Abstract
Introduction:
Type 2 diabetes is increasing in prevalence worldwide and is a leading cause of morbidity and mortality, mainly due to the development of complications. Vildagliptin is an inhibitor of dipeptidyl peptidase 4 (DPP-4), a new class of oral antidiabetic agents.
Aims:
To evaluate the role of vildagliptin in the management of type 2 diabetes.
Evidence review:
Clear evidence shows that vildagliptin improves glycemic control (measured by glycosylated hemoglobin and blood glucose levels) more than placebo in adults with type 2 diabetes, either as monotherapy or in combination with metformin. Vildagliptin is as effective as pioglitazone and rosiglitazone, and slightly less effective than metformin, although better tolerated. Further glycemic control is achieved when adding vildagliptin to metformin, pioglitazone, or glimepride. There is evidence that vildagliptin improves beta-cell function and insulin sensitivity. Vildagliptin does not appear to be associated with weight gain or with a higher risk of hypoglycemia than placebo or other commonly used oral antidiabetic agents. Economic evidence is currently lacking.
Place in therapy:
Vildagliptin improves glycemic control with little if any weight gain or hypoglycemia in adult patients with type 2 diabetes when given alone or in combination with metformin, thiazolidinediones, or sulfonylureas. Since many diabetic patients require combination therapy, the complementary mechanism of action of vildagliptin and other commonly prescribed antidiabetic drugs represents an important new therapeutic option in diabetes management.
Keywords: dipeptidyl peptidase IV (dipeptidyl peptidase 4) inhibition, glycemic control, LAF 237, type 2 diabetes, vildagliptin
Core evidence place in therapy summary for vildagliptin 50 mg once or twice daily in adults with type 2 diabetes
| Outcome measure | Evidence | Implications |
|---|---|---|
| Disease-oriented evidence | ||
| Reduction in glycylated hemoglobin | Clear | Vildagliptin improves glycemic control more than placebo; 50 mg bid less effective than metformin 1 g bid; comparable to pioglitazone and rosiglitazone |
| Reduction in fasting and postprandial plasma glucose | Clear | Greater reduction with vildagliptin than with placebo; more sustained effect than rosiglitazone |
| Glycemic control in combination with metformin, pioglitazone, or glimepride | Clear | Adding vildagliptin to metformin, thiazolidinediones, or sulfonylureas causes a further improvement in glycemic control |
| Increase in postprandial GLP-1 | Clear | Greater increase with vildagliptin than placebo |
| Effects on postprandial insulin | Substantial | Similar with vildagliptin and placebo. Implies improved insulin secretion and sensitivity, shown by no change in insulin with lower glucose |
| Improvement in beta-cell function | Clear | Greater improvement with vildagliptin than placebo |
| Improvement in insulin sensitivity | Substantial | Greater improvement with vildagliptin than placebo |
| Patient-oriented evidence | ||
| Hypoglycemia | Clear | Similar frequency with vildagliptin and placebo, and with metformin or thiazolidinediones |
| Weight gain | Clear | Similar with vildagliptin and placebo; weight gain less than with thiazolidinediones, but greater than with metformin |
| Tolerability | Clear | Well tolerated with few adverse effects; nasopharyngitis, cough, and headache |
| Liver function | Limited | Unpublished data reveal similar elevations in liver enzymes compared with metformin, a thiazolidinedione, a sulfonylurea, or placebo. Liver function tests necessary before and during treatment with vildagliptin, and the drug should not be used in patients with liver impairment |
| Economic evidence | ||
| Cost effectiveness | No evidence | Studies required to verify the impact of vildagliptin alone or in combination with other oral agents on costs of illness |
bid, twice daily; GLP-1, glucagon-like peptide.
Scope, aims, and objectives
Vildagliptin (Galvus®, LAF 237, Novartis) is an inhibitor of dipeptidyl peptidase 4 (DPP-4) that has been developed for the treatment of type 2 diabetes. The drug was approved for use in the EU in February 2008 and is available in Mexico and Brazil.
DPP-4 is a new therapeutic target for the treatment of type 2 diabetes, and its role and the mechanism of action of vildagliptin are discussed in the Disease overview section below.
This article reviews the currently available published evidence on the effects of vildagliptin in type 2 diabetes, and considers its potential for clinical and economic benefit. The primary emphasis is given to clinical studies, but results of preclinical and clinical pharmacology investigations are also summarized.
Methods
English language literature searches were conducted on October 10, 2005 in the following databases, searching from the beginning of the database to date unless otherwise stated. The search strategy was “(vildagliptin OR laf 237) AND diabetes” unless otherwise stated.
PubMed, http://www.ncbi.nlm.nih.gov/entrez/query.fcgi, 1966 to date. Search strategy, “(vildagliptin OR laf 237) AND diabetes,” was expanded by Automatic Term Mapping to “(((“Vildagliptin” [TIAB] NOT Medline[SB]) OR “Vildagliptin”[Substance Name] OR vildagliptin[Text Word]) OR (“1-(((3-hydroxy-1-adamantyl)amino) acetyl)-2-cyanopyrrolidine”[Substance Name] OR laf 237[Text Word])) AND ((“diabetes mellitus”[TIAB] NOT Medline[SB]) OR “diabetes mellitus”[MeSH Terms] OR (“diabetes insipidus”[TIAB] NOT Medline[SB]) OR “diabetes insipidus”[MeSH Terms] OR diabetes[Text Word]) AND English[Lang].”
EMBASE, http://www.datastarweb.com, 1974 to date. Search strategy: “(vildagliptin OR laf 237) AND diabetes”.
Database of Abstracts of Reviews of Effectiveness (DARE), National Health Service (NHS) Economic Evaluations Database (NHSEED), Health Technology Assessment (HTA), www.york.ac.uk/inst/crd/darehp.htm. All three databases were searched together. All fields searched.
NHS HTA, www.ncchta.org.
National Guideline Clearing House, www.guideline.gov.
National Institute for Health and Clinical Excellence (NICE), www.nice.org.uk.
Cochrane Database of Systematic Reviews (CDSR), www.cochrane.org/index0.htm. Entire site searched.
Clinical Evidence (BMJ), www.clinicalevidence.com.
After removal of duplicates, a total of 69 records were identified. Records were manually reviewed and 61 were excluded for the following reasons: nonsystematic reviews (n=45); letters, editorials, news items, notes, comments, and corrections (n=13); articles about other drugs or treatments (n=2); articles on other subjects (n=1). Eight papers remained and were included in the evidence base (Table 1).
Table 1.
Evidence base included in the review
| Category |
Number of records |
|
|---|---|---|
| Full papers | Abstracts | |
| Initial search | 69 | 19 |
| records excluded | 61 | 6 |
| records included | 8 | 13 |
| Additional papers identified | 0 | 0 |
| Search update | ||
| records excluded | 5 | 35 |
| records included | 8 | 12 |
| Publications not available on databases and supplied by manufacturer | 0 | 3a |
| Publications identified from additional sources | 4 | 0 |
| Level 1 clinical evidence (systematic review, meta analysis) | 1 | 0 |
| Level 2 clinical evidence (RCT) | 15 | 13 |
| Level ≥3 clinical evidence | ||
| trials other than RCT | 1 | 6 |
| case reports | ||
| Evidence from animal and in vitro studies | 3 | 9 |
| Economic evidence | 0 | 0 |
| Total records included | 20 | 28 |
Four were supplied but one was a duplicate of data presented in a full paper and was excluded. For definitions of levels of evidence, see Editorial Information inside back cover or on the Core Evidence website (http://www.coremedicalpublishing.com).
RCT, randomized controlled trial.
Meeting abstracts from 2002 or later were identified by searching BIOSIS Previews, http://www.datastarweb.com, 1996 to date, using the search strategy “(vildagliptin OR laf 237) AND diabetes AND PT=MEETING$ AND LG=EN AND (YEAR=2002 OR YEAR=2003 OR YEAR=2004 OR YEAR=2005).” One record was retrieved.
Abstracts from the American Diabetes Association (ADA) Scientific Sessions for 2003–2005 inclusive were searched online at http://scientificsessions.diabetes.org/Abstracts/index.cfm using a text search for the terms “vildagliptin” or “laf 237”. A further 19 abstracts were obtained, one of which was a duplicate of the abstract on BIOSIS (Table 1). The manufacturer supplied citation details for four additional abstracts that were not available on the databases searched, and these four were also included (Table 1). This produced a total of 23 abstracts, of which three were excluded as duplicate presentations of data already published as full papers and a further four were excluded because their main focus was another compound, thus leaving 16 abstracts for inclusion (Table 1).
The PubMed literature search was repeated on October 11, 2007 with search limits “humans, clinical trial, meta-analysis, randomized controlled trial, English language” and yielded 13 records, of which eight were included, the remainder being pharmacokinetic or pharmacodynamics studies. Abstracts from the 2006 and 2007 annual meetings of the ADA and European Association for the Study of Diabetes (EASD) were also searched and 12 were included after removal of duplicates, subanalyses, and out-of-scope records. Finally, four full papers were identified from a meta analysis (Amori et al. 2007) that were not present in the PubMed search.
Disease overview
The increasing prevalence of type 2 diabetes
It is estimated that there are currently approximately 194 million people with diabetes worldwide and the prevalence is projected to exceed 333 million by 2025 (IDF 2005a). Over 90% of those affected have type 2 diabetes. Furthermore, due to its asymptomatic nature, a substantial proportion of individuals with type 2 diabetes are unaware that they have the disease (WHO 2003). Global estimates of the number of individuals with impaired glucose tolerance (IGT) or impaired fasting glucose (IFG) (often referred to as prediabetes) are unavailable. However, the World Health Organization (WHO) has suggested that it is likely to be even greater than the number with diabetes (WHO 2003).
Type 2 diabetes usually occurs in adults over the age of 40 years and is associated with a number of factors, including obesity, family history of diabetes, physical inactivity, and race/ethnicity. However, over the last two decades the increasing prevalence of type 2 diabetes in children and adolescents has been recognized as a global health problem (CDC 2005a; Pinhas-Hamiel & Zeitler 2005). Diabetes is one of the most common chronic diseases among children in the US with approximately 150 000 young people under the age of 18 years having the condition. It is estimated that 8–43% of those affected have type 2 diabetes (CDC 2005a). To examine the prevalence of diabetes among children and adolescents in the US, a 5-year study, SEARCH for Diabetes in Youth, has been initiated by the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health.
Type 2 diabetes is most commonly observed in American Indian, African American, Asian, and Hispanic/Latino youths. However, the obesity epidemic and a sedentary lifestyle are thought to be contributing factors. Generally, children and adolescents diagnosed with type 2 diabetes are between 10 and 19 years old, obese, and have insulin resistance and a strong family history of type 2 diabetes (Bloomgarden 2004; CDC 2005b).
Medical and socioeconomic burden of type 2 diabetes
Type 2 diabetes is associated with significant morbidity and mortality because of the resulting complications. Diabetes is the leading cause of end-stage renal disease, accounting for 44% of new cases and is the leading cause of blindness in the Western world (CDC 2003). People with diabetes are also likely to have a clustering of cardiovascular risk factors such as obesity, hypertension, and dyslipidemia, which is often referred to as the “metabolic syndrome” (Kahn et al. 2005). Individuals with diabetes are two to four times more likely to develop cardiovascular disease and have a greater risk of heart attack or stroke than individuals without diabetes (IDF 2005a). Indeed, a recent meta analysis of prospective cohort studies in patients with diabetes showed that hyperglycemia is associated with an increased risk of cardiovascular disease (Selvin et al. 2004).
There is also increasing evidence that cardiovascular risk factors are more prevalent in individuals with IFG or IGT compared with those with normal glucose levels, and that these individuals have an increased risk of cardiovascular disease and death (Coutinho et al. 1999; DECODE Study Group 2001; Saydah et al. 2001). Recent data have shown that abnormal glucose tolerance is a strong risk factor for future cardiovascular events after myocardial infarction (Bartnik et al 2004). In addition, the presence of cardiovascular disease risk factors explains the relationship between prediabetes and the development of chronic kidney disease (Fox et al. 2005).
These complications place a huge burden on healthcare services. It is estimated that the total direct and indirect expenditure for diabetes in the US alone in 2002 was $US132 billion (Hogan et al. 2003). The European Cost of Diabetes in Europe − type 2 (CODE-2) study estimated that three times the healthcare resources are spent on treating the complications of type 2 diabetes than those spent on controlling the disease before the onset of complications (Jonsson 2002). Hospitalization accounted for over 50% of the total cost. In contrast, management of glucose control with oral drugs and insulin accounted for only 7% of healthcare costs. It is evident that preventing the development of type 2 diabetes complications could have a beneficial effect on the huge economic and healthcare burden of type 2 diabetes.
The UK Prospective Diabetes Study (UKPDS) 41 compared the cost effectiveness of intensive blood glucose control (sulfonylurea or insulin) with conventional glucose control (primarily diet) in patients with type 2 diabetes (Gray et al. 2000). This study showed that although intensive blood glucose control significantly increased treatment costs, it substantially reduced the cost of complications and increased the time free of complications.
The role of incretin hormones in the pathophysiology of type 2 diabetes
Type 2 diabetes is a complex metabolic disease that has a multifactorial pathogenesis (Stumvoll et al. 2005). Factors involved in the development and progression of type 2 diabetes include insulin resistance, reduced pancreatic beta-cell response, and inappropriately elevated glucagon levels. These in turn can be affected by secondary factors such as overeating, lack of exercise, liver damage, hyperlipidemia, and impaired secretion of incretin hormones.
Beta-cell dysfunction is a major factor in the pathogenesis of type 2 diabetes. Insulin from the pancreas normally reduces glucose output by the liver, enhances glucose uptake by skeletal muscle, and suppresses fatty acid release from fat tissue. Individuals develop insulin resistance in peripheral tissues and to maintain normal glucose levels pancreatic beta cells increase the amount of insulin they produce. However, over time there is a progressive deterioration of pancreatic beta-cell function and these cells are unable to meet the demand for further increases in insulin levels, resulting in elevated glucose levels and subsequently type 2 diabetes (Stumvoll et al. 2005). It has been suggested that there is approximately a 50% loss of beta-cell function in patients with newly diagnosed type 2 diabetes (Turner et al. 1999). Furthermore, it has been shown that beta-cell response continues to decline (by as much as 4% per year) despite the use of insulin or oral glucose-lowering drugs (Holman 1998).
Glucagon is a hormone that stimulates the release of glucose from the liver during fasting to maintain normal glucose homeostasis. During periods of hyperglycemia glucagon secretion is suppressed. In patients with type 2 diabetes, glucagon concentrations are elevated resulting in increased hepatic glucose output and postprandial glucose spikes (excursions).
The role of the incretins, glucagon-like peptide (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), in the regulation of postprandial insulin secretion have recently been elucidated (Holst & Gromada 2004). GLP-1 is an incretin hormone secreted into the bloodstream from endocrine cells located in the intestinal mucosa in response to a meal. It mediates its insulintropic activity via GLP-1 receptors on pancreatic beta cells and other tissues. The biological activities of GLP-1 include mediating glucose-dependent insulin secretion; suppression of postprandial glucagon secretion; regulation of gastric emptying; and suppression of appetite leading to reduction of food intake (Nauck et al. 1996; Zaunder et al. 2002). In individuals with type 2 diabetes or IGT, the release of GLP-1 in response to a meal is defective, resulting in reduced circulating concentrations of postprandial GLP-1 and a blunted insulin secretory response to food intake (Nauck et al. 1986). The action of GLP-1 is inactivated by the enzyme DPP-4.
Current therapy options
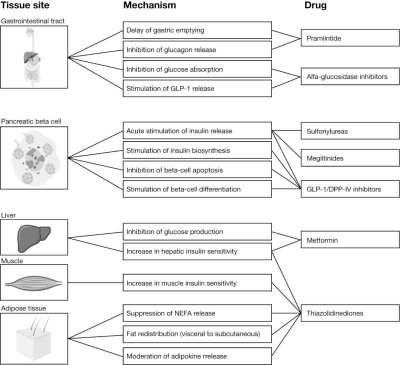
Initially, patients with type 2 diabetes may be able to control their blood glucose levels with diet and exercise alone. However, many people with diabetes progress to require antidiabetic medications. Currently, there are five classes of oral glucose-lowering drugs available that may be used as a monotherapy or in combination. The mechanism of action and the risks and benefits of each of these therapy classes are indicated in Fig. 1 and Table 2, respectively.
Fig.1.
Mechanism of action of current oral glucose-lowering drugs (reprinted from Stumvoll et al. 2005, with permission from Elsevier). DPP-lV, dipephidyl peptidase lV; GLP-1 glucagon-like peptide 1; NEFA, nonesterified fatty acid.
Table 2.
Overview of current therapies for the treatment of type 2 diabetes
| Advantages | Disadvantages | UK Guidelines (NICE 2002) | |
|---|---|---|---|
| Biguanide (metformin) | Only agent associated with potential weight loss Modest lowering of lipid levels Improvements in fibrinolysis, inflammatory markers, and endothelial function Prevents development of T2D in patients with IGT Relatively inexpensive |
GI adverse effects common − minimized by slow titration Rare risk of lactic acidosis limits its use in contraindicated patients e.g. patients with impaired renal function |
Recommended as a first-line glucose-lowering therapy in patients whose blood glucose is inadequately controlled using lifestyle intervention alone |
| Insulin secretagogues (sulfonylureas) | Relatively inexpensive | Drug interactions Weight gain Hypoglycemia − requires self-monitoring of blood glucose by the patient Potential effect on myocardial ischemic preconditioning |
First-line therapy if the patient is not overweight or metformin is not tolerated or contraindicated Use in combination with metformin in overweight or obese patients when glucose control becomes unsatisfactory |
| Fast-acting insulin secretagogues (meglitinides) | Rapid onset and shorter duration of action than SUs due to more physiologically appropriate control of postprandial glucose levels | Drug interactions Weight gain − to a lesser degree than SUs Less risk for hypoglycemia than SUs Cost is higher than SUs More frequent dosing schedule than most agents − must be taken shortly before each meal |
|
| Thiazolidinediones (glitazones/PPAR-gamma agonists) | Beneficial effects on cardiovascular risk determinants: cytokines, inflammatory markers, lipids, blood pressure, endothelial function Reduced insulin resistance Can be used in patients with reduced renal function |
Weight gain Edema ADA/AHA and FDA recommend that glitazones are not used in patients with advanced heart failure symptoms (class III or IV NYHA classification) Increased risk of edema and cardiac failure in combination with insulin Relatively high cost |
Offered as combination therapy if unable to take, or if HbA1c remains unsatisfactory with, metformin and insulin secretagogues as combination therapy |
| Alfa-glucosidase inhibitors | STOP-NIDDM trial demonstrated reduced risk of myocardial infarction with acarbose Delay/prevent the development of T2D Do not cause weight gain No clinically significant drug interactions |
GI adverse effects e.g. abdominal bloating and cramping Less efficacious than other classes Relatively high cost |
Acarbose considered as alternative when unable to use other oral drugs |
| Insulin | Ability to achieve tight glycemic control Newer noninjectable formulations becoming available |
Hypoglycemia Weight gain Daily injections Self-monitoring (cost and convenience) |
Offered when inadequate blood glucose control on optimized oral drugs |
ADA, American Diabetes Association; AHA, American Heart Association; GI, gastrointestinal; HbA1c, glycated hemoglobin A1c; IGT, impaired glucose tolerance; NICE, National Institute for Health and Clinical Excellence; NYHA, New York Heart Association; PPAR, peroxisome proliferators-activated receptor; STOP-NIDDM, noninsulin dependent diabetes mellitus; SU, sulfonylurea; T2D, type 2 diabetes.
The choice of antidiabetic medication is a complex decision that requires several factors to be taken into consideration, therefore a decision is often made on a case by case basis.
Metformin is widely regarded as the drug of choice for patients with type 2 diabetes (IDF 2005b), especially for people who are overweight or obese. Indeed, the UK National Institute for Health and Clinical Excellence (NICE) guidelines recommend metformin as a first-line therapy option for the treatment of type 2 diabetes (NICE 2002) (Table 2). In comparison, the European Diabetes Policy Group (EDPG) guidelines, The American Association of Clinical Endocrinologists (AACE) guidelines, and the ADA guidelines do not specify their recommendation for first-line therapy with oral glucose-lowering drugs (EDPG 1999; AACE 2002; ADA 2005). With the continuous deterioration of glucose control over time, the use of combination therapy is recommended and many combination preparations are available. After the failure of multiple oral agents, people with diabetes additionally require insulin therapy.
The aims of treatment with currently available antidiabetic agents are to control hyperglycemia and to reduce cardiovascular risk and prevent the development of complications. Recent European, UK, and US guidelines for type 2 diabetes recommend glycemic targets, with a glycated hemoglobin A1c (HbA1c) target range below 6.5–7.5% (EDPG 1999; AACE 2002; NICE 2002; ADA 2005). However, the recently completed Action to Control Cardiovascular Risk in Diabetes (ACCORD) study in patients with type 2 diabetes revealed increased mortality with treatment to get HbA1c levels below 6% (ACCORD Study Group 2008). The AACE have published a series of road maps that suggest treatment algorithms to achieve AACE glycemic goals for patients with type 2 diabetes (HbA1c ≤6.5%) naïve to therapy or those previously treated with monotherapy or combination therapy (AACE 2005; Jellinger et al. 2007).
Unmet needs
Long-term glycemic control
The AACE has reported that 67% of individuals with type 2 diabetes do not have adequately controlled blood glucose (AACE 2005). Furthermore, the National Health and Nutrition Examination Survey (NHANES III) has reported that from 1998 to 2000 the frequency of poor glycemic control increased among US adults with type 2 diabetes (Koro et al. 2004).
In general, for every 1.0% unit reduction in HbA1c, the risk of developing microvascular diabetic complications is reduced by 40% (CDC 2003). The UKPDS demonstrated that the risk of developing, and the progression of, microvascular complications are substantially reduced with intensive blood glucose control (HbA1c approximately 7%) (UKPDS 1998a,b). Most of the commonly used oral glucose-lowering drugs are only able to reduce HbA1c by approximately 1% (Kimmel & Inzucchi 2005). This reduction is not sufficient for patients who have HbA1c levels much greater than the recommended target.
Long-term glycemic control is difficult to sustain with oral glucose-lowering drugs. One of the main reasons for the lack of long-term glycemic control with oral medications is that they target the tissue abnormalities of the disease (i.e. sensitize specific tissues to the effects of insulin or enhance the insulin-producing activity of the pancreas) rather than treating the underlying causes of these abnormalities, which is the declining function of pancreatic beta cells that results in a reduction in endogenous insulin secretion. Antidiabetic medications are required that can preserve beta-cell function and prevent continuing loss and disease progression. Despite the ability of insulin to achieve tight glycemic control, physicians and people with diabetes are reluctant to use it to treat type 2 diabetes until after the failure of multiple oral glucose-lowering drugs. This is partly due to the burden of multiple daily injections that are associated with poor patient compliance. Therefore, there is a need for oral medications that can achieve similar prolonged glycemic control to that of insulin. Furthermore, the emerging epidemic of type 2 diabetes in children and adolescents presents a new challenge, as this population will have a lifetime risk of complications and may require effective glycemic control over a prolonged period of time.
Safety and tolerability
Current treatments for type 2 diabetes are also often limited by their contraindications and side-effect profile that may affect patient compliance and quality of life (Table 2).
One of the most common side effects with sulfonylureas, thiazolidinediones, and insulin is weight gain. The UKPDS 33 study demonstrated that weight gain was significantly higher (mean 2.9 kg) in patients with type 2 diabetes receiving blood glucose control with sulfonylureas or insulin compared with conventional treatment with diet (P<0.001) (UKPDS 1998a). This is of particular concern given that people with diabetes are often overweight before they commence therapy.
Another common side effect of the sulfonylureas and insulin is hypoglycemia. Regular self-monitoring of blood glucose levels by fingertip glucose measurements is required to try and avoid hypoglycemic events. However, this extensive monitoring is inconvenient to the patient and incurs extra costs.
Finally, agonists of peroxisome proliferators-activated receptor (PPAR) gamma have been associated with fluid retention and concerns over cardiac failure, and metformin is associated with gastrointestinal intolerance and is contraindicated in patients with renal impairment or poor left ventricular function due to the risk of lactic acidosis.
Diabetes prevention
An emerging concept is the earlier treatment of glucose control in the prediabetes state. Recent clinical trials have shown that the progression of IGT to type 2 diabetes can be delayed or prevented. These trials demonstrated that in patients with IGT, lifestyle intervention (weight loss and modest exercise), treatment with the weight loss agent orlistat, or treatment with antidiabetic medications (metformin or acarbose) can reduce the risk of developing type 2 diabetes by 25–58% (Pan et al. 1997; Tuomilehto et al. 2001; Chiasson et al. 2002; Knowler et al. 2002; Torgerson et al. 2004).
The ADA recommends screening men and women aged ≥45 years, particularly those with a body mass index (BMI) ≥25 kg/m2 every 3 years to detect IGT or IFG (ADA/ NIDDKD 2002). Currently, it is recommended that patients diagnosed with IGT or IFG are given counseling on weight loss and increasing physical activity. The ADA and National Institute of Diabetes, Digestive and Kidney Disease (NIDDKD) have suggested that additional research is required to determine the cost effectiveness of drug therapy as a preventative measure (ADA/NIDDKD 2002). No antidiabetic drugs are currently licensed for use in individuals with prediabetes and existing medications have several disadvantages including unwanted side effects. Therefore, there may be a need for further treatments to delay or prevent the progression of IGT to type 2 diabetes.
Addressing unmet need: new classes of antidiabetic medications
To address some of these unmet needs in patients with type 2 diabetes, new classes of noninsulin antidiabetic drugs been developed, including the dual PPAR agonists (e.g. muraglitizar, subsequently discontinued), amylin analogs (e.g. pramlintide acetate, an injectable adjunct to insulin), incretin mimetics (GLP-1 analogues), and the DPP-4 inhibitors, that act as incretin enhancers. In addition, a number of alternative methods of delivery of insulin are being studied.
Incretin mimetics and DPP-4 inhibitors address the need to act on the underlying disease rather than on its symptoms. They enhance glucose-dependent insulin secretion by pancreatic beta cells, and consequently there is little risk of hypoglycemia. They also suppress elevated glucagon secretion and increase satiety. It has been hypothesized that they may restore beta-cell sensitivity to glucose, which could mean that they may be able to delay the onset of type 2 diabetes, slow its progression, and reduce its cardiovascular and metabolic complications (Holst & Gromada 2004).
The GLP-1 analog exenatide (Byetta®; Amylin Pharmaceuticals and Eli Lilly) was the first in this new class of diabetes treatment to be approved by the FDA in June 2005. It is approved as an adjunctive therapy in patients with type 2 diabetes who have not achieved adequate control on metformin and/or a sulfonylurea. All GLP-1 analogs are injectable, which may pose a barrier to their use as many doctors and people with diabetes are reluctant to use injected therapies.
A review of the dual-PPAR agonists, amylin analogs, and GLP-1 analogs is beyond the scope of this article, and therefore, these drugs will not be discussed in further detail.
DPP-4 inhibitors
Vildagliptin (Galvus) is one of a new class of DPP-4 inhibitors that was approved for use in type 2 diabetes in the EU in February 2008. Other DPP-4 inhibitors include sitagliptin phosphate (Januvia®; MRK-0431; Merck Sharp & Dohme/Merck & Co. Inc), which was approved in the US in 2006, and saxagliptin (BMS-477118; Bristol-Myers Squibb). Inhibition of DPP-4 delays the degradation of GLP-1 therefore reducing glycemia, sustaining insulin levels, and reducing glucagon levels in patients with type 2 diabetes (Ahrén et al. 2004b). DPP-4 inhibitors were developed to meet existing unmet needs in the treatment of type 2 diabetes, and an ideal agent would possess the following attributes: good glycemic control, low incidence of hypoglycemia, beta-cell preservation and improvement in beta-cell function, no weight gain, oral route of administration, and good tolerability with minimal gastrointestinal events. One of the main advantages of DPP-4 inhibitors, in contrast to the GLP-1 agonists, will be their mode of administration, which is via the oral route, and may lead to higher levels of adherence to therapy.
Clinical evidence with vildagliptin
In-vitro studies have shown that vildagliptin is a potent, reversible, competitive inhibitor of human and rat DPP-4, with high selectivity for DPP-4 over other peptidase enzymes (Hughes et al. 2002; Villhauer et al. 2003; Brandt et al. 2005). In vivo, vildagliptin has been shown to inhibit plasma DPP-4 activity in rats (Villhauer et al. 2003) and in cynomolgus monkeys for up to 10 hours (Hughes et al. 2002).
In obese, insulin-resistant cynomolgus monkeys, vildagliptin treatment for 10 weeks significantly (P<0.05) reduced HbA1c and fasting plasma insulin (Dardik et al. 2003a). Plasma fibrinogen and plasminogen activator inhibitor-1 (PAI-1), two thrombogenic risk factors, were also significantly (P<0.05) reduced during vildagliptin treatment (Dardik et al. 2003a). Acute administration of vildagliptin significantly (P<0.05) delayed gastric emptying (Dardik et al. 2003b), although this was unaffected in 14 patients with type 2 diabetes given vildagliptin 50 mg twice daily for 10 days (Vella et al. 2007).
A study in obese Zucker rats suggests that pioglitazone and vildagliptin may have synergistic effects. When coadministered, pioglitazone and vildagliptin increased the glucose clearance rate after an oral glucose tolerance test, but neither drug alone had this effect (Burkey et al. 2002).
Oral administration of vildagliptin improved glucose tolerance in various animal models of diabetes, including obese Zucker rats (Villhauer et al. 2003), obese, insulin-resistant cynomolgus monkeys (Dardik et al. 2003b), and ob/ob mice (Mika et al. 2003). Chronic dosing in obese Zucker rats produced similar effects to those of acute dosing, and vildagliptin had no effect on the animals’ bodyweight (Burkey et al. 2005).
There is some evidence from animal studies that vildagliptin may have beneficial effects on beta-cell function. A study in mice showed that the improvement in glucose tolerance was paralleled by an increase in glucose-stimulated insulin secretion from isolated islet cells, and was lost in transgenic mice with a defect in the islet beta-cell response to glucose (Ahrén et al. 2005a). Vildagliptin has also been shown to increase beta-cell mass in newborn rats, apparently by stimulating beta-cell genesis and inhibiting beta-cell apoptosis (Duttaroy et al. 2005a). A separate study in mice showed that vildagliptin improved glucose tolerance in mice subjected to streptozocin-induced beta-cell damage, but in this case there was no effect on apoptosis and the effect appeared to be mediated via enhanced differentiation of pancreatic progenitor cells (Duttaroy et al. 2005b). However, vildagliptin was unable to prevent progression of diabetes in the ZDF rat, a model of rapidly-developing diabetes (Rolin et al. 2004).
Vildagliptin’s major antidiabetic mechanism of action appears to be an enhancement of glucose-stimulated insulin release, mediated via an increase in GLP-1 levels (Burkey et al. 2005; Holst & Deacon 2005). It has also been argued that increased GLP-1 may not be the only mediator of the clinical effects of DPP-4 inhibitors, and that other neuropeptides may be implicated (Nauck & El-Ouaghlidi 2005). Recently, however, it has been shown that insulin secretion stimulated by the neuropeptide pituitary adenylate cyclase-activating peptide was unaffected by vildagliptin, which appears to act by prolonging the half life of L- and K-cell derived incretin hormones (Hjøllund et al. 2007).
The clinical evidence on vildagliptin published to date is mainly concerned with disease-oriented outcomes (such as HbA1c, blood glucose, and insulin levels), and patient-oriented evidence on weight gain and adverse events.
Effects on glycated HbA1c
HbA1c is an established measure of glycemic control. Evidence shows that treatment for up to 52 weeks with vildagliptin at doses of 50 or 100 mg/day is effective in reducing HbA1c (Tables 3 and 4). The mean reduction in HbA1c with vildagliptin was typically up to 1 percentage point (Table 3). As discussed earlier (Unmet needs section), most of the commonly used oral glucose-lowering drugs are also able to reduce HbA1c by approximately 1 percentage point. One early study showed that addition of vildagliptin 50 mg/day to metformin treatment resulted in improved glycemic control at 12 weeks compared with metformin alone (Ahrén et al. 2004a; Table 4). An optional 40-week extension to this study provided limited evidence that adding vildagliptin 50 mg/day to metformin treatment may help to maintain long-term glycemic control. There was a slight increase in HbA1c during the extension phase, but this was less in the patients receiving vildagliptin plus metformin than in the patients receiving metformin alone, and was not statistically significantly different from zero (Ahrén et al. 2004a; Table 4). More robust evidence has substantiated the beneficial effects of adding vildagliptin to patients whose diabetes is poorly controlled by metformin alone. After 24 weeks, HbA1c was significantly improved by vildagliptin 50 or 100 mg/day compared with placebo in patients receiving metformin ≥1.5 g/day (Bosi et al. 2007; Dejager et al. 2007b; Table 4). Vildagliptin 50 mg once or twice daily also caused a further reduction in HbA1c of 0.7% compared with placebo in patients taking glimepride 4 mg once daily (Garber et al. 2007a; Table 4). Similarly, adding vildagliptin 50 mg twice daily reduced HbA1c compared with placebo in patients poorly controlled on insulin therapy after 24 weeks (Fonseca et al. 2007; Table 4). Patients receiving placebo were switched to vildagliptin 50 mg once daily and after a further 28 week extension HbA1c decreased significantly by 0.4% (Fonseca et al. 2006).
Table 3.
Effects of vildagliptin on blood glucose and glycosylated hemoglobin (HbA1c) in active-controlled comparative studies (all level 2 evidence)
| Design | Treatment and dose |
Outcome |
Reference | |||
|---|---|---|---|---|---|---|
| Mean change from baseline in HbA1c (percentage points) | % patients reaching HbA1c <7.0%a | Mean change from baseline in fasting plasma glucose (mmol/L) | Mean change from baseline in 4 h-postprandial plasma glucose (mmol/L) | |||
| Double-blind, placebo-controlled RCT, 24 weeks | Placebo + P 30 mg qd (n=157) | Placebo + P 30 mg qd −1.4 | Placebo + P 30 mg qd 43% | Placebo + P 30 mg qd −1.9 | NR | Rosenstock et al. 2007a |
| Placebo + V 100 mg qd (n=150) | Placebo + V 100 mg qd −1.1 (P<0.001 vs V 100 mg qd + P 30 mg qd) | Placebo + V 100 mg qd 43% | Placebo + V 100 mg qd −1.3 (P<0.001 vs V 100 mg qd + P 30 mg qd) | NR | ||
| V 50 mg qd + P 15 mg qd (n=139) | V 50 mg qd + P 15 mg qd −1.7 (P=0.039 vs placebo + P) | V 50 mg qd + P 15 mg qd 54% | V 50 mg qd + P 15 mg qd −2.4 (P=0.022 vs placebo + P) | V 50 mg qd + P 15 mg qd −3.8 (P=0.024 vs placebo + P)b | ||
| V 100 mg qd + P 30 mg qd (n=146) | V 100 mg qd + P 30 mg qd −1.9 (P<0.001 vs placebo + P) | V 100 mg qd + P 30 mg qd 65% | V 100 mg qd + P 30 mg qd −2.8 (P<0.001 vs placebo + P) | V 100 mg qd + P 30 mg qd −5.2 (P<0.001 vs placebo + P)b | ||
| (P<0.001 vs placebo + V and placebo + P) | ||||||
| Double-blind RCT, 24 weeks | V 50 mg bid (n=459) | V 50 mg bid −1.1 | NR | V 50 mg bid −1.3 | NR | Rosenstock et al. 2007b |
| R 8 mg qd (n=238) | R 8 mg qd −1.3 | NR | R 8 mg qd −2.3 (P<0.001 vs V) | NR | ||
| Double-blind RCT, 52 weeks | V 50 mg bid (n=526) | V 50 mg bid −1.0 | V 50 mg bid 35% | V 50 mg bid −0.9 | NR | Goeke et al. 2006; Schweizer et al. 2007 |
| M 1 g bid (n=254) | M 1 g bid −1.4 | M 1 g bid 45% | M 1 g bid −1.9 (P<0.001 vs V) | NR | ||
| Double-blind, placebo-controlled RCT, 24 weeks | Placebo + P 45 mg qd (n=138) | Placebo + P 45 mg qd −0.3 | Placebo + P 45 mg qd 14.8% | Placebo + P 45 mg qd −0.5 | Baron et al. 2006; Garber et al. 2007b | |
| V 50 mg qd + P 45 mg qd (n=124) | V 50 mg qd + P 45 mg qd −0.8 (P=0.001 vs placebo) | V 50 mg qd + P 45 mg qd 28.7% (P=0.007 vs placebo) | V 50 mg qd + P 45 mg qd −0.8 | V 50 mg qd + P 45 mg qd −1.2b,c | ||
| V 50 mg bid + P 45 mg qd (n=136) | V 50 mg bid + P 45 mg qd −1.0 (P<0.001 vs placebo) | V 50 mg bid + P 45 mg qd 36.4% (P<0.001 vs placebo) | V 50 mg bid + P 45 mg qd −1.1 | V 50 mg bid + P 45 mg qd −1.9 (P=0.008 vs placebo)b,c | ||
Of patients with HbA1c ≥7.0% at baseline.
Value relative to placebo + P.
2h-postprandial plasma glucose.
bid, twice daily; M, metformin; NR, not reported; P, pioglitazone; qd, once daily; R, rosiglitazone; RCT, randomized controlled trial; V, vildagliptin.
Table 4.
Effects of vildagliptin on blood glucose and glycosylated hemoglobin (HbA1c) in placebo-controlled studies (all level 2 evidence)
| Design | Treatment and dose |
Outcome |
Reference | |||
|---|---|---|---|---|---|---|
| Mean change from baseline in HbA1c (percentage points) | % patients reaching HbA1c <7.0%a | Mean change from baseline in fasting plasma glucose (mmol/L) | Mean change from baseline in 4 h-postprandial plasma glucose (mmol/L) | |||
| Double-blind, placebo-controlled RCT, 24 weeks | Placebo + M ≥1.5 g (n=130) | Placebo + M ≥1.5 g +0.2 | Placebo + M ≥1.5 g 9.4% | NR | NR | Bosi et al. 2007; Dejager et al. 2007b |
| V 50 mg qd + M ≥1.5 g (n=143) | V 50 mg qd + M ≥1.5 g −0.5 (P<0.001 vs placebo) | NR | NR | NR | ||
| V 100 mg qd + M ≥1.5 g (n=143) | V 100 mg qd + M ≥1.5 g −0.9 (P<0.001 vs placebo) | V 100 mg qd + M ≥1.5 g 35.5% (P<0.001 vs placebo) | NR | NR | ||
| Double-blind, placebo-controlled RCT, 24 weeks | Placebo (n=94) | Placebo −0.3 | NR | Placebo −0.1 | NR | Dejager et al. 2007a |
| V 50 mg qd (n=104) | V 50 mg qd −0.8 | NR | V 50 mg qd −1.0 | NR | ||
| V 50 mg bid (n=90) | V 50 mg bid −0.8 | NR | V 50 mg bid −0.8 | NR | ||
| V 100 mg qd (n=92) | V 100 mg qd −0.9 (all P<0.01 vs placebo) | NR | V 100 mg qd −0.8 | NR | ||
| Double-blind, placebo-controlled RCT, 24 weeks | Placebo + G 4 mg qd (n=144) | Placebo + G 4 mg qd 0b | NR | Greater decrease with V than with placebo at (P=0.118)c | NR | Garber et al. 2007a |
| V 50 mg qd or bid + G 4 mg qd (n=132) | V 50 mg qd or bid + G 4 mg qd −0.7b (P<0.001 vs placebo) | NR | NR | NR | ||
| Double-blind, placebo-controlled RCT, 24 weeks | Placebo (n=88) | Placebo 0 | Placebo 13.6% | Placebo +0.1 | NR | Pi-Sunyer et al. 2007 |
| V 50 mg bid (n=84) | V 50 mg qd −0.5 | V 50 mg qd 25%c | V 50 mg qd −0.5 | NR | ||
| V 50 mg bid (n=79) | V 50 mg bid −0.7 (P<0.001 vs placebo) | V 50 mg bid 30.4% (P<0.01 vs placebo) | V 50 mg bid −1.2 (P<0.001 vs placebo) | NR | ||
| V 100 mg bid (n=89) | V 100 mg qd −0.8 (P<0.001 vs placebo) | V 100 mg qd 39.1% (P<0.001 vs placebo) | V 100 mg qd −1.1 (P<0.001 vs placebo) | NR | ||
| Double-blind, placebo-controlled RCT, 24 weeks | Placebo + I >30 U/day (n=149) | Placebo + I >30 U/day −0.2 | NR | Placebo + I >30 U/day −0.8 | NR | Fonseca et al. 2007 |
| V 50 mg bid + I >30 U/day (n=140) | V 50 mg bid + I >30 U/day −0.5 (P=0.01 vs placebo) | NR | V 50 mg bid + I >30 U/day −0.2 | NR | ||
| Double-blind, placebo-controlled RCT, 12 weeks | Placebo (n=72) | Placebo +0.28 | NR | Placebo +0.13 | Placebo +0.2d | Kikuchi et al. 2006; Mimori et al. 2006 |
| V 10 mg bid (n=71) | V 10 mg bid −0.53 | NR | V 10 mg bid −0.62 | V 10 mg bid −3.5d | ||
| V 25 mg bid (n=72) | V 25 mg bid −0.67 | NR | V 25 mg bid −0.78 | V 25 mg bid −3.2d | ||
| V 50 mg bid (n=76) | V 50 mg bid −0.92 (all P<0.001 vs placebo) | NR | V 50 mg bid −1.37 (all P<0.001 vs placebo) | V 50 mg bid −3.4d (all P<0.001 vs placebo) | ||
| Double-blind, placebo-controlled RCT, 12 weeks | Placebo (n=28) | Placebo −0.6 | Placebo NR | Placebo +0.23 | Placebo +0.2 | Pratley et al. 2006; Pratley & Galbreath 2004 |
| V 25 mg bid (n=70) | V −0.6 (P=0.0012) | V 47% | V −0.9 (P=0.0043) | V −1.7 (P<0.0001) | ||
| Double-blind, placebo-controlled, multicenter RCT, 12 weeks | Placebo (n=58) | NR | Placebo 23% | Placebo −0.41 | Placebo −0.61 | Ristic et al. 2005 |
| V 25 mg bid (n=51) | NR | V 25 mg bid 44% | V 25 mg bid −0.44 | V 25 mg bid −1.03 | ||
| V 25 mg qd (n=54) | NR | V 25 mg qd 28% | V 25 mg qd −0.30 | V 25 mg qd −1.50 | ||
| V 50 mg qd (n=53) | V 50 mg qd −0.56 (P=0.003 vs placebo) | V 50 mg qd 40% | V 50 mg qd −0.97 | V 50 mg qd −2.00 (P=0.012 vs placebo) | ||
| V 100 mg qd (n=63) | V 100 mg qd −0.53 (P=0.004 vs placebo) | V 100 mg qd 46% | V 100 mg qd −0.95 | V 100 mg qd −1.50 | ||
| Other doses NSD vs placebo | P values NR | All doses NSD vs placebo | All other doses NSD vs placebo | |||
| Double-blind, placebo-controlled, multicenter RCT, 12 weeks | Placebo (n=51, of whom 29 completed extension) | At 12 weeks: V −0.6 Placebo 0.1 (P<0.0001) | Placebo 10.7% V 41.7% P value NR | Greater decrease with V than with placebo at 12 weeks (P=0.0057) | Greater decrease with V than with placebo at 12 weeks (P<0.0001) | Ahrén et al. 2004a |
| Optional extension for further 40 weeks | V 50 mg qd (n=56, of whom 42 completed extension) All patients were also taking stable doses of M 1.5–3 g/day |
In extension study: V +0.0128 per month Placebo +0.0656 per month (P=0.0243) |
Greater decrease with V than with placebo at 52 weeks (P=0.031) | Greater decrease with V than with placebo at 52 weeks (P=0.0001) | ||
| Double-blind, placebo-controlled, multicenter RCT, 4 weeks | Placebo (n=19) | Placebo −0.15 | NR | Placebo −0.4 | Placebo −0.4 | Ahrén et al. 2004b |
| V 100 mg qd (n=18) | V −0.53 (P<0.001) | NR | V −1.1 (P=0.037) | V −1.9 (P<0.001) | ||
| Double-blind, placebo-controlled RCT, 4 weeks | Placebo (n=20) | NR | NR | Placebo −0.3 | NR | Ahrén et al. 2003 |
| V 100 mg qd (n=20) | NR | NR | V −1.0 (P<0.05) | NR | ||
| Double-blind, placebo-controlled, single center RCT, 4 weeks | Placebo (n=11) | NR | NR | Greater decrease with V than placebo (P<0.05) | NR | Mari et al. 2005 |
| V 100 mg bid (n=9) | NR | NR | NR | NR | ||
Of patients with HbA1c ≥7.0% at baseline.
Placebo subtracted value.
Results presented graphically, not stated.
2 h-postprandial plasma glucose.
bid, twice daily; G, glimepiride; I, insulin; M, metformin; NR, not reported; NSD, not statistically significantly different; qd, once daily; RCT, randomized controlled trial; V, vildagliptin.
The percentage of patients reaching a target of <7.0% HbA1c was consistently in the range 40–47% with vildagliptin at 50 or 100 mg/day, compared with only up to 23% with placebo (Tables 3 and 4). It appears that 25 mg/day is little more effective than placebo, and that 50 mg/day and 100 mg/day have approximately similar effectiveness (Tables 3 and 4). However, in patients with a higher baseline HbA1c (>8.0%) the effect of vildagliptin 100 mg given either as a single dose or as 50 mg twice daily was greater than that of vildagliptin 50 mg once daily (Dejager et al. 2007a; Pi-Sunyer et al. 2007). Similarly, the reduction in HbA1c was greater in patients with higher (mean 8.5%) versus lower (mean 7.5% baseline HbA1c with vildagliptin 25 mg twice daily (Pratley et al. 2006). Nearly four times as many patients achieved HbA1c <7.0% when vildagliptin was added to metformin, and nearly half as many again when vildagliptin 100 mg once daily was combined with pioglitazone 30 mg once daily compared with either agent alone (Rosenstock et al. 2007a; Table 3). Fewer patients with a higher baseline HbA1c (>8.5%) achieved the target of <7.0% when vildagliptin 50 or 100 mg/day was added to metformin compared with those with intermediate (7.9–8.5%) baseline levels (7.5% and 16.3% versus 22.2% and 31.4%) (Bosi et al. 2007).
Although a similar reduction in HbA1c was achieved, vildagliptin 50 mg twice daily was not found to be noninferior to metformin 2 g daily (Goeke et al. 2006; Schweizer et al. 2007; Table 3). Vildagliptin appears to be as effective as pioglitazone (Baron et al. 2006; Garber et al. 2007b), and rosiglitazone (Rosenstock et al. 2007b) at reducing HbA1c (Table 3).
Effects on blood glucose
Most of the published evidence shows that vildagliptin 50 mg/day or 100 mg/day is more effective than placebo in reducing both fasting plasma glucose and 2 and 4 hours postprandial plasma glucose (Tables 3 and 4). In the dose-ranging study, the changes from baseline did not reach statistical significance compared with placebo (except for the change in 4 hours postprandial glucose with vildagliptin 50 mg once daily), but the numerical values were broadly consistent with a dose-related effect reaching a plateau at 50 or 100 mg/day (Ristic et al. 2005; Table 4).
Between-group changes in fasting plasma glucose (FPG) with vildagliptin 50 and 100 mg/day (−0.8 and −1.7 mmol/L, respectively) and in 2 hours postprandial glucose (−1.9 and −2.3 mmol/L) also showed significant improvements compared with placebo in patients receiving metformin ≥1.5 g/day (Bosi et al. 2007). Vildagliptin caused a significantly smaller reduction in FPG than rosiglitazone (Table 4), suggesting a more sustained effect throughout the day (Rosenstock et al. 2007b).
Effects on glucagon, insulin, and GLP-1
As expected from its mechanism of action, evidence consistently shows that vildagliptin increases postprandial GLP-1 levels relative to placebo (Table 5). Vildagliptin also consistently lowers plasma glucagon relative to placebo, but most of the evidence appears to show little effect on plasma insulin levels (Table 5). However, it has been argued that plasma insulin levels are not necessarily a direct measure of plasma insulin secretion, and that beta-cell function can be improved without appreciable changes in plasma insulin (Mari et al. 2005). Moreover, this observation probably reflects improved insulin secretion and sensitivity, shown by the same insulin level in response to lower glucose levels. Inhibition of DPP-4 might not be expected to have a significant effect on fasting GLP-1 or GIP because incretin release depends on nutrient intake. However, there is some evidence from a small study that a high dose of vildagliptin, 100 mg twice daily, significantly increased GLP-1 and GIP in patients with type 1 (n=11) or type 2 (n=9) diabetes after 4 weeks (Kelley et al. 2006).
Table 5.
Effects of vildagliptin on plasma insulin, glucagon, and GLP-1 levels (all level 2 evidence).
| Design | Treatment and dose |
Outcome |
Reference | ||
|---|---|---|---|---|---|
| Mean change from baseline in 4 h-postprandial plasma insulin (pmol/L) | Mean change from baseline in 30 min postprandial glucagon (ng/mL) | Mean change from baseline in 30 min postprandial GLP-1 (pmol/L) | |||
| Double-blind, placebo-controlled, multicenter RCT, 12 weeks | Placebo (n=58) | Placebo −30.1 | NR | NR | Ristic et al. 2005 |
| V 25 mg bid (n=51) | V 25 mg bid +3.0 | ||||
| V 25 mg qd (n=54) | V 25 mg qd −48.2 | ||||
| V 50 mg qd (n=53) | V 50 mg qd +2.8 | ||||
| V 100 mg qd (n=63) | V 100 mg qd +19.7 (P=0.022 vs placebo) | ||||
| All other doses NSD vs placebo | |||||
| Double-blind, placebo-controlled, multicenter RCT, 12 weeks Optional extension for further 40 weeks |
Placebo (n=51, of whom 29 completed extension) V 50 mg qd (n=56, of whom 42 completed extension) All patients were also taking stable doses of metformin 1.5–3 g/day |
NSD between groups at 12 weeks Greater increase with V than placebo at 52 weeks (P=0.015) |
NR | NR | Ahrén et al. 2004a |
| Double-blind, placebo-controlled, multicenter RCT, 4 weeks | Placebo (n=19) V 100 mg qd (n=18) |
Insulin response to meal ingestion NSD between groups | Significant reduction in V group (P=0.005) No significant change in placebo group |
Significant increase in V group (P<0.001) Placebo NR |
Ahrén et al. 2004b |
| Double-blind, placebo-controlled RCT, 4 weeks | Placebo (n=20) V 100 mg qd (n=20) |
Insulin response to meal ingestion NSD between groups | Placebo +2 V 12 (P<0.01) |
Placebo +0.9 V +4.7 (P<0.001) |
Ahrén et al. 2003 |
| Double-blind, placebo-controlled, single center RCT, 4 weeks | Placebo (n=11) V 100 mg bid (n=9) |
NR | 3.5 h mean glucagon: lower with V than placebo but NSD | Greater increase with V than placebo in 13.5 h mean GLP-1 (P<0.001) | Mari et al. 2005 |
bid, twice daily; NR, not reported; NSD, not statistically significantly different; qd, once daily; RCT, randomized controlled trial; V, vildagliptin.
Effects on beta-cell function
Evidence suggests that vildagliptin can increase the secretion of insulin in response to glucose, indicating an improvement in beta-cell function (Table 6). The dose-ranging study found that only the highest dose of vildagliptin (100 mg/day) was significantly superior to placebo, whereas other studies have shown statistically significant differences from placebo at lower doses (Table 6). However, this may reflect the variety of measures used to assess beta-cell function, which makes it difficult to compare results across different studies. In patients inadequately controlled with metformin ≥1.5 g/day, the insulin secretory response increased by 5.2 and 5.7 pmol/min/m2 with vildagliptin 50 and 100 mg/day, respectively, compared with placebo (Bosi et al. 2007). Adding vildagliptin to pioglitazone also improved beta-cell function (Rosenstock et al. 2007a; Table 6).
Table 6.
Effects of vildagliptin on beta-cell function (all level 2 evidence)
| Design | Treatment and dose |
Outcome |
||||
|---|---|---|---|---|---|---|
| Mean change from baseline in HOMA-Ba | Insulin response corrected for peak glucose | Insulin secretion after meal divided by increase in plasma glucose | Insulin secretory response at 7 mmol/L glucose (pmol/min/m2) | Reference | ||
| Double-blind, placebo-controlled RCT, 24 weeks | Placebo + P 30 mg qd (n=157) | NR | NR | NR | NR | Rosenstock et al. 2007a |
| Placebo + V 100 mg qd (n=150) | NR | NR | NR | NR | ||
| V 50 mg qd + P 15 mg qd (n=139) | NR | NR | V 50 mg qd + P 15 mg qd +8.0 (P=0.046 vs placebo + P) | NR | ||
| V 100 mg qd + P 30 mg qd (n=146) | NR | NR | V 100 mg qd + P 30 mg qd +5.5 | NR | ||
| Double-blind, placebo-controlled RCT, 24 weeks | Placebo + P 45 mg qd (n=138) | NR | NR | Placebo + P 45 mg qd +2 | NR | Garber et al. 2007b |
| V 50 mg qd + P 45 mg qd (n=124) | V 50 mg qd + P 45 mg qd +6 (P<0.01 vs placebo)b | |||||
| V 50 mg bid + P 45 mg qd (n=136) | NR | NR | V 50 mg bid + P 45 mg qd +7 (P<0.01 vs placebo)b | NR | ||
| Placebo-controlled RCT, 52 weeks | Placebo (n=150) | NR | NR | NR | NR | Scherbaum et al. 2007 |
| V 50 mg qd (n=156) | NR | NR | NR | V 50 mg qd +5 vs placebo (P<0.001) | ||
| Double-blind, placebo-controlled, multicenter RCT, 12 weeks | Placebo (n=58) | Placebo −4.3 | NR | NR | NR | Ristic et al. 2005 |
| V 25 mg bid (n=51) | V 25 mg bid +16.9 | NR | NR | NR | ||
| V 25 mg qd (n=54) | V 25 mg qd +2.9 | NR | NR | NR | ||
| V 50 mg qd (n=53) | V 50 mg qd +6.4 | NR | NR | NR | ||
| V 100 mg qd (n=63) | V 100 mg qd +22.5 (P=0.007 vs placebo) | NR | NR | NR | ||
| All other doses NSD vs placebo | ||||||
| Double-blind, placebo-controlled RCT, 12 weeks | Placebo (n=28) | NR | Significant increase with V vs placebo (P<0.05) | NR | NR | Pratley & Galbreath 2004 |
| V 25 mg bid (n=72) | ||||||
| Double-blind, placebo-controlled, multicenter RCT, 12 weeks Optional extension for further 40 weeks |
Placebo (n=51, of whom 29 completed extension) V 50 mg qd (n=56, of whom 42 completed extension) All patients were also taking stable doses of metformin 1.5–3 g/day |
NR | Greater increase with V than placebo at 12 weeks (P=0.0007) and 52 weeks (P=0.0005) | Greater with V than placebo at 12, 24, and 52 weeks (P<0.05) | NR | Ahrén et al. 2004a, 2005b |
| Double-blind, placebo-controlled, single center RCT, 4 weeks | Placebo (n=11) | NR | NR | NR | Greater increase with V than placebo (P<0.005) | Mari et al. 2005 |
| V 100 mg bid (n=9) | ||||||
Defined as: HOMA-B (homeostasis model of beta-cell function) = [20 x fasting insulin (mU/L)]/(fasting glucose [mmol/L] −3.5).
Data presented graphically.
bid, twice daily; NR, not reported; P, pioglitazone; qd, once daily; RCT, randomized controlled trial; V, vildagliptin.
Effects on insulin sensitivity
Evidence from one randomized controlled trial (Ahrén et al. 2005b) indicates that long-term vildagliptin treatment can increase insulin sensitivity during oral glucose intake (Table 7). The same study also found that vildagliptin increased the adaptation index (Table 7), which is considered by the authors to be a measure of the ability of beta cells to adapt insulin secretion to the prevailing insulin sensitivity (Ahrén et al. 2005b).
Table 7.
Effects of vildagliptin on insulin sensitivity (level 2 evidence)
| Design | Treatment and dose |
Outcome |
Reference | |
|---|---|---|---|---|
| Oral glucose insulin sensitivity (mL/min/m2) | Adaptation index (insulin secretion x insulin sensitivity) | |||
| Double-blind, placebo-controlled, multicenter RCT, 12 weeks Optional extension for further 40 weeks |
Placebo (n=51, of whom 29 completed extension) V 50 mg qd (n=56, of whom 42 completed extension All patients were also taking stable doses of metformin 1.5–3 g/day |
Greater with V than placebo at 24 and 52 weeks (P<0.05) | Greater with V than placebo at 12, 24 (P<0.05), and 52 weeks (P<0.01) | Ahrén et al. 2005b |
qd, once daily; RCT, randomized controlled trial; V, vildagliptin.
Effects on bodyweight
Excess bodyweight is a major risk factor in type 2 diabetes (see Disease overview section). Thus, it is important that medications for the treatment of diabetes should not cause an increase in bodyweight.
Vildagliptin is not associated with significantly more weight gain than placebo according to level 1 evidence from a meta analysis; there was a small increase of 0.42 kg [95% confidence interval (CI): 0.12, 0.72] (Amori et al. 2007). Weight reduction was greater with vildagliptin compared with pioglitazone or rosiglitazone (mean 1.7 kg difference), but less than that with metformin (mean 2.2 kg difference). The effects of vildagliptin on bodyweight are shown in Table 8.
Table 8.
Effects of vildagliptin on body weight (all level 2 evidence)
| Design | Treatment and dose | Mean change from baseline in body weight (kg) | Reference |
|---|---|---|---|
| Double-blind RCT, 52 weeks | V 50 mg bid (n=526) | V 50 mg bid +0.3 | Schweizer et al. 2007 |
| M 1 g bid (n=254) | M 1 g bid −1.9 (P<0.001 vs V) | ||
| Double-blind, placebo-controlled RCT, 24 weeks | Placebo + P 45 mg qd (n=138) | V 50 mg qd + P 45 mg qd +0.1a | Garber et al. 2007b |
| V 50 mg qd + P 45 mg qd (n=124) | V 50 mg bid + P 45 mg qd +1.3 (P=0.003 vs placebo)a | ||
| V 50 mg bid + P 45 mg qd (n=136) | |||
| Double-blind, placebo-controlled, multicenter RCT, 12 weeks | Placebo (n=58) | Placebo −0.73 | Ristic et al. 2005 |
| V 25 mg bid (n=51) | V 25 mg bid +0.05 | ||
| V 25 mg qd (n=54) | V 25 mg qd −0.55 | ||
| V 50 mg qd (n=53) | V 50 mg qd +0.04 | ||
| V 100 mg qd (n=63) | V 100 mg qd −0.07 | ||
| All V doses NSD vs placebo | |||
| Double-blind, placebo-controlled, multicenter RCT, 12 weeks Optional extension for further 40 weeks |
Placebo (n=51, of whom 29 completed extension) V 50 mg qd (n=56, of whom 42 completed extension) All patients were also taking stable doses of metformin 1.5–3.0 g/day |
At 12 weeks: Placebo −0.5 V −0.4 Extension phase: Placebo −0.2 V −0.2 (P values NR) |
Ahrén et al. 2004a |
| Double-blind, placebo-controlled, multicenter RCT, 4 weeks | Placebo (n=19) | Placebo +0.12 | Ahrén et al. 2004b |
| V 100 mg qd (n=18) | V +0.21 | ||
| NSD vs placebo |
Change versus placebo + P.
bid, twice daily; M, metformin; NR, not reported; NSD, not statistically significantly different; P, pioglitazone; qd, once daily; RCT, randomized controlled trial; V, vildagliptin.
Combination with other agents
Some evidence from animal models suggested that pioglitazone and vildagliptin could have synergistic effects on glucose clearance after an oral glucose load (Burkey et al. 2002). The combination has subsequently been studied in humans. One study investigated the combination in 12 patients with type 2 diabetes (Serra et al. 2005). Patients received pioglitazone 45 mg once daily for 8 weeks, and were then randomized to receive add-on placebo or vildagliptin 100 mg once daily for 28 days, followed by vildagliptin alone for 7 days. Coadministration did not alter the pharmacokinetic behavior of either compound, but the combination reduced postprandial glucose significantly (P<0.05) more than pioglitazone alone (Serra et al. 2005).
A crossover study in 15 patients with type 2 diabetes found that coadministration of glyburide 10 mg once daily and vildagliptin 100 mg twice daily did not modify the peak plasma concentration, area-under-the-curve, or half-life of either agent. Two hypoglycemic events occurred in patients receiving glyburide alone, but none in patients receiving the combination (Barilla et al. 2004).
A study in 16 healthy volunteers reported that vildagliptin significantly enhanced glibenclamide-stimulated insulin secretion, but did not increase the risk of reactive hypoglycemia (El-Ouaghlidi et al. 2003, 2004).
These early studies have been verified in larger, randomized placebo-controlled trials. The combination of vildagliptin 50 mg once or twice daily plus metformin caused a significant decrease of HbA1c compared with metformin alone, and a significantly greater proportion of patients achieved levels <7.0% (Dejager et al. 2007b; Table 3). Similarly, there was a significantly greater reduction in HbA1c when vildagliptin 50 mg or 100 mg daily was added to pioglitazone 15, 30, or 45 mg daily compared with pioglitazone 30 or 45 mg alone (Garber et al. 2007b; Rosenstock et al. 2007a; Table 3).
Effects in prediabetes
There is some preliminary evidence that vildagliptin is effective in people with prediabetes. In a 12-week randomized trial, vildagliptin 50 mg once daily (n=90) significantly (P<0.001) increased GLP-1 and GIP compared with placebo (n=89), and improved beta-cell function (Rosenstock et al. 2007c). Furthermore, vildagliptin reduced prandial glucose excursions by 22% compared with baseline, and by 32% compared with placebo. Vildagliptin 100 mg once daily also improved insulin sensitivity and beta-cell function in 22 patients with prediabetes after 6 weeks (Utzschneider et al. 2007).
Safety and tolerability
Level 1 evidence from a meta analysis of 12 randomized controlled trials comparing vildagliptin with placebo (Ristic et al. 2005; Mimori et al. 2006; Pratley et al. 2006; Pi-Sunyer et al. 2007; Dejager et al. 2007), metformin with or without placebo (Ahrén et al. 2004a; Bosi et al. 2007; Schweitzer et al. 2007), pioglitazone with or without placebo (Garber et al. 2007b; Rosenstock et al. 2007a), rosiglitazone (Rosenstock et al. 2007b), or insulin (Fonseca et al. 2007) revealed that vildagliptin was well tolerated with few adverse effects (Amori et al. 2007). There was no risk of gastrointestinal adverse effects compared with placebo; urinary tract infection and headache were more frequent with vildagliptin versus comparator (3.6% versus 1.3%, and 6.3% versus 4.4%, respectively). Nasopharyngitis occurred in 7.3% of vildagliptin recipients and upper respiratory tract infection in 6.8%, compared with 7.3% and 8.0%, respectively, with comparator.
Mild to moderate hypoglycemia occurred with the same frequency in patients taking vildagliptin (1.4%) or comparator drugs (1.2%) (Amori et el. 2007).
Liver function
A pooled analysis of clinical trial data from more than 8000 patients was submitted by the manufacturer to European regulatory authorities in November 2007 following concerns over liver function with vildagliptin. Elevations more than three times the upper limit of normal levels of aspartate aminotransferase and alanine aminotransferase were revealed in 0.86%, 0.34%, and 0.21% of patients taking vildagliptin 100 mg once daily, 50 mg twice daily, and 50 mg once daily, respectively (Anon 2007). The corresponding rates were 0.20% in approximately 4400 patients receiving metformin, a thiazolidinedione, a sulfonylurea, or placebo, and 0.40% for placebo alone.
Resource utilization
Type 2 diabetes is a disease with a high social and economic cost, and is steadily increasing in prevalence (see Disease overview section). The bulk of this cost is attributable to the complications of diabetes. As discussed earlier, intensive control of blood glucose increases short-term costs compared with conventional management, but may reduce the long-term complication rate. This reduction in complications should offset the additional cost of intensive blood glucose management. A new antidiabetic agent that achieves better glycemic control, and in turn lowers complication rates, would have the potential to make significant savings in the total resource utilization for diabetes management. Economic evidence for vildagliptin is not currently available and its effect on healthcare resources has not been assessed.
Vildagliptin has shown some evidence of improved glycemic control (measured by HbA1c) compared with placebo, either as monotherapy or as an add-on to metformin (Tables 3 and 4). The magnitude of the reduction in HbA1c demonstrated by the evidence so far is up to 1 percentage point (Tables 3 and 4), comparable to that achieved with other oral glucose-lowering drugs. Vildagliptin improves beta-cell function (Table 6) and insulin sensitivity (Table 7), which in turn improves control of blood glucose. In theory, since vildagliptin acts by enhancing the physiologic response to ingestion of a meal, it may provide finer control of blood glucose than is possible with agents such as sulfonylureas or insulin that act more directly.
There is evidence that glycemic control is maintained in the long term (52 weeks) with vildagliptin used as monotherapy or in combination with metformin (Ahrén et al. 2004a; Schweizer et al. 2007). Successful long-term glycemic control is an important clinical benefit, as it should contribute to a lower risk of complications, but this needs to be confirmed by further studies.
While long-term glycemic control is clearly important, if vildagliptin (and other DPP-4 inhibitors) is shown to have disease-modifying effects by protecting against the prolonged decline in beta-cell function that continues with other drugs, this could be of greater potential benefit in people with diabetes. Inhibiting disease progression and hence reducing or delaying the development of diabetic complications could provide substantial economic benefits.
Evidence is also needed to compare vildagliptin with injected insulin in type 2 diabetes. An oral glucose-lowering drug that could reduce HbA1c as effectively as insulin, without weight gain or hypoglycemia, would have a considerable advantage over injected insulin in terms of patient convenience.
However, direct evidence will be necessary to assess the likely impact of vildagliptin on resource utilization.
Patient group/population
Vildagliptin acts by prolonging the life of GLP-1 and thereby enhancing its effect on beta cells, so vildagliptin relies on the patient having an intact GLP-1 response to food and at least some beta-cell function. Vildagliptin would therefore not be expected to work in people with diabetes with little or no beta-cell function, such as those with type 1 diabetes, and these patients have been excluded from the trial populations. It is possible that vildagliptin may work better in patients with less advanced type 2 diabetes, and there is some evidence that it is effective in patients with prediabetes (Rosenstock et al. 2007c).
Patients with significant diabetic or cardiovascular complications were excluded from the published studies, so there is no evidence relating to the use of vildagliptin in this population with more severe disease.
Vildagliptin has been investigated in patients not previously treated for diabetes, and as an add-on to metformin in patients with an inadequate response to metformin therapy. All studies of vildagliptin were conducted in adults (aged ≥18 years). Pooled analyses indicate that the drug is effective in different ethnicities (Rosenstock et al. 2007d) and in elderly patients (Pratley et al. 2007b), and has a tolerability profile similar to that of metformin, pioglitazone, and rosiglitazone in patients with moderate renal insufficiency [glomerular filtration rate (GFR) 30 to <60 mL/min/1.73 m2), but better than metformin in those with mild dysfunction (GFR 60 to <90 mL/min/1.73 m2) (Thuren et al. 2007).
Thus, the majority of the present evidence base relates to the use of vildagliptin in adult patients with type 2 diabetes but without diabetic or cardiovascular complications and who may be either drug-naïve or who have not responded adequately to metformin therapy. Further results may extend this population to patients with prediabetes.
Dosage, administration, and formulations
Vildagliptin (Galvus) can be prescribed to patients with type 2 diabetes at a dosage of 50 mg once daily in combination with a sulfonylurea, or 50 mg twice daily with metformin or a thiazolidinedione such as pioglitazone. A 100 mg formulation of vildagliptin is available in Brazil and Mexico. A fixed dose combination of vildagliptin 50 mg plus metformin 850 mg or vildagliptin 50 mg plus metformin 1000 mg (Eucreas®) has also been approved for twice-daily use in patients inadequately controlled with metformin alone or who are already receiving each drug separately.
Liver function tests should be conducted before beginning treatment with vildagliptin, every 3 months in the first year of treatment, and periodically thereafter. Vildagliptin should not be used in patients with liver impairment, those with type 1 diabetes, patients with moderate to severe renal impairment, or those with congestive heart failure.
Place in therapy
Metformin is currently first line treatment for people with type 2 diabetes, with sulfonylureas established as second line, until newer agents such as DPP-4 inhibitors and PPAR agonists can demonstrate a clear advantage in terms of efficacy, safety, disease progression, or cost.
There is clear evidence of improved glycemic control (measured by HbA1c, blood glucose or numbers of patients achieving HbA1c <7.0%) with vildagliptin compared with placebo, either as monotherapy or in combination with metformin, the thiazolidinediones pioglitazone and rosiglitazone, or the sulfonylurea glimepride. Since most patients with type 2 diabetes ultimately require more than one drug for effective glycemic control, this evidence indicates a valuable place for vildagliptin in combination therapy. The drug is effective in drug-naïve patients, those poorly controlled with current treatment, in the elderly, and in different ethnicities. There is also evidence of improved beta-cell function and improved insulin sensitivity compared with placebo.
Vildagliptin was not associated with significantly more weight gain than placebo, and is not associated with a higher rate of hypoglycemia (Amori et al. 2007). The drug is well tolerated with few adverse effects. The incidence of liver enzyme elevations with vildagliptin 50 mg once daily in clinical trials is comparable to that seen with metformin, a thiazolidinedione, or a sulfonylurea; the incidence with 50 mg twice daily is less than that with placebo.
The present evidence base supports the use of vildagliptin in adults with type 2 diabetes and no significant diabetic or cardiovascular complications. It can be inferred from the drug’s mechanism of action that it may be of most benefit in patients in the early stages of type 2 diabetes. As some authorities advocate earlier use of insulin in people with diabetes at this stage, studies comparing vildagliptin with early insulin would be valuable. There is evidence that vildagliptin is effective in patients with prediabetes.
The main area where further evidence is required is on the effect of vildagliptin on important patient-oriented outcomes such as disease progression, impact on cardiovascular risk, long-term complications, health-related quality of life, and adherence to therapy. As the majority of the costs of managing diabetes are attributable to managing complications, this evidence would also be important in assessing the potential economic impact of the agent.
In summary, vildagliptin has shown evidence of improved glycemic control and a potentially favorable tolerability profile in adult patients with type 2 diabetes. This has potential for important clinical and/or economic benefits in the management of diabetes, as improved glycemic control is likely to reduce the risk of complications and thus improve resource utilization. Vildagliptin could be a useful option for use in combination therapy in patients with type 2 diabetes.
Acknowledgments
The authors declare that they have no conflicts of interest.
References
- AACE (American Association of Clinical Endocrinologists) Diabetic Guidelines. Endocrine Practice. 2002;8(Suppl 1):41–65. [Google Scholar]
- AACE (American Association of Clinical Endocrinologists) State of Diabetes in America. Available at: http://www.stateofdiabetes.com (accessed November 8, 2005) [Google Scholar]
- ACCORD Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADA (American Diabetes Association) and NIDDKD (National Institute of Diabetes, Digestive and Kidney Diseases) The prevention or delay of type 2 diabetes. Diabetes Care. 2002;25:742–749. doi: 10.2337/diacare.25.4.742. [DOI] [PubMed] [Google Scholar]
- ADA (American Diabetes Association) Standards of medical care in diabetes. Diabetes Care. 2005;28(Suppl. 1):S4–S36. [PubMed] [Google Scholar]
- Ahrén B, Landin-Olsson M, Jansson P-A, et al. The DPPIV inhibitor, LAF237, reduces fasting and postprandial glucose in subjects with type 2 diabetes over a 4 week period by increasing active GLP-1, sustaining insulin and reducing glucagon. Diabetes. 2003;52(Suppl. 1):A15. Abstract 65-OR. [Google Scholar]
- Ahrén B, Mills D, Gomis R, Standl E, Mills D, Schweizer A. Twelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetes. Diabetes Care. 2004a;27:2874–2880. doi: 10.2337/diacare.27.12.2874. [DOI] [PubMed] [Google Scholar]
- Ahrén B, Landin-Olsson M, Jansson P-A, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagons levels in type 2 diabetes. J Clin Endocrinol Metab. 2004b;89:2078–2084. doi: 10.1210/jc.2003-031907. [DOI] [PubMed] [Google Scholar]
- Ahrén B, Gomis R, Mills D, Schweizer A. The DPP-4 inhibitor, LAF237, improves glycemic control in patients with type 2 diabetes (T2DM) inadequately treated with metformin. Presented at the American Diabetes Association Scientific Sessions, 2004c; Abstract 354-OR. Available at: http://scientificsessions.diabetes.org/Abstracts/index.cfm (accessed December 12, 2005) [Google Scholar]
- Ahrén B, Winzell MS, Burkey B, Hughes TE. Beta-cell expression of a dominant-negative hepatocyte nuclear factor (HNF)-1alfa compromises the ability of DPP-4 inhibition to elicit a long-term augmentation of insulin secretion in mice. Presented at the American Diabetes Association Scientific Sessions, 2005a; Abstract 1559-P. Available at: http://scientificsessions.diabetes.org/Abstracts/index.cfm (accessed December 12, 2005) [Google Scholar]
- Ahrén B, Foley JE, Pacin G, Schweizer A. Improved meal-related beta-cell function and insulin sensitivity by the dipeptidyl peptidase-IV inhibitor vildagliptin in metformin-treated patients with type 2 diabetes over 1 year. Diabetes Care. 2005b;28:1936–1940. doi: 10.2337/diacare.28.8.1936. [DOI] [PubMed] [Google Scholar]
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- Anon New Galvus® clinical data reinforces efficacy profile; safety update provided to regulatory agencies. Available at http://cws.huginonline.com/N/134323/PR/200711/1166139_5_2.html (accessed November 12, 2007) [Google Scholar]
- Barilla D, He Y, Balez S, et al. No pharmacokinetic interactions or acute clinical safety issues preclude combination of the DPP-4 inhibitor LAF237 with glyburide. Presented at the American Diabetes Association Scientific Sessions, 2004. Abstract 1967-PO; Available at: http://scientificsessions.diabetes.org/Abstracts/index.cfm (accessed December 12, 2005) [Google Scholar]
- Baron MA, Rosenstock J, Bassiri B, et al. Efficacy of vildagliptin combined with pioglitazone in patients with type 2 diabetes. Presented at the European Association for the Study of Diabetes Annual Meeting, 2006. Abstract 0801; Available at: http://www.easd.org/ (accessed October 11, 2007) [Google Scholar]
- Bartnik M, Malmberg K, Norhammar A, Tenerz A, Ohrvik J, Rydén L. Newly detected abnormal glucose tolerance: an important predictor of long-term outcome after myocardial infarction. Eur Heart J. 2004;25:1990–1997. doi: 10.1016/j.ehj.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Bloomgarden ZT. Type 2 diabetes in the young: the evolving epidemic. Diabetes Care. 2004;27:998–1010. doi: 10.2337/diacare.27.4.998. [DOI] [PubMed] [Google Scholar]
- Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890–895. doi: 10.2337/dc06-1732. [DOI] [PubMed] [Google Scholar]
- Brandt I, Joossens J, Chen X, et al. Inhibition of dipeptidyl-peptidase IV catalyzed peptide truncation by vildagliptin ((2S)-{[3-hydroxyadamantan-1-yl)amino]acetyl]}-pyrrolidine-2-carbonitrile) Biochem Pharmacol. 2005;70:134–143. doi: 10.1016/j.bcp.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Burkey B, Li X, Bolognese L, et al. Combination treatment of a DPP-IV inhibitor NVP-LAF237 with pioglitazone completely normalized glucose tolerance in adult obese Zucker rats. Diabetes. 2002;51(Suppl. 2):A338. Abstract 1383-P. [Google Scholar]
- Burkey BP, Li X, Bolognese L, et al. Acute and chronic effects of the incretin enhancer vildagliptin in insulin resistant rats. J Pharmacol Exp Ther. 2005;315:688–695. doi: 10.1124/jpet.105.087064. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Diabetes Projects, 2005b. Available at: http://www.cdc.gov/diabetes/projects/cda2.htm (accessed November 7, 2005) [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Fact Sheet. SEARCH for Diabetes in Youth, 2005a. Available at: http://www.cdc.gov/diabetes/pubs/pdf/search.pdf (accessed November 7, 2005) [Google Scholar]
- CDC (Centers for Disease Control and Prevention) National Diabetes Fact Sheet, United States, 2003. General Information. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2003.pdf (accessed November 7, 2005) [Google Scholar]
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, STOP-NIDDM Trail Research Group Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- Dardik B, Schwartzkopf C, Stevens D, et al. The dipeptidyl peptidase IV inhibitor NVP-LAF237 improves metabolic control in diabetic and nondiabetic cynomolgus monkeys. Diabetes. 2003a;52(Suppl.1):A322. Abstract 1391-P. [Google Scholar]
- Dardik B, Valentin M, Schwartzkopf C, et al. NVP-LAF237, a dipeptidyl peptidase IV inhibitor, improves glucose tolerance and delays gastric emptying in obese insulin resistant cynomolgus monkeys. Diabetes. 2003b;52(Suppl.1):A322. Abstract 1392-P. [Google Scholar]
- DECODE Study Group, the European Diabetes Epidemiology Group Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161:397–405. doi: 10.1001/archinte.161.3.397. [DOI] [PubMed] [Google Scholar]
- Dejager S, Razac S, Foley JE, Schweizer A. Vildagliptin in drug-naïve patients with type 2 diabetes: a 24-week, double-blind, randomized, placebo-controlled, multiple-dose study. Horm Metab Res. 2007a;39:218–223. doi: 10.1055/s-2007-970422. [DOI] [PubMed] [Google Scholar]
- Dejager S, Schweizer A, Couturier A, Pratley RE. Achievement of glycaemic targets with vildagliptin. Presented at the European Association for the Study of Diabetes Annual Meeting, 2007b. Abstract 0885; Available at: http://www.easd.org/ (accessed October 11, 2007) [Google Scholar]
- Duttaroy A, Voelker F, Merriam K, Zhang X, Ren X, Burkey B. The DPP-4 inhibitor vildagliptin increases pancreatic beta-cell neogenesis and decreases apoptosis. Presented at the American Diabetes Association Scientific Sessions, 2005a. Abstract 572-P; Available at: http://scientificsessions.diabetes.org/Abstracts/index.cfm (accessed December 12, 2005) [Google Scholar]
- Duttaroy A, Voelker F, Ren X, et al. Head-to-head comparison of the DPP-4 inhibitor vildagliptin with exendin-4 in a model of pancreatic beta-cell injury. Presented at the American Diabetes Association Scientific Sessions, 2005b; Abstract 267-OR. Available at: http://scientificsessions.diabetes.org/Abstracts/index.cfm (accessed December 12, 2005) [Google Scholar]
- EDPG (European Diabetes Policy Group) A desktop guide to type 2 diabetes. International Diabetes Federation European Region. Diabetic Med. 1999;16:716–730. [PubMed] [Google Scholar]
- El-Ouaghlidi A, Rehring E, Holst JJ, Schweizer A, Holmes D, Nauck MA. Reduced increments in total glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP) in plasma after a single dose of the dipeptidyl peptidase-4 inhibitor LAF237 before an oral glucose load in healthy subjects. Presented at the European Association for the Study of Diabetes Annual Meeting, 2004. Abstract 786; Available at: http://212.144.4.93/easd/UpApplications/UpTables/provisionalprogram/groupviewpage.asp (accessed December 12, 2005) [Google Scholar]
- El-Ouaghlidi A, Rehring E, Schweizer A, Holmes D, Nauck M. The dipeptidyl peptidase IV inhibitor LAF 237 does not accentuate reactive hypoglycemia caused by the sulfonylurea glibenclamide administered before an oral glucose load in healthy subjects. Diabetes. 2003;52(Suppl. 1):A118. Abstract 507-P. [Google Scholar]
- Fonseca V, Dejager S, Albrecht D, Shao Q, Schweizer A. Sustained efficacy and reduced hypoglycaemia with vildagliptin added to insulin in patients with type 2 diabetes. Presented at the European Association for the Study of Diabetes Annual Meeting, 2006. Abstract 0802; Available at: http://www.easd.org/ (accessed October 11, 2007) [Google Scholar]
- Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia. 2007;50:1148–1155. doi: 10.1007/s00125-007-0633-0. [DOI] [PubMed] [Google Scholar]
- Fox CS, Larson MG, Leip EP, Meigs JB, Wilson PW, Levy D. Glycemic status and development of kidney disease: the Framingham Heart Study. Diabetes Care. 2005;28:2436–2440. doi: 10.2337/diacare.28.10.2436. [DOI] [PubMed] [Google Scholar]
- Garber AJ, Camisasca R-P, Jauffret S, Baron MA. Efficacy and tolerability of vildagliptin added to a sulfonylurea (SU) in patients with type 2 diabetes (T2DM). Presented at the American Diabetes Association Annual Meeting, 2007a. Abstract 0501-P; Available at: http://scientificsessions.diabetes.org/Abstracts/index.cfm (accessed October 11, 2007) [Google Scholar]
- Garber AJ, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes, Obesity and Metabolism. 2007b;9:166–174. doi: 10.1111/j.1463-1326.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- Goeke B, Schweizer A, Couturier A, LeBeaut A, Dejager S. Sustained efficacy and tolerability of vildagliptin monotherapy during one-year treatment of drug-naïve patients with type 2 diabetes. Presented at the European Association for the Study of Diabetes Annual Meeting, 2006. Abstract 0042; Available at: http://www.easd.org/ (accessed October 11, 2007) [Google Scholar]
- Gray A, Raikou M, McGuire A, et al. United Kingdom Prospective Diabetes Study Group Cost effectiveness of an intensive blood glucose control policy in patients with type 2 diabetes: economic analysis alongside randomised controlled trial (UKPDS 41) BMJ. 2000;320:1373–1378. doi: 10.1136/bmj.320.7246.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjøllund KR, Hughes TE, Holst JJ. The dipeptidyl peptidase 4 inhibitor vildagliptin has no acute effect on the endocrine secretion in the isolated perfused porcine pancreas. Presented at the European Association for the Study of Diabetes Annual Meeting, 2007. Abstract 0691; Available at: http://www.easd.org/ (accessed March 4, 2008) [Google Scholar]
- Hogan P, Dall T, Nikolov P, American Diabetes Association Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–932. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- Holman RR. Assessing the potential for alpha-glucosidase inhibitors in prediabetic states. Diabetes Res Clin Pract. 1998;40(Suppl):S21–S25. doi: 10.1016/s0168-8227(98)00038-2. [DOI] [PubMed] [Google Scholar]
- Holst JJ, Deacon CF. Glucagon-like peptide-1 mediates the therapeutic actions of DPP-IV inhibitors. Diabetologia. 2005;48:612–615. doi: 10.1007/s00125-005-1705-7. [DOI] [PubMed] [Google Scholar]
- Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab. 2004;287:E199–E206. doi: 10.1152/ajpendo.00545.2003. [DOI] [PubMed] [Google Scholar]
- Hughes TE, Russell ME, Bolognese L, et al. NVP-LAF237, a highly selective and long-acting dipeptidyl peptidase IV inhibitor. Diabetes. 2002;51(Suppl. 2):A67. Abstract 272-OR. [Google Scholar]
- IDF (International Diabetes Federation). Diabetes Prevelance 2005a Available at http://www.idf.org/home/index.cfm?node=264 (accessed November 7, 2005) [Google Scholar]
- IDF Global guideline for type 2 diabetes. 2005b. Available at http://www.idf.org/home/index.cfm?node=1457 (accessed October 16, 2007). [Google Scholar]
- Jellinger PS, Davidson JA, Blonde L, et al. Road maps to achieve glycemic control in type 2 diabetes mellitus: ACE/AACE Diabetes Road Map Task Force. Endocr Pract. 2007;13:260–268. doi: 10.4158/EP.13.3.260. [DOI] [PubMed] [Google Scholar]
- Jonsson B. CODE-2 Advisory Board. Revealing the cost of Type II diabetes in Europe. Diabetologia. 2002;45:S5–S12. doi: 10.1007/s00125-002-0858-x. [DOI] [PubMed] [Google Scholar]
- Kahn R, Buse J, Ferrannini E, Stern M, American Diabetes Association. European Association for the Study of Diabetes The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- Kelley DE, Dunning BE, Ligueros-Saylan M, Holst JJ, Deacon CF, Foley JE. Four-week treatment with vildagliptin increases fasting plasma levels of intact incretin hormones both in patients with type 1 (T1DM) and type 2 (T2DM) diabetes. Presented at the American Diabetes Association Annual Meeting, 2006. Abstract 1481-P; Available at: http://scientificsessions.diabetes.org/Abstracts/index.cfm (accessed October 11, 2007) [Google Scholar]
- Kikuchi M, Abe N, Kato M, Terao S, Holmes D, Mimori N. Vildagliptin decreases HbA1c after 12 weeks treatment in Japanese patients with type 2 diabetes. Presented at the European Association for the Study of Diabetes Annual Meeting, 2006. Abstract 0789; Available at: http://www.easd.org/ (accessed October 11, 2007) [Google Scholar]
- Kimmel B, Inzucchi SE. Oral agents for type 2 diabetes: an update. Clinical Diabetes. 2005;23:64–76. [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic control from 1988 to 2000 among US adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care. 2004;27:17–20. doi: 10.2337/diacare.27.1.17. [DOI] [PubMed] [Google Scholar]
- Mari A, Sallas M, He YL, et al. Vildagliptin, a dipeptidyl peptidase-IV inhibitor, improves model-assessed beta-cell function in patients with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:4888–4894. doi: 10.1210/jc.2004-2460. [DOI] [PubMed] [Google Scholar]
- Mika A, Stashko M, Lubben T, et al. Acute DPP-IV inhibition minimizes glucose and insulin responses to a glucose challenge in a diabetic mouse. Presented at the American Diabetes Association Scientific Sessions, 2003. Abstract 1516-P; Available at: http://scientificsessions.diabetes.org/Abstracts/index.cfm (accessed December 12, 2005) [Google Scholar]
- Mimori N, Terao S, Holmes D. Vildagliptin improves glucose control as evidenced by HbA1c after 12 weeks therapy in Japanese patients with type 2 diabetes. Presented at the American Diabetes Association Annual Meeting, 2006. Abstract 527-P; Available at: http://scientificsessions.diabetes.org/Abstracts/index.cfm (accessed October 11, 2007) [Google Scholar]
- Nauck MA, El-Ouaghlidi A. The therapeutic actions of DPP-IV inhibition are not mediated by glucagon-like peptide-1. Diabetologia. 2005;48:608–611. doi: 10.1007/s00125-005-1704-8. [DOI] [PubMed] [Google Scholar]
- Nauck M, Stockmann F, Ebert R, et al. Reduced incretin effect in type 2 (non-insulin dependent) diabetes. Diabetologia. 1986;29:46–52. doi: 10.1007/BF02427280. [DOI] [PubMed] [Google Scholar]
- Nauck MA, Wollschlager D, Werner J, et al. Effects of subcutaneous glucagon-like peptide 1 (GLP-1[7–36 amide]) in patients with NIDDM. Diabetologia. 1996;39:1546–1553. doi: 10.1007/s001250050613. [DOI] [PubMed] [Google Scholar]
- NICE (National Institute for Health and Clinical Excellence) Clinical guidelines for type 2 diabetes: Management of blood glucose. September 2002. Available at: http://www.nice.org.uk (accessed November 7, 2005) [Google Scholar]
- Pan XR, Li GW, Hu YH, et al. The Da Qing IGT Diabetes Study Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy in drug-naïve patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;76:132–138. doi: 10.1016/j.diabres.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146:693–700. doi: 10.1016/j.jpeds.2004.12.042. [DOI] [PubMed] [Google Scholar]
- Pratley R, Galbreath E. Twelve-week monotherapy with the DPP-4 inhibitor LAF237 improves glycemic control in patients with type 2 diabetes. Diabetes. 2004;53(Suppl. 2):A83. doi: 10.1055/s-2006-944546. Abstract 355-OR. [DOI] [PubMed] [Google Scholar]
- Pratley RE, Jauffret-Kamel S, Galbreath E, Holmes D. Twelve-week monotherapy with the DPP-4 inhibitor vildagliptin improves glycemic control in subjects with type 2 diabetes. Horm Metab Res. 2006;38:423–428. doi: 10.1055/s-2006-944546. [DOI] [PubMed] [Google Scholar]
- Pratley RE, Rosenstock J, Pi-Sunyer FX, Couturier A, Schweizer A, Dejager S. Efficacy and safety of vildagliptin in the elderly: pooled analysis of 5 monotherapy studies. Presented at the European Association for the Study of Diabetes Annual Meeting, 2007b. Abstract 0887; Available at: http://www.easd.org/ (accessed October 11, 2007) [Google Scholar]
- Ristic S, Byiers S, Foley J, Holmes D. Improved glycaemic control with dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes: vildagliptin (LAF237) dose response. Diabetes Obes Metab. 2005;7:692–698. doi: 10.1111/j.1463-1326.2005.00539.x. [DOI] [PubMed] [Google Scholar]
- Rolin B, Nygaard H, Wilken M, Kanstrup AB, Carr RD. The novel, xanthine-based, DPPIV inhibitor NN7201 and LAF237 improve glucose tolerance but do not prevent progression of diabetes in Zucker diabetic fatty rats. Presented at the American Diabetes Association Scientific Sessions, 2004. Abstract 1417-P; Available at: http://scientificsessions.diabetes.org/Abstracts/index.cfm (accessed December 12, 2005) [Google Scholar]
- Rosenstock J, Baron MA, Camisasca RP, Cressier F, Cautier A, Dejager S. Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetes. Diabetes Obes Metab. 2007a;9:175–185. doi: 10.1111/j.1463-1326.2006.00698.x. [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Baron MA, Dejager S, Mills D, Schweizer A. Comparison of vildagliptin and rosiglitazone monotherapy in patients with type 2 diabetes. Diabetes Care. 2007b;30:217–223. doi: 10.2337/dc06-1815. [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Rendell MS, Landin-Olsson M, Foley JE, Rochette E, Baron MA, Dejager S. Effects of vildagliptin in subjects with IGT. Presented at the American Diabetes Association Annual Meeting, 2007c. Abstract 0505-P; Available at: http://scientificsessions.diabetes.org/Abstracts/index.cfm (accessed October 11, 2007) [Google Scholar]
- Rosenstock J, Pi-Sunyer FX, Banerji MA, Couturier A, Schweizer A, Dejager S. Consistent efficacy and safety of vildagliptin monotherapy across ethnicities. Presented at the American Diabetes Association Annual Meeting, 2007d. Abstract 2141-PO; Available at: http://scientificsessions.diabetes.org/Abstracts/index.cfm (accessed October 11, 2007) [Google Scholar]
- Saydah SH, Eberhardt MS, Loria CM, Brancati FL. Subclinical states of glucose intolerance and risk of death in the US. Diabetes Care. 2001;24:447–453. doi: 10.2337/diacare.24.3.447. [DOI] [PubMed] [Google Scholar]
- Scherbaum WA, Schweizer A, Mari A, et al. Efficacy and tolerability of vildagliptin in drug-naïve patients with type 2 diabetes (T2DM) and mild hyperglycemia. Presented at the American Diabetes Association Annual Meeting, 2007. Abstract 0503-P; Available at: http://scientificsessions.diabetes.org/Abstracts/index.cfm (accessed October 11, 2007) [Google Scholar]
- Schweizer A, Couturiert A, Foley JE, Dejager S. Comparison between vildagliptin and metformin to sustain reductions in HbA1c over 1 year in drug-naïve patients with type 2 diabetes. Diabetic Med. 2007;24:955–961. doi: 10.1111/j.1464-5491.2007.02191.x. [DOI] [PubMed] [Google Scholar]
- Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421–431. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- Serra DB, He Y-L, Wang Y, et al. Combination of the DPP-4 inhibitor vildagliptin (LAF237) with pioglitazone is safe and well tolerated with no pharmacokinetic interaction. Presented at the American Diabetes Association Scientific Sessions, 2005. Abstract 2192-PO; Available at: http://scientificsessions.diabetes.org/Abstracts/index.cfm (accessed December 12, 2005) [Google Scholar]
- Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- Thuren T, Byiers S, Mohideen P. Vildagliptin is safe and well tolerated in patients with mild or moderate renal impairment. Presented at the European Association for the Study of Diabetes Annual Meeting, 2007. Abstract 0881; Available at: http://www.easd.org/ (accessed October 11, 2007) [Google Scholar]
- Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155–161. doi: 10.2337/diacare.27.1.155. Erratum in: Diabetes Care 2004; 27:856. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Lindström J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- Turner RC, Cull CA, Frighi V, Homan R, The UKPDS Group Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS49) JAMA. 1999;281:2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- UKPDS (UK Prospective Diabetes Study) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998a;352:854–865. [PubMed] [Google Scholar]
- UKPDS (UK Prospective Diabetes Study) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998b;352:837–853. [PubMed] [Google Scholar]
- Utzschneider KM, Tong J, Udayasankar J, et al. The dipeptidyl peptidase-4 (DPP-4) inhibitor vildagliptin improves insulin sensitivity and beta-cell function in subjects with impaired fasting glucose. Presented at the American Diabetes Association Annual Meeting, 2007. Abstract 0515-P; Available at: http://scientificsessions.diabetes.org/Abstracts/index.cfm (accessed October 11, 2007) [DOI] [PubMed] [Google Scholar]
- Vella A, Bock G, Giesler PD, et al. Effects of dipeptidyl peptidase-4 inhibition on gastrointestinal function, meal appearance, and glucose metabolism in type 2 diabetes. Diabetes. 2007;56:1475–1480. doi: 10.2337/db07-0136. [DOI] [PubMed] [Google Scholar]
- Villhauer EB, Brinkman JA, Naderi GB, et al. 1-[[(3-hydroxy-1-adamantyl)amino] acetyl]-2-cyano-(S)-pyrrolidine: a potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties. J Med Chem. 2003;46:2774–2789. doi: 10.1021/jm030091l. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) Screening for type 2 diabetes. Report of a World Health Organization and International Federation Meeting 2003. Available at http://www.who.int/diabetes/publications/en/screening_mnc03.pdf (accessed November 7, 2005) [Google Scholar]
- Zander M, Madsbad S, Madsen JL, Holst J. Effect of a 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359:824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]