, serum calcium, interleukin-6 (IL-6) level, bone marrow plasma cell labeling index, and morphological features.18,19 However, in the current era of high-dose chemotherapy, novel immunomodulatory agents, and new small molecule inhibitors, a number of other prognostic markers relating to mechanisms of disease progression are now considered to be important.17
Abnormal cytogenetics play a dominant role in predicting the outcome of patients with acute leukemia, and the evidence now suggests that cytogenetics have a similar role in MM. Tricot and colleagues20,21 observed, using standard cytogenetic techniques, that in patients with newly diagnosed or previously treated disease, the presence of partial or complete deletions of chromosome 13 (del13q) and 11q abnormalities were associated with inferior event-free survival (EFS) and OS. In addition, they noted a significant association between the unfavorable karyotypes and immunoglobulin A (IgA) isotype, elevated levels of β2-microglobulin, and age >60 years.20
Conventional cytogenetic analysis is hampered by low mitotic activity of myeloma cells and may miss up to half of chromosome 13 abnormalities. Using FISH, Facon and colleagues22 demonstrated that in MM patients receiving first-line high-dose chemotherapy, the presence of chromosome 13 abnormalities was strongly predictive of poor survival, especially when associated with a β2-microglobulin level of ≥2.5 mg/L. FISH has since been used to identify patients with poor, intermediate, and better prognosis according to immunoglobulin heavy chain translocations and chromosome 13 abnormalities with other abnormalities such as t(4,14), t(14,16), and del17q, emerging as prognostically unfavorable.23 However, as combinations of independent prognostic factors provide greater power than any one prognostic factor alone, the technique with potentially the highest utility in the future is gene expression profiling, which allows the simultaneous characterization of many different cytogenetic markers.24
Evaluation of response
Evaluation of tumor response to treatment is based on the assessment of changes in serum and/or urinary M-protein level. The most commonly used criteria for evaluating response are those introduced in 1998 by the European Group for Blood and Marrow Transplant (EBMT).4 The criteria for a complete response (CR) require <5% plasma cells in the bone marrow and the complete absence of M-protein by immunofixation and electrophoresis, with the response maintained for a minimum of six weeks. A partial response (PR) is defined as a reduction in serum M-protein levels of ≥50% and a reduction in 24-hour urinary light chain excretion either by ≥90% or to <200 mg, maintained for a minimum of six weeks. Near CR (nCR), a subset of PR, is defined as a CR with a positive immunofixation test but otherwise satisfies the criteria for CR.25 A minimal response (MR) is defined as a reduction in serum M-protein levels of 25%–49% and a 50%–89% reduction in 24-hour urinary light chain excretion that still exceeds 200 mg, maintained for a minimum of six weeks.
The International Myeloma Working Group (IMWG) has recently proposed changes to the original EBMT criteria in order to facilitate precise comparisons between new treatment strategies and to provide clarification of response in the clinical setting.26,27 For patients with measurable levels of serum and urine M-protein, the criteria for CR and PR remain unchanged. The most important changes are the inclusion of a new category of stringent CR (sCR) to reflect recent advances in therapy, and the inclusion of the serum-free light chain (FLC) assay to allow evaluation of patients with oligosecretory disease. The subcategories of nCR and very good PR (VGPR) have been integrated into a single category, VGPR, with sCR defined as CR based on EBMT criteria with the additional requirement for a normal FLC ratio and the absence of clonal cells in bone marrow by immunohistochemistry or immunofluorescence. VGPR is defined as serum and urine M-protein levels detectable by immunofixation, but not on electrophoresis, or a ≥90% reduction in serum M-protein plus urinary M-protein level <100 mg per 24 hours. The IMWG criteria eliminate the mandatory six-week period to confirm response and instead have a non-time-dependent confirmation for relapse and/or disease progression.26 Further modifications to this (including the restoration of MR as a response parameter associated with clinical benefit) as well as validation of key aspects, such as the assessment of serum FLC are anticipated.28
Goals of therapy
Treatment prolongs survival in MM, although remissions are inevitably followed by relapse.4 Therefore, the aim of treatment includes controlling disease by safely achieving a sequence of durable responses, without compromising quality of life.29 Given that current assessment techniques may not reflect true molecular remission, even using sCR or molecular CR criteria, and effective suppression of abnormal karyotype has been linked with long-term survival, suppression of abnormal karyotype may represent a part of the treatment goal to eradicate the myeloma clone.30 As the choice of therapy is influenced by patient factors, such as age and comorbidities, the goals of therapy are individual to the patient. Thus, CR may be the primary goal in a younger patient whereas control of disease activity to prevent progressive organ damage and to preserve performance status may be the goal in an older, more frail patient. The advent of novel therapies has dramatically expanded the options available for both younger and older patients in this context, especially given the favorable tolerability profiles seen with newer combinations, including bortezomib-based therapy as well as immunomodulatory approaches.
Current therapy options
Treatment recommendations for MM are dynamic and there is currently no single standard therapy for active myeloma. For patients with asymptomatic disease, a watch-and-wait approach is adopted because at present there is no evidence of benefit for early treatment in this population.31,32 Patients with symptomatic disease involving at least one of the following: hypercalcemia, renal insufficiency, anemia, or bone lesions require active treatment for which there are multiple options.12 These include proteasome inhibition (such as bortezomib), immunomodulating agents (such as thalidomide and lenalidomide), corticosteroids, bisphosphonates, conventional chemotherapy, radiotherapy, and autologous SCT.
Newly diagnosed disease
In patients with newly diagnosed disease who are eligible for autologous SCT, the initial goal of treatment is to reduce tumor burden with induction therapy. Induction regimens that are sufficiently nontoxic to hematopoietic stem cells include single-agent dexamethasone, combination vincristine + doxorubicin + dexamethasone (VAD), and novel regimens such as bortezomib-based treatments, thalidomide + dexamethasone, and lenalidomide + dexamethasone.7,27 More recent data suggest VAD has little or no role in induction given its inferiority to novel regimens demonstrated in numerous randomized trials.27 Following stem cell harvest, high-dose therapy is the standard of care for those undergoing autologous SCT given its survival advantage over conventional chemotherapy,33 which may involve a single autologous SCT, tandem autologous SCT, allogeneic SCT or syngeneic SCT. Interim data suggest there is no survival advantage of tandem over single autologous SCT, with the latter also being preferred over allogeneic SCT due to its superior efficacy in the absence of a syngeneic donor, its safety, and the absence of biological age-related disease differences.34 However, preliminary results for nonmyeloablative allogeneic transplantation are encouraging and support the feasibility of this approach.34 As almost all patients relapse, maintenance treatments that help prolong the duration of remission and survival are used, including thalidomide.35–37
Patients ineligible for SCT due to their age, performance status, comorbidities, or other factors have in the past received melphalan plus prednisone as the standard of care for induction therapy.38 However, other combinations have emerged, with the evidence base, in particular, supporting the combination of melphalan, prednisone, and thalidomide27,39 and most recently melphalan, prednisone, and bortezomib.40 Indeed, combination approaches with bortezomib as the first in class proteosome inhibitor, have shown particular promise both in autologous SCT eligible and nontransplantation populations, with high-quality responses seen.27 Other first-line options include melphalan, prednisone, and lenalidomide,41 lenalidomide plus dexamethasone,42,43 or dexamethasone plus thalidomide or bortezomib.39,44 The combination of lenalidomide and dexamethasone is now recognized by the National Comprehensive Cancer Network (NCCN) practice guidelines as an option for primary induction therapy in transplantation candidates based on category of evidence 2B (lower-level evidence including clinical experience and nonuniform consensus),27 together with bortezomib-based therapies.27
Relapsed or refractory disease
An ongoing effort toward understanding the molecular pathogenesis of MM has led to the rational development of novel therapeutic agents, such as the immunomodulatory agents thalidomide and lenalidomide, and the proteasome inhibitor bortezomib, in this setting. The combination of these agents with dexamethasone in particular has shown impressive activity in relapsed or refractory MM and adds to the wide range of therapeutic options available.45 Other options include conventional chemotherapy, melphalan plus prednisone, dexamethasone alone in good-risk patients and, in patients with early stem cell harvest, autologous SCT may be considered as salvage therapy.27
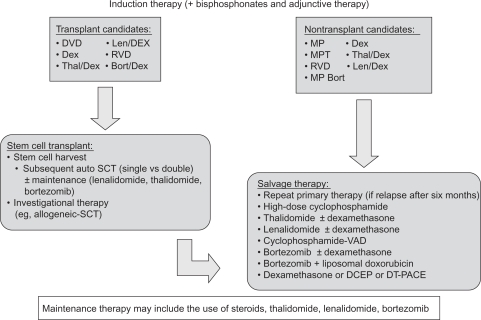
According to NCCN guidelines, patients who relapse after more than six months may benefit from reduction with the primary induction therapy.27 Conventional dose salvage therapy in combination with novel agents can be considered in patients with progressive disease following allogeneic or autologous SCT, in patients with primary progressive disease following initial allogeneic or autologous SCT, and in patients who are not candidates for transplantation with progressive or relapsing disease. Possible salvage therapies with category 1 evidence (uniform NCCN consensus based on high-level evidence) or 2A (uniform NCCN consensus based on lower-level evidence including clinical experience) are summarized in Figure 1, together with recommended options for induction and maintenance therapies.27 As an example, lenalidomide combined with dexamethasone has received US Food and Drug Administration (FDA) approval, based on two studies of 692 patients, for use in MM patients with at least one prior treatment and so is assigned a category 1 recommendation.46 The NCCN recommends anticoagulation therapy in patients treated with lenalidomide plus dexamethasone with lenalidomide monotherapy as a category 2A recommendation.27
Figure 1.
Treatment options in multiple myeloma.27
Abbreviations: Bort, bortezomib; Bort/Dex, bortezomib and dexamethasone; DCEP, dexamethasone, cyclophosphamide, etoposide, and cisplatin; Dex, dexamethasone; DT-PACE, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide; DVD, liposomal doxorubicin, vincristine, and dexamethasone; Len/Dex, lenalidomide plus dexamethasone; MP, melphalan and prednisone; MPT, melphalan, prednisone, and thalidomide; SCT, stem cell transplantation; RVD, lenalidomide, bortezomib and dexamethasone; VAD, vincristine, doxorubicin, and dexamethasone; Thal/Dex, thalidomide plus dexamethasone.
Thalidomide
As a salvage therapy for patients with relapsed or refractory MM, thalidomide has been investigated as monotherapy, in combination with dexamethasone (Thal + Dex), with bortezomib and dexamethasone, and in combination with dexamethasone, cisplatin, doxorubicin, cyclophosphamide, and etoposide.47–49 As a single-agent therapy, thalidomide has demonstrated an overall response rate (ORR) approaching 30%, with a relatively low CR rate of 1.6%, and an incidence of venous thromboembolism (VTE) of 3%, and a rate of discontinuation due to intolerance of 15%.50 The combination of thalidomide and dexamethasone offers significantly greater activity than respective single-agent therapies, with a rate of PR or better in the order of 55%–59% (CR 0%–23%), and a median survival of 13–26 months in relapsed or refractory disease.51–53 Low-dose thalidomide has been investigated in combination with cyclophosphamide and dexamethasone, yielding an ORR in one study of 79%, including a CR rate of 17%.54 Two-year OS and EFS were 73% and 34%, respectively.
Bortezomib
Bortezomib was first studied in the setting of relapsed or refractory MM, and showed an overall response rate of 28% (PR or better) including 10% CR/nCR in heavily pretreated patients,25 leading to its accelerated approval by the FDA in 2003. In a recent systematic analysis, single-agent bortezomib was compared with single-agent thalidomide in patients with relapsed or refractory MM.55 The ORR was 41% for patients receiving bortezomib versus 22% for thalidomide. Similarly, bortezomib monotherapy yielded a higher ORR than single-agent dexamethasone in the relapse setting (38% vs 18%, respectively) and a higher CR rate (6% vs 1%).56 Bortezomib was associated with improved TTP compared with single-agent dexamethasone (6.2 months vs 3.5 months, respectively) and one-year survival (80% vs 66%). A recent update showed an ORR of 43% (PR or better) and a median OS of 29.8 months.57 There is also evidence showing increased response rates for bortezomib in combination with dexamethasone.25,58,59 In combination with low-dose melphalan and dexamethasone, bortezomib yielded an ORR of 69%, including 29% with VGPR or better.60 The recent FDA approval of a novel bortezomib combination with pegylated liposomal doxorubicin was based on a priority review of interim data from a phase III clinical trial, which showed that this combination significantly extended TTP compared with bortezomib alone (9.3 months vs 6.5 months, respectively). OS was also significantly improved compared with bortezomib alone.61 Bortezomib is currently being investigated in the relapsed or refractory disease setting in combination with numerous novel agents, including tanespimycin (an inhibitor of heat-shock protein 90), perifosine (an AKT inhibitor), and oral vorinostat and related histone deacetylase inhibitors.57,62,64,65 Importantly, a four-drug combination has shown particular promise, with a phase I/II trial of bortezomib, melphalan, prednisone, and thalidomide yielding an ORR of 67% (all PR), including 43% with a VGPR.66
Unmet needs
Corticosteroids and alkylating agents have formed the mainstay of therapy for decades and continue to be used in combination regimens, where drugs with different mechanisms of action can offer important synergistic effects. However, more effective targeted therapies are beginning to emerge as a result of an improved understanding of the biology of MM.13 The rational development of these therapies, which include lenalidomide, thalidomide, and bortezomib, provides an opportunity to treat patients more effectively with fewer side-effects while aiming for durable responses. With mechanisms of action that are distinct from cytotoxic chemotherapies, these novel treatments (ORR) will continue to offer synergistic effects with conventional treatments and so offer potential survival benefit.
Thalidomide was the first immunomodulatory drug to demonstrate significant activity in newly diagnosed and relapsed disease, particularly in combination with dexamethasone. Its anti-MM effects are directed by multiple mechanisms that include antiangiogenesis, immunomodulation of the tumor microenvironment, and induction of apoptosis in tumor cells.49 However, in addition to having teratogenic potential, thalidomide is associated with many possible side effects, including sedation, fatigue, skin rash, and constipation; less common side effects include bradycardia, impotence, neutropenia, dysmenorrhea, and edema. Importantly, long term use can cause peripheral neuropathy.9 In addition to neuropathy, perhaps the most worrying side effect is VTE, including deep vein thrombosis (DVT), which is particularly problematic in combination with multiagent chemotherapy and dexamethasone.67,68
Lenalidomide
As a means of enhancing the immunomodulatory effects and overcoming the nonhematological adverse events of thalidomide, analogs such as lenalidomide have been developed.9,69 Like thalidomide, lenalidomide exerts pleiotropic effects, which include immunomodulatory, antiangiogenic, and antineoplastic activities.69 In preclinical studies, lenalidomide has demonstrated more potent anti-MM activity than its parent compound and its toxicity profile is more favorable.9,69 After comprehensive phase I and phase II trials in advanced MM, followed by two pivotal phase III trials, lenalidomide was approved by the US FDA and by the European Medicines Agency (EMEA) in June 200770 for use in combination with dexamethasone in the treatment of MM in patients who have received at least one prior therapy.
Mechanism of action in MM
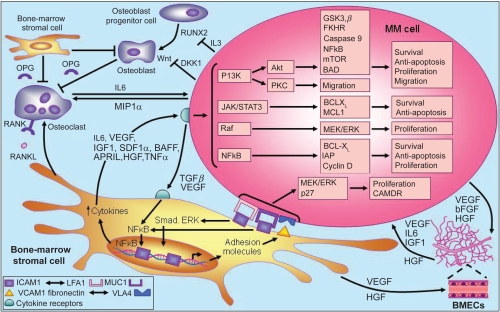
The molecular mechanisms associated with disease progression in MM are dependent on the interaction between MM cells and the bone marrow microenvironment.71 Briefly, the adhesion of MM cells to bone marrow stromal cells triggers the release of cytokines that mediate separate pathways of MM cell growth and survival, including proliferation, antiapoptosis, cell cycle progression, and migration. Stromal cell-derived IL-6, tumor necrosis factor-alpha (TNF-α) and vascular endothelial growth factor (VEGF), for example, are involved in the activation of several MM cell signaling pathways, including phosphatidylinositol 3-kinase (PI3K)/Akt, Janus kinase (JAK)/signal transducer and activator of transcription 3 (STAT3), Raf/Mek/mitogen-activated protein kinase (MAPK), and NF-κB, together with their downstream targets (Figure 2).72
Figure 2.
Pathogenesis of multiple myeloma. Copyright © 2007. Adapted with permission from Macmillan Publishers Ltd. Hideshima T, Mitsiades C, Tonon G, et al Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–598.
Abbreviations: BAD, BCL-XL associated death promoter; BCL-XL, basal cell lymphoma-extra large; bFGF, basic fibroblast growth factor; BMECs, bone marrow endothelial cells; CAMDR, cell adhesion-mediated drug resistance; DKK1, Dickkopf-1; FKHR, forkhead transcription factor; GSK3β, glycogen synthase kinase 3-beta; HGF, hepatocyte growth factor; IAP, inhibitor of apoptosis proteins; ICAM, intercellular adhesion molecule;IGF-1, insulin-like growth factor;IL, interleukin; JAK/STAT, janus kinases/signal transducers, and activators of transcription; LFA, lymphocyte function-associated antigen; MCL, myeloid cell leukemia; MEK/ERK, mitogen-activated protein kinase/extracellular regulated kinase; MIP1 α, macrophage inflammatory protein 1alpha; mTOR, mammalian target of rapamycin; MUC, mucin; NFκB, nuclear factor kappa B; OPG, osteoprotegerin; PKC, protein kinase C; RANKL, receptor activated NFκB ligand; RUNX, runt-related transcription factor; SDF1α, stromal cell derived factor 1-alpha; TGFβ, transforming growth factor beta; TNF-α, tumor necrosis factor-alpha; VCAM, vascular cell adhesion molecule; VEGF, vascular endothelial growth factor; VLA4, very late antigen-4.
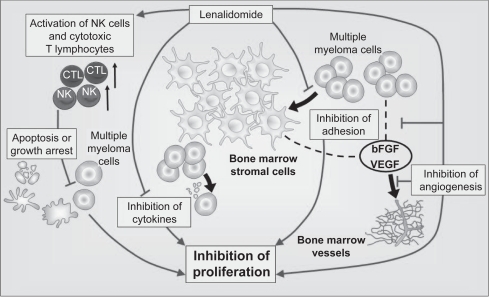
Lenalidomide has been shown to affect many of the interactions central to myeloma development by both direct and indirect mechanisms.73 The direct effects of lenalidomide include induction of apoptosis or cell cycle arrest of the tumor cell71,74,75 and indirect effects involving alteration of the tumor microenvironment and augmentation of the innate and acquired immune responses. Combined, these effects result in effective tumor cell reduction and suppression. This duality of action may be important in the treatment of MM.76
The rational development of lenalidomide as an anticancer agent followed the success of thalidomide, a potent inhibitor of TNF-α with antiangiogenesis activity and T-cell costimulatory activity.73 Compared with its parent compound, lenalidomide is a more powerful inhibitor of TNF-α secretion by activated monocytes.77,78 In addition to TNF-α, lenalidomide also inhibits transforming growth factor-beta (TGF-β) and the proinflammatory cytokines, IL-1β, IL-6, and IL-12, whereas secretion of the antiinflammatory cytokine IL-10 appears to be increased by lenalidomide.78,79 This differential regulation of cytokine activity, and particularly IL-6 activity, provides the basis for lenalidomide altering the bone marrow microenvironment in which the aberrant expression of proinflammatory cytokines is important for the growth and survival of MM cells.71 Moreover, inhibition of VEGF by lenalidomide may alter the bone marrow microvasculature, thereby making the tumor microenvironment less hospitable for MM cell growth, migration, and survival.71,78 VEGF inhibition likely occurs via the PI3K/Akt signaling pathway, which normally becomes phosphorylated in response to VEGF stimulation.80,81
Lenalidomide is up to 2,000 times more potent than thalidomide in stimulating the proliferation of T-cells and up to 100 times more potent at increasing the release of IL-2 and interferon-γ (IFN-γ).77 This T-cell costimulatory activity suggests that lenalidomide is able to act as an adjuvant to promote type 1 cell-mediated antitumor immune responses involving both CD4+ T-helper cells and CD8+ cytotoxic T-cells.73 The ability of lenalidomide to enhance activator protein-1 and NF-κB activity in antigen-primed T-cells has been proposed as a T-cell costimulatory mechanism, which may not only overcome T-cell anergy, but also potentiates any non-T-cell receptor-mediated signaling.78 In addition to bolstering the adaptive immune response, there is also evidence that lenalidomide can enhance innate immunity and natural killer cell-mediated lysis of MM cells in particular via its effects on IL-2 production by T-cells.71,73,82
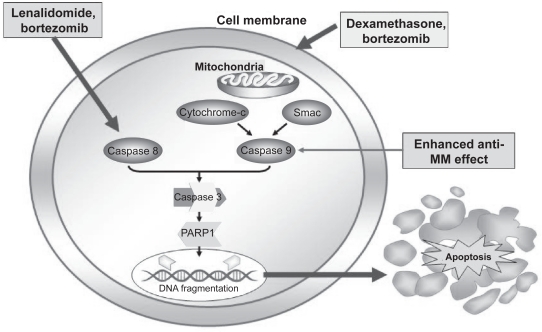
Lenalidomide has been shown to directly potentiate apoptosis of MM cells via several pathways. These include inhibition of expression of the cellular inhibitor of apoptosis protein-2, potentiation of the activities of other apoptosis inducers such as TNF-related apoptosis-inducing ligand (TRAIL), increased sensitivity to Fas induction, and enhanced caspase 8 activation.78 Caspase 8, an integral component of Fas-mediated apoptosis, is sharply upregulated by lenalidomide (Figure 3).63 In addition, dexamethasone activates caspase 9 indicating that the two drugs in combination generate dual signals capable of enhanced cell death.71 Lenalidomide has been associated with direct antiproliferative activity against MM cells in the absence of immune cells or proapoptotic mechanisms by inducing G1 growth arrest.74,78 Importantly, lenalidomide inhibits the proliferation of malignant B cells while protecting normal CD34+ progenitor cells.75 The various mechanisms of action of lenalidomide are summarized in Figure 4.
Figure 3.
Caspase-mediated pathway. Copyright © 2007. Reproduced with permission by American Society of Hematology. Richardon P, Mitsiades C, Schlossman R, et al. The treatment of relapsed and refractory multiple myeloma. Hematology Am Soc Hematol Educ Program. 2007:317–323.
Abbreviations: MM, multiple myeloma; PARP1, Poly(adenine diphosphate-ribose) polymerase 1; Smac, second mitochondria-derived activator of caspase; Caspase, cysteine-aspartic acid proteases.
Figure 4.
Mechanism of action of lenalidomide in multiple myeloma. Copyright © 2009. Adapted with permission from Richardson P, Jagannanth S, Hussein M, et al. Safety and efficacy of single-agent lenalidomide in patients with relapsed and refractory multiple myeloma. Blood. 2009;114:772–778.
Abbreviations: bFGF, basic fibroblast growth factor; CTL, cytotoxic T lymphocytes; NK, natural killer cells; VEGF, vascular endothelial growth factor.
Clinical evidence for lenalidomide in MM
Newly diagnosed disease
Lenalidomide is not yet approved for use in patients with previously untreated disease. However, several clinical studies have reported promising response and survival outcomes in this group of patients.
Response rates and duration of response
Lenalidomide plus dexamethasone
In a phase III study, which had a planned enrollment of 500 patients with newly diagnosed MM but subsequently closed after 198 patients were enrolled due to external data affecting the acceptability of the control arm, patients were randomized to lenalidomide 25 mg/day plus high-dose dexamethasone, or high-dose dexamethasone 40 mg/day plus placebo.83 Lenalidomide was administered on 28 of 35 days for three induction cycles, and then 21 of 28 days as maintenance thereafter. High-dose dexamethasone was administered on days 1–4, 9–12, and 17–20 during induction, and then days 1–4 and 15–18 during maintenance. Treatment with lenalidomide plus high-dose dexamethasone yielded an ORR of 85.3% and a CR rate of 22.1% versus treatment with high-dose dexamethasone alone (51.3% and 3.8%, respectively; P = 0.001).
A second phase III randomized study compared lenalidomide plus high-dose dexamethasone with lenalidomide plus low-dose dexamethasone in 445 patients with newly diagnosed MM.84 Lenalidomide was dosed at 25 mg/day on days 1–21 every 28 days. Patients in the high-dose arm received dexamethasone 40 mg/day on days 1–4, 9–12, and 17–20 every 28 days, whereas patients in the low-dose arm received dexamethasone 40 mg/day on days 1, 8, 15, and 22 every 28 days. Within the first four cycles of treatment, a response of PR or higher was seen in 82% of patients treated with lenalidomide plus high-dose dexamethasone versus 70% of patients in the lenalidomide plus low-dose dexamethasone arm (P = 0.007). CR plus VGPR rates were 52% versus 42%, respectively (P = 0.06).
In a phase II study, 34 previously untreated MM patients (mean age 64 years) were administered lenalidomide 25 mg/day on days 1–21 of a 28-day cycle and dexamethasone 40 mg/day on days 1–4, 9–12, and 17–20 of each cycle for at least four cycles.42 Treatment with lenalidomide plus dexamethasone yielded an TTP of 91%, including six patients (18%) with a CR and 13 (38%) who met the criteria for VGPR and nCR.42,43 Among 21 patients who did not subsequently receive SCT and were eligible for treatment beyond four cycles at the discretion of the investigator, 14 (67%) achieved either a CR or VGPR.43
Lenalidomide/cyclophosphamide/dexamethasone
A phase II study of 33 patients (median age 63 years) with newly diagnosed MM evaluated the combination of lenalidomide 25 mg/day on days 1–28 of every 28-day cycle, cyclophosphamide 300 mg/m2 on days 1, 8, and 15 of each cycle, and dexamethasone 40 mg/day on days 1, 8, 15, and 22 of each cycle.85 Among 19 of 33 evaluable patients, two achieved a VGPR (10.5%) and 13 achieved a PR (68.4%), giving an ORR of 78.9%.
Lenalidomide/bortezomib/dexamethasone (RVd)
In a phase I/II study, the combination of lenalidomide 15–25 mg/day on days 1–14, bortezomib 1.0–1.3 mg/m2 on days 1, 4, 8, and 11, and dexamethasone 40/20 mg/day (cycles 1–4/5–8) on day of and day after bortezomib administration for up to eight 21-day cycles produced an ORR of 98% in 42 evaluable patients with newly-diagnosed MM.86 Nine of 42 (21%) patients had a CR, 3 (7%) had nCR, 10 (24%) had VGPR, and 19 (45%) had PR, giving an ORR of 98% at the time of this analysis. All 11 patients who received treatment with lenalidomide/bortezomib/dexamethasone RVd at the phase II dose level of lenalidomide 25 mg, bortezomib 1.3 mg, and dexamethasone 20 mg achieved PR or better (100% ORR).
Clarithromycin/lenalidomide/dexamethasone (BiRD)
In a phase II study of 72 patients (median age 63 years) with newly diagnosed MM, induction therapy with clarithromycin 500 mg twice -daily, lenalidomide 25 mg/day on days 1–21 of a 28-day cycle, and dexamethasone 40 mg/day once weekly was associated with an objective response of PR or better in 65 (90.3%) patients (90.3%), including a CR rate of 38.9%.87 Fifty-three patients (73.6%) achieved at least a 90% decrease in M-protein levels. The mean duration of response was 333 days and the mean time to response was 54 days, with a mean time to maximum response of 209 days. Patients with atypical serum immunofixation pattern (ASIP) development during induction therapy with BiRD had significantly better response than patients without ASIP, with a CR rate of 71% versus 23%, respectively (P = 0.00002).88
Lenalidomide/melphalan/prednisone (RMP)
In a phase I/II study conducted by the Italian Multiple Myeloma Network, nine monthly cycles of lenalidomide 5–10 mg/day administered on days 1–21, melphalan 0.18–0.25 mg/kg given on days 1–4, and prednisone 2 mg/kg given on days 1–4 yielded an ORR of 81% in 53 elderly patients (median age 71 years) with newly diagnosed MM.41 Seven patients (13%) in total had a CR, including 5 of 21 (24%) patients assigned to lenalidomide 10 mg plus melphalan 0.18 mg/kg, and 2 (10%) of 20 (10%) patients assigned to lenalidomide 10 mg plus melphalan 0.25 mg/kg. Another 13 patients (25%) in total had a VGPR. The median time to best response was four months and PR was achieved in 53% of patients after the first cycle of treatment.
Time to progression
Lenalidomide plus dexamethasone
In a phase II study, lenalidomide plus dexamethasone was associated with a median TTP of 32.4 months in patients who did not undergo SCT, whereas median TTP was not reached at the time of publication in patients who underwent SCT.43 The two-year TTP rates were 71% for the entire cohort, 66% in the nontransplantation group, and 83% in the transplantation group.
RVd
In a phase I/II study, median TTP was not reached after a median follow-up of four months in 42 patients who received lenalidomide in combination with bortezomib and dexamethasone.86
RMP
Among 21 elderly patients (median age 69 years) in a phase I/II study who received the maximum tolerated dose of lenalidomide 10 mg/day for 21 days, melphalan 0.18 mg/kg for four days, and prednisone 2 mg/kg for four days of every 28 days for a maximum of nine cycles, followed by lenalidomide 10 mg/day for 21 of every 28 days as maintenance after a median follow-up of 29.5 months. The median TTP was 28.5 months.89
Overall survival
Lenalidomide plus dexamethasone
In a phase III study comparing lenalidomide in combination with either high-dose or low-dose dexamethasone, OS was superior for the low-dose dexamethasone combination (P = 0.006).84
RVd
In a phase I/II study, median OS was not reached after a median follow-up of four months.86
BiRD
Among 72 evaluable patients treated with BiRD in a phase II study, actuarial EFS at two years was 97.2%.87 The median EFS duration was not yet reached.
One-, two-, and three-year survival
Lenalidomide plus dexamethasone
Lenalidomide plus dexamethasone has recently been evaluated in a randomized controlled phase III study of 445 patients with previously untreated MM.84,90 Survival significantly favored lenalidomide plus low-dose dexamethasone, with a one-year survival rate of 96% compared with 88% for lenalidomide plus high-dose dexamethasone (P = 0.006).84 Among patients aged <65 years, one-year survival rates for low- versus high-dose dexamethasone were 97% versus 92%, respectively (P = 0.022); the respective data for patients aged ≥65 years were 94% versus 83% (P = 0.002).90 Two-year OS rates were 87% versus 75%, respectively.84 In a landmark analysis of the 210 patients who were alive and went off study after four months, the one- and two-year OS rates among the 102 patients who underwent SCT were 99% and 94%, respectively. In contrast, among the 108 patients who did not undergo SCT, one- and two-year OS rates were 85% and 70%, respectively.
In a second randomized controlled phase III study, one-year OS rates were 93% and 91% in patients assigned to lenalidomide plus high-dose dexamethasone and high-dose dexamethasone alone, respectively.83 In a subgroup analysis that considered patients with and without abnormal karyotypes at baseline, one-year OS rates among those with abnormal karyotypes were 82% and 77% in patients treated with lenalidomide plus high-dose dexamethasone and dexamethasone alone, respectively.91
Among a cohort of 34 patients treated with lenalidomide plus dexamethasone in a phase II study, two- and three-year OS was approximately 91% and 88%, respectively.43
RMP
Among 53 elderly patients treated with RMP in a phase II study, the one-year OS rate was 100%.41 Among 21 patients treated with the maximum tolerated dose in this study followed by lenalidomide 10 mg/day on 21 of every 28 days as maintenance therapy, the two-year OS rate was 90.5%.92
Adverse events
Lenalidomide plus dexamethasone
A phase III study conducted by the Eastern Cooperative Oncology Group (ECOG) reported a lower rate of grade 3 or 4 adverse events among patients who were randomized to lenalidomide plus low-dose dexamethasone than in patients randomized to lenalidomide plus high-dose dexamethasone.90 In patients assigned to high- versus low-dose dexamethasone, major grade 3 or 4 toxicities and their respective rates were: neutropenia (10% vs 19%, respectively; P = 0.01); VTE (25% vs 9%; P < 0.001); and infection/pneumonia (16% vs 6%; P < 0.001). Grade 3 or 4 nonhematological toxicities occurred in 49% and 32% of patients assigned to high- versus low-dose dexamethasone, respectively in combination with lenalidomide (P < 0.001). Of verified deaths in the high-dose dexamethasone arm, 13 were due to disease progression, six cases were related to VTE, three were due to infection, and another five cases were due to cardiac ischemia, stroke, and respiratory failure. Of nine verified deaths in the low-dose dexamethasone arm, five were due to disease progression, two to infection, one to VTE, and one to cardiac arrest. In the first four months of therapy, the mortality rate was 5% in the high-dose dexamethasone group compared with 0.5% in the low-dose group.
In a second randomized, double-blind, phase III study, lenalidomide plus high-dose dexamethasone was associated with a higher rate of adverse events than treatment with high-dose dexamethasone alone.83 Grade 3 or 4 neutropenia was reported by 13.5% of patients treated with lenalidomide plus high-dose dexamethasone compared with 2.4% of patients treated with high-dose dexamethasone alone (P = 0.01). There were 20 VTE events in the lenalidomide plus dexamethasone group including 14 events associated with aspirin prophylaxis; there were 12 thromboembolic events in the dexamethasone-only group all of which were associated with aspirin prophylaxis.
In phase II studies of lenalidomide plus dexamethasone, 47%–55% of patients experienced a grade 3 or 4 nonhematological toxicity during therapy, most commonly fatigue (15%–21%), anxiety (6%), pneumonitis (6%), muscle weakness (6%), and rash (6%).42,43 Grade 3 or 4 hematological adverse events included neutropenia (12%–21%), leucopenia (9%), lymphopenia (6%), and anemia (6%). All patients received aspirin once daily as thromboprophylaxis. However, although one patient developed a grade 4 pulmonary embolism they recovered with therapy. Two patients died from infection that was deemed to be possibly related to study therapy.42,43
RVd
In a phase I/II dose-finding study, among 53 evaluable patients who completed a median of six treatment cycles, 14 patients discontinued treatment.86 Two dose-limiting toxicities of grade 3 hyperglycemia due to high-dose dexamethasone were seen at dose level 4 (lenalidomide 25 mg/day, bortezomib 1.3 mg/m2, and dexamethasone 40 mg/day), with subsequent recruitment into phase II involving a reduction in dexamethasone dose to 20 mg/day. Dose reductions in cycle 2 and beyond occurred for lenalidomide in 12 patients, bortezomib in 11 patients, and dexamethasone in 18 patients. Adverse events were manageable with no unexpected events, no grade 4 peripheral neuropathy, two episodes of DVT, and no treatment-related mortality.
BiRD
In a phase II study, 17 of 72 patients treated with BiRD required at least one lenalidomide dose reduction for a grade 3 or 4 adverse event.87 Grade 3 or 4 hematological toxicities included neutropenia (19.4%), anemia (13.8%), and thrombocytopenia (22.2%). Nonhematological grade 3 or 4 toxicities included myopathy (11.1%), thrombosis (9.7%), rash (5.6%), and diverticular abscess (5.6%). VTE occurred in nine patients (12.5%), of which five events were associated with aspirin interruption or poor compliance.87
RMP
In a phase II study of RMP in 53 elderly patients, at the maximum tolerated dose, grade 3 or 4 hematological toxicities were neutropenia (52%), thrombocytopenia (24%), and anemia (5%).41 Grade 3 febrile neutropenia, vasculitis, and VTE were reported in 10%, 10%, and 5% of patients, respectively. In a subgroup of 21 patients who were followed for a median of 29.5 months, grade 3 and 4 neutropenia were reported in 38% and 14% of patients, respectively, during initial therapy.92 Grade 3 and 4 thrombocytopenia were reported in 14% and 10% of patients, respectively. Whereas the incidence and depth of neutropenia did not increase with the number of cycles, thrombocytopenia was more pronounced after nine cycles. One patient required a lenalidomide dose reduction for severe neutropenia and three patients discontinued due to severe thrombocytopenia and neutropenia.
Stem cell transplantation
Stem cell collection
Lenalidomide plus dexamethasone
In MM patients who received initial therapy with lenalidomide plus dexamethasone, a retrospective analysis of a five-year treatment period at a single institution indicated there was a trend towards decreased peripheral blood stem cell yield with increasing duration of lenalidomide therapy.93 A retrospective study by Paripati and colleagues comparing lenalidomide plus dexamethasone induction therapy versus other induction therapy showed that the first attempt at stem cell collection was unsuccessful significantly more frequently in lenalidomide plus dexamethasone recipients compared with those who had received other induction therapy (7% vs 45%, respectively; P = 0.001).94 Lenalidomide plus dexamethasone recipients had lower mean peripheral blood CD34+ cell counts compared with those who received other induction therapies (14.0 cells/μL vs 28.9 cells/μL; P < 0.0002) and mean total stem cells collected (5.1 × 106 cells/kg vs 7.4 × 106 cells/kg; P = 0.0025) compared with those who received other induction therapies. However, compared with single-agent dexamethasone, thalidomide plus dexamethasone or vincristine/adriamycin/dexamethasone, there was no effect on quality of yield in patients receiving lenalidomide based on similar engraftment.93
Lenalidomide-based induction therapy
In a recent study where 21 patients with MM received lenalidomide-based induction therapy prior to stem cell mobilization, lenalidomide did not prevent the harvest of adequate numbers of CD34+ cells for autologous SCT (median 6.3 cells × 106/kg; range 2.4–19.7 cells × 106/kg).95 Patients were mobilized with cyclophosphamide plus granulocyte colonystimulating factor (G-CSF) (n = 17), G-CSF and AMD3100 (n = 2), or G-CSF alone (n = 2). Repeat mobilization was required in patients who received G-CSF alone and was successful on the second attempt with the addition of AMD3100. The median number of collections was 3 (range 1–8) in patients mobilized with cyclophosphamide plus G-CSF and 4.5 (range 2–6) in those mobilized with G-CSF plus AMD3100. The respective median CD34+ cell counts were 6.3 × 106/kg (range 3.0–19.7 × 106/kg) and 8.4 × 106/kg (range 5.6–12.3 × 106/kg). No correlation between the number of lenalidomide cycles (median 4, range 1–16) and the number of stem cell collections or total CD34+ cell counts was reported.
BiRD plus G-CSF or G-CSF plus cyclophosphamide for stem cell mobilization
In a subset of 28 treatment-naïve MM patients who were treated with the BiRD regimen in a phase II trial, the effect of cyclophosphamide plus G-CSF as a stem cell mobilization regimen compared with G-CSF alone was investigated.96 Successful stem cell harvest sufficient for two autologous SCTs was achieved in all patients who received mobilization with cyclophosphamide plus G-CSF, compared with only 33% of patients who were mobilized with G-CSF alone (P < 0.0001). No correlation between duration of lenalidomide and stem cell collection was observed.
Response
Bortezomib/doxorubicin/dexamethasone followed by lenalidomide and prednisone
In a phase II study, 94 patients aged 65–75 years with newly diagnosed MM were treated with bortezomib and doxorubicin plus dexamethasone (PAD) induction (bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11, pegylated liposomal doxrorubicin 20 mg/m2 on day 4, and dexamethasone 40 mg on days 1–4, 8–11, and 15–18 for cycle 1 and days 1–4 for cycles 2–4) prior to reduced intensity autologous SCT.89 Cyclophosphamide 3 mg/m2 plus G-CSF was used to harvest stem cells, with patients then conditioned with tandem melphalan 100 mg/m2 and stem cell support (MEL100). Following autologous SCT, patients received consolidation therapy with lenalidomide 25 mg/day on days 1–21 plus prednisone 50 mg/day every other day, and then maintenance therapy with lenalidomide alone (10 mg/day on days 1–21 every 28 days). After four cycles of PAD therapy, 96% of patients had at least PR (60% had at least VGPR, 23% had at least nCR, and 13% had CR), after tandem MEL100, 95% had at least PR (80% at least VGPR, 60% at least nCR, and 33% had CR), and after lenalidomide plus prednisone consolidation all patients had at least PR (89% had at least VGPR, 78% had at least nCR, and 56% had CR).
Adverse events
Lenalidomide plus prednisone consolidation therapy
In a study of 94 elderly patients with newly diagnosed MM who received lenalidomide plus prednisone as consolidation therapy following PAD induction therapy and autologous SCT, one case of DVT and one discontinuation because of prolonged thrombocytopenia and anemia were reported during consolidation therapy.89
Relapsed or refractory disease
Two multicenter, double-blind, randomized, placebo-controlled phase III studies (MM-009/and MM-010) investigated the efficacy and safety of lenalidomide plus dexamethasone versus dexamethasone alone in the treatment of patients with relapsed or refractory MM; they provided the basis for the approval of lenalidomide in this indication.46 Patients with relapsed or refractory MM and ≤3 previous regimens were eligible. Based on the findings of phase I and II studies, lenalidomide was administered at 25 mg/day on days 1–21 of each 28-day cycle. Patients were randomized to either four 28-day cycles of lenalidomide plus high-dose dexamethasone 40 mg/day on days 1–4, 9–12, and 17–20 of each cycle, or matched placebo plus dexamethasone as for the group assigned to active treatment. After four cycles of therapy, treatment was continued until disease progression, but with dexamethasone 40 mg administered only on days 1–4 of every 28-day cycle. The primary end point of TTP was evaluated according to EBMT criteria.4 A total of 353 patients in MM-009 and 351 patients in MM-010 were randomized and received study medication.
Response rates and duration of response
Lenalidomide plus dexamethasone
In the MM-009 and MM-010 studies, lenalidomide in combination with high-dose dexamethasone led to a significantly better ORR compared with dexamethasone alone.2,3 In these studies, 108 patients (61.0%) in MM-009 and 106 patients (60.2%) in MM-010 assigned to lenalidomide plus dexamethasone achieved a response of PR or better. In comparison, 35 patients (19.9%) in MM-009 and 42 patients (24.0%) in MM-010 assigned to dexamethasone alone had a response to therapy (P < 0.001 versus lenalidomide plus dexamethasone). In both studies, the CR rate in response to lenalidomide plus dexamethasone was approximately 15% (MM-009: 14.1%; MM-010: 15.9%) and the nCR rate was approximately 9% (MM-009: 10.2%; MM-010: 8.5%) (Table 3).2,3 In a pooled analysis that included data from all 704 patients enrolled in both trials, the ORR in the lenalidomide plus dexamethasone group and the dexamethasone-only group was 60.6% and 21.9%, respectively (P < 0.001).97 The respective data for CR rate were 15.0% and 2.0% (P < 0.001). Among patients who received lenalidomide plus dexamethasone, the median duration of response was significantly higher for those who achieved a CR or nCR compared with those who achieved a PR (not yet reached vs 8.8 months; P < 0.001).98
Table 3.
|
MM-009 (N = 353) |
MM-010 (N = 351) |
|||||
|---|---|---|---|---|---|---|
| Leni + Dex | Dex | Pvalue | Leni + Dex | Dex | Pvalue | |
| Randomized, n | 177 | 176 | 176 | 175 | ||
| ORR (CR, nCR, PR), % | 61.0 | 19.9 | <0.001 | 60.2 | 24.0 | <0.001 |
| CR, % | 14.1 | 0.6 | <0.001 | 15.9 | 3.4 | <0.001 |
| Median TTP, months | 11.1 | 4.7 | <0.001 | 11.3 | 4.7 | <0.001 |
| Median OS, months | 29.6 | 20.2 | <0.001 | NR | 20.6 | 0.03 |
Abbreviations: CR, complete response; Dex, placebo plus dexamethasone; Leni + Dex, lenalidomide plus dexamethasone; nCR, near complete response; NR, not reached; ORR, overall response rate; OS, overall survival; PR, partial response; TTP, time-to-progression.
Patients in the MM-009 and MM-010 studies were stratified according to β2-microglobulin (≤2.5 mg/L vs >2.5 mg/L), prior SCT (none vs ≥1), and number of prior regimens (1 vs >1).2,3 In both studies lenalidomide plus dexamethasone was associated with significantly higher response rates than dexamethasone alone, irrespective of β2-microglobulin level, prior SCT, or number of prior therapies (Table 4). In addition, lenalidomide plus dexamethasone yielded higher response rates than dexamethasone alone irrespective of prior bortezomib or thalidomide therapy.2,3
Table 4.
| Subgroup analysis |
MM-009 (N = 353) |
MM-010 (N = 351) |
||||
|---|---|---|---|---|---|---|
| Leni + Dex | Dex | Pvalue | Leni + Dex | Dex | Pvalue | |
| Number of prior therapies | ||||||
| 1 prior therapy | ||||||
| ORR, % | 64.7 | 22.4 | <0.001 | 66.1 | 29.8 | <0.001 |
| Median TTP, months | NR | 5.1 | <0.001 | NR | 4.7 | <0.001 |
| ≥2 prior therapies | ||||||
| ORR, % | 58.7 | 18.3 | <0.001 | 57.5 | 21.2 | <0.001 |
| Median TTP, months | 10.2 | 4.6 | <0.001 | 11.1 | 4.7 | <0.001 |
| Prior therapies | ||||||
| Prior thalidomide | ||||||
| ORR, % | 56.8 | 12.5 | <0.001 | 49.1 | 16.4 | 0.002 |
| Median TTP, months | 8.5 | 4.1 | <0.001 | 8.4 | 4.6 | <0.001 |
| No prior thalidomide | ||||||
| ORR, % | 64.1 | 26.0 | <0.001 | 65.0 | 28.7 | <0.001 |
| Prior bortezomib | ||||||
| ORR, % | 68.4 | 10.0 | <0.001 | |||
| Median TTP, months | 10.3 | 3.3 | <0.001 | |||
| No prior bortezomib | ||||||
| ORR, % | 60.1 | 21.2 | <0.001 | |||
| β2-microglobulin level | ||||||
| <2.5 mg/L | ||||||
| ORR, % | 75.0 | 27.5 | <0.001 | 70.6 | 37.5 | <0.001 |
| ≥2.5 mg/L | ||||||
| ORR, % | 55.2 | 16.8 | <0.001 | 56.0 | 18.9 | <0.001 |
| Prior SCT | ||||||
| Yes | ||||||
| ORR, % | 66.1 | 19.4 | <0.001 | 61.9 | 28.4 | <0.001 |
| No | ||||||
| ORR, % | 52.9 | 20.6 | <0.001 | 58.2 | 18.8 | <0.001 |
Abbreviations: Dex, placebo plus dexamethasone; Leni + Dex, lenalidomide plus dexamethasone; NR, not reached; ORR, overall response rate; SCT, stem cell transplantation; TTP, time to progression.
In a prospective, pooled subgroup analysis of 704 patients enrolled in the MM-009/and MM-010 studies, the ORR was significantly higher with lenalidomide plus dexamethasone treatment compared with dexamethasone alone in patients who had received prior thalidomide (60% vs 18%, respectively; P < 0.01) or in patients who had not had prior thalidomide (64% vs 28%; P < 0.01).99 When patients who had received prior thalidomide were divided into three subgroups based on the degree of thalidomide resistance, the ORR was similar across resistance groups. Even the group with the strongest resistance to thalidomide (ie, never responded nor had stable disease) had a higher response rate (P < 0.01).
In another prospective subgroup analysis, the benefits of starting lenalidomide therapy at first relapse were assessed by comparing outcomes with lenalidomide plus dexamethasone versus dexamethasone alone among patients who had received one versus ≥2 prior therapies.100 Among the 248 of 692 patients who had received only one prior therapy, those assigned to second-line lenalidomide plus dexamethasone had a significantly higher ORR than those receiving dexamethasone alone (65% vs 26%, respectively). Among the 456 patients who had received ≥2 prior therapies, those treated with lenalidomide plus dexamethasone also had a significantly higher ORR than those treated with dexamethasone alone (58% vs 20%, respectively). Comparing patients who received lenalidomide plus dexamethasone as second-line versus later salvage therapy, the ORR appeared higher with early treatment. A higher proportion of patients receiving second-line therapy had previously had SCT (66% vs 54%), whereas more patients receiving later salvage therapy had previously received thalidomide (53.2% vs 12.5%) and bortezomib (11.6% vs 0.4%).
In further subanalyses of MM-009 and MM-010, Foa and colleagues reported that among 154 patients with IgA disease at baseline, lenalidomide plus dexamethasone was associated with a significantly higher ORR than dexamethasone alone (68.1% vs 18.3%, respectively; P < 0.001).101 The CR rate in patients with IgA disease who were treated with lenalidomide plus dexamethasone, versus dexamethasone alone, was 18.1% and 0%, respectively (P = ns). Similarly, in patients without IgA disease at baseline, lenalidomide plus dexamethasone achieved a higher ORR compared with dexamethasone alone (57.7% vs 23.0%, respectively; P < 0.001). A separate analysis demonstrated that the superiority of lenalidomide plus dexamethasone compared with dexamethasone alone was independent of baseline ECOG performance status.102 In this analysis, patients with an ECOG scores of 0 or ≥1 had significantly higher ORR with lenalidomide plus dexamethasone (59% and 62%, respectively) compared with dexamethasone alone (22% and 22%, respectively; P < 0.001 for both). Also, age did not determine response to lenalidomide, with another subanalysis showing that ORR was significantly higher for lenalidomide plus dexamethasone compared with dexamethasone alone for patients aged <65 years (61.5% vs 22.2%, respectively), 65–75 years (58.4% vs 21.4%), and >75 years (63.9% vs 29.9%).103
In a pooled subgroup analysis of 682 patients with serum creatinine levels of ≤2.5 mg/dL at baseline, lenalidomide plus dexamethasone significantly improved response rate compared with dexamethasone alone in patients with normal renal function (creatinine clearance [CrCl] >80 mL/min: 63.9% vs 27.0%, respectively; P < 0.001) and in those with mild (CrCl ≥ 50 mL/min to <80 mL/min: 64.0% vs 19.8%; P < 0.001) and moderate (CrCl ≥ 30 mL/min to <50 mL/min: 61.9% vs 20.6%; P = 0.001) renal impairment104 (Table 5). The ORR was not significantly different between lenalidomide plus dexamethasone and dexamethasone alone in the 28 patients with severe renal impairment (CrCl <30 mL/min: 50.0% vs 25.0%, respectively; P = 0.205), with CR rates following a similar trend to ORR.
Table 5.
Treatment response, time-to-progression and overall survival in MM-009 and MM-010: pooled subgroup analysis according to baseline renal impairment104
|
MM-009 and MM-010 (N = 682) |
|||
|---|---|---|---|
| Leni + Dex | Dex | Pvalue | |
| CRCl >80 mL/min | |||
| ORR, % | 63.9 | 27.0 | <0.001 |
| CR, % | 16.5 | 1.8 | |
| Median TTP, months | 11.3 | 4.7 | <0.001 |
| Median OS, months | NR | 101.2 | 0.142 |
| CRCl ≥50 to <80 mL/min | |||
| ORR, % | 64.0 | 19.8 | <0.001 |
| CR, % | 12.8 | 2.3 | |
| Median TTP, months | 12.1 | 4.7 | <0.001 |
| Median OS, months | 34.7 | 27.2 | 0.131 |
| CRCl ≥30 to <50 mL/min | |||
| ORR, % | 61.9 | 20.6 | 0.001 |
| CR, % | 21.4 | 0 | |
| Median TTP, months | 11.4 | 2.8 | <0.001 |
| Median OS, months | 30.4 | 12.5 | 0.068 |
| CRCl <30 mL/min | |||
| ORR, % | 50.0 | 25.0 | 0.205 |
| CR, % | 6.3 | 8.3 | |
| Median TTP, months | 7.9 | 4.7 | 0.031 |
| Median OS, months | 18.6 | 16.9 | 0.849 |
Abbreviations: CR, complete response; CRCl, creatinine clearance; Dex, placebo plus dexamethasone; Leni + Dex, lenalidomide plus dexamethasone; NR, not reached; ORR, overall response rate.
Finally, a post-hoc analysis of data from the MM-009 and MM-010 trials indicated that dexamethasone dose reductions improved the efficacy of lenalidomide plus dexamethasone treatment compared with patients who continued to receive dexamethasone at the planned dose.105 Patients assigned to lenalidomide plus dexamethasone and who had a subsequent dexamethasone dose reduction experienced a significantly higher ORR and CR rate (69.6% and 23.9%, respectively) compared with patients who continued to receive the standard dexamethasone regimen in combination with lenalidomide (50.8% and 13.0%, respectively; P < 0.05 for both).
In an ongoing Dutch compassionate need program, patients with relapsed or refractory MM were treated with lenalidomide 25 mg/day on days 1–21 every 28 days, in combination with dexamethasone 40 mg/day on days 1–4 and 15–18 until disease progression, unacceptable toxicity, or for a maximum of eight courses. Fifteen patients received lenalidomide 10 mg/day maintenance therapy without dexamethasone after 6–8 courses of therapy.106 The preliminary response data of the first 42 patients showed an ORR of 83% (CR 5%, VGPR 45%, PR 45%, and MR 5%).
Single-agent lenalidomide
In a multicenter, open-label phase II study of single-agent lenalidomide in relapsed or refractory MM, 102 patients were treated with either lenalidomide 30 mg once daily or 15 mg twice daily for 21 days of every 28-day cycle.107 A total of 56% of patients had received at least four prior lines of therapy, 61% had received prior high-dose chemotherapy followed by SCT, 76% had received prior thalidomide, and 18% had previously received bortezomib. In the entire cohort, the ORR to lenalidomide was 25% (24% for once daily and 29% for twice daily), and a further 29% of patients responded with the addition of low-dose dexamethasone, which was permitted after two cycles for progressive or stable disease. The median duration of response, with censoring at the time that dexamethasone was added, was 19 months (range 2–22 months). In the twice-daily group, the median duration of response was 23 months (2–25 months). In a long-term follow-up of 15 patients who remained on therapy for a median of 4.1 years, 11 had achieved either CR or PR and continued to respond, including four of six patients receiving lenalidomide monotherapy (including a patient who progressed after 3.7 years), and seven of nine patients receiving concomitant dexamethasone.108 The remaining four patients maintained stable disease during this long-term follow-up.
A second multicenter, open-label study evaluated single-agent lenalidomide in 222 patients with relapsed or refractory MM (MM-014).111,112 Lenalidomide was administered at 30 mg once daily on days 1–21 every 28 days until disease progression or intolerance. Concomitant dexamethasone was not permitted. All patients had received at least two prior therapies, including bortezomib (43%), thalidomide (80%), and stem cell transplantation (45%). The ORR was 26%, with an additional 66% of patients achieving stable disease. The median duration of response was 13 months.
In a phase I dose-escalation study of 27 patients who received lenalidomide as a single daily dose, 24 patients received at least 28 days of therapy and were considered evaluable for response.113 Seventeen patients (71%) had a best response of ≥25% reduction in M-protein, including seven patients (29%) who achieved ≥50% reduction. The median duration of response was six months and the median time to response was two months.
Lenalidomide plus doxorubicin
In the relapsed or refractory MM setting, lenalidomide has been investigated in a phase I/II study in combination with pegylated liposomal doxorubicin-based chemotherapy.114 Sixty-two patients (median age 62 years) received liposomal doxorubicin 40 mg/m2 and vincristine 2 mg on day 1, dexamethasone 40 mg/day on days 1–4, and lenalidomide 5–15 mg/day on days 1–21 of every 28-day cycle. Among 52 evaluable patients, the ORR of the combination was 75%, including 29% of patients with either a CR or nCR. Best response occurred after a median of 115 days and four cycles of therapy.
Lenalidomide/cyclophosphamide/dexamethasone
In a retrospective analysis of 21 patients who were administered lenalidomide 25 mg/day on days 1–21, cyclophosphamide 500 mg/day on days 1, 8, 15, and 21, and dexamethasone 40 mg/day on days 1–4 and 12–15 of every 28-day cycle for a maximum of nine cycles, 15 of 20 (75%) evaluable patients had a response, including one CR, three VGPR, and nine PR.115 The median time to response was 31 days. There was no difference in response rate between patients who required a dose reduction compared with those who tolerated the full treatment schedule.
Lenalidomide/doxorubicin/dexamethasone (RAD)
In a phase I/II study, lenalidomide was evaluated in combination with doxorubicin and dexamethasone.116,117 A total of 69 patients (median age 65 years) received six 28-day cycles of lenalidomide 10–25 mg/day on days 1–21, doxorubicin 4–9 mg/m2 as a 24-hour infusion on days 1–4, and dexamethasone 40 mg/day on days 1–4 and 17–20, including 20 patients who received treatment at five lenalidomide and doxorubicin dose levels during phase I. In phase II of the study, all patients received the fifth dose level of lenalidomide 25 mg on days 1–21, doxorubicin 9 mg/m2 on days 1–4, and dexamethasone 40 mg on days 1–4 and 17–20.117 G-CSF support was given at 6 mg on day 6. ORR for patients receiving treatment at dose levels 1–4 in the phase I study was 60%, including five patients (25%) with nCR. ORR for the 41 patients receiving the highest dose level in phase II of the study was 85%, including 10 patients (24%) with CR and 24 patients (59%) with VGPR.
Lenalidomide plus prednisone
In a study of 69 patients who received lenalidomide plus corticosteroids (pulsed dexamethasone or prednisone) as part of an Expanded Access Program in Canada, the ORR was 58% in patients aged ≥65 years and older, and 56% in patients aged <65 years.118
Lenalidomide plus bortezomib
In the relapsed or refractory disease setting, the combination of lenalidomide and bortezomib in a phase I dose-escalation study of 36 patients yielded an ORR of 58%, including 6% with CR or nCR.110 Lenalidomide was administered at a dose of 5, 10, 15, or 20 mg on days 1–14, and bortezomib was given at either 1.0 or 1.3 mg/m2 on days 1, 4, 8, and 11 of every 21-day cycle for a median of six cycles. The median duration of response was six months, with 11 patients remaining on therapy beyond one year. Dexamethasone was added in 14 patients with progressive disease, with an objective response subsequently achieved in 10 patients.
RVd
Lenalidomide may sensitize MM cells to bortezomib and dexamethasone, suggesting combination therapy may enhance clinical activity. In a recently completed phase II trial of 65 patients, 43 patients (median age 67 years) with relapsed or refractory MM have to date received up to eight cycles of lenalidomide 15 mg on days 1–14 of a 21-day cycle, bortezomib 1.0 mg/m2 on days 1, 4, 8, and 11 of a 21-day cycle, and dexamethasone 40 mg (cycles 1–4) or 20 mg (cycles 5–8) twice weekly for two weeks of every 21-day cycle.62,119 Based on safety data, dexamethasone dosing was subsequently reduced to 20 mg for cycles 1–4 and 10 mg for cycles 5–8. In 33 evaluable patients with a median of two prior therapies including dexamethasone (90%), thalidomide (78%), and bortezomib (68%), the ORR (minimal response or better) of major response or better was 73%, including 36% with CR, unconfirmed CR or VGPR. The median duration of response was 39 weeks.119
Terpos and colleagues compared lenalidomide 25 mg/day on days 1–21 every 28 days plus either high- (n = 38) or low-dose (n = 20) dexamethasone with the combination of lenalidomide 15 mg/day on days 1–14 every 21 days plus bortezomib 1.0 mg/m2 on days 1, 4, 8, and 11, and low-dose dexamethasone (n = 13).120 Currently, 50 patients have completed three cycles of therapy, including 38 of 58 patients assigned to lenalidomide plus either high- or low-dose dexamethasone and 12 of 13 patients assigned to RVd. A total of 26 patients have received six cycles of therapy, including 19 of 58 patients assigned to lenalidomide and dexamethasone and seven of 13 patients assigned to RVd. The ORR was 58% in patients treated with lenalidomide and dexamethasone compared with 53% in patients treated with RVd.
Bevacizumab/lenalidomide/dexamethasone (Bev/Rev/Dex)
In a phase II study, 17 patients received four-weekly cycles of lenalidomide 25 mg/day on days 1–21, bevacizumab 10 mg/kg as a two-hour infusion every two weeks, and dexamethasone 40 mg once a week.121 Among 10 evaluable patients who have completed at least four cycles of therapy, seven patients (70%) achieved a PR after a median of two cycles and have maintained their response.
Lenalidomide/melphalan/prednisone/thalidomide (RMPT)
In a phase II study, 43 patients (median age 69 years) were administered six cycles of lenalidomide 10 mg/day on days 1–21 every 28 days, melphalan 0.18 mg/kg on days 1–4, prednisone 2 mg/kg on days 1–4, and thalidomide 50–100 mg/day on days 1–28 followed by maintenance therapy of lenalidomide 10 mg/day.122 Therapy was administered as second-line in 61% of patients and third-line in 39%. After two cycles, 52% of patients achieved at least PR and after a median of four cycles, 91% achieved at least PR including 45% with VGPR.
Time to progression
Lenalidomide plus dexamethasone
In the MM-009 and MM-010 studies, TTP was the primary end point. The median TTP was significantly longer in patients assigned to lenalidomide plus dexamethasone compared with dexamethasone alone (MM-009: 11.1 months vs 4.7 months, respectively; P < 0.001; MM-010: 11.3 months vs 4.7 months; P < 0.001).2,97 TTP was also significantly longer in the lenalidomide plus dexamethasone group compared with dexamethasone alone in patients who had received prior thalidomide or bortezomib therapy, and in patients with 1 or ≥2 prior therapies (Table 4). In a pooled analysis of all 704 patients in both studies, the median TTP in patients treated with lenalidomide plus dexamethasone versus dexamethasone alone was 11.2 months versus 4.7 months (P < 0.001).97 Response was related to TTP as among the patients treated with lenalidomide plus dexamethasone who achieved a CR or nCR, median TTP was significantly longer than those who achieved a PR (<15.1 months vs 10.7 months; P < 0.001).98
Among the 154 patients with IgA disease at baseline, median TTP was significantly longer in the lenalidomide plus dexamethasone group than in the dexamethasone-only group (10.3 months vs 3.8 months, respectively; P < 0.001).101 In patients without IgA disease, median TTP was again significantly longer in the lenalidomide plus dexamethasone group compared with dexamethasone alone (12.0 months vs 4.7 months, respectively; P < 0.001). Patients with a baseline ECOG score of 0 or ≥1 also had a significantly longer median TTP on lenalidomide plus dexamethasone (10.3 months and 13.3 months, respectively) than dexamethasone alone (4.7 months and 4.7 months, respectively; P < 0.001 for both comparisons).102 Dexamethasone dose reduction was similarly associated with a longer TTP. In the pooled subgroup analysis of patients with renal impairment, median TTP was significantly longer for lenalidomide plus dexamethasone compared with dexamethasone alone in patients with normal renal function (11.3 months vs 4.7 months, respectively; P < 0.001), and mild (12.1 months vs 4.7 months; P < 0.001), moderate (11.4 months vs 2.8 months; P < 0.001), and severe (7.9 months vs 4.7 months; P = 0.031) renal impairment104 (Table 5). In another subgroup analysis of patients who received lenalidomide plus dexamethasone, dose reduction of dexamethasone was associated with significantly longer TTP than continuing dexamethasone according to the planned dosing schedule (>13.9 months vs >5.6 months, respectively; P = 0.002).105
Single-agent lenalidomide
Among 222 patients enrolled in the multicenter, open-label phase II MM-014 study, 69% of patients had disease progression by the end of the study with a median TTP of 5.4 months.112
RAD
In a phase I/II study of 41 patients treated for six 28-day cycles with lenalidomide 25 mg/day on days 1–21, doxorubicin 9 mg/m2 on days 1–4, dexamethasone 40 mg/day on days 1–4 and 17–20, and G-CSF 6 mg on day 6, median TTP after a median follow-up of five months was 9.3 weeks.117
Overall survival
Lenalidomide plus dexamethasone
Relative to dexamethasone alone, median OS was significantly prolonged in patients assigned to lenalidomide plus dexamethasone in both the MM-009 and MM-010 studies.2,3 At a median follow-up post-randomization of 17.1 months in the MM-009 study, the median OS in patients assigned to lenalidomide plus dexamethasone was 29.6 months versus 20.2 months for dexamethasone alone (P < 0.001).3 Similarly, at a median follow-up of 16.5 months in the MM-010 study, the median OS in patients assigned to lenalidomide plus dexamethasone had not been reached, whereas in patients assigned to dexamethasone-only median OS was estimated at 20.6 months (P = 0.03).2 With an extended follow-up of 31.3 months, the median OS for all 704 patients pooled from both studies was 35.0 months for those receiving lenalidomide plus dexamethasone and 31.0 months for those on dexamethasone alone (P < < 0.05).97 It should be noted that this significant difference in OS was maintained despite 47% of patients receiving dexamethasone alone crossing over to lenalidomide plus dexamethasone therapy.97 Response was correlated with survival because among patients assigned to lenalidomide plus dexamethasone the median OS was significantly higher for those who achieved CR or nCR, than for patients who achieved a PR (>30.9 months vs >27.5 months, respectively; P < 0.01).98
In both MM-009 and MM-010, OS was significantly improved in the lenalidomide plus dexamethasone group compared with the dexamethasone-only group, among patients who had previously been treated with thalidomide.2,3 In addition, lenalidomide plus dexamethasone was associated with significantly longer OS compared with dexamethasone alone, irrespective of the number of prior therapies.97,123 In a pooled analysis of all 704 patients, the median OS was not yet reached in patients assigned to the lenalidomide plus dexamethasone group who had received one prior therapy compared with 35.3 months in patients assigned to dexamethasone alone (P = 0.24).97 In patients who had received >1 prior therapy, median OS was 32.4 months in those assigned to lenalidomide plus dexamethasone compared with 27.3 months in those assigned to dexamethasone alone (P < 0.05).
In patients with IgA disease at baseline, there was a trend towards improved OS with lenalidomide plus dexamethasone treatment compared with dexamethasone alone (30.3 months vs 23.8 months, respectively; P = not significant).101 In patients without IgA disease at baseline, there was a significant benefit in terms of OS for lenalidomide plus dexamethasone versus dexamethasone alone (36.3 months vs 31.7 months, respectively; P < 0.05). Similarly, patients with an ECOG performance status of 0 at baseline had a similar median OS with lenalidomide plus dexamethasone relative to dexamethasone alone (36.3 months vs 37.0 months, respectively; P = not significant).102 However, among patients with an ECOG score ≥1, median OS was significantly higher in patients assigned to lenalidomide plus dexamethasone versus dexamethasone alone (32.9 months vs 24.1 months, respectively; P < 0.01). When patients were stratified according to renal function, there was a trend towards improved OS with lenalidomide plus dexamethasone compared with dexamethasone alone in patients with moderate renal impairment (30.4 months vs 12.5 months, respectively; P = 0.068)104 (Table 5). However, OS was not significantly different for those with normal renal function (not reached vs 101.2 months, respectively; P = 0.142), mild renal impairment (34.7 months vs 27.2 months; P = 0.131) or severe renal impairment (18.6 months vs 16.9 months; P = 0.849). Among patients who were assigned to lenalidomide plus dexamethasone, dose reduction of dexamethasone was associated with a trend towards improved OS compared with patients who were maintained on the planned dexamethasone dose regimen (>28.3 months vs >25.5 months, respectively; P = 0.19).105
In the MM-009/and MM-010 studies, 47% of patients randomized to dexamethasone alone later switched to lenalidomide plus dexamethasone at disease progression or following ethical study unblinding.124 In a survival analysis that adjusted for the overestimation of survival in the group treated with dexamethasone alone, Morgan and colleagues reported that treatment of patients who had one prior therapy with single-agent dexamethasone yielded a median survival of 16.2 months compared with 33.6 months following crossover to lenalidomide plus dexamethasone.124 The median survival for patients with multiple prior therapies was 12.6 months compared with 27.3 months with crossover to lenalidomide plus dexamethasone. Using a lifetime simulation model, Morgan and colleagues estimated a mean survival of 2.2 life-years with dexamethasone alone compared with 5.6 life-years with lenalidomide plus dexamethasone for patients with one prior therapy. For patients with multiple prior therapies, lifetime simulation yielded an estimated mean survival of 1.5 life-years for dexamethasone alone compared with 4.2 life-years for lenalidomide plus dexamethasone.
The MM-016 study was a multicenter, single-arm, open-label expanded access program for lenalidomide in relapsed and refractory MM that reported on the efficacy of lenalidomide plus dexamethasone in patients according to their del13q, t(4; 14), and del17p13 status. Patients received lenalidomide 25 mg/day on days 1–21 of a 28-day cycle, plus dexamethasone 40 mg/day on days 1–4, 9–12 and 17–20 for four cycles, then days 1–4 only beginning with cycle 5.125 In the entire group, progression-free survival (PFS) was 10.6 months and the median OS was not reached at a median follow-up of 16 months. Compared with the overall cohort, treatment with lenalidomide plus dexamethasone overcame poor prognosis conferred by del13q and t(4; 14) cytogenetic abnormalities, with no increased risk of a reduction in OS (del13q: hazard ratio [HR], 0.56, 95% confidence interval [CI] 0.25–1.29; P = 0.179; and t(4; 14): HR, 1.26, 95% CI 0.46–3.42; P = 0.641). However, patients with del17p13 had a reduced OS despite a rapid initial response to therapy (HR, 3.83; 95% CI 1.34–10.93; P = 0.012).
In a preliminary analysis of 42 patients with relapsed or refractory MM treated with lenalidomide and dexamethasone in an ongoing Dutch compassionate need program, the median OS has not been reached (median PFS 10 months).106
Single-agent lenalidomide
In an open-label, phase II study of 102 patients, at a median follow-up of 31 months, lenalidomide 30 mg/day was associated with a median OS of 27 months. There was no significant survival advantage reported for patients who received 30 mg once-daily dosing versus 15 mg twice daily.107 In the multicenter, open-label, phase II MM-014 study of 222 patients, in which concomitant dexamethasone was not permitted, three-year OS was 41%, with a median OS of 1.9 years.112
Lenalidomide and bortezomib
Overall survival in the lenalidomide plus bortezomib is emerging at 37 months.
Lenalidomide plus prednisone
Among 69 patients who received lenalidomide plus corticosteroids (pulsed dexamethasone or prednisone) as part of an Expanded Access Program in Canada, OS was 74% in patients aged ≥65 years compared with 76% in patients <65 years.118
RAD
In a phase I/II study of 41 patients treated for six 28-day cycles with lenalidomide 25 mg/day on days 1–21, doxorubicin 9 mg/m2 on days 1–4, dexamethasone 40 mg/day on days 1–4 and 17–20, and G-CSF 6 mg on day 6, after a median follow-up of five months OS was 79%.117
Safety and tolerability
In the two pivotal phase III studies of relapsed or refractory MM, grade 3 or 4 adverse events were reported more frequently in patients assigned to lenalidomide plus dexamethasone compared with dexamethasone alone.2,3 In the MM-009 study, grade 3 or 4 hematologic adverse events in the lenalidomide plus dexamethasone versus dexamethasone-only groups were neutropenia (41.2% vs 4.5%, respectively), anemia (13.0% vs 5.1%), thrombocytopenia (14.7% vs 6.9%), and febrile neutropenia (3.4% vs 0%). Other commonly occurring grade 3 or 4 adverse events were any infection (21.4% vs 12.0%, respectively), pneumonia (12.4% vs 7.4%), hyperglycemia (10.8% vs 8.6%), hypokalemia (6.2% vs 1.1%), and fatigue (6.2% vs 6.3%). VTE events occurred in 14.7% of patients in the lenalidomide plus dexamethasone group compared with 3.4% of patients in the dexamethasone-only group (P < 0.001).3 In the MM-010 study, grade 3 or 4 hematologic adverse events in the lenalidomide plus dexamethasone versus dexamethasone-only groups were neutropenia (29.5% vs 2.3%, respectively), anemia (8.6% vs 6.9%), thrombocytopenia (11.4% vs 5.7%), and febrile neutropenia (3.4% vs 0%). Other commonly occurring grade 3 or 4 adverse events were any infection (11.3% vs 6.2%, respectively), muscle weakness (7.4% vs 4.6%), asthenia (6.2% vs 5.7%), and fatigue (6.8% vs 3.4%). Grade 3 or 4 VTE events occurred in 11.4% of patients in the lenalidomide plus dexamethasone group compared with 4.6% of patients in the dexamethasone-only group.2
The increased incidence of VTE in patients receiving lenalidomide plus dexamethasone compared with dexamethasone alone does not appear to affect survival. In an analysis of 177 patients assigned to receive lenalidomide plus dexamethasone in the MM-009 study, OS (P = 0.4) and TTP (P = 0.7) were not significantly different for the 31 patients who experienced DVT compared with patients who did not experience DVT.126 In the MM-009 and MM-010 studies, multivariate analysis indicated that lenalidomide plus dexamethasone treatment with adjunctive erythropoietin was independently correlated with thrombosis; older age, lower plasma cell involvement in the bone marrow, and better ECOG performance status had a weaker association with thrombosis.127 None of the 23 patients who used aspirin during the first month of treatment developed thromboses; all events occurred in patients with rising M-protein levels at baseline.
In the MM-009 and MM-010 studies, the predominant reason for adjusting dexamethasone dose among patients assigned to lenalidomide plus dexamethasone was for an adverse event (41 of 46 patients).105 In this group of patients, reducing dexamethasone dose yielded a similar safety profile to those who did not require dose reductions. Grade 3 or 4 hematological events in patients who received dexamethasone dose reductions relative to those who maintained the planned dexamethasone dose were: neutropenia (23.7% vs 32.6%, respectively), thrombocytopenia (8.5% vs 6.8%), and anemia (6.8% vs 6.2%).
Among 1,400 patients with relapsed or refractory MM who were administered lenalidomide 25 mg plus high-dose dexamethasone in 28-day cycles as part of an expanded access program in North America, the most commonly reported grade 3 or 4 adverse events were: neutropenia (7.9%), thrombocytopenia (6.0%), fatigue (3.6%), anemia (3.5%), pneumonia (3.1%), and hyperglycemia (2.0%).128 Although the grade 3 or 4 adverse events were the same as those reported in the two phase III studies, their frequencies were lower. Likewise, the most commonly reported adverse events of all grades were the same as those reported in the two pivotal studies.
The findings of a recent analysis of 72 patients receiving lenalidomide plus dexamethasone as first-line therapy indicate that myelosuppression is associated with renal dysfunction.129 In this analysis, eight of 14 patients with grade 3 or 4 myelosuppression had a baseline CrCl of ≤40 mL/min, with Kaplan–Meier analysis showing a significant association between renal insufficiency and time to myelosuppression. In the subgroup analysis of patients in the MM-009 and MM-010 studies, patients with renal impairment at baseline tended to have an increased incidence of thrombocytopenia compared with those with normal renal function.104 In patients treated with lenalidomide plus dexamethasone, the incidences of neutropenia and thrombocytopenia increased among those with normal renal function from 31.0% and 7.0%, respectively, to 39.2% and 16.0% for mild renal impairment, and to 42.9% and 19.0% for moderate renal impairment, respectively. The incidences of neutropenia and thrombocytopenia among the 16 patients with severe renal impairment were 37.5% and 37.5%, respectively. In the dexamethasone-only arm, the incidences of neutropenia and thrombocytopenia among those with normal renal function were 4.3% and 5.5%, respectively (P < 0.001 and P = 0.649 relative to the lenalidomide plus dexamethasone arm), compared with 1.5% and 5.3% in patients with mild renal impairment (P < 0.001 and P = 0.007), 5.9% and 17.6% for moderate renal impairment (P < 0.001 and P = 1.00), and 8.3% and >0% in the 12 patients with severe renal impairment (P = 0.184 and P = 0.024), respectively. There were no significant differences in the incidences of thrombotic episodes in lenalidomide plus dexamethasone versus dexamethasone-only patients with mild (12.0% vs 6.1%, respectively; P = 0.126), moderate (14.3% vs 2.9%; P = 0.122), or severe (6.3% vs 8.3%; P = 1.00) renal impairment.
In a pooled analysis of the MM-009 and MM-010 studies, the incidence of diarrhea was 39% in the lenalidomide plus dexamethasone arm compared with 28% in the dexamethasone-only arm.130 Multivariate analysis found that therapy duration but not treatment assignment predicted diarrhea. Among a cohort of patients who received 9–15 months of therapy, the incidence of diarrhea after adjustment for treatment duration was similar for lenalidomide plus dexamethasone versus dexamethasone alone (42.3% vs 42.5%, respectively), suggesting that the risk of unexpected diarrhea with long-term therapy may be partly attributable to dexamethasone, but it is important to note that mild-to-moderate diarrhea is a well recognized effect of lenalidomide monotherapy, particularly with prolonged use.
As a single-agent therapy in the relapsed or refractory MM setting, lenalidomide is again associated with myelosuppression. In a phase I dose-escalation study of lenalidomide 5–50 mg/day, neutropenia was the most common adverse event, with grade 3 neutropenia occurring in 15 of 25 (60%) patients and grade 4 neutropenia in four of 25 (16%) patients.113 Grade 3 thrombocytopenia occurred in five of 25 (20%) patients. In a phase II study evaluating lenalidomide 30 mg once-daily versus 15 mg twice-daily, an increased incidence of cytopenia was noted in the twice-daily group, prompting a once-daily schedule moving forward.107 In a long-term follow-up of 15 patients treated initially with either 30 mg once daily (n = 11) or 15 mg twice daily (n = 4), with or without the addition of dexamethasone, the most common grade 3 or 4 toxicity was neutropenia, which occurred in 10 patients.108 No grade 3 or 4 thrombocytopenia, anemia, peripheral neuropathy, or DVT was reported. In a subsequent phase II study of 222 patients with relapsed or refractory MM, the most frequent grade 3 or 4 toxicities with single-agent lenalidomide 30 mg once daily given on days 1–21 of every 28-day cycle were neutropenia (60%), thrombocytopenia (39%), and anemia (20%).112 However, the incidence of DVT and febrile neutropenia was low (both 4%).
Prior to receiving regulatory approval, both thalidomide and lenalidomide were associated with VTE incidences >20% when combined with dexamethasone for use as an off-label treatment for MM.131 In a systematic review of VTE rates, a search of the US FDA’s MedWatch program found reports of VTE among eight lenalidomide-treated cancer patients, including three receiving aspirin prophylaxis, two on warfarin, and one on low-molecular-weight heparins.131 Clinical trials identified VTE in 38 of 278 (13.7%) previously untreated patients and 48 of 346 (13.9%) relapsed or refractory MM patients receiving lenalidomide plus dexamethasone. None of these patients received routine thromboprophylaxis. In another systematic review, VTE rates ranged from 8.5%–75% in MM patients treated with lenalidomide and dexamethasone or erythropoietin. However, with the addition of aspirin this rate was <3.4%.132 NCCN guidelines currently recommend anticoagulation therapy in patients treated with lenalidomide plus dexamethasone.27 However, controlled studies may be needed to identify optimal thromboprophylaxis for patients treated with lenalidomide and dexamethasone.
In combination with bortezomib 1.0 mg/m2 and dexamethasone 20 mg/10 mg, lenalidomide 15 mg administered for up to eight cycles is associated with manageable toxicities consisting mainly of grade 1 or 2 myelosuppression.119 Attributable nonhematologic toxicities were DVT in two of 41 patients, grade 3 atrial fibrillation in two patients, and grade 3 peripheral neuropathy in one patient. Dose reductions were required for lenalidomide in nine patients, bortezomib in five patients, and dexamethasone in 14 patients.
The combination of lenalidomide 10 mg/day with melphalan 0.18 mg/kg, prednisone 2 mg/kg and thalidomide 50–100 mg was generally well tolerated in patients who received up to six cycles of therapy as second- or third-line treatment.122 The most frequent adverse events were hematologic, with 48% of patients experiencing grade 3 neutropenia and 16% experiencing grade 4 neutropenia. Grade 3 and 4 thrombocytopenia were reported in 26% and 10% of patients, respectively. Growth factor support was required in 39% of patients and one1 patient required platelet transfusion. The most frequent nonhematologic toxicity was infection in 19% of patients. No VTE events were detected.
In 41 patients treated with lenalidomide 25 mg (days 1–21) in combination with doxorubicin 9 mg/m2 (days 1–4), dexamethasone 40 mg (days 1–4 and 17–20), and G-CSF 6 mg, grade 3 or 4 infection occurred in 10% of patients and VTE occurred in 5%.117 Eight patients prematurely discontinued due to catheter-related septicemia (n = 2), thrombosis of basal artery (n = 1), prolonged pneumonia (n = 1), or withdrawal of consent (n = 4). Adverse events were generally of moderate severity and manageable.
Ongoing clinical development
The encouraging results of the two pivotal phase III studies demonstrating that lenalidomide in combination with dexamethasone significantly prolongs survival compared with dexamethasone alone, has led to further studies in previously treated MM patients. Among the phase III or IV studies currently being conducted in this setting, lenalidomide is being evaluated in combination with: dexamethasone; bortezomib and dexamethasone; and dexamethasone with or without thalidomide. Lenalidomide is additionally being evaluated as maintenance therapy following ASCT. Other investigational combinations currently being investigated in phase I and II trials include lenalidomide plus dexamethasone in combination with each of the following: panobinostat, bevacizumab, SGN-40, perifosine, vorinostat, dasatinib, NPI-0002, and carfilzomib. Lenalidomide is also being studied in combination with everolimus, and as monotherapy in patients who have relapsed on prior SCT.
The finding that lenalidomide in combination with dexamethasone yields high objective response and survival rates at one-, two-, and three-year follow-up has also encouraged further research in newly diagnosed MM. In this setting, lenalidomide is being evaluated in phase III studies as single-agent therapy (melphalan/prednisone/thalidomide and single-agent dexamethasone as comparators), and for use in combination with dexamethasone, and melphalan and prednisone in patients aged >65 years. There is now evidence that initial induction therapy with a lenalidomide-based regimen does not prevent harvest of adequate numbers of CD34+ positive stem cells for autologous SCT, but appears to be dependent on mobilization using a combination of G-CSF and cyclophosphamide, or similar.94–96 Numerous phase I and II studies are currently investigating lenalidomide combination regimens in previously untreated patients including lenalidomide and bortezomib plus dexamethasone, with or without cyclophosphamide, and lenalidomide and bortezomib plus dexamethasone and doxorubicin. Lenalidomide is additionally being evaluated as maintenance therapy following autologous SCT.
Economic evidence and resource utilization
Limited information on the health economics of lenalidomide in MM comes from a budget impact model comparing resource utilization of four approved therapies in the US.133 This study used a managed-care payer perspective to assess resource utilization in MM associated with each of single-agent bortezomib, bortezomib plus pegylated liposomal doxorubicin, thalidomide plus dexamethasone, and lenalidomide plus dexamethasone. Drug costs were calculated based on average wholesale price less 15%, with a 10% patient coinsurance contribution for thalidomide plus dexamethasone and lenalidomide plus dexamethasone, and a 20% patient contribution for single-agent bortezomib and bortezomib plus doxorubicin. Costs of therapy and costs of treating adverse events were based on standard sources or from peer-reviewed publications and/or meeting presentations. Incidences of adverse events, and assumptions for supportive care and prophylaxis were obtained from the prescribing information for each of the approved therapies and from published reports of pivotal phase III trials. Duration of therapy was based on the published median duration of therapy.
In this model, total costs for each of the four regimens were primarily driven by direct drug costs, with an acquisition cost of US $64,806 for the combination of lenalidomide plus dexamethasone.133 This represented a 1.7-fold increase on drug costs for the thalidomide plus dexamethasone combination, and a 1.9-fold increase on drug costs for the bortezomib plus doxorubicin combination. However, associated medical costs of lenalidomide plus dexamethasone (US $1,623) were comparable to thalidomide plus dexamethasone, and less than a quarter of that of bortezomib plus doxorubicin. Costs attributable to adverse events were again favorable for the lenalidomide plus dexamethasone combination (US $5,243), representing a cost-saving of US $2,667 compared with thalidomide plus dexamethasone, and US $851 compared with bortezomib plus doxorubicin. The total cost of the lenalidomide plus dexamethasone regimen including cost of prophylaxis for DVT and pulmonary embolism was US $72,822, which represents a 1.5-fold higher total cost compared with either thalidomide plus dexamethasone, or bortezomib plus doxorubicin.
This study has several weaknesses of which the most important is that it does not account for differences in efficacy as a function of cost. No consideration was given to the patient populations, which in the pivotal phase III trials of lenalidomide involved a heavily pretreated population with relapsed or refractory disease, with a consequent impact on duration of therapy and adverse events. As oral drugs, lenalidomide and thalidomide in combination with dexamethasone would be expected to offer an improvement in health-related quality of life compared with combination bortezomib plus doxorubicin, which are administered as intravenous infusions. However, this study offers a starting point for comparisons between MM therapies and is a valid approach to economic analysis from the viewpoint of the payer. Additional health economic studies of lenalidomide are required that include quality of life measures and data on the cost utility of treatment.
In a chart review conducted in five university hospitals in France during the period 2004–2007, the total direct costs of usual care of patients with relapsed or refractory MM were estimated at €73, 000 per patient from first relapse until death or last follow-up.134 The study included a total of 102 patients with a mean age at diagnosis of 59 years and a mean of 2.8 lines of therapy since first relapse. Novel agents were used in 205 of 281 lines (73%) and consisted of thalidomide combination therapy (28%), bortezomib (22%), lenalidomide (13%), and bortezomib plus thalidomide (10%). The average cost per line was €26, 510 including €17,525 for drugs. With respect to the third-line of treatment, lenalidomide-based therapy was similar to bortezomib: mean duration and cost of treatment for lenalidomide was 7.4 months and €46,724 compared with 6.9 months and €46,321 for bortezomib.
Deniz and colleagues used a discrete event simulation model to estimate the long-term health and cost consequences of lenalidomide plus dexamethasone versus dexamethasone-alone in MM patients who received either 1 or ≥2 prior therapies.135 The model used patient responses to treatment and time-to-event data based on Weibull functions derived from pooled data from the MM-009 and MM-010 clinical studies. Long-term results from UK Medical Research Council-sponsored trials and Mayo Clinic data were used to calculate dexamethasone survival given that 47% of patients in the dexamethasone arm of the MM-009 and MM-010 trials crossed over to receive lenalidomide treatment following disease progression or ethical unblinding. Disease management costs were reflective of clinical practice in Wales, UK. Cost and health outcomes were discounted at 3.5% per annum to adjust to present values. Events and costs were considered over two years to reflect trial follow-up, whereas survival and quality-adjusted life years (QALYs) were modeled to end of life to avoid truncation bias. In patients with one prior therapy, lenalidomide plus dexamethasone was associated with improvements in both survival and QALYs (4.54 projected mean life years and 3.20 QALYs) compared with dexamethasone alone (2.00 and 1.39, respectively). This equated to an incremental cost per life year gained of £20,617 and per QALY gained of £28,943 in patients receiving lenalidomide. Similarly, in patients with at least two prior therapies, lenalidomide plus dexamethasone was associated with a projected mean survival of 3.61 life years and 2.50 QALYs compared with 1.41 life years and 1.00 QALYs for dexamethasone alone. The incremental cost of lenalidomide per life year gained in this group of patients was £19,218 and £28,184 per incremental QALY gained.
Patient group/population
The evidence to support lenalidomide in its licensed indication for use in combination with dexamethasone for the treatment of patients with relapsed or refractory MM who have undergone at least one prior therapy was predominantly derived from two pivotal phase III studies that compared lenalidomide plus high-dose dexamethasone with dexamethasone alone.2,3 MM-009 was conducted in 48 centers in the USA and Canada, and MM-010 was conducted in 51 centers in Europe, Australia, and Israel. The median age of patients was 63 years, most were male, and most had an ECOG performance status of 0 or 1. Approximately 65% of patients had Durie–Salmon stage III disease at diagnosis, three-quarters of patients had lytic disease, and a third had bone marrow involvement. A total of 61% of patients enrolled in MM-009 and 55% of patients in MM-010 had previously received at least one prior SCT, and most had received ≥2 previous lines of treatment. In MM-009, 10% of patients had previously received bortezomib, 44% had received thalidomide, and 60% had received dexamethasone. The respective data for patients enrolled in MM-010 were 4%, 34%, and 67%.
The patients enrolled in these studies represented a heavily pretreated population with advanced disease. A high proportion of patients (40.5%) were aged >65 years.136 Of the elderly group, the median time to diagnosis was approximately 3.3 years, and three-quarters had received ≥2 prior therapies, including dexamethasone in 69% and thalidomide in 32% of patients. However, the clinical benefit of lenalidomide plus dexamethasone in terms of response, TTP, and OS was comparable with younger patients.
Of the 353 patients in MM-009 and MM-010 who were randomized to receive lenalidomide plus dexamethasone, 210 (59.5%) had previously undergone autologous SCT.137 In a subgroup analysis comparing outcomes in patients with prior autologous SCT and no prior autologous SCT, there were no significant differences in ORR (63% vs 55%, respectively) or CR rate (13% vs 16%; P = 0.12). There was a trend towards prolonged TTP in patients without prior autologous SCT (14.2 vs 10.2 months for previous autologous SCT; P = 0.13). An interesting observation was that the median time from first pathologic diagnosis was similar for the two groups (3.4 years in the prior autologous SCT group vs 2.9 years in the no prior autologous SCT group). Based on the TTP trend, this observation implies that patients who have not had a chance to benefit from autologous SCT may receive an advantage from lenalidomide plus dexamethasone therapy, and provides a rationale for commencing lenalidomide-based therapy early in the disease course.137 This is further supported by the findings of a subgroup analysis, which suggested there was an advantage for second-line compared with later salvage treatment in terms of response rate and TTP.100
Thus, in patients with relapsed or refractory disease, the data indicate that treatment with lenalidomide plus dexamethasone is suitable as early or later salvage therapy in a broad group of patients. In particular, lenalidomide plus dexamethasone is effective at prolonging TTP independently of patient age, number or type of previous therapies including previous autologous SCT, and β2-microglobulin status.2,3 Moreover, the combination of lenalidomide plus dexamethasone is effective at prolonging OS irrespective of prior thalidomide use or the number of previous therapies; OS is also improved in patients with IgA disease at baseline and in patients with an ECOG performance status >0.2,3,101,102 In the relapsed or refractory setting, lenalidomide is emerging as a suitable partner for bortezomib, with nonoverlapping toxicities and a high rate of response.62,119
There is now increasing evidence to support a role for lenalidomide-based regimens as a first-line option where ORR >90% have been reported, including CR rates of 18%–25%.43,83,129 In newly diagnosed patients with ASIPs, the BiRD combination of lenalidomide, clarithromycin, and dexamethasone (BiRD) is associated with a CR rate of 71% and a VGPR or better rate of 96%.88
Dosage, administration, and formulation
Lenalidomide (CC-5013, Revlimid®), an immunomodulatory drug with antitumor, antiangiogenesis, and apoptotic activities, is an analog of thalidomide with more potent activity and a different tolerability profile. It is available for oral administration in 5 mg, 10 mg, 15 mg, and 25 mg capsules. Lenalidomide is indicated in combination with dexamethasone for the treatment of patients with MM who have received at least one prior therapy. The recommended starting dose is 25 mg/day with water, administered as a single 25 mg capsule on days 1–21 of a repeated 28-day cycle. The recommended dose of dexamethasone is 40 mg/day on days 1–4, 9–12, and 17–20 of each 28-day cycle for the first four cycles of therapy, and then at a dose of 40 mg/day on days 1–4 every 28 days. Dose modifications and interruptions are recommended to manage grade 3 or 4 neutropenia or thrombocytopenia, or other grade 3 or 4 lenalidomide-associated toxicities.138 In thrombocytopenia, when platelets fall to <30,000 per μL, lenalidomide treatment should be interrupted and follow-up complete blood counts performed weekly until recovery is confirmed (≥30,000 per μL). Treatment should be restarted at 15 mg/day. For each subsequent platelet fall to <30,000 per μL, treatment should again be interrupted and resumed at 5 mg less than the previous dose when platelet levels recover to 30,000 per μL. In neutropenia, when the absolute neutrophil count (ANC) falls to <1000 per μL, lenalidomide treatment should be interrupted and treatment with G-CSF initiated with weekly follow-up complete blood counts. When the ANC increases to ≥1000 per μL and neutropenia is the only toxicity, lenalidomide should be resumed at 25 mg/day or at 15 mg/day if there is another toxicity. For each subsequent fall to <1000 per μL, treatment should again be interrupted and resumed at 5 mg less than the previous dose when the ANC recovers to ≥1000 per μL. For other grade 3 or 4 toxicities related to lenalidomide, treatment should be interrupted and restarted at the next lower dose level when the toxicity has resolved to grade 2 or lower. Lenalidomide should not be dosed below 5 mg/day.138
Place in therapy
In patients with relapsed or refractory MM who have received 1–3 prior lines of therapy, lenalidomide in combination with high-dose dexamethasone produces significant prolongation of TTP and OS compared with high-dose dexamethasone alone.2,3 In patients with previously untreated MM, lenalidomide in combination with low-dose dexamethasone produces a significant survival advantage compared with lenalidomide plus high-dose dexamethasone.84,90 In both the newly diagnosed and relapsed or refractory settings, the addition of lenalidomide to high-dose dexamethasone is associated with a higher rate of grade 3 or 4 myelosuppression, and in the absence of appropriate thromboprophylaxis, a higher rate of VTE events compared with high-dose dexamethasone alone.2,3,43,139 However, there is level 2 evidence from the MM-009 study that survival is not affected by occurrence of DVT.126
The MM-009 and MM-010 pivotal phase III studies provided level 2 evidence in support of lenalidomide plus dexamethasone in the relapsed or refractory setting. After a median follow-up of 17.1 months post-randomization, median OS in the lenalidomide plus high-dose dexamethasone group was 29.6 months in MM-009 and not yet reached in MM-010.2,3 In comparison, median OS in the placebo plus high-dose dexamethasone group was 20.2 months in MM-009 and 20.6 months in MM-010. Thus, the addition of lenalidomide to high-dose dexamethasone in patients who have received 1–3 prior therapies is likely to prolong median survival by approximately nine months. After adjusting for crossover of patients initially assigned to dexamethasone alone into the lenalidomide arm, prolongation of survival is likely to be further enhanced.124 Level 2 evidence is available in support of this regimen in patients with one or more than one prior lines of therapy,100 in patients with or without previous thalidomide exposure,99 in patients with or without prior autologous SCT,137 and in patients with mild-to-moderate renal impairment.104 Furthermore, there is level 2 evidence in support of this regimen in patients aged ≥65 years and in those aged <65 years.136
There is level 2 evidence in support of lenalidomide plus dexamethasone in patients with newly diagnosed MM from two phase III studies.83,84,90,91 In one study, the investigators did not directly compare the lenalidomide-based regimen with a recognized therapy (ie, dexamethasone alone).84,90 Instead, patients in each arm received lenalidomide with either high-dose or low-dose dexamethasone. OS was significantly superior in the low-dose dexamethasone group (one-year OS 96% vs 88% in the high-dose dexamethasone group; two-year OS 87% vs 75% in the high-dose group).84 These data compare favorably with other regimens in this setting, including bortezomib monotherapy (one-year survival 80%).109 In the second study, which compared lenalidomide plus dexamethasone with dexamethasone alone, patient accrual was stopped early due to external data affecting the acceptability of the control arm.83 The one-year survival data did not favor either treatment arm (93% for lenalidomide plus dexamethasone vs 91% for dexamethasone alone). A subgroup analysis suggested the presence of abnormal cytogenetics at baseline was associated with a reduced one-year OS rate compared with no abnormal karyotype (one-year OS 82% vs 97%, respectively). High-risk cytogenetic abnormalities (HRCA) did not appear to account for this difference (one-year OS in patients with HRCA 100% vs 92% without HRCA); however, sample size limited the statistical power of this study.91 Significant differences between the two treatment arms were observed in terms of response rates with lenalidomide plus dexamethasone yielding an ORR of 85.3% and a CR rate of 22.1% compared with 51.3% and 3.8%, respectively, for dexamethasone alone (P = 0.001).83
During clinical development of lenalidomide, it became apparent that addition of the drug to dexamethasone resulted in a higher rate of VTE events than dexamethasone alone. Although early trial protocols did not include thromboprophylaxis, anticoagulation therapy with aspirin or low-molecular weight heparin is now recommended. Given that anticoagulation therapy has been inconsistently applied during the lenalidomide clinical development program, it is difficult to assess the impact of anticoagulation therapy on VTE. However, level 4 evidence is available from a systematic review of published literature, abstracts, and package inserts to support the hypothesis that aspirin therapy reduces the incidence of VTE events to <5% of patients.132 A similar review that captured data for thalidomide as well as lenalidomide suggested that lower rates of VTE may be obtained using low-molecular-weight heparins.131 The authors of this review concluded that randomized clinical trials of anticoagulation therapies are needed in order to identify appropriate prophylaxis when MM patients receive either lenalidomide or thalidomide with dexamethasone.
Overall, the current evidence base presented herein suggests that lenalidomide has significantly impacted the treatment of MM, and delivered survival benefits to both patients with newly diagnosed, or relapsed or refractory disease. Although lenalidomide is associated with an increased risk of grade 3 or 4 myelosuppression when combined with dexamethasone, these risks can be mitigated through routine monitoring, dose interruptions, and growth factor support where appropriate. Adequate anticoagulation therapy is needed to minimize the risk of VTE, and in this regard further investigation is necessary to determine optimal treatment. The ability to combine lenalidomide with other agents (eg, bortezomib) is an important feature, and as such lenalidomide, together with bortezomib, thalidomide, and glucocorticoids, can be considered “backbone” agents as part of combination therapy in the treatment of MM.140
Acknowledgments
The authors gratefully acknowledge the assistance of Katherine Redman in preparing this paper and also received editorial support in the preparation of this manuscript, funded in part by Celgene. The authors, however, were fully responsible for content and editorial decisions for this manuscript. The authors disclose the following potential conflicts of interest: Drs Richardson, Ghobrial, and Munshi have participated in advisory boards for Millennium and Celgene; Drs Richardson, Schlossman, Ghobrial, and Munshi have received honoraria from Millennium and Celgene; Dr Anderson has acted as consultant, participated in advisory boards, and received honoraria and grants from Millennium, Celgene, and Novartis; Drs Mitsiades, Laubach, and Teru Hideshima declare no potential conflicts of interest.
References
- 1.Celgene Corporation Revlimid® Prescribing Information. 2009. Accessed April 2009. Available from: http://www.revlimid.com/pdf/REVLIMID_PI.pdf
- 2.Dimopoulos M, Spencer A, Attal M, et al. Multiple Myeloma (010) Study Investigators Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 3.Weber DM, Chen C, Niesvizky R, et al. Multiple Myeloma (009) Study Investigators Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007a;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 4.Bladé J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 5.Kuehl WM, Bergsagel PL. Multiple myeloma: evolving genetic events and host interactions. Nat Rev Cancer. 2002;2:175–187. doi: 10.1038/nrc746. [DOI] [PubMed] [Google Scholar]
- 6.Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 7.[ACS] American Cancer Society ). Detailed Guide: Multiple Myeloma. 2008. Accessed April 2008. Available from: http://www.cancer.org/docroot/CRI/CRI_2_3x.asp?dt=30
- 8.Piazza FA, Gurrieri C, Trentin L, Semenzato G. Towards a new age in the treatment of multiple myeloma. Ann Hematol. 2007;86:159–172. doi: 10.1007/s00277-006-0239-5. [DOI] [PubMed] [Google Scholar]
- 9.Kumar S, Rajkumar SV. Thalidomide and lenalidomide in the treatment of multiple myeloma. Eur J Cancer. 2006;42:1612–1622. doi: 10.1016/j.ejca.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 11.[NCI] National Cancer Institute Surveillance Epidemiology and End Results (SEER). 2008. Accessed April 2008. Available from: http://seer.cancer.gov/at: http://seer.cancer.gov/statfacts/html/mulmy.html
- 12.[IMWG] International Myeloma Working Group Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–757. [PubMed] [Google Scholar]
- 13.Durie BG. New approaches to treatment for multiple myeloma: durable remission and quality of life as primary goals. Clin Lymphoma Myeloma. 2005;6:181–190. doi: 10.3816/CLM.2005.n.045. [DOI] [PubMed] [Google Scholar]
- 14.Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–854. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 15.[MMRF] Multiple Myeloma Research Foundation Multiple myeloma: disease overview. 2008. Accessed April 2008. Available from: http://www.multiplemyeloma.org/downloads/about_myeloma/Disease_Overview.pdf
- 16.Mihou D, Katodritou I, Zervas K. Evaluation of five staging systems in 470 patients with multiple myeloma. Haematologica. 2006;91:1149–1150. [PubMed] [Google Scholar]
- 17.Gertz MA. Relevant prognostic features of multiple myeloma and the new International Staging System. Leuk Lymphoma. 2007;48:458–468. doi: 10.1080/10428190601059753. [DOI] [PubMed] [Google Scholar]
- 18.Rajkumar SV, Greipp PR. Prognostic factors in multiple myeloma. Hematol Oncol Clin North Am. 1999;13:1295–1314. doi: 10.1016/s0889-8588(05)70128-3. [DOI] [PubMed] [Google Scholar]
- 19.Turesson I, Abildgaard N, Ahlgren T, et al. Prognostic evaluation in multiple myeloma: an analysis of the impact of new prognostic factors. Br J Haematol. 1999;106:1005–1012. doi: 10.1046/j.1365-2141.1999.01651.x. [DOI] [PubMed] [Google Scholar]
- 20.Tricot G, Barlogie B, Jagannath S, et al. Poor prognosis in multiple myeloma is associated only with partial or complete deletions of chromosome 13 or abnormalities involving 11q and not with other karyotype abnormalities. Blood. 1995;86:4250–4256. [PubMed] [Google Scholar]
- 21.Tricot G, Sawyer JR, Jagannath S, et al. Unique role of cytogenetics in the prognosis of patients with myeloma receiving high-dose therapy and autotransplants. J Clin Oncol. 1997;15:2659–2666. doi: 10.1200/JCO.1997.15.7.2659. [DOI] [PubMed] [Google Scholar]
- 22.Facon T, Avet-Loiseau H, Guillerm G, et al. Intergroupe Francophone du Myélome Chromosome 13 abnormalities identified by FISH analysis and serum beta2-microglobulin produce a powerful myeloma staging system for patients receiving high-dose therapy. Blood. 2001;97:1566–1571. doi: 10.1182/blood.v97.6.1566. [DOI] [PubMed] [Google Scholar]
- 23.Fonseca R, Blood E, Rue M, et al. Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood. 2003;101:4569–4575. doi: 10.1182/blood-2002-10-3017. [DOI] [PubMed] [Google Scholar]
- 24.Fonseca R, Barlogie B, Bataille R, et al. Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Res. 2004;64:1546–1558. doi: 10.1158/0008-5472.can-03-2876. [DOI] [PubMed] [Google Scholar]
- 25.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 26.Durie BG, Harousseau JL, Miguel JS, et al. International Myeloma Working Group International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 27.[NCCN] National Comprehensive Cancer Network® ). NCCN Clinical Practice Guidelines in Oncology™. Multiple myeloma. V.2. Accessed August 2009. Available from: http://www.nccn.org/professionals/physician_gls/PDF/myeloma.pdf
- 28.Anderson KC, Kyle RA, Rajkumar SV, et al. ASH/FDA Panel on Clinical Endpoints in Multiple Myeloma Clinically relevant end points and new drug approvals for myeloma. Leukemia. 2007;22:231–239. doi: 10.1038/sj.leu.2405016. [DOI] [PubMed] [Google Scholar]
- 29.Smith A, Wisloff F, Samson D, UK Myeloma Forum. Nordic Myeloma Study Group. British Committee for Standards in Haematology Guidelines on the diagnosis and management of multiple myeloma 2005. Br J Haematol. 2006;132:410–451. doi: 10.1111/j.1365-2141.2005.05867.x. [DOI] [PubMed] [Google Scholar]
- 30.Bergsagel PL. A kinder, gentler way: control of the proliferative tumor compartment, not cosmetic complete response, should be the goal of myeloma therapy. Leukemia. 2008;22:673–675. doi: 10.1038/leu.2008.2. [DOI] [PubMed] [Google Scholar]
- 31.Hjorth M, Hellquist L, Holmberg E, Magnusson B, Rödjer S, Westin J. Initial versus deferred melphalan-prednisone therapy for asymptomatic multiple myeloma stage I – a randomized study. Myeloma Group of Western Sweden. Eur J Haematol. 1993;50:95–102. doi: 10.1111/j.1600-0609.1993.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 32.Grignani G, Gobbi PG, Formisano R, et al. A prognostic index for multiple myeloma. Br J Cancer. 1996;73:1101–1107. doi: 10.1038/bjc.1996.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar S, Lacy MQ, Dispenzieri A, et al. Single agent dexamethasone for pre-stem cell transplant induction therapy for multiple myeloma. Bone Marrow Transplant. 2004;34:485–490. doi: 10.1038/sj.bmt.1704633. [DOI] [PubMed] [Google Scholar]
- 34.Kumar A, Loughran T, Alsina M, Durie BG, Djulbegovic B. Management of multiple myeloma: a systematic review and critical appraisal of published studies. Lancet Oncol. 2003;4:293–304. doi: 10.1016/s1470-2045(03)01077-5. [DOI] [PubMed] [Google Scholar]
- 35.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 36.Attal M, Harousseau JL, Leyvraz S, et al. Inter-Groupe Francophone du Myélome (IFM) Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108:3289–3294. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 37.Mihelic R, Kaufman JL, Lonial S. Maintenance therapy in multiple myeloma. Leukemia. 2007;21:1150–1157. doi: 10.1038/sj.leu.2404633. [DOI] [PubMed] [Google Scholar]
- 38.Orlowski RZ. Initial therapy of multiple myeloma patients who are not candidates for stem cell transplantation. Hematology Am Soc Hematol Educ Program. 2006:338–347. doi: 10.1182/asheducation-2006.1.338. [DOI] [PubMed] [Google Scholar]
- 39.Jagannath S. Treatment of myeloma in patients not eligible for transplantation. Curr Treat Options Oncol. 2005;6:241–253. doi: 10.1007/s11864-005-0007-0. [DOI] [PubMed] [Google Scholar]
- 40.San Miguel JF, Schlag R, Khuageva NK, et al. VISTA Trial Investigators Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 41.Palumbo A, Falco P, Corradini P, et al. GIMEMA – Italian Multiple Myeloma Network Melphalan, prednisone, and lenalidomide treatment for newly diagnosed myeloma: a report from the GIMEMA – Italian Multiple Myeloma Network. J Clin Oncol. 2007;25:4459–4465. doi: 10.1200/JCO.2007.12.3463. [DOI] [PubMed] [Google Scholar]
- 42.Rajkumar SV, Hayman SR, Lacy MQ, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106:4050–4053. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lacy MQ, Gertz MA, Dispenzieri A, et al. Long-term results of response to therapy, time to progression, and survival with lenalidomide plus dexamethasone in newly diagnosed myeloma. Mayo Clin Proc. 2007;82:1179–1184. doi: 10.4065/82.10.1179. [DOI] [PubMed] [Google Scholar]
- 44.Thomas S, Alexanian R. Current treatment strategies for multiple myeloma. Clin Lymphoma Myeloma. 2007;7(Suppl 4):S139–S144. doi: 10.3816/clm.2007.s.014. [DOI] [PubMed] [Google Scholar]
- 45.Voorhees PM, Orlowski RZ. Emerging role of novel combinations for induction therapy in multiple myeloma. Clin Lymphoma Myeloma. 2006;7:33–41. doi: 10.3816/CLM.2006.n.037. [DOI] [PubMed] [Google Scholar]
- 46.[FDA] US Food and Drug Administration Accessed October 2008. FDA approves lenalidomide oral capsules (Revlimid) for use in combination with dexamethasone in patients with multiple myeloma. 2006. Available from: http://www.fda.gov/cder/Offices/OODP/whatsnew/lenalidomide.htm
- 47.Rajkumar SV, Fonseca R, Dispenzieri A, et al. Thalidomide in the treatment of relapsed multiple myeloma. Mayo Clin Proc. 2000;75:897–901. doi: 10.4065/75.9.897. [DOI] [PubMed] [Google Scholar]
- 48.Lee CK, Barlogie B, Munshi N, et al. DTPACE: an effective, novel combination chemotherapy with thalidomide for previously treated patients with myeloma. J Clin Oncol. 2003;21:2732–2739. doi: 10.1200/JCO.2003.01.055. [DOI] [PubMed] [Google Scholar]
- 49.Kumar S, Rajkumar SV. Thalidomide and dexamethasone: therapy for multiple myeloma. Expert Rev Anticancer Ther. 2005;5:759–766. doi: 10.1586/14737140.5.5.759. [DOI] [PubMed] [Google Scholar]
- 50.Prince HM, Schenkel B, Mileshkin L. An analysis of clinical trials assessing the efficacy and safety of single-agent thalidomide in patients with relapsed or refractory multiple myeloma. Leuk Lymphoma. 2007b;48:46–55. doi: 10.1080/10428190601001904. [DOI] [PubMed] [Google Scholar]
- 51.Dimopoulos MA, Zervas K, Kouvatseas G, et al. Thalidomide and dexamethasone combination for refractory multiple myeloma. Ann Oncol. 2001;12:991–995. doi: 10.1023/a:1011132808904. [DOI] [PubMed] [Google Scholar]
- 52.Schütt P, Ebeling P, Buttkereit U, et al. Thalidomide in combination with dexamethasone for pretreated patients with multiple myeloma: serum level of soluble interleukin-2 receptor as a predictive factor for response rate and for survival. Ann Hematol. 2005;84:594–600. doi: 10.1007/s00277-005-1007-7. [DOI] [PubMed] [Google Scholar]
- 53.Terpos E, Mihou D, Szydlo R, et al. The combination of intermediate doses of thalidomide with dexamethasone is an effective treatment for patients with refractory/relapsed multiple myeloma and normalizes abnormal bone remodeling, through the reduction of sRANKL/osteoprotegerin ratio. Leukemia. 2005;19:1969–1976. doi: 10.1038/sj.leu.2403890. [DOI] [PubMed] [Google Scholar]
- 54.Kyriakou C, Thomson K, D’Sa S, et al. Low-dose thalidomide in combination with oral weekly cyclophosphamide and pulsed dexamethasone is a well tolerated and effective regimen in patients with relapsed and refractory multiple myeloma. Br J Haematol. 2005;129:763–770. doi: 10.1111/j.1365-2141.2005.05521.x. [DOI] [PubMed] [Google Scholar]
- 55.Prince HM, Adena M, Smith DK, Hertel J. Efficacy of single-agent bortezomib vs single-agent thalidomide in patients with relapsed or refractory multiple myeloma: a systematic comparison. Eur J Haematol. 2007;79:93–99. doi: 10.1111/j.1600-0609.2007.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richardson PG, Sonneveld P, Schuster MW, et al. Assessment of Proteasome Inhibition for Extending Remissions (APEX) Investigators Bortezomib or high-dose dexamethasone for relapsed multiple myeloma N Engl J Med 20051062547352:2487–2498. [DOI] [PubMed] [Google Scholar]
- 57.Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110:3557–3560. doi: 10.1182/blood-2006-08-036947. [DOI] [PubMed] [Google Scholar]
- 58.Jagannath S, Barlogie B, Berenson J, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127:165–172. doi: 10.1111/j.1365-2141.2004.05188.x. [DOI] [PubMed] [Google Scholar]
- 59.Kropff MH, Bisping G, Wenning D, et al. Bortezomib in combination with dexamethasone for relapsed multiple myeloma. Leuk Res. 2005;29:587–590. doi: 10.1016/j.leukres.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 60.Popat R, Oakervee H, Williams C, et al. Bortezomib, low dose intravenous melphalan and dexamethasone for patients with relapsed multiple myeloma [abstract] Haematologica. 2008;93(Suppl 1):0918. doi: 10.1111/j.1365-2141.2008.07572.x. [DOI] [PubMed] [Google Scholar]
- 61.Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 62.Richardson P, Jagannath S, Raje N, et al. Lenalidomide, bortezomib, and dexamethasone (Rev/Vel/Dex) in patients with relapsed or relapsed/refractory multiple myeloma (MM): preliminary results of a phase II study [abstract] Blood 2007110 Abstract 2714. [Google Scholar]
- 63.Richardson P, Mitsiades C, Schlossman R, et al. The treatment of relapsed and refractory multiple myeloma. Hematology Am Soc Hematol Educ Program. 2007:317–323. doi: 10.1182/asheducation-2007.1.317. [DOI] [PubMed] [Google Scholar]
- 64.Badros Z, Philip S, Niesvizky R, et al. Phase I trial of vorinostat plus bortezomib (Bort) in relapsed and refractory multiple myeloma (MM) patients (pts) [abstract] Haematologica. 2008;93(Suppl 1):0642. [Google Scholar]
- 65.Weber DM, Jagannath S, Mazumder A, et al. Phase I trial of oral vorinostat in combination with bortezomib in advanced multiple myeloma [abstract] Haematologica 200893Suppl 1 Abstract 0640. [Google Scholar]
- 66.Palumbo A, Ambrosini MT, Benevolo G, et al. Italian Multiple Myeloma Network; Gruppo Italiano Malattie Ematologicche dell’Adulto Bortezomib, melphalan, prednisone, and thalidomide for relapsed multiple myeloma. Blood. 2007;109:2767–2772. doi: 10.1182/blood-2006-08-042275. [DOI] [PubMed] [Google Scholar]
- 67.Zangari M, Anaissie E, Barlogie B, et al. Increased risk of deep-vein thrombosis in patients with multiple myeloma receiving thalidomide and chemotherapy. Blood. 2001;98:1614–1615. doi: 10.1182/blood.v98.5.1614. [DOI] [PubMed] [Google Scholar]
- 68.Zangari M, Barlogie B, Thertulien R, et al. Thalidomide and deep vein thrombosis in multiple myeloma: risk factors and effect on survival. Clin Lymphoma. 2003;4:32–35. doi: 10.3816/clm.2003.n.011. [DOI] [PubMed] [Google Scholar]
- 69.Kastritis E, Dimopoulos MA. The evolving role of lenalidomide in the treatment of hematologic malignancies. Expert Opin Pharmacother. 2007;8:497–509. doi: 10.1517/14656566.8.4.497. [DOI] [PubMed] [Google Scholar]
- 70.[EMEA] European Medicines Agency Scientific Discussion, 2007. Available from: http://www.emea.europa.eu/humandocs/PDFs/EPAR/revlimid/H-717-en6.pdf Accessed October 2008.
- 71.Anderson KC. Lenalidomide and thalidomide: mechanisms of action – similarities and differences. Semin Hematol. 2005;42(4 Suppl 4):S3–S8. doi: 10.1053/j.seminhematol.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 72.Hideshima T, Mitsiades C, Tonon G, et al. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 73.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4:314–322. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 74.Hideshima T, Chauhan D, Shima Y, Richardson PG, Anderson KC. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96:2943–2950. [PubMed] [Google Scholar]
- 75.Verhelle D, Corral LG, Wong K, et al. Lenalidomide and CC-4047 inhibit the proliferation of malignant B cells while expanding normal CD34+ progenitor cells. Cancer Res. 2007;67:746–755. doi: 10.1158/0008-5472.CAN-06-2317. [DOI] [PubMed] [Google Scholar]
- 76.List AF. Lenalidomide – the phoenix rises. N Engl J Med. 2007;357:2183–2186. doi: 10.1056/NEJMe078203. [DOI] [PubMed] [Google Scholar]
- 77.Bruno B, Rotta M, Giaccone L, et al. New drugs for treatment of multiple myeloma. Lancet Oncol. 2004;5:430–442. doi: 10.1016/S1470-2045(04)01511-6. [DOI] [PubMed] [Google Scholar]
- 78.Knight R. IMiDs: a novel class of immunomodulators. Semin Oncol. 2005;32(4 Suppl 5):S24–S30. doi: 10.1053/j.seminoncol.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 79.Corral LG, Haslett PA, Muller GW, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163:380–386. [PubMed] [Google Scholar]
- 80.Mitsiades CS, Mitsiades N, Koutsilieris M. The Akt pathway: molecular targets for anti-cancer drug development. Curr Cancer Drug Targets. 2004;4:235–256. doi: 10.2174/1568009043333032. [DOI] [PubMed] [Google Scholar]
- 81.Dredge K, Horsfall R, Robinson SP, et al. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc Res. 2005;69:56–63. doi: 10.1016/j.mvr.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 82.Davies FE, Raje N, Hideshima T, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–216. doi: 10.1182/blood.v98.1.210. [DOI] [PubMed] [Google Scholar]
- 83.Zonder JA, Crowley J, Hussein MA, et al. Superiority of lenalidomide (Len) plus high-dose dexamethasone (HD) compared to HD alone as treatment of newly-diagnosed multiple myeloma (NDMM): results of the randomized, double-blinded, placebo-controlled SWOG trial S0232 [abstract] Blood. 2007;110:77. [Google Scholar]
- 84.Rajkumar SV, Jacobus S, Callander N, et al. Randomized trial of lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone in newly diagnosed myeloma (E4A03), a trial coordinated by the Eastern Cooperative Oncology Group: Analysis of response, survival, and outcome [abstract] J Clin Oncol. 2008;26(Suppl):8504. [Google Scholar]
- 85.Kumar S, Hayman SR, Buadi FK, et al. Phase II trial of lenalidomide, cyclophosphamide, and dexamethasone (CRd) for newly diagnosed myeloma [abstract] Blood. 2007;110:190. [Google Scholar]
- 86.Richardson PG, Lonial S, Jakubowiak A, et al. Safety and efficacy of lenalidomide (Len), bortezomib (Bz), and dexamethasone (Dex) in patients (pts) with newly diagnosed multiple myeloma (MM): A phase I/II study [abstract] J Clin Oncol. 2008;26(Suppl):8520. [Google Scholar]
- 87.Niesvizky R, Jayabalan DS, Christos PJ, et al. BiRD (Biaxin [clarithromycin]/Revlimid [lenalidomide]/dexamethasone) combination therapy results in high complete- and overall-response rates in treatment-naive symptomatic multiple myeloma. Blood. 2008;111:1101–1109. doi: 10.1182/blood-2007-05-090258. [DOI] [PubMed] [Google Scholar]
- 88.Mark T, Jayabalan D, Pearse RN, et al. Atypical serum immunofixation pattern (ASIP) development during induction therapy with BiRD for newly diagnosed multiple myeloma correlates with a high rate of complete remission [abstract] Blood. 2007;110:2737. [Google Scholar]
- 89.Palumbo AP, Falco T, Corradini P, et al. Bortezomib, pegylated-lyposomal-doxorubicin and dexamethasone (PAD) as induction therapy prior to reduced intensity autologous stem cell transplant (ASCT) followed by lenalidomide and prednisone (LP) as consolidation and lenalidomide alone as maintenance [abstract] J Clin Oncol. 2008;26(Suppl):8518. [Google Scholar]
- 90.Rajkumar SV, Jacobus S, Callander N, et al. A randomized trial of lenalidomide plus high-dose dexamethasone (RD) versus lenalidomide plus low-dose dexamethasone (Rd) in newly diagnosed multiple myeloma (E4A03): a trial coordinated by the Eastern Cooperative Oncology Group [abstract] Blood 20071107417371947 [Google Scholar]
- 91.Zonder JA, Crowley JJ, Bolejack V, et al. A randomized Southwest Oncology Group study comparing dexamethasone (D) to lenalidomide + dexamethasone (LD) as treatment of newly-diagnosed multiple myeloma (NDMM): Impact of cytogenetic abnormalities on efficacy of LD, and updated overall study results [abstract] J Clin Oncol. 2008;26(Suppl):8521. [Google Scholar]
- 92.Palumbo A, Falco P, Corradini P, et al. Melphalan, prednisone and lenalidomide for newly diagnosed myeloma: kinetics of neutropenia/thrombocytopenia and time to event results [abstract] Haematologica. 2008;93(Suppl 1):0213. doi: 10.3816/CLM.2009.n.035. [DOI] [PubMed] [Google Scholar]
- 93.Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21:2035–2042. doi: 10.1038/sj.leu.2404801. [DOI] [PubMed] [Google Scholar]
- 94.Paripati H, Stewart AK, Fonseca R, et al. Impact of lenalidomide therapy on stem cell mobilization in myeloma [abstract] J Clin Oncol. 2008;26(Suppl):8543. [Google Scholar]
- 95.Cook RJ, Vogl D, Mangan PA, et al. Lenalidomide and stem cell collection in patients with multiple myeloma [abstract] J Clin Oncol. 2008;26(Suppl):8547. [Google Scholar]
- 96.Mark T, Stern J, Furst J, et al. Stem cell mobilization with cylophosphamide overcomes the suppressive effect of lenalidomide therapy on stem cell collection in multiple myeloma. Biol Blood Marrow Transplant. 2008;14:795–798. doi: 10.1016/j.bbmt.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weber D, Knight R, Chen C, et al. Prolonged overall survival with lenalidomide plus dexamethasone compared with dexamethasone alone in patients with relapsed or refractory multiple myeloma [abstract] Blood. 2007;110:412. [Google Scholar]
- 98.Harousseau J–L, Weber D, Dimopoulos M, et al. Relapsed/refractory multiple myeloma patients treated with lenalidomide/dexamethasone who achieve a complete or near complete response have longer overall survival and time to progression compared with patients achieving a partial response [abstract] Blood. 2007;110:3598. [Google Scholar]
- 99.Wang M, Knight R, Dimopoulos D, et al. Effect of Len/Dex in MM despite Thal resistance [abstract] Haematol Hematol J Haematologica 200792s26 Suppl 2):PO-662. [Google Scholar]
- 100.Stadtmauer E, Weber D, Dimopoulos M, et al. Lenalidomide in combination with dexamethasone is more effective than dexamethasone at first relapse in relapsed multiple myeloma [abstract] Blood. 2006;108:3552. [Google Scholar]
- 101.Foa R, Weber D, Dimopoulos M, et al. Lenalidomide/dexamethasone improves response and prolongs time to progression, even in patients with IgA multiple myeloma: a sub-analysis of the MM-009/010 studies [abstract] Blood. 2007;110:4839. [Google Scholar]
- 102.Chanan-Khan AA, Dimopoulos M, Weber D, et al. ECOG performance status affects efficacy, but not safety, of lenalidomide/dexamethasone in relapsed/refractory multiple myeloma (MM009/010 sub-analysis) [abstract] Blood. 2007;110:2721. [Google Scholar]
- 103.Lonial S, Knight R, Dimopoulos M, et al. Effect of Len/Dex in MM in different age groups. Haematologica. 2007;92(6 Suppl 2):PO-663. [Google Scholar]
- 104.Weber DM, Spencer A, Wang M, et al. MM-009 and MM-010 Investigators The efficacy and safety of lenalidomide plus dexamethasone in relapsed or refractory multiple myeloma patients with impaired renal function [abstract] J Clin Oncol. 2008;26(Suppl):8542. [Google Scholar]
- 105.San Miguel JF, Dimopoulos M, Weber D, et al. Dexamethasone dose adjustments seem to result in better efficacy and improved tolerability in patients with relapsed/refractory multiple myeloma who are treated with lenalidomide/dexamethasone (MM-009/010 sub-analysis) [abstract] Blood. 2007;110:2712. [Google Scholar]
- 106.Kneppers E, Lokhorst HM, Eeltink C, et al. Pretreatment with thalidomide and bortezomib does not influence response to lenalidomide in relapsed myeloma. Preliminary results from patients included in Dutch compassionate need programme [abstract] Haematologica. 2008;93(Suppl 1):0651. [Google Scholar]
- 107.Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458–3464. doi: 10.1182/blood-2006-04-015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jagannath S, Richardson PG, Zeldenrust S, et al. Long-term responses observed with lenalidomide therapy for patients with relapsed or refractory multiple myeloma [abstract] J Clin Oncol. 2008;26(Suppl):8525. [Google Scholar]
- 109.Richardson P, Sonneveld P, Schuster M, et al. Bortezomib continues to demonstrate superior efficacy compared with high-dose dexamethasone in relapsed multiple myeloma: updated results of the APEX trial [abstract] Blood. 2005;106:2547. [Google Scholar]
- 110.Richardson PG, Jagannath S, Avigan DE, et al. Lenalidomide plus bortezomib (Rev-Vel) in relapsed and/or refractory multiple myeloma (MM): final results of a multicenter phase 1 trial [abstract] Blood 200610840516790588 [Google Scholar]
- 111.Richardson P, Jagannanth S, Hussein M, et al. Safety and efficacy of single-agent lenalidomide in patients with relapsed and refractory multiple myeloma. Blood. 2009;114:772–778. doi: 10.1182/blood-2008-12-196238. [DOI] [PubMed] [Google Scholar]
- 112.Hussein MA, Richardson PG, Jagannath S, et al. Final analysis of MM-014: single-agent lenalidomide in patients with relapsed and refractory multiple myeloma [abstract] J Clin Oncol. 2008;26(Suppl):8524. [Google Scholar]
- 113.Richardson PG, Schlossman RL, Weller E, et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002;100:3063–3067. doi: 10.1182/blood-2002-03-0996. [DOI] [PubMed] [Google Scholar]
- 114.Baz R, Walker E, Karam MA, et al. Lenalidomide and pegylated liposomal doxorubicin-based chemotherapy for relapsed or refractory multiple myeloma: safety and efficacy. Ann Oncol. 2006;17:1766–1771. doi: 10.1093/annonc/mdl313. [DOI] [PubMed] [Google Scholar]
- 115.Morgan GJ, Schey SA, Wu P, et al. Lenalidomide (Revlimid), in combination with cyclophosphamide and dexamethasone (RCD), is an effective and tolerated regimen for myeloma patients. Br J Haematol. 2007;137:268–269. doi: 10.1111/j.1365-2141.2007.06538.x. [DOI] [PubMed] [Google Scholar]
- 116.Knop S, Gerecke C, Liebisch P, et al. The efficacy and toxicity of the RAD regimen (Revlimid®, Adriamycin®, dexamethasone) in relapsed and refractory multiple myeloma – a phase I/II trial of “Deutsche Studiengruppe Multiples Myelom” [abstract] Blood. 2007;110:2716. doi: 10.1182/blood-2008-10-184135. [DOI] [PubMed] [Google Scholar]
- 117.Knop S, Gerecke C, Liebisch P, et al. Results of a phase I/II trial of Deutsche Studiengruppe Multiples Myelom, showing efficacy and safety of RAD regimen (Revlimid®, Adriamycin®, dexamethasone) in relapsed/refractory multiple myeloma [abstract] Haematologica. 2008;93(Suppl 1):0637. [Google Scholar]
- 118.Reece DE, Masih-Khan E, Chen C, et al. Lenalidomide (Revlimid®) +/− corticosteroids in elderly patients with relapsed/refractory multiple myeloma [abstract] Blood 20061081013a–1014a.#. 3550.16614247 [Google Scholar]
- 119.Anderson KC, Jagannath S, Jakubowiak A, et al. Phase II study of lenalidomide (Len), bortezomib (Bz), and dexamethasone (Dex) in patients (pts) with relapsed or relapsed and refractory multiple myeloma (MM) [abstract] J Clin Oncol. 2008;26(Suppl):8545. [Google Scholar]
- 120.Terpos E, Christoulas D, Kastritis E, et al. Effect of lenalidomide-based regimens on bone remodeling in patients with relapsed/refractory multiple myeloma [abstract] Haematologica. 2008;93(Suppl 1):0192. [Google Scholar]
- 121.Raschko M, Markovina S, Miyamoto S, et al. Phase II trial of bevacizumab combined with low dose dexamethasone and lenalidomide (Bev/Rev/Dex) for relapsed or refractory myeloma (MM) [abstract] Blood. 2007;110:1173. [Google Scholar]
- 122.Palumbo A, Falco P, Sanpaolo G, et al. Lenalidomide, melphalan, prednisone and thalidomide (RMPT) for relapsed/refractory multiple myeloma [abstract] Haematologica. 2008;93(Suppl 1):0636. [Google Scholar]
- 123.Stadtmauer EA, Lonial S, Vesole DH, et al. A practical guide to achieving and maintaining the best response to lenalidomide in multiple myeloma: roundtable proceedings. Clin Adv Hematol Oncol. 2007;5(10 Suppl 15):7–19. [PubMed] [Google Scholar]
- 124.Morgan J, Ishak K, Deniz B, et al. Overall survival with dexamethasone in phase III multiple myeloma trials after adjustment for cross-over to lenalidomide [abstract] Haematologica. 2008;93(Suppl 1):0441. [Google Scholar]
- 125.Bahlis NJ, Song K, Trieu Y, et al. Lenalidomide overcomes poor prognosis conferred by del13q and t(4;14) but not del17p13 in multiple myeloma: results of the Canadian MM016 trial [abstract] Blood. 2007;110:3597. [Google Scholar]
- 126.Zangari M, Fink L, Knight R, et al. Effect on survival of lenalidomide and dexamethasone associated deep vein thrombosis (DVT) in relapsed multiple myeloma patients [abstract] Haematologica. 2008;93(Suppl 1):0638. [Google Scholar]
- 127.Niesvizky R, Spencer A, Wang M, et al. Increased risk of thrombosis with lenalidomide in combination with dexamethasone and erythropoietin [abstract] J Clin Oncol. 2006;24(Suppl):7506. [Google Scholar]
- 128.Chen C, Reece DE, Siegel D, et al. Expanded Access Program (EAP) for lenalidomide (Revlimid®) plus dexamethasone in over 1400 subjects with relapsed or refractory multiple myeloma [abstract] Blood 2006108355616873673 [Google Scholar]
- 129.Niesvizky R, Naib T, Christos PJ, et al. Lenalidomide-induced myelosuppression is associated with renal dysfunction: adverse events evaluation of treatment-naïve patients undergoing front-line lenalidomide and dexamethasone therapy. Br J Haematol. 2007;138:640–643. doi: 10.1111/j.1365-2141.2007.06698.x. [DOI] [PubMed] [Google Scholar]
- 130.Simpson L, Rajkumar SV, Dispenzieri A, et al. High incidence of diarrhea in patients on long term therapy with lenalidomide and dexamethasone for multiple myeloma [abstract] J Clin Oncol. 2008;26(Suppl):8586. [Google Scholar]
- 131.Bennett CL, Hussain Z, Courtney M, Yarnold P, Raisch D, McKoy JM. RADAR update on thalidomide (Thal)- and lenalidomide (Len)-associated venous thromboembolism (VTE): safety concerns persist for multiple myeloma (MM) despite FDA approvals in this setting [abstract] Blood. 2006;108:3310. [Google Scholar]
- 132.Angelotta C, Lurie AJ, Yarnold PR, et al. Black box warning on lenalidomide-associated venous thromboembolism (VTE) in off-label setting: A preemptive and unusual safety initiative [abstract] J Clin Oncol. 2006;24(Suppl):6074. [Google Scholar]
- 133.Fullerton DSP, Trautman H, Huang H, et al. A budget impact model comparing resource utilization of four approved therapies for multiple myeloma (MM) in the US [abstract] Blood. 2007;110:3324. [Google Scholar]
- 134.Armoiry X, Fagnani F, Benboubker L, et al. Estimating direct costs of care for patients with relapsed or refractory multiple myeloma in French hospitals [abstract] J Clin Oncol. 2008;26(Suppl):8596. [Google Scholar]
- 135.Deniz HB, Ishak KJ, Edwards DR, Shearer A, Dale P, Caro JJ. Economic evaluation of lenalidomide for the treatment of multiple myeloma in Wales in patients who have received at least one prior therapy [abstract] Haematologica. 2008;93(Suppl 1):0804. [Google Scholar]
- 136.Chanan-Khan AA, Weber D, Dimopoulos M, et al. Lenalidomide (L) in combination with dexamethasone (D) improves survival and time to progression in elderly patients (pts) with relapsed or refractory (rel/ref) multiple myeloma (MM) [abstract] Blood. 2006a;108:3551. doi: 10.1007/s12185-012-1125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chanan-Khan AA, Yu Z, Weber D, et al. Lenalidomide (L) in combination with dexamethasone (D) significantly improves time to progression (TTP) in non-stem cell transplant patients (pts) with relapsed or refractory (rel/ref) multiple myeloma (MM): analysis from MM-009 and MM-010 randomized phase III clinical trials [abstract] Blood. 2006;108:3554. [Google Scholar]
- 138.Anon Revlimid (lenalidomide) Prescribing information. 2008. Available from: http://www.revlimid.com/pdf/REVLIMID_PI.pdf. Accessed October 2, 2008.
- 139.Zonder JA, Durie BG, McCoy J, et al. High incidence of thrombotic events observed in patients receiving lenalidomide (L) + dexamethasone (D) (LD) as first-line therapy for multiple myeloma (MM) without aspirin (ASA) prophylaxis [abstract] Blood. 2005;106:3455. [Google Scholar]
- 140.Richardson RG, Mitsiades C, Schlossman R, et al. New drugs for myeloma. Oncologist. 2007;12:664. doi: 10.1634/theoncologist.12-6-664. [DOI] [PubMed] [Google Scholar]