Abstract
Cervical total disc replacement (CTDR) aims to decrease the incidence of adjacent segment disease through motion preservation in the operated disc space. Ongoing data collection and increasing number of studies describing heterotopic ossification (HO) resulting in decreased mobility of implants, forced us to carefully evaluate our long-term clinical and morphological results of patients with CTDR. We present the first 54 consecutive patients treated with 65 ProdiscC™ prostheses during a 12-month period (2/2004–3/2005). All patients signed an informed consent and were included in prospective long-term study approved by hospital ethical committee. The 1- and 2-year follow-up analysis were available for all patients included and 4-year results for 50 patients (60 implants). Clinical (neck disability index-NDI, visual analog scale-VAS) and radiological follow-up was conducted at 1-, 2- and 4-years after the procedure. The Mehren/Suchomel modification of McAfee scale was used to classify the appearance of HO. Mean preoperative NDI was 34.5%, VAS for neck pain intensity 4.6 and VAS for arm pain intensity 5.0. At 1-, 2- and 4-year follow-up, the mean NDI was 30.7, 27.2, and 30.4, mean VAS for neck pain intensity 2.5, 2.1 and 2.9 and mean VAS for arm pain intensity pain 2.2, 1.9 and 2.3, respectively. Significant HO (grade III) was present in 45% of implants and segmental ankylosis (grade IV) in another 18% 4 years after intervention. This finding had no clinical consequences and 92% of patients would undergo the same surgery again. Our clinical results (NDI, VAS) are comparable with fusion techniques. Although, advanced non-fusion technology is used, a significant frequency of HO formation and spontaneous fusion in cervical disc replacement surgery must be anticipated during long-term follow-up.
Keywords: Total cervical disc replacement, Heterotopic ossification, Spontaneous fusion, Ankylosis, ProdiscC™
Introduction
Anterior cervical discectomy and fusion (ACDF) has been the mainstay treatment of cervical degenerative disc disease (CDDD) refractory to conservative therapy for several decades [7, 16, 30]. While highly successful in the diseased segment, a fusion procedure is likely detrimental to the remaining motion segments [14, 15]. On the other hand, cervical total disc replacement (CTDR) technique aims to decrease the incidence of adjacent segment disease (ASD) through motion pattern preservation at both the operated level and the adjacent ones [10, 27]. Several biomechanical studies in human cadaveric models have demonstrated increased intradiscal pressure or hypermobility in adjacent segments following fusion [9, 12, 32]. These pressure patterns and decreased motion cumulatively translate into increased stress on adjacent non-operated discs which can accelerate the rate of disc degeneration [1, 10, 14]. Hilibrand et al. [15] suggested that symptomatic ASD occurs at a relatively constant annual rate of 2.9% and thus more than one-fourth of fused patients can potentially be affected at 10 years. However, a portion of these patients certainly represented those with progression of their disease due to natural history of cervical spondylosis. Treatment of ASD by ACDF is associated with higher rates of pseudoarthrosis and swallowing problems [11, 16]. Several studies have been performed evaluating ProdiscC™ and comparing it with ACDF. Initial results were highly promising [5, 23, 24]. Nevertheless, ongoing data collection and increasing number of studies describing heterotopic ossification (HO) resulting in decreased mobility of implants [19, 21, 31] forced us to carefully evaluate our long-term clinical and especially morphological results of CTDR. Our prospective non-controlled single center study describes morphological development of HOs and clinical results at 1, 2, and 4 years after the CTDR with ProdiscC™.
Patients and methods
Study design
The aim of this prospective study was to evaluate safety and efficacy of ProdiscC™ in the treatment of CDDD. The study was approved by local hospital ethical committee and included all consecutive patients treated from February 2004 to March 2005. All patients were fully educated and signed informed consent. The purpose of this paper is to report long-term (4-year) follow-up results.
Inclusion criteria were patients with disc herniations and/or minor degenerative changes between C3/4 and C6/7 levels causing arm with or without neck pain, with or without motor or sensory deficit. All patients were conservatively treated for at least 6 weeks, unless there was neurologic deterioration.
General exclusion criteria were infection, pregnancy, osteoporosis, rheumatoid arthritis, allergy to ProdiscC™ metal components, malignancy in last 5 years, metabolic or systemic disease, chronic use of steroids and other medications influencing bone or soft tissues and also patient’s non-compliance with treatment.
Specific exclusion criteria were target segment instability, ossification of the posterior longitudinal ligament, bridging osteophytes, segmental motion range less than two degrees, disc height loss larger than 70% in comparison to adjacent levels.
Clinical status of each patient was assessed preoperatively, at 3, 6 months and 1-, 2- and 4-years postoperatively. The evaluation at every visit included self-assessment questionnaires [Neck Disability Index (NDI), Visual Analogue Scale (VAS) of neck and arm pain] and neurologic examination (reflexes, presence of sensory or motor deficits). Radiographic examination was performed at 6 months, 1-, 2- and 4-years postoperatively and included routine anteroposterior, lateral and dynamic cervical spine films to assess position of the implant and motion of the operated segment. Presence of HOs was also evaluated at these time points according to the Mehren/Suchomel modification [21] of McAfee classification [20] used for lumbar spine: grade 0—segments without any new HO formation after prosthesis implantation (Fig. 1); grade I—segments with new HO formation not reaching the intervertebral space (Fig. 2); grade II—HO reaches the intervertebral space but segmental movement is not limited (Fig. 3); grade III—important bridging ossifications with limited, but possible movement (Fig. 4); grade IV—segmental fusion (Fig. 5). Two neurosurgeons, not involved in the original surgery, independently evaluated these films for the presence of HO. Any discrepancies were resolved through a consensus; however, intra- or inter-observer reliability was not assessed.
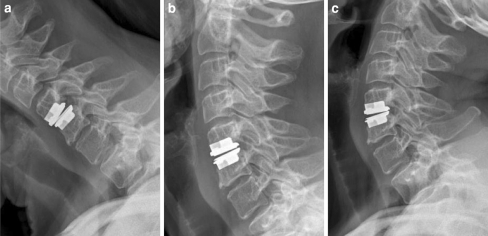
Fig. 1.
HO grade 0: No signs of heterotopic ossification and full range of motion as seen on flexion (a), neutral (b) and extension (c) lateral cervical spine films at 4-year follow-up
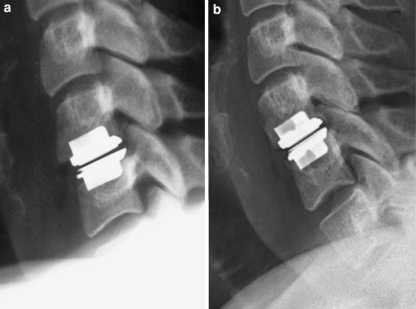
Fig. 2.
HO grade I: No signs of HO postoperatively (a) and a clear sign of heterotopic ossification grade I (b) on lateral cervical spine films at 4-year follow-up
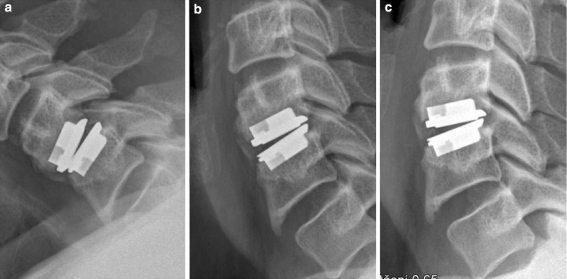
Fig. 3.
HO grade II: Clear signs of HO with no significant reduction in motion of the implant on flexion (a), neutral (b) and extension (c). Lateral cervical spine films, 4-year follow-up
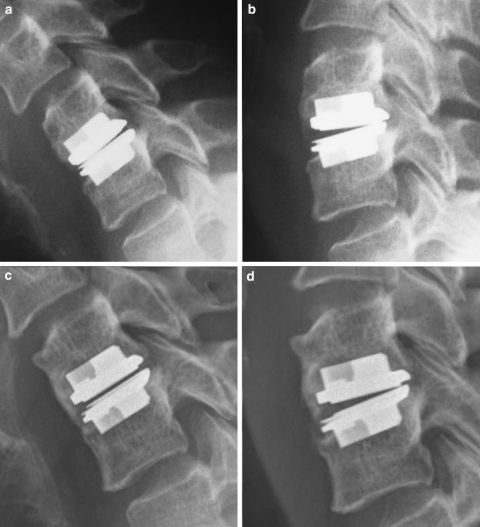
Fig. 4.
HO grade III: No signs of HO with satisfactory range of motion on postoperative lateral flexion (a) and extension (b) cervical spine films. The same patient on 4-year follow-up with significant heterotopic ossification limiting range of motion on flexion (c) and extension (d)
Fig. 5.
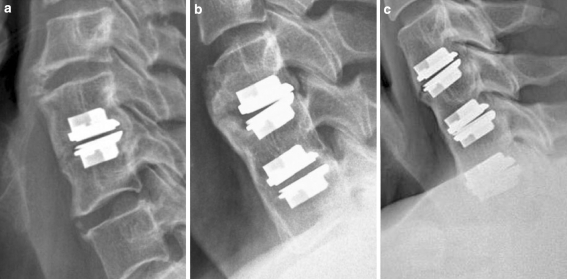
HO grade IV: Fusion without any motion of treated segment(s) as seen on lateral cervical spine films of single-level (a), double-level (b), and triple-level (c) implantations
The implant and surgical technique
The ProdiscC™ prosthesis manufactured by Synthes, Inc., (Paoli, PA) is a metal-on polyethylene ball-and-socket type articulating device. It consists of two cobalt–chrome–molybdenum alloy endplates and ultra-high molecular weight polyethylene (UHMWPE) inlay fixed to the lower one. The implant endplates are primarily secured to the vertebral bodies by central keels and are coated with plasma-sprayed titanium to allow secondary bone in-growth important for long-term stability. The implant is inserted en-bloc as a one-piece device.
The standard right-sided anterolateral approach was performed in all cases. Discectomy and decompression were performed with the use of an operating microscope and the involved segment was distracted and held in position by retaining Caspar posts. An appropriate size of trial implant was inserted respecting the sagittal midline. The slots for keels were chiseled, along the trial implant followed by the impaction of the ProdiscC™ prosthesis into the prepared disc space. All surgeries were performed by three board-certified neurosurgeons with a minimum of 100 routine ACDF procedures and 5 years of experience with cervical spine surgery.
All patients wore a Philadelphia collar until verification cervical spine films on the first postoperative day confirmed appropriate implant position. All patients were educated about the importance of physical therapy and learnt to perform exercises under the supervision of an experienced physiotherapist. Non-steroidal anti-inflammatory medications were not used regularly and pain management after discharge was left to the discretion of the local neurologist or referring physician.
Statistical analysis
Statistical analysis was performed using STATISTICA 8.0 software. Repeated measures design ANOVA with post hoc Fisher LSD tests were used for evaluation of effect of operation on NDI, VAS for neck pain and VAS for arm pain scales. For other comparisons t tests were used. Homogenities of variances were assessed using Levene’s tests. Level of statistical significance was determined as p = 0.05.
Results
Patient characteristics
During the study period, 54 consecutive patients underwent surgery for CDDD with ProdiscC™ implantation. The mean age of all patients was 45.3 years (range 30–60 years). There were 27 women and 27 men. Motor deficit was present in 17 patients (31%), sensory in 18 patients (33%) and reflex abnormality was seen in 26 patients (48%). A single-level surgery was performed in 44 patients (81%), double-level in 9 (17%) and triple-level in 1 patient (2%). The intervertebral disc C3/4 was replaced in 2 (3%), C4/5 in 6 (10%), C5/6 in 38 (59%) and C6/7 in 19 (28%) operated levels. Altogether, 65 ProdiscC™ prostheses were implanted. All patients underwent radiological follow-up at 6-month, 1- and 2-year follow-up, however, 4-year follow-up was only available in 50 patients (93%) with 60 implants (92%). Thus, four patients (five implants) did not attend the final visit. Basic patient and surgical data are summarized in Table 1.
Table 1.
Basic patient characteristics
| Number of patients | 54 |
| Women:Men | 27:27 |
| Mean age (range), years | 45.3 (30–60) |
| Motor deficit | 17 (31%) |
| Sensory deficit | 18 (33%) |
| Reflex abnormality | 26 (48%) |
| Single-level surgery | 44 (81%) |
| Two-level surgery | 9 (17%) |
| Three levels | 1 (2%) |
| Level operated | |
| C3/4 | 2 (3%) |
| C4/5 | 6 (10%) |
| C5/6 | 38 (59%) |
| C6/7 | 19 (28%) |
| Total number of implants | 65 |
| Follow-up (patients/implants) | |
| 6 months | 54/65 |
| 1 year | 54/65 |
| 2 years | 54/65 |
| 4 years | 50/60 |
Neurological status
Among 17 patients presenting a preoperative motor deficit, gradual improvement up to full-strength was noted in ten during follow-up, while the remaining seven patients experienced only a limited improvement. One patient suffered late deterioration due to newly diagnosed amyotrophic lateral sclerosis. One temporary C5 root paresis occurred, probably due to prolonged shoulder traction, and resolving by 3 months.
Among 18 patients with sensory deficit, gradual improvement up to normal was noted during follow-up visits in 13 with only limited improvement in the remaining 5. Out of 26 patients, 22 improved their initial reflex abnormality by their final follow-up check.
Neck disability and visual analog scores
ANOVA results for 50 cases with completed follow-up revealed significant overall effect of operation in improving results on all three scales used for clinical evaluation. The mean preoperative NDI was 34.5% [standard deviation (SD) = 19.6%]. The mean preoperative VAS for neck pain intensity was 4.6 (SD = 3.0) and for arm pain intensity 5.0 (SD = 3.2) on a 10-point visual scale.
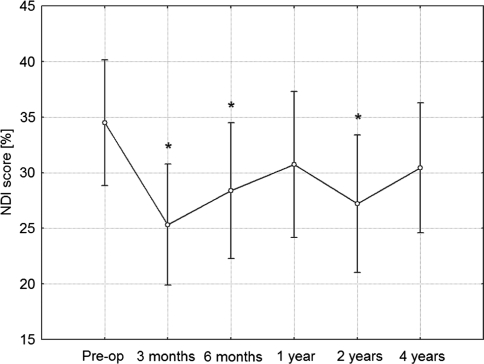
Mean NDI was 25.3% (SD = 18.9%) at 3 months, 28.4% (SD = 21.3%) at 6 months, 30.7% (SD = 22.9%) at 1 year, 27.2% (SD = 21.5%) at 2 years, and finally 30.4% (SD = 20.3%) at 4 years. In comparison to preoperative values using post hoc Fisher LSD test, the improvement was statistically significant at 3 months (p < 0.001), 6 months (p = 0.015) and 2 years (p = 0.004), however, this difference was not significant at 1 year and 4 years (Fig. 6).
Fig. 6.
Neck Disability Index (NDI) scores during investigated time period. Vertical bars denote 0.95 confidence intervals for means. Significant effect of operation in improvement of results was revealed using ANOVA. Post hoc analyses revealed significant difference from preoperative value at 3 months, 6 months and 2 years follow-up controls. *Significant difference from preoperative value
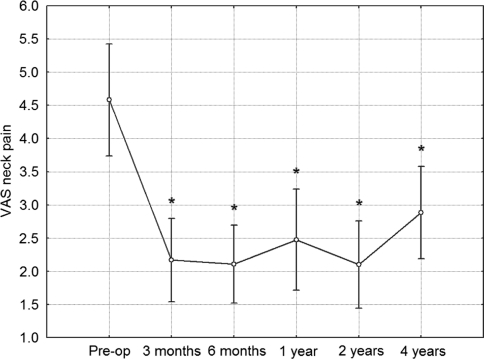
Mean VAS for neck pain was 2.2 (SD = 2.2) at 3 months, 2.1 (SD = 2.1) at 6 months, 2.5 (SD = 2.7) at 1 year, 2.1 (SD = 2.3) at 2 years and finally 2.9 (SD = 2.4) at 4 years. In post hoc comparison to preoperative values, the VAS improvement was highly statistically significant at all follow-up observations (p < 0.001 in all cases) (Fig. 7).
Fig. 7.
VAS score for neck pain intensity during investigated time period. Vertical bars denote 0.95 confidence intervals for means. Significant effect of operation in improvement of results was revealed using ANOVA. Post hoc analyses revealed significant difference from preoperative value at all follow-up controls. *Significant difference from preoperative value
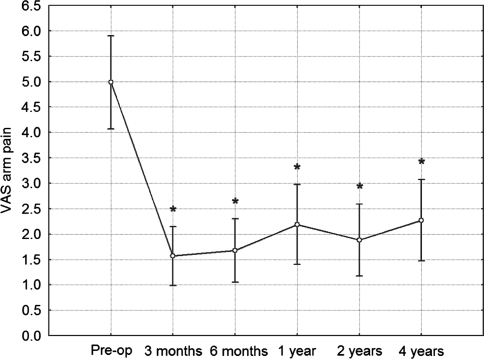
Mean VAS for arm pain intensity was 1.6 (SD = 2.0) at 3 months, 1.7 (SD = 2.2) at 6 months, 2.2 (SD = 2.8) at 1 year, 1.9 (SD = 2.5) at 2 years, and finally 2.3 (SD = 2.8) at 4 years. In post hoc comparison to preoperative values, the VAS improvement was highly statistically significant at all follow-up observations (p < 0.001 in all cases) (Fig. 8). At 4 years, 46 (92%) patients answered that they would undergo the same surgery again.
Fig. 8.
VAS score for arm pain intensity during investigated time period. Vertical bars denote 0.95 confidence intervals for means. Significant effect of operation in improvement of results was revealed using ANOVA. Post hoc analyses revealed significant difference from preoperative value at all follow-up controls. *Significant difference from preoperative value
Radiological evaluation
At 6-months follow-up, HO of grade 0–II was present in 59 (91%) of treated levels and significant HO (grade III) in another 6 (9%). Segmental fusion (grade IV) was not observed.
At 1-year follow-up, HO of grade 0–II was present in 50 (77%) of treated levels, important HO (grade III) in 10 (15%) and segmental ankylosis (grade IV) in another 5 (8%).
At 2-year follow-up, HO of grade 0–II was present in 44 (67%) of treated levels, important HO (grade III) in 9 (14%) and segmental ankylosis (grade IV) in another 12 (19%).
At 4-year follow-up (50 patients/60 implants), HO of grade 0–II was present in 22 (37%), important HO (grade III) in 27 (45%) and segmental ankylosis (grade IV) in 11 (18%) treated levels.
Development of HO is summarized in Table 2.
Table 2.
Development of heterotopic ossifications; operated segments (%)
| 6 months | 1 year | 2 years | 4 years | |
|---|---|---|---|---|
| Grade 0 | 30 (46) | 18 (28) | 14 (21) | 7 (12) |
| Grade I | 20 (31) | 10 (15) | 8 (12) | 8 (13) |
| Grade II | 9 (14) | 22 (34) | 22 (34) | 7 (12) |
| Grade 0–II | 59 (91) | 50 (77) | 44 (67) | 22 (37) |
| Grade III | 6 (9) | 10 (15) | 9 (14) | 27 (45) |
| Grade IV | 0 (0) | 5 (8) | 12 (19) | 11 (18) |
| Grade III–IV | 6 (9) | 15 (23) | 21 (33) | 38 (63) |
| Total | 65 (100) | 65 (100) | 65 (100) | 60a (100) |
aFour patients (five implants) were lost to 4-year follow-up
When divided according to presence of HO (groups HO grade 0–II vs. groups HO grade III–IV) at follow-up at 2 and 4 years, there is no statistically significant difference between mean improvement (difference between preoperative and postoperative score) on NDI and both VAS scales. Results are summarized in Table 3.
Table 3.
Comparison of mean improvement between groups divided according to HO development at 2- and 4-year follow-up
| Follow-up | Scale | Mean improvement Group HO 0–II ± SD |
Mean improvement Group HO III–IV ± SD |
t test p value |
|---|---|---|---|---|
| 2-years, N = 54 | NDI [%] | 8.7 ± 18.1 | 4.5 ± 26.2 | 0.50 |
| VAS neck | 2.9 ± 2.9 | 1.2 ± 3.4 | 0.07 | |
| VAS arm | 3.1 ± 3.6 | 2.9 ± 3.5 | 0.88 | |
| 4-years, N = 50 | NDI [%] | 5.3 ± 16.9 | 4.2 ± 23.7 | 0.86 |
| VAS neck | 2.4 ± 2.4 | 1.2 ± 3.6 | 0.19 | |
| VAS arm | 2.2 ± 3.5 | 3.0 ± 3.5 | 0.42 |
Surgery and surgical complications
The mean operative time and blood loss were 82 min (range 35–200 min) and 81 ml (range 5–310 ml), respectively. Mean length of hospital stay was 3.7 days (range 1–7 days).
Splitting of vertebral bodies occurred on two occasions. Both were caused by chiseling of keel grooves in double-level procedures C5/6–C6/7 with the split of C6 body being discovered intraoperatively. In the first patient, the split resulted in the lower ProdiscC™ implant being unstable and therefore a C6/7 hybrid fusion was performed. In the second case, both ProdiscC™ implants held in place steadily and no surgery modification was needed. Hard cervical collar was prescribed for 6 weeks without additional consequences. No other complications (infection, implant migration/subsidence or vocal paresis) were encountered and no surgical revisions were necessary.
Discussion
Overall results of CTDR implantations are very good and promising [13, 23, 26, 28]. Patients are usually rapidly mobilized without major restrictions and the clinical results are, in general, comparable to or better than the control groups treated with ACDF. Furthermore, revision rates and secondary procedures for ASD are less frequent when motion preservation techniques are utilized [22, 23, 27].
The clinical results in our series of 54 consecutive patients treated with ProdiscC™ are similar. The visual analog scores for intensity of neck and arm pain show statistically significant improvement during the entire follow-up period. The neurologic status has also improved. The insignificant improvement in NDI scores 1 and 4 years after surgery is slightly surprising. This phenomenon could be explained by low mean entry NDI score (34.5%). This probably reflects a patient population presenting predominantly with radicular symptoms or neurological deficit rather than neck pain.
We observed two cases of split vertebral bodies caused by chiseling during prosthesis keel groove cutting in double-level procedures. This complication was solved without clinical consequences. Sagittal splits [8] as well as avulsion fracture of posterior vertebral wall caused by chiseling [29] were described in literature as an exceptional but possible specific complication of ProdiscC™ implantation.
The fundamental point in the decision process whether to use a more sophisticated, but also more expensive, mobile implant is whether motion in the affected segment is to prevail in the long-term and thus prevent the adjacent segment overload with all known consequences.
The frequency of significant HO (grade III and IV) in our cohort of CTDR patients appears higher than we would expect from published series with a 2-year follow-up [3, 22, 23].
Fusion as a result of CTDR was initially described only rarely and often as a case report [2, 25]. Certain publications focus on description of very good clinical results and the occurrence of HO or limited implant motion is either not mentioned or not analyzed [5, 27]. Commonly, in large series, individual patient data are not available and the motion follow-up analysis is summarized in average or mean values [22, 23, 26]. A further point of contention is the fact that the segmental movement greater than 2° is considered as sufficient motion preservation in most published series [13, 19]. This value is difficult to measure even with digitalization of radiographic data and inclusion of those with 2° of segmental motion in the group with preserved mobility is questionable not only in our opinion [23]. Certain series describe a surprisingly low rate of HO. Pimenta et al. [26] described only one case of HO in his series of 229 PCM implantations with follow-up longer than 1 year. Mummaneni et al. [22] report only a single case of HO in a randomized controlled multicenter trial (IDE study) with a 2 year follow-up. Unfortunately, only 65% of patients underwent radiographic evaluation at the time of publication. Murrey et al. [23], in ProdiscC™ final IDE study, reported 2.9% of bridging bone at the operated level after 2 years, but no other types of HO were analyzed.
Bertagnoli et al. [5] reported results of 27 ProdiscC™ patients with 1 year follow-up without any appearance of fusion. Later, the same author [4] observed a 9.4% incidence of HO among 117 patients treated with ProdiscC™ and followed-up for more than 2 years. He proposed a new classification system and emphasized the clinical irrelevance of HO appearance.
Contrary to previously mentioned works, Sola et al. [31] presented a 60% fusion rate 5 years after implantation of Bryan™ disc. Our previously reported series [21] with ProdiscC™ implants demonstrated that only 33.8% of patients did not show any sign of HO at 1 year. There were 49.4% of the patients with HO of Class II and III and the fusion rate was 9.1%.
There are only a few reports focusing on frequency of HO and its influence on segmental motion.
Leung et al. [19], in his analysis of first multicenter series of Bryan™ disc implantations, described a 17.8% overall rate of HO occurrence with 6.7% being grade II or III at 1 year follow-up evaluation of 90 out of 100 available patients with single-level procedures. In 11% of treated patients segmental motion was less than 2°. The authors noticed a strong association between HO occurrence and subsequent loss of movement of the implanted disc. Furthermore, they found male sex and older age as risks factors for the development of HO. There are many theories as to what is the main reason for HO formation after CTDR. In our opinion, this process is multifactorial.
Surgical indication for CTDR is one of the most important factors. Natural aging of the disc ultimately leads to fusion. It makes sense that the further the natural fusion process progresses, the lesser time it will take to fuse, even in a segment with temporarily increased motion through CTDR. Although, our surgical indication criteria have not changed prospectively during the learning curve, approximately halfway in our series, we were more likely to include patients with less pronounced bony degenerative changes and excluded those with chronic kyphosis and those with preoperative flexion–extension motion of less than 4°. There appeared to be a decreased incidence of HO in the latter half of our cohort of patients.
Another important issue may be the surgical bone work during CTDR procedure. Excessive drilling and opening of bone canals during chiseling could result in new bone formation [19]. Some currently available prostheses require extensive bone work during preparation of the endplate due to a non-physiological shape of the contact surface. Usually, bony degenerative changes (osteophytes, uncinate hypertrophy, etc.,) require more extensive bony work to achieve sufficient neural decompression, therefore making “hard disc” disease a contraindication to motion preservation technology.
In an effort to prevent HO formation, many orthopedic surgeons recommend to use non-steroidal anti-inflammatory medications during the first 14 days after total hip or knee replacement [6, 18]. However, there is a lack of evidence that NSAIDs can prevent HO formation in CTDR.
The quality, intensity and periodicity of rehabilitation could be of relative importance in retention of segmental motion in the early postoperative period. The design of prostheses may also play an important role. Motion characteristics of each individual design are important. The extent of end-plate coverage, necessity to adapt the vertebral endplate shape to prosthesis, inadequate position of the center of rotation or motion characteristics that are too constrictive or too mobile to replicate the natural disc movement. These are some of the questions raised during evaluation of CTDR technology and HO occurrence.
Chronic segmental kyphosis cannot be corrected by any type of mobile prosthesis. Kyphotic position of the implant will lead to abnormal motion pattern, chronic irritation of surrounding soft structures and subsequent HO formation. The natural, genetically given speed of disc degeneration is another point of interest that may be hindering our motion preservation efforts.
Some authors [4, 17] conclude that HO is not important in the absence of clinical deterioration. In our opinion, it certainly creates a strong argument against the theory of adjacent segment protection. A fused implant in an incorrect position (e.g., kyphosis) will likely result in overload of the adjacent segment, probably to a greater degree than ACDF segment.
Conclusion
Our clinical results (NDI, VAS) are comparable with fusion techniques. Despite using advanced non-fusion technology, an unpredictable frequency of spontaneous fusion (in our view up to 20–30%) can be expected during long-term follow-up. Although our results show no evidence of new morbidity connected with spontaneous fusion, the expected benefit of motion preservation may be less than initially anticipated. The verdict on adjacent segment protection with motion preservation is still to be determined. Only a careful, objective and continuous audit of clinical results will lead to answers regarding CTDR technology.
Acknowledgment
This work was presented in part at the Eurospine 2009 meeting in Warsaw, October 21–24.
Conflict of interest statement None.
References
- 1.Baba H, Furusawa N, Imura S, Kawahara N, Tsuchiya H, Tomita K. Late radiographic findings after anterior cervical fusion for spondylotic myeloradiculopathy. Spine (Phila Pa 1976) 1993;18:2167–2173. doi: 10.1097/00007632-199311000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Bartels RH, Donk R. Fusion around cervical disc prosthesis: case report. Neurosurgery. 2005;57:E194. doi: 10.1227/01.NEU.0000163419.59635.78. [DOI] [PubMed] [Google Scholar]
- 3.Beaurain J, Bernard P, Dufour T, Fuentes JM, Hovorka I, Huppert J, Steib JP, Vital JM, Aubourg L, Vila T. Intermediate clinical and radiological results of cervical TDR (Mobi-C) with up to 2 years of follow-up. Eur Spine J. 2009;18:841–850. doi: 10.1007/s00586-009-1017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertagnoli R. Heterotopic ossification at the index level after Prodisc-C surgery: what is the clinical relevance? Spine J. 2008;8:123S. doi: 10.1016/j.spinee.2008.06.687. [DOI] [Google Scholar]
- 5.Bertagnoli R, Duggal N, Pickett GE, Wigfield CC, Gill SS, Karg A, Voigt S. Cervical total disc replacement, part two: clinical results. Orthop Clin North Am. 2005;36:355–362. doi: 10.1016/j.ocl.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Brooker AF, Bowerman JW, Robinson RA, Riley LH., Jr Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Joint Surg Am. 1973;55:1629–1632. [PubMed] [Google Scholar]
- 7.Cloward RB. The treatment of ruptured lumbar intervertebral disc by vertebral body fusion. III. Method of use of banked bone. Ann Surg. 1952;136:987–992. doi: 10.1097/00000658-195212000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datta JC, Janssen ME, Beckham R, Ponce C. Sagittal split fractures in multilevel cervical arthroplasty using a keeled prosthesis. J Spinal Disord Tech. 2007;20:89–92. doi: 10.1097/01.bsd.0000211258.90378.10. [DOI] [PubMed] [Google Scholar]
- 9.DiAngelo DJ, Roberston JT, Metcalf NH, McVay BJ, Davis RC. Biomechanical testing of an artificial cervical joint and an anterior cervical plate. J Spinal Disord Tech. 2003;16:314–323. doi: 10.1097/00024720-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Dmitriev AE, Cunningham BW, Hu N, Sell G, Vigna F, McAfee PC. Adjacent level intradiscal pressure and segmental kinematics following a cervical total disc arthroplasty: an in vitro human cadaveric model. Spine (Phila Pa 1976) 2005;30:1165–1172. doi: 10.1097/01.brs.0000162441.23824.95. [DOI] [PubMed] [Google Scholar]
- 11.Doran SE, Walsh J, Kinkaid A, Cutler D (2003) Prospective analysis of dysphagia following anterior cervical spine fusion. In: 31st annual meeting of the Cervical Spine Research Society. Scottsdale, Arizona, December 11–13, 2003
- 12.Eck JC, Humphreys SC, Lim TH, Jeong ST, Kim JG, Hodges SD, An HS. Biomechanical study on the effect of cervical spine fusion on adjacent-level intradiscal pressure and segmental motion. Spine (Phila Pa 1976) 2002;27:2431–2434. doi: 10.1097/00007632-200211150-00003. [DOI] [PubMed] [Google Scholar]
- 13.Goffin J, Calenbergh F, Loon J, Casey A, Kehr P, Liebig K, Lind B, Logroscino C, Sgrambiglia R, Pointillart V. Intermediate follow-up after treatment of degenerative disc disease with the Bryan Cervical Disc Prosthesis: single-level and bi-level. Spine (Phila Pa 1976) 2003;28:2673–2678. doi: 10.1097/01.BRS.0000099392.90849.AA. [DOI] [PubMed] [Google Scholar]
- 14.Goffin J, Loon J, Calenbergh F, Plets C. Long-term results after anterior cervical fusion and osteosynthetic stabilization for fractures and/or dislocations of the cervical spine. J Spinal Disord. 1995;8:500–508. doi: 10.1097/00002517-199512000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519–528. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Hilibrand AS, Yoo JU, Carlson GD, Bohlman HH. The success of anterior cervical arthrodesis adjacent to a previous fusion. Spine (Phila Pa 1976) 1997;22:1574–1579. doi: 10.1097/00007632-199707150-00009. [DOI] [PubMed] [Google Scholar]
- 17.Janssen M, Goldstein J, Murrey D, Delamarter R. Heterotopic ossification at the index level after Prodisc-C: what is the clinical significance? Spine J. 2007;7:48S–49S. doi: 10.1016/j.spinee.2007.07.117. [DOI] [Google Scholar]
- 18.Kjaersgaard-Andersen P, Schmidt SA. Total hip arthroplasty. The role of antiinflammatory medications in the prevention of heterotopic ossification. Clin Orthop Relat Res. 1991;263:78–86. [PubMed] [Google Scholar]
- 19.Leung C, Casey AT, Goffin J, Kehr P, Liebig K, Lind B, Logroscino C, Pointillart V. Clinical significance of heterotopic ossification in cervical disc replacement: a prospective multicenter clinical trial. Neurosurgery. 2005;57:759–763. doi: 10.1227/01.NEU.0000175856.31210.58. [DOI] [PubMed] [Google Scholar]
- 20.McAfee PC, Cunningham BW, Devine J, Williams E, Yu-Yahiro J. Classification of heterotopic ossification (HO) in artificial disk replacement. J Spinal Disord Tech. 2003;16:384–389. doi: 10.1097/00024720-200308000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Mehren C, Suchomel P, Grochulla F, Barsa P, Sourkova P, Hradil J, Korge A, Mayer HM. Heterotopic ossification in total cervical artificial disc replacement. Spine (Phila Pa 1976) 2006;31:2802–2806. doi: 10.1097/01.brs.0000245852.70594.d5. [DOI] [PubMed] [Google Scholar]
- 22.Mummaneni PV, Burkus JK, Haid RW, Traynelis VC, Zdeblick TA. Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: a randomized controlled clinical trial. J Neurosurg Spine. 2007;6:198–209. doi: 10.3171/spi.2007.6.3.198. [DOI] [PubMed] [Google Scholar]
- 23.Murrey D, Janssen M, Delamarter R, Goldstein J, Zigler J, Tay B, Darden B. Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. Spine J. 2009;9:275–286. doi: 10.1016/j.spinee.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Nabhan A, Ahlhelm F, Pitzen T, Steudel WI, Jung J, Shariat K, Steimer O, Bachelier F, Pape D. Disc replacement using Pro-Disc C versus fusion: a prospective randomised and controlled radiographic and clinical study. Eur Spine J. 2007;16:423–430. doi: 10.1007/s00586-006-0226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkinson JF, Sekhon LH. Cervical arthroplasty complicated by delayed spontaneous fusion. Case report. J Neurosurg Spine. 2005;2:377–380. doi: 10.3171/spi.2005.2.3.0377. [DOI] [PubMed] [Google Scholar]
- 26.Pimenta L, McAfee PC, Cappuccino A, Bellera FP, Link HD. Clinical experience with the new artificial cervical PCM (Cervitech) disc. Spine J. 2004;4:315S–321S. doi: 10.1016/j.spinee.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 27.Robertson JT, Papadopoulos SM, Traynelis VC. Assessment of adjacent-segment disease in patients treated with cervical fusion or arthroplasty: a prospective 2-year study. J Neurosurg Spine. 2005;3:417–423. doi: 10.3171/spi.2005.3.6.0417. [DOI] [PubMed] [Google Scholar]
- 28.Sasso RC, Smucker JD, Hacker RJ, Heller JG. Clinical outcomes of BRYAN cervical disc arthroplasty: a prospective, randomized, controlled, multicenter trial with 24-month follow-up. J Spinal Disord Tech. 2007;20:481–491. doi: 10.1097/BSD.0b013e3180310534. [DOI] [PubMed] [Google Scholar]
- 29.Shim CS, Shin HD, Lee SH. Posterior avulsion fracture at adjacent vertebral body during cervical disc replacement with ProDisc-C: a case report. J Spinal Disord Tech. 2007;20:468–472. doi: 10.1097/BSD.0b013e31803b95db. [DOI] [PubMed] [Google Scholar]
- 30.Smith GW, Robinson RA. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am. 1958;40-A:607–624. [PubMed] [Google Scholar]
- 31.Sola S, Hebecker R, Mann S (2008) Bryan cervical disc prosthesis: 5 years follow-up. Motion preservation technology 8th annual meeting. Miami, Florida, May 6–9, 2008
- 32.Weinhoffer SL, Guyer RD, Herbert M, Griffith SL. Intradiscal pressure measurements above an instrumented fusion. A cadaveric study. Spine (Phila Pa 1976) 1995;20:526–531. doi: 10.1097/00007632-199503010-00004. [DOI] [PubMed] [Google Scholar]