Abstract
We set out to determine whether a minimally invasive approach for one-level instrumented posterior lumbar interbody fusion reduced undesirable changes in the multifidus muscle, compared to a conventional open approach. We also investigated associations between muscle injury during surgery (creatinine kinase levels), clinical outcome and changes in the multifidus at follow-up. We studied 59 patients treated by one team of surgeons at a single institution (minimally invasive approach in 28 and conventional open approach in 31, voluntarily chosen by patients). More than 1 year postoperatively, all the patients were followed up with the visual analogue scale (VAS) and Oswestry disability index (ODI), and 16 patients from each group were evaluated using MRI. This enabled the cross-sectional area (CSA) of lean multifidus muscle, and the T2 signal intensity ratio of multifidus to psoas muscle, to be compared at the operative and adjacent levels. The minimally invasive group had less postoperative back pain (P < 0.001) and lower postoperative ODI scores (P = 0.001). Multifidus atrophy was less in the minimally invasive group (P < 0.001), with mean reductions in CSA of 12.2% at the operative and 8.5% at the adjacent levels, compared to 36.8% and 29.3% in the conventional open group. The increase in the multifidus:psoas T2 signal intensity ratio was similarly less marked in the minimally invasive group where values increased by 10.6% at the operative and 8.3% at the adjacent levels, compared to 34.4 and 22.7% in the conventional open group (P < 0.001). These changes in multifidus CSA and T2 signal intensity ratio were significantly correlated with postoperative creatinine kinase levels, VAS scores and ODI scores (P < 0.01). The minimally invasive approach caused less change in multifidus, less postoperative back pain and functional disability than conventional open approach. Muscle damage during surgery was significantly correlated with long-term multifidus muscle atrophy and fatty infiltration. Furthermore these degenerative changes of multifidus were also significantly correlated with long-term clinical outcome.
Keywords: Minimally invasive, Lumbar spine, Multifidus muscle, Interbody fusion
Introduction
Conventional open techniques for instrumented posterior lumbar interbody fusion (PLIF) are still widely accepted methods for the management of a variety of spinal disorders which need spinal stabilization. However, the approach-related morbidity resulting from iatrogenic muscle and soft tissue injury has become an increasing concern for many surgeons. The long incisions, extensive detachment of muscle from the spinal processes, and subsequently prolonged wide retraction can result in ischemic necrosis and denervation of the paraspinal musculature [24, 27]. Spinous processes, supraspinal and interspinal ligaments are all resected for decompression, severely compromising the architecture of the spinal posterior column. The paraspinal muscles are physiologically abnormal following operation due to scar healing and denervation, [24] and there is evidence that both the resection and the changes in the paraspinal muscles may lead to decreased trunk muscle strength, [12, 16] late onset of spinal instability, [21] and potentially to severe back pain or “failed back syndrome” [18, 24].
In order to reduce the iatrogenic muscle injury and the approach-related morbidity, minimally invasive techniques have been developed. However, the concept of “minimally invasive” means not only short incisions, but also less extensive soft tissue injury and maximum therapeutic result [3, 15]. In 1986, Leu and Hauser [14] developed a biportal percutaneous endoscopic approach to interbody fusion which was carried out through a 7.5-mm diameter cannula inserted into the disc space via a posterolateral percutaneous approach. Based on such approaches, Foley and Smith [4] developed a tubular retractor system, which was initially applied to the treatment of herniated lumbar discs and lateral recess stenosis in 1994. Later, with modifications of the tubular retractor system, this approach was utilized for posterior lumbar interbody fusion (PLIF), transforaminal LIF, and posterolateral LF. Preliminary results suggested that the minimally invasive approach is superior to the conventional open approach in terms of reduced intramuscular pressure and paraspinal muscle edema, [25] less blood loss, [8, 11, 20, 22] lower serum creatinine kinase (CK) and inflammatory cytokine levels, [13] and less postoperative back pain [20, 22]. Particularly, all of the midline supporting musculo-ligamentous structures are left intact with this technique. But there is still no long-term evaluation of undesirable changes in the multifidus muscle and their potential effects. So, this study was designed to determine whether a minimally invasive approach for one-level instrumented PLIF reduces changes in the multifidus, compared to a conventional open approach. We also investigated associations between muscle injury during surgery (assessed by CK level), clinical outcome, and changes in the composition of multifidus at follow-up.
Materials and methods
Patient population
From March 2006 to August 2007, we studied a consecutive series of 59 patients who underwent one-level instrumented PLIF using two different approaches: a minimally invasive (MI) approach in 28 and a conventional open (CO) approach in 31. These procedures were carried out by one team of surgeons at our institution. All patients consented to participate in the study. They complained mainly of low back pain and varying degrees of radiating pain and neurologic symptoms and underwent conservative therapy for at least 6 months before surgery. Indications for surgery were segmental instability at the level of spinal stenosis, huge lumbar disc herniation, or low-grade spondylolisthesis confirmed by anteroposterior, lateral, oblique, and flexion–extension plain radiographs, CT scans, and MRIs. During preoperative consultation, we informed the patients of the theoretical superiority of the MI approach, but that it was an innovative technique, and the long-term results needed to be justified. Each patient voluntarily chose the method to be used. Every patient had preoperative MRI to measure the cross-sectional area (CSA) and the T2-weighted signal intensity of the multifidus muscle. The visual analogue scale (VAS) score and Oswestry disability index (ODI) were evaluated 3 days preoperatively. The patients’ demographic characteristics and procedure data of the two groups are listed in Table 1. All patients were followed up (mean 14.4 months in the MI group, 14.2 months in the CO group, P > 0.05). At the final follow-up, 16 patients in each group consented to have MRI examinations. The mean period of follow-up for these 32 patients was not significantly different from the entire cohort (14.0 months in the MI group, 13.5 months in the CO group).
Table 1.
Patient demographic and clinical outcome data
| MI group | CO group | P | |
|---|---|---|---|
| No. of patients | 28 | 31 | |
| Age (years) | 53.3 ± 9.9 | 53.0 ± 11.6 | 0.920 |
| Gender [M/F (%male)] | 10/18 (35.7) | 16/15 (51.6) | 0.219 |
| Preoperative diagnosis [no. (%) of patients] | 0.862 | ||
| Isthmic spondylolisthesis | 7 (25.0) | 6 (19.4) | |
| Degenerative spondylolisthesis | 12 (42.9) | 15 (48.4) | |
| Lumbar disc herniation | 3 (10.7) | 2 (6.5) | |
| Spinal stenosis with instability | 6 (21.4) | 8 (25.8) | |
| Height (cm) | 164.1 ± 6.9 | 164.2 ± 7.4 | 0.934 |
| Weight (kg) | 68.3 ± 8.3 | 66.5 ± 8.3 | 0.408 |
| BMI | 25.6 ± 3.1 | 24.6 ± 1.8 | 0.108 |
| No. (%) of patients with obesity (BMI of ≥25) | 14 (50.0) | 10 (32.3) | 0.166 |
| No. (%) of preoperative ASA score (class1/2/3/4) | 10 (36)/18 (64)/0/0 | 14 (45)/17 (55)/0/0 | 0.461 |
| Level of fusion [no. (%) of patients] | 0.495 | ||
| L3–L4 | 1 | 0 | |
| L4–L5 | 20 | 21 | |
| L5–S1 | 7 | 10 | |
| Average time of last follow up [months (range)] | 14.4 (12–16) | 14.2 (12–17) | 0.480 |
| VAS score | |||
| Preoperative | 7.2 ± 1.5 | 6.6 ± 1.8 | 0.233 |
| Postoperative 6 months | 1.7 ± 0.4 | 3.1 ± 0.5 | <0.001 |
| Last follow-up | 1.8 ± 0.6 | 3.4 ± 0.5 | <0.001 |
| ODI score | |||
| Pre-operative | 69.2 ± 17.6 | 69.2 ± 17.7 | 0.988 |
| Postoperative 6 months | 15.9 ± 4.2 | 25.7 ± 5.5 | <0.001 |
| Last follow-up | 10.7 ± 4.0 | 21.2 ± 6.4 | <0.001 |
BMI body mass index = weight (kg)/height (m2)
ASA American Society of Anesthesiologists, a preoperative risk score based on the presence of co-morbidities at the time of surgery
ANOVA was used to identify mean differences between groups at each time of measurement for VAS and ODI
Surgical techniques
After induction of general anesthesia, the patients were positioned prone on a radiolucent table. Lateral and anteroposterior C-arm fluoroscopic images were obtained to ensure the body surface positions of two pairs of pedicles bilaterally at the operative level, and the ipsilateral pedicles were marked with a straight line. Two paramedian skin incisions about 2.5 cm long were made 3.0 cm lateral to the midline according to previous positioning. Two K-wires were used to penetrate the multifidus muscles bilaterally, after which serial dilators were used to create two muscle-sparing surgical corridors. Bilateral appropriate-length 22-mm-diameter METRx (Medtronic Sofamor Danek, Memphis, TN) tubular retractors were docked on the facet joint complex. Pedicle screw instrumentation was performed first. Then bilateral hemilaminectomies and medial facetectomies were performed under microscopic visualization. Adequate decompression was achieved by partially cutting the lamina, and hypertrophied superior and inferior articular processes, while the lateral recess or nerve root canal was also decompressed if necessary. The ligamentum flavum was resected and the nerve roots were retracted medially. A complete discectomy was performed, after which the disc space was sequentially distracted. The endplates were then prepared for fusion. The anterior disc space was packed with autologous bone graft, then, interbody cages packed with autograft were placed. Once the interbody fusion had been finished, the tubular retractors were removed and pedicle rod instrumentation was placed through the same incisions. Compression was applied to the instrumentation before final tightening, providing compression of the bone graft to guarantee a stable anchor and to recreate lordosis.
In the CO group, after one skin incision about 12 cm long was made on the midline, paravertebral muscles were dissected from the bony structures in the standard fashion. After complete bony exposure, pedicle screw instrumentation was performed first, then laminectomies and medial facetectomies were performed. The remaining steps were the same as those used for the MI approach.
Clinical assessment
Data concerning operative and clinical parameters were collected prospectively for comparison. Operative measures were: length of operation time, intraoperative and postoperative estimated blood loss, total blood replacement, duration of postoperative hospital stay, and time to ambulation after surgery. For clinical outcome assessment, the VAS score was determined for back pain, along with ODI evaluation, in which the section about sexual life was deleted for cultural reasons, so the score was calculated as: ODI score = total scored/45 (total possible score) × 100% [2]. A questionnaire introduced by Kim et al. [12] was modified for the last follow-up. Patients were asked to provide their opinion regarding the outcome of the surgery by answering two questions. The Satisfaction question was “How satisfied were you with your operation?” The answers were categorized as: 1, very unsatisfactory; 2, unsatisfactory; 3, fair; 4, satisfactory; 5, very satisfactory. The Recommendation question was “Would you recommend the same operation to a family member for the same problem?” The answers were categorized as: 1, definitely no; 2, probably no; 3, neither yes nor no; 4, probably yes; 5, definitely yes. A satisfactory outcome was defined as a score of 4 or 5 on each question.
Evaluation of back muscle injury
The CK level was measured one day preoperatively, and on days 1, 3, 5, and 7 postoperatively. The CK activity was determined with a Synchron Clinical System LX20 (Beckman Coulter, USA).
MRI was performed on a 1.5 Tesla System (Siemens, Germany) preoperatively and at the final follow-up, more than 1 year postoperatively. All images were obtained using a T2-weighted fast spin echo pulse sequence, with matrix size 255 × 512, field of view 240 × 240 mm, bandwidth 120 Hz/Px, and echo factor 15. Slice thickness was 4 mm and interslice gap was 1 mm. Patients were placed supine with a pillow positioned underneath the knees, ensuring that they were lying symmetrically with weight evenly distributed across both sides. The experienced musculoskeletal radiologists who took the MRIs were blinded to the operation method. They used anatomic markers and locating lines on sagittal plane scans to select the most similar preoperative and follow-up axial images, at the same spinal level, for comparison.
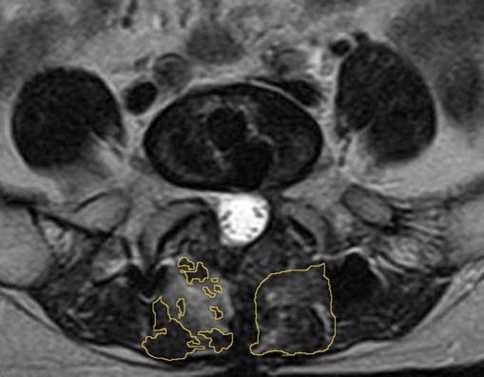
Measurements were obtained with a picture archiving and communication system, or PACS, workstation (Jin YeXiang, Beijing, China) and embedded region of interest (ROI) and grayscale histogram software. To determine the lean multifidus muscle CSA, the ROI was drawn around the multifidus muscles bilaterally, taking care to avoid nearby fat, bony structures, and other soft tissues (Fig. 1, left). The sum of CSAs of bilateral lean multifidus was calculated. To determine the mean signal intensity of multifidus, the ROI was drawn around the outer perimeter of the muscle unilaterally, to include any areas of intramuscular fat (Fig. 1, right). Mean signal intensity of unilateral gross multifidus muscle on a T2-weighted axial image was evaluated quantitatively by the grayscale histogram software of PACS, in which a higher score means higher signal intensity. The mean signal intensity of psoas muscle in the same axial image was also evaluated as control from a 100 mm2 circular ROI placed in the center of the muscle. The signal intensity ratio of gross multifidus to psoas was calculated.
Fig. 1.
Measurement of cross-sectional area of multifidus muscle in a highly atrophied muscle (left). Lean muscle CSA was the sum of all the areas outlined in yellow. The ROI outlined for T2 signal intensity of gross multifidus muscle is shown on the right
Three experienced musculoskeletal radiologists blinded to the operation method analyzed two selected axial images (preoperative and follow-up) at the operative and adjacent levels (superior and inferior to the operative level) by measurements repeated after two weeks with the same protocol. Inter- and intra-observer repeatability were calculated using an intra-class correlation coefficient, ICC(3, 1), formula [23]. ICCs for inter- and intra- observer repeatability of the CSA, and the T2-weighted signal intensity ratio ranged from 0.89 to 0.96. Because the ICCs indicated good intra- and inter-observer reliability, the mean of the readings was used.
Data analysis
Data from the 32 patients with MRI follow-up were analyzed separately. The associations between the postoperative CK level (on the first day postoperatively, when it was highest) and changes in multifidus (percentage changes in CSA and T2 signal intensity ratio of all the bilateral six or four masses of the muscles both at the operative and adjacent levels) were analyzed. The associations between multifidus muscle measurements and the clinical effects represented by VAS and ODI scores were analyzed as well.
Statistical assessments
Student’s t test was used to make comparisons between groups for continuous variables measured at a discrete time point (e.g. operative measures or physical characteristics such as age, height etc.). Dichotomous values were compared with the χ2 contingency table. Fisher’s exact test was used to determine the differences between the groups for the Satisfaction and Recommendation questions.
Repeated-measures analysis of variance (ANOVA) was applied to test for the effects of group, time and group × time interaction for VAS and ODI scores, and to determine differences among the three time points in each group. ANOVA was used to identify mean differences between groups at each time of measurement for VAS and ODI. Changes in the CSA of lean multifidus and T2 signal intensity ratio of gross multifidus to psoas were compared with the Mann–Whitney U test. Spearman’s test for correlation was used to assess associations of postoperative CK levels, VAS scores and ODI scores with the percentage changes in both multifidus CSA and the signal intensity ratio.
In all analyses, a P value of <0.05 was considered significant. All tests were performed with SPSS 13.0 software.
Ethical considerations
The Ethical Committee of our institution approved the study.
Results
Clinical results for entire cohort (n = 59)
There were no statistically significant differences in the demographic data or preoperative VAS and ODI scores between the MI and CO groups (Table 1). All details of the perioperative data are listed in Table 2.
Table 2.
Perioperative data (mean ± SD)
| Variable | MI Group | CO Group | P |
|---|---|---|---|
| Intraoperative blood loss (mL) | 496.4 ± 217.2 | 887.7 ± 555.3 | 0.001 |
| Postoperative drainage (mL) | 146.7 ± 61.9 | 344.3 ± 114.0 | <0.001 |
| Total blood replacement (U) | 0.43 ± 0.8 | 1.16 ± 1.3 | 0.015 |
| Time to ambulation (days) | 3.8 ± 0.8 | 13.4 ± 5.7 | <0.001 |
| Length of hospital stay (days) | 9.5 ± 2.0 | 15.2 ± 3.4 | <0.001 |
| Surgical time (min) | 203.6 ± 36.6 | 194.5 ± 47.2 | 0.417 |
All patients were available for the 6-month postoperative and final follow-up assessments using VAS and ODI, and interactions were found between group and time (P < 0.001), while differences occurred between the groups and among the time points (P < 0.001). After the data were split by group, the VAS and ODI scores at 6 months after surgery were markedly lower than the preoperative scores (P < 0.001), but did not differ from the scores at the final follow-up. At the 6-month and the last follow-ups, the scores were significantly lower in the MI group than in the CO group (Table 1).
In response to the satisfaction question, a satisfactory outcome was achieved in 89.3% of the MI group and 61.3% of the CO group (P = 0.018). A satisfactory outcome of the Recommendation question was achieved in 89.3% of the MI group and 54.8% of the CO group (P = 0.004) (Table 3).
Table 3.
Answers to satisfaction and recommendation questions (revised from the questionnaire introduced by Kim [12])
| Answer number | Satisfaction questiona | Recommendation questionb | ||
|---|---|---|---|---|
| MI Group | CO Group | MI Group | CO Group | |
| 1 | 0 | 2 | 0 | 2 |
| 2 | 1 | 4 | 0 | 6 |
| 3 | 2 | 6 | 3 | 6 |
| 4 | 10 | 7 | 8 | 8 |
| 5 | 15 | 12 | 17 | 9 |
aHow satisfied were you with your operation?
bWould you recommend the same operation to your family member for the same problem?
The number of patients answering either 4 or 5 to each question was greater in the MI Group (P = 0.018 for satisfaction question, and P = 0.004 for recommendation question)
Creatinine kinase levels in entire cohort (n = 59)
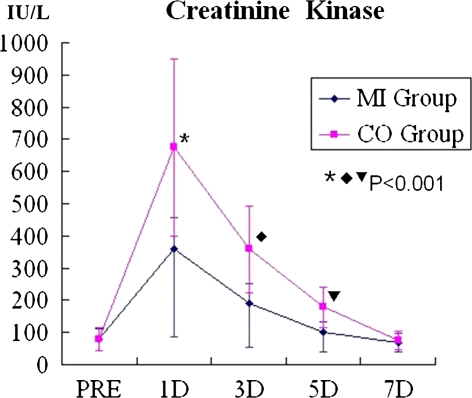
The mean CK level was higher in the CO group than in the MI group on days 1, 3, and 5 postoperatively (P < 0.001). The levels peaked on day 1 and in both groups returned to baseline by 7 days (Fig. 2).
Fig. 2.
Serum creatinine kinase (CK) concentrations for both groups. CK levels were significantly lower in the MI group than the CO group at 1, 3 and 5 days after surgery. CK concentrations of both groups returned to baseline after 7 days
Follow-up group (n = 32)
At the last follow-up, 16 patients in the MI group and 16 in the CO group had MRI. Analyses showed no significant differences between patients with MRI follow-up and those without in terms of VAS scores, ODI scores and CK levels, except for CK levels on day 5 postoperatively (data not shown). The differences in VAS and ODI scores of these 32 patients were similar to those in the population of all patients (Table 4). When gender was taken into account as a covariate, there were no interactions between VAS, ODI and gender (P > 0.05, repeated-measures ANOVA).
Table 4.
Patients with MRI follow-up: demographic and clinical outcome data
| MI group | CO group | P | |
|---|---|---|---|
| No. of patients | 16 | 16 | |
| Age (years) | 53.6 ± 8.6 | 55.0 ± 10.9 | 0.695 |
| Gender [M/F (%male)] | 5/11 (31.3) | 10/6 (62.5) | 0.077 |
| Preoperative diagnosis [no. (%) of patients] | 0.865 | ||
| Isthmic spondylolisthesis | 1 (6.3) | 2 (12.5) | |
| Degenerative spondylolisthesis | 8 (50.0) | 9 (56.3) | |
| Lumbar disc herniation | 3 (18.8) | 2 (12.5) | |
| Spinal stenosis with instability | 4 (25.0) | 3 (18.8) | |
| Height (cm) | 164.7 ± 7.1 | 166.1 ± 7.3 | 0.576 |
| Weight (kg) | 67.5 ± 9.2 | 66.9 ± 7.8 | 0.837 |
| BMI | 25.3 ± 3.0 | 24.1 ± 1.3 | 0.173 |
| No. (%) of patients with obesity (BMI of ≥ 25) | 5 (31) | 4 (25) | 0.694 |
| No. (%) of preoperative ASA score (class 1/2/3/4) | 6 (38)/10 (62)/0/0 | 7 (44)/9 (56)/0/0 | 0.719 |
| Level of fusion [no. (%) of patients] | 0.085 | ||
| L3–L4 | 1 | 0 | |
| L4–L5 | 9 | 12 | |
| L5–S1 | 6 | 4 | |
| Time of follow up [months (range)] | 14.0 (12–15) | 13.5 (12–16) | 0.219 |
| VAS Score | |||
| Preoperative | 7.1 ± 1.5 | 6.5 ± 1.9 | 0.395 |
| Postoperative 6 months | 1.6 ± 0.4 | 3.2 ± 0.6 | <0.001 |
| Last follow-up | 1.7 ± 0.6 | 3.5 ± 0.6 | <0.001 |
| ODI Score | |||
| Pre-operative | 68.5 ± 19.1 | 67.1 ± 21.6 | 0.849 |
| Postoperative 6 months | 14.3 ± 3.2 | 27.2 ± 5.7 | <0.001 |
| Last follow-up | 9.7 ± 3.2 | 23.1 ± 6.4 | <0.001 |
BMI body mass index = weight (kg)/height (m)2
ASA American Society of Anesthesiologists, a preoperative risk score based on the presence of co-morbidities at the time of surgery
ANOVA was used to identify mean differences between groups at each time of measurement for VAS and ODI. Repeated-measures ANOVA analysis showed interaction between group and time (P < 0.001), and significant differences between the groups and among the time points
Lean multifidus muscle cross-sectional area in follow-up group (n = 32)
The CSA of lean multifidus muscle at the operative and adjacent levels had decreased at final follow-up in both MI and CO groups (Table 5; since the L5-S1 level has no adjacent level below, there were 28 adjacent levels in the CO group and 26 in the MI group). The percentage changes were greater in the CO group not only at the operative level, but also at adjacent levels (P < 0.001). When further analyses were run separately for the men and women in each group, the differences between groups were still evident (P < 0.001).
Table 5.
Multifidus muscle evaluation: atrophy and fatty infiltration
| CO Group | MI Group | P | |||||
|---|---|---|---|---|---|---|---|
| Preoperative | Follow-up | Change (%) | Preoperative | Follow-up | Change (%) | ||
| Operative level (no.) | 16 | 16 | |||||
| Mcsa (mm2) | 1162.4 ± 211.4 | 734.6 ± 207.6 | −36.8 ± 12.3 | 1058.6 ± 274.4 | 929.8 ± 254.0 | −12.2 ± 4.5 | <0.001 |
| T2 ratio | 2.11 ± 0.23 | 2.83 ± 0.37 | 34.4 ± 12.9 | 2.17 ± 0.34 | 2.40 ± 0.36 | 10.6 ± 4.3 | <0.001 |
| Adjacent level (no.) | 28 | 26 | |||||
| Mcsa (mm2) | 1087.1 ± 225.8 | 773.5 ± 253.0 | −29.3 ± 13.3 | 956.2 ± 258.8 | 874.9 ± 241.8 | −8.5 ± 3.9 | <0.001 |
| T2 ratio | 2.13 ± 0.26 | 2.62 ± 0.49 | 22.7 ± 17.4 | 2.10 ± 0.30 | 2.28 ± 0.32 | 8.3 ± 3.5 | <0.001 |
Mcsa: cross-sectional area of lean multifidus muscle. T2 ratio: T2-weighted signal intensity ratio of gross multifidus to psoas muscle. Statistical assessments for percentage change between two groups: Mann–Whitney U test
T2-weighted signal intensity of gross multifidus muscle in follow-up group (n = 32)
The signal intensity ratio of gross multifidus to psoas had increased at the operative and adjacent levels in both groups at final follow-up (Table 5). The percentage change in the ratio postoperatively was larger in the CO group, not only at the operative level, but also at adjacent levels (P < 0.001). When further analyses were run separately for the men and women in each group, the differences remained (P < 0.001).
Associations among CK levels, changes in multifidus muscle and clinical effects in follow-up group (n = 32)
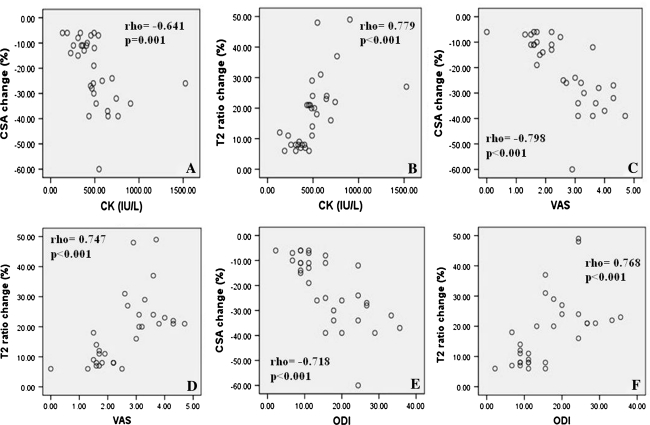
The serum level of CK on postoperative day 1 (the peak value) was significantly correlated with the percentage change in the CSA of all the measured multifidus (Fig. 3a), and with the percentage change in the T2 signal intensity ratio of multifidus to psoas (Fig. 3b). VAS score at final follow-up was significantly correlated with the percentage change in the CSA of all the measured multifidus (Fig. 3c), and with the percentage change in the ratio of multifidus to psoas (Fig. 3d). Similar correlations were found for the ODI scores at final follow-up (Fig. 3e, f).
Fig. 3.
Scatterplots showing associations among CK Level, VAS and ODI at the final follow-up, and changes of multifidus CSA and T2 signal intensity ratio of multifidus to psoas muscle. CK level versus CSA (a) and intensity ratio (b); VAS score versus CSA (c) and intensity ratio (d); and ODI score versus CSA (e) and intensity ratio (f). All the associations were significant
Discussion
Minimally invasive spinal surgery was developed to reduce the degree of iatrogenic muscle injury while still accomplishing the conventional goals of the open procedure. Preliminary results suggested that the MI posterior lumbar fusion procedures hold the promise of decreased iatrogenic muscle injury and approach-related morbidity, while allowing the surgeon to perform the operation as effectively as the conventional open surgery [8, 11, 13, 20, 22, 25]. However, there was no long-term evaluation of changes in multifidus muscle and their potential effects.
Similar to previous reports, [8, 11, 20, 22] we showed that the minimally invasive PLIF lessened the intra-operative blood loss, postoperative drainage, and the need for transfusion compared with conventional open PLIF. Moreover patients in the MI group began to walk 3.8 days postoperatively, much shorter than the 13.4 days in the CO group. Patients in the MI group were discharged 9.5 days postoperatively, but the average hospital stay in the CO group was 15.2 days. The comparatively long hospital stay for the ambulatory patients can be explained by their preference for discharge after the “stitch-out” procedure in China.
In the PLIF procedure, multifidus was the muscle most affected. Muscle injury during spinal surgery increases the serum concentration of CK, which is routinely used for muscle injury evaluation at the early postoperative stage [10, 13, 17]. CK activity increased after surgery, reached a maximum on day 1, and subsequently declined to the normal value by 7 days [10]. Similar to the study by Kim et al. [13] our results showed serum CK levels significantly lower in the MI group than in the CO group on postoperative days 1, 3 and 5. The long-term effects of muscle injury are assessed on MRI by a decrease in the muscle CSA and deposition of fat and connective tissue, which give high signal intensity in T2-weighted images in the advanced stages [12]. Previous studies have reported muscle swelling due to edema up to 10 months postoperatively [12, 26] indicating the need for a long-term follow-up in order to detect such chronic changes. Kawaguchi et al. [9, 10] determined that back muscle injury, in terms of elevated serum levels of creatine phosphokinase, is directly related to the retraction pressure, time, and extent of exposure. Gejo et al. [5] concluded that an increase in T2 signal intensity on serial MRIs is correlated with longer muscle retraction time. In the current study, the percentage change of lean multifidus muscle CSA and T2 signal intensity ratio of gross multifidus to psoas were significantly less in the MI group than in the CO group. These results, combined with those in the literature, [13, 25] confirm that the MI approach causes less muscle injury than the conventional open approach. Furthermore, we found significant correlations between the postoperative CK level on day 1 and the percentage change of lean multifidus muscle CSA or T2 signal intensity ratio of gross multifidus to psoas. These results confirm the hypothesis of Suwa et al. [26] that direct paraspinal muscle damage during lumbar surgery may be one of the most important causes of back muscle atrophy.
Many studies have highlighted the importance of the multifidus muscle in providing dynamic control [6, 7, 19, 28]. Wilke et al. [29] examined the effect of the simulated muscle force of multifidus on motion segment stiffness. Relative to sacrospinalis and psoas, multifidus contributed two-thirds of the increased stiffness imparted by the simulated contraction of these muscles. Therefore, wasting of this muscle would be expected to have direct effects on lumbar segmental stability, and thus predispose to further damage [1]. Chronic back pain is associated with more fatty infiltration in the muscles of patients operated for low back pain [6]. Patients suffering from severe postoperative failed back syndrome have dorsal ramus lesions in one or more segments covered by the scar and local paraspinal muscle atrophy at the corresponding segments [22]. In our patients, the VAS and ODI scores decreased in both groups postoperatively. However, the CO group showed higher scores than the MI group, suggesting that patients in the CO group experienced less pain relief and more inconvenience in daily life postoperatively. Furthermore, the percentage changes in multifidus CSA and T2 signal intensity ratio were positively correlated with VAS and ODI scores at the last follow-up, which confirmed that less multifidus atrophy and fatty infiltration are associated with less back pain and functional disability. However, after lumbar posterior surgery, whether multifidus atrophy and fatty infiltration are the causes of back pain and functional disability or vice versa, need to be further investigated.
Whether the new operative approach is better than the widely accepted conventional approach should be decided not only by doctors but also by patients, so, we used the satisfaction and recommendation questions to compare the MI and CO approaches as perceived by patients. Satisfactory outcomes were reported more often in the MI group. Two patients in the CO group had satisfactory outcomes in the Satisfaction question compared to their preoperative severe symptoms, but they had unsatisfactory outcomes in the Recommendation question because of postoperative moderate back pain and functional disability. However, the satisfactory values for “satisfaction” and “recommendation” for the CO procedure for one-level PLIF were much lower than the outcome data from similar studies reported in the literature [12, 20].
This study had limitations. (1) The sample size was small, although comparable with other similar studies [12, 25]. (2) The study was not randomized because of our preoperative consultation “MI approach theoretically better but unproven in the long-term”, which might have made some patients think the CO approach more trustworthy and attract more optimistic and positive individuals to the MI group. (3) While intra-class correlation coefficients indicated good intra- and inter- observer reliability for the method of measuring CSA and T2 signal intensity, there was a potential for error in constructing irregular line type circles around the outer perimeters of lean and gross multifidus muscle (Fig. 1). (4) Only some patients were willing to undergo MRI scans for cultural reasons (e.g. some thought MRI examinations may be harmful, some could not wait a few days to take examinations because of the busy queue, and some even thought the free MRI examinations may influence their limited health insurance). However, the demographic variables of the patients who had MRI were not statistically different between the two groups (Table 4).
Conclusion
The MI approach induced less multifidus muscle damage in terms of lower CK levels in the early postoperative period, less change in CSA of lean multifidus and less change in T2 signal intensity ratio of gross multifidus to psoas in the long-term evaluation. For the clinical outcomes, the MI approach was associated with less back pain and functional disability, and might therefore be preferred by patients.
Acknowledgments
Sponsored by Zhejiang Provincial Program for the cultivation of High-level Innovative Health talents. The Institutional ethical board of this university has reviewed this study and approved this report contributed to the Eur Spine J.
References
- 1.Danneels LA, Vanderstraeten GG, Cambier DC, Witvrouw EE, Cuyper HJ. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J. 2000;9:266–272. doi: 10.1007/s005860000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25(22):2940–2952. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 3.Fitzpatrick JM, Wickham JE. Minimal invasive surgery. Br J Surg. 1990;77:721–722. doi: 10.1002/bjs.1800770702. [DOI] [PubMed] [Google Scholar]
- 4.Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine. 2003;28(Suppl 15):S26–S35. doi: 10.1097/00007632-200308011-00006. [DOI] [PubMed] [Google Scholar]
- 5.Gejo R, Matsui H, Kawaguchi Y, Ishihara H, Tsuji H. Serial changes in trunk muscle performance after posterior lumbar surgery. Spine. 1997;24:1023–1028. doi: 10.1097/00007632-199905150-00017. [DOI] [PubMed] [Google Scholar]
- 6.Hides JA, Richardson CA, Jull GA. Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine. 1996;21(23):2763–2769. doi: 10.1097/00007632-199612010-00011. [DOI] [PubMed] [Google Scholar]
- 7.Hides JA, Stokes MJ, Saide M, Jull GA, Cooper DH. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine. 1994;19(2):165–172. doi: 10.1097/00007632-199401001-00009. [DOI] [PubMed] [Google Scholar]
- 8.Isaacs RE, Podichetty VK, Santiago P, Sandhu FA, Spears J, Kelly K, et al. Minimally invasive microendoscopy-assisted transforaminal lumbar interbody fusion with instrumentation. J Neurosurg Spine. 2005;3:98–105. doi: 10.3171/spi.2005.3.2.0098. [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. A histologic and enzymatic analysis. Spine. 1996;21(8):941–944. doi: 10.1097/00007632-199604150-00007. [DOI] [PubMed] [Google Scholar]
- 10.Kawaguchi Y, Matsui H, Tsuji H. Changes in serum creatine phosphokinase MM isoenzyme after lumbar spine surgery. Spine. 1997;22(9):1018–1023. doi: 10.1097/00007632-199705010-00015. [DOI] [PubMed] [Google Scholar]
- 11.Khoo LT, Palmer S, Laich DT, Fessler RG. Minimally invasive percutaneous posterior lumbar interbody fusion. Neurosurgery. 2002;51:S166-1. [PubMed] [Google Scholar]
- 12.Kim DY, Lee SH, Chung SK, Lee HY. Comparison of multifidus muscle atrophy and trunk extension muscle strength: percutaneous versus open pedicle screw fixation. Spine. 2005;30:123–129. doi: 10.1097/01.brs.0000157172.00635.3a. [DOI] [PubMed] [Google Scholar]
- 13.Kim KT, Lee SH, Suk KS, Bae SC. The quantitative analysis of tissue injury markers after mini-open lumbar fusion. Spine. 2006;31:712–716. doi: 10.1097/01.brs.0000202533.05906.ea. [DOI] [PubMed] [Google Scholar]
- 14.Leu HF, Hauser RK. Percutaneous endoscopic lumbar spine fusion. Neurosurg Clin North Am. 1996;7(1):107–117. [PubMed] [Google Scholar]
- 15.Mayer HM. The ALIF concept. Eur Spine J. 2000;9(Suppl):35–43. doi: 10.1007/PL00010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer TG, Vanharanta H, Gatchel RJ, Mooney V, Barnes D, Judge L. Comparison of CT scan muscle measurements and isokinetic trunk strength in postoperative patients. Spine. 1989;14(1):33–36. doi: 10.1097/00007632-198901000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Nanji AA. Serum creatine kinase isoenzymes: a review. Muscle Nerve. 1983;6:83–90. doi: 10.1002/mus.880060203. [DOI] [PubMed] [Google Scholar]
- 18.Onesti ST. Failed back syndrome. Neurologist. 2004;10(5):259–264. doi: 10.1097/01.nrl.0000138733.09406.39. [DOI] [PubMed] [Google Scholar]
- 19.Panjabi MM. The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. J Spinal Disord. 1992;5(4):383–389. doi: 10.1097/00002517-199212000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Park Y, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine. 2007;32(5):537–543. doi: 10.1097/01.brs.0000256473.49791.f4. [DOI] [PubMed] [Google Scholar]
- 21.Quint U, Wilke HJ, Shirazi-Adl A, Parnianpour M, Löer F, Claes LE. Importance of the intersegmental trunk muscles for the stability of the lumbar spine: a biomechanical study in vitro. Spine. 1998;23(18):1937–1945. doi: 10.1097/00007632-199809150-00003. [DOI] [PubMed] [Google Scholar]
- 22.Schwender JD, Holly LT, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech. 2005;18(Suppl):S1–S6. doi: 10.1097/01.bsd.0000132291.50455.d0. [DOI] [PubMed] [Google Scholar]
- 23.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428. doi: 10.1037/0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 24.Sihvonen T, Herno A, Paljärvi L, Airaksinen O, Partanen J, Tapaninaho A. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine. 1993;18:575–581. doi: 10.1097/00007632-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Stevens KJ, Spenciner DB, Griffiths KL, Kim KD, Zwienenberg-Lee M, Alamin T, et al. Comparison of minimally invasive and conventional open posterolateral lumbar fusion using magnetic resonance imaging and retraction pressure studies. J Spinal Disord Tech. 2006;19:77–86. doi: 10.1097/01.bsd.0000193820.42522.d9. [DOI] [PubMed] [Google Scholar]
- 26.Suwa H, Hanakita J, Ohshita N, Gotoh K, Matsuoka N, Morizane A. Postoperative changes in paraspinal muscle thickness after various lumbar back surgery procedures. Neurol Med Chir (Tokyo) 2000;40(3):151–154. doi: 10.2176/nmc.40.151. [DOI] [PubMed] [Google Scholar]
- 27.Taylor H, McGregor AH, Medhi-Zadeh S, Richards S, Kahn N, Zadeh JA, et al. The impact of self-retaining retractors on the paraspinal muscles during posterior spinal surgery. Spine. 2002;27:2758–2762. doi: 10.1097/00007632-200212150-00004. [DOI] [PubMed] [Google Scholar]
- 28.Waddell G. A new clinical model for the treatment of low-back pain. Spine. 1987;12:632–644. doi: 10.1097/00007632-198709000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Wilke HJ, Wolf S, Claes LE, Arand M, Wiesend A. Stability increase of the lumbar spine with different muscle groups. A biomechanical in vitro study. Spine. 1995;20:192–198. doi: 10.1097/00007632-199501150-00011. [DOI] [PubMed] [Google Scholar]