Abstract
The objective of the study was to investigate how patients with sciatica due to disc herniation rate the bothersomeness of paresthesia and weakness as compared to leg pain, and how these symptoms are associated with socio-demographic and clinical characteristics. A cross-sectional study was conducted on 411 patients with clinical signs of radiculopathy. Items from the Sciatica Bothersomeness Index (0 = none to 6 = extremely) were used to establish values for paresthesia, weakness and leg pain. Associations with socio-demographic and clinical variables were analyzed by multiple linear regression. Mean scores (SD) were 4.5 (1.5) for leg pain, 3.4 (1.8) for paresthesia and 2.6 (2.0) for weakness. Women reported higher levels of bothersomeness for all three symptoms with mean scores approximately 10% higher than men. In the multivariate models, more severe symptoms were associated with lower physical function and higher emotional distress. Muscular paresis explained 19% of the variability in self-reported weakness, sensory findings explained 10% of the variability in paresthesia, and straight leg raising test explained 9% of the variability in leg pain. In addition to leg pain, paresthesia and weakness should be assessed when measuring symptom severity in sciatica.
Keywords: Sciatica, Disc herniation, Lumbosacral radicular syndrome, Neuromuscular manifestations, Pain measurement
Introduction
The 1-year incidence of sciatica, defined as radicular pain below the knee, is estimated at 1–2% [43], with a point prevalence of approximately 2–5% [15, 21]. Disc herniation is the most common cause of sciatica. Herniated discs may cause mechanical compression and inflammation of the nearby nerve roots and dorsal root ganglia [20, 24, 33, 34, 36, 42], which may result in radicular pain, sensory disturbances, muscular weakness and back pain. Some patients also experience bladder, bowel and genital dysfunction. The condition can vary from short-lasting, single episodes to a remitting or permanent course over months or years. Socioeconomic costs are high, mainly due to work absenteeism [14, 15, 43].
Traditionally, leg pain, and to some extent back pain, have been utilized to rate symptom severity in sciatica. We have not found any data describing how sciatica patients rate the severity of sensory disturbances and muscular weakness.
The introduction of the Sciatica Bothersomeness Index (SBI) [26] provides an opportunity to investigate patient perceptions of these symptoms using a standardized methodology. The index includes self-reported ratings of symptom intensity of (1) leg pain, (2) numbness or tingling in the leg, foot or groin, (3) weakness in the leg/foot, and (4) back or leg pain while sitting (See “Appendix”). A composite score can be calculated by summing up the ratings across the four symptom scales. The SBI has been used in several studies [3, 27, 40], and three of the items have been incorporated in the North American Spine Society outcome instrument [8]. To date, results generated from the SBI have only been published as composite scores, resulting in a dearth of data for individual items such as numbness/tingling (paresthesia) and weakness.
The Sciatica Frequency Index is a questionnaire in which patients rate the frequency of the SBI items. We have previously shown that patients largely fail to distinguish between sciatica bothersomeness and frequency [12]. The term “bothersomeness” has been increasingly utilized to measure symptoms, not only in sciatica and low back pain [10, 17], but also in a broad spectrum of conditions such as prostatism [4], migraine [7], sinusitis [2] and pneumonia [22]. Therefore, in the present study, the SBI was chosen as the most appropriate instrument to assess how patients perceive their sciatica symptoms.
The objectives of the study were (1) to investigate how patients with sciatica due to disc herniation rate the bothersomeness of paresthesia and weakness as compared to leg pain, and (2) to investigate how these symptoms are associated with socio-demographic and clinical characteristics.
Patients and methods
Setting
This study was part of an observational longitudinal study of patients with sciatica and disc herniation referred to back clinics at four hospitals in southeastern Norway (Sykehuset Østfold, Sørlandet Sykehus, Ullevaal Universitetssykehus, and Sykehuset Innlandet). Data presented in this paper were cross-sectional and obtained at baseline.
Patients were invited to participate by the clinic staff. Study participation did not involve any specific type of intervention; patients received treatment as usual at each center. The protocol was approved by the Regional Committee for Medical Research Ethics and The Ombudsman for Privacy in Research at the Norwegian Social Science Data Services.
Patients
Included patients were 18 years of age or older, had radiating pain or paresis below the knee and a lumbar disc herniation verified by MRI or CT at the corresponding level and side. Exclusion criteria were pregnancy, spinal fracture, tumor, infection, previous surgery in the affected disc or inability to communicate in written Norwegian. A total of 466 patients were enrolled in the observational study. To ensure that the reported symptoms were related to the herniated disc, 55 patients without clinical signs of radiculopathy were excluded from these analyses. Thus, 411 patients with at least one abnormal clinical test result for straight leg raising test (SLR), sensibility, reflexes or muscle strength were included in this study.
The patients’ mean age was 43.7 years, and 41.8% were women. At the time of enrollment, 37 patients (9.0%) were hospitalized, whereas the majority were outpatients. A total of 46% of patients reported a first episode of sciatica, 29% had experienced one or two prior episodes and 25% reported three or more previous episodes. Median duration of the current sciatica episode was 14 weeks for men and 17 weeks for women.
Procedure and measurements
The validated Norwegian version of the SBI [12] was used. Each symptom item is rated on a scale from 0 to 6, with anchors at 0 (not bothersome), 3 (somewhat bothersome) and 6 (extremely bothersome). Patients were instructed to rate the severity of symptoms that occurred during the past week.
Level of education was recorded as the total number of years completed at school. Duration of the current sciatica episode was measured in weeks, and participants were categorized as smokers (daily or occasionally) or non-smokers. Work status was dichotomized as either “working full time” or “not working full time.”
Emotional distress (symptoms of anxiety, depression and somatization) was measured by the Hopkins Symptom Checklist (HSCL-25) [9, 16]. Each item includes four response categories ranging from “not at all = 1” to “extremely = 4”. The total score was calculated as the sum of the score divided by the number of the items answered.
Additionally, the generic health status questionnaire SF-36 [39] items for general health and physical functioning were used. Each domain is scored from 0 (poor health) to 100 (optimal health).
A clinical examination was conducted by a physician or physiotherapist and included: SLR (deemed abnormal if <60°), sensibility (abnormal if reduced light touch), reflexes (abnormal if depressed patellar or Achilles), and muscular paresis (present if one of the following were abnormal: single limb stance, tiptoe or heel walking, supine knee or ankle flexion/extension or big toe extension).
Statistical analyses
Gender differences for socio-demographic characteristics, sciatica history, clinical findings, emotional distress, items of SF-36 and symptoms at baseline were tested with Mann–Whitney U test, t tests, and χ2 tests. Bivariate associations were analyzed between the SBI items, which were categorized as low (score 0–1), medium (score 2–4) or high (score 5–6), and the socio-demographic variables (age, gender, work status, length of education/school, smoking status, duration of current sciatica episode), level of emotional distress (HSCL-25), general health, physical functioning and clinical findings (SLR, sensibility, muscular paresis and reflexes). Statistical significance was determined by χ2 for trend (linear by linear association). Multivariate analyses were carried out by multiple linear regression to estimate the effects of these variables on each of the reported symptoms. The assumptions underlying linear regression analysis, i.e., collinearity, normality, and linearity were adequately met. When assessing how large percentage of the variability of a dependent variable was explained by one independent variable, a linear regression including only this independent variable was performed. The level of statistical significance was set at 5%. All data analyses were performed using SPSS version 14.0 (SPSS, Inc., Chicago, IL).
Results
Mean bothersomeness scores (SD) for the total cohort were 4.5 (1.5) for leg pain, 3.4 (1.8) for paresthesia and 2.6 (2.0) for weakness. Corresponding mean scores (SD) for the subgroup of hospitalized patients were 5.4 (1.0), 4.0 (1.9) and 3.5 (2.2), respectively. Patient characteristics according to gender are presented in Table 1. Men generally reported lower symptom scores than women. On average, men’s leg pain scores were 10% lower (P < 0.01), paresthesia scores were 11% lower (P < 0.01), and weakness scores were 11% lower (P = 0.23) than women’s scores.
Table 1.
Patient characteristics and sciatica symptom bothersomeness scores by gender
| Men | Women | P | |
|---|---|---|---|
| (n = 239) | (n = 172) | ||
| Age years, mean (SD) | 43.8 (10.9) | 43.6 (12.0) | 0.85 |
| Education years, mean (SD) | 13.0 (2.9) | 12.9 (3.0) | 0.81 |
| Work status, n (%) | |||
| Working full time | 54 (22.6%) | 25 (14.5%) | 0.12 |
| Partly sick leave | 24 (10.0%) | 20 (11.6%) | |
| Sick leave, disability pension, others | 161 (67.4%) | 127 (73.8%) | |
| Current smoker, n (%) | 114 (42.2%) | 86 (42.7%) | 0.92 |
| Duration of current episode, n (%) | |||
| 0–3 months | 116 (48.5%) | 63 (37.1%) | 0.01 |
| 4–6 months | 74 (31.0%) | 58 (34.1%) | |
| >6 months | 49 (20.5%) | 49 (28.8%) | |
| Clinical findings, n (%) | |||
| Positive straight leg raising test | 148 (62.4%) | 119 (69.6%) | 0.13 |
| Sensory impairment | 159 (66.5%) | 114 (66.3%) | 0.96 |
| Muscular paresis | 125 (53.0%) | 78 (45.9%) | 0.16 |
| Reflexes depressed | 129 (54.9%) | 84 (49.1%) | 0.25 |
| Hopkins symptom checklist-25 (1–4) | 1.49 (0.34) | 1.69 (0.50) | <0.01 |
| SF-36 scale (0–100)a | |||
| General health | 71.9 (18.6) | 65.5 (22.2) | <0.01 |
| Physical functioning | 50.2 (25.8) | 45.4 (24.9) | 0.06 |
| Sciatica bothersomeness (0–6) | |||
| Leg pain | 4.3 (1.6) | 4.8 (1.3) | <0.01 |
| Paresthesia (numbness and tingling) | 3.2 (1.7) | 3.6 (1.8) | <0.01 |
| Weakness | 2.5 (1.9) | 2.8 (2.1) | 0.23 |
Group values are given as means (SD) if not otherwise stated
aHigher values indicate better health
Women reported significantly longer duration of the current sciatica episode, higher emotional distress and lower general health, compared to men. No significant gender differences were found for the other socio-demographic and clinical variables.
Table 2 shows the bivariate associations between symptoms (leg pain, paresthesia and weakness) and the socio-demographic, psychological and clinical variables. Results demonstrated that patients with higher levels of emotional distress, lower levels of physical functioning and general health reported significantly more paresthesia, weakness and leg pain. Work status was also significantly related to all three symptoms.
Table 2.
Association between self-reported bothersomeness and socio-demographic/clinical characteristics
| Paresthesia | Weakness | Leg pain | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Medium | High | P* | Low | Medium | High | P* | Low | Medium | High | P* | |
| All patients (n = 411) | 18.9 | 48.9 | 32.2 | 35.1 | 43.2 | 21.6 | 5.4 | 33.3 | 61.4 | |||
| Socio-demographic characteristics | ||||||||||||
| Age > median (41.8 years) | 15.2 | 50.5 | 34.3 | 0.09 | 32.2 | 42.4 | 25.4 | 0.07 | 5.3 | 34.5 | 60.2 | 0.70 |
| Female sex | 16.4 | 43.3 | 40.4 | 0.01 | 33.9 | 39.8 | 26.3 | 0.17 | 2.9 | 29.7 | 67.4 | 0.01 |
| Smokers | 14.7 | 48.8 | 36.5 | 0.04 | 35.7 | 43.3 | 21.1 | 0.82 | 2.9 | 32.2 | 64.9 | 0.07 |
| Education >12 years | 20.3 | 51.3 | 28.4 | 0.13 | 35.9 | 44.4 | 19.7 | 0.67 | 8.1 | 34.3 | 57.6 | 0.03 |
| Working full time | 31.6 | 51.9 | 16.5 | <0.01 | 50.6 | 36.7 | 12.7 | <0.01 | 12.7 | 39.2 | 48.1 | <0.01 |
| Duration of current episode >12 weeks | 18.8 | 49.0 | 32.2 | 0.98 | 39.2 | 42.7 | 18.1 | <0.01 | 5.4 | 32.2 | 62.5 | 0.63 |
| Emotional distress | ||||||||||||
| HSCL-25 >1.75 | 9.4 | 49.1 | 41.5 | <0.01 | 27.6 | 42.9 | 29.5 | 0.01 | 3.8 | 18.9 | 77.4 | <0.01 |
| SF-36 | ||||||||||||
| General health < median (72) | 11.3 | 54.6 | 34.0 | 0.01 | 28.9 | 43.3 | 27.8 | <0.01 | 3.1 | 32.3 | 64.6 | 0.07 |
| Physical functioning < median (50) | 10.7 | 48.2 | 41.1 | <0.01 | 24.4 | 43.7 | 32.0 | <0.01 | 2.0 | 19.2 | 78.8 | <0.01 |
| Clinical findings | ||||||||||||
| Abnormal straight leg raising test | 15.8 | 48.9 | 35.3 | 0.01 | 32.7 | 45.9 | 21.4 | 0.38 | 3.0 | 25.9 | 71.1 | <0.01 |
| Abnormal sensibility | 11.9 | 49.6 | 38.5 | <0.01 | 31.2 | 45.4 | 23.4 | 0.03 | 6.3 | 31.4 | 62.4 | 0.96 |
| Abnormal strength | 11.4 | 49.0 | 39.6 | <0.01 | 20.2 | 44.8 | 35.0 | <0.01 | 5.9 | 34.0 | 60.1 | 0.50 |
| Abnormal reflexes | 19.0 | 46.9 | 34.1 | 0.65 | 29.7 | 46.2 | 24.1 | 0.03 | 7.1 | 31.6 | 61.3 | 0.55 |
Results are presented as percent distribution of low (score 0–1), medium (score 2–4) and high (score 5–6) symptom scores for the total cohort and according to socio-demographic/clinical variables
* Determined by χ2 for trend (linear by linear association) among low, medium and high for paresthesia, weakness and leg pain, respectively
In the multivariate analyses (Table 3), the relatively strong bivariate associations between symptoms and gender and between symptoms and general health lost significance when emotional distress was entered into the three models. The associations between leg pain and work status and between weakness and work status lost significance when physical functioning was entered. Among the clinical tests, SLR remained significantly associated with leg pain and paresthesia, but not with weakness. Both sensibility and muscular paresis remained significantly associated with paresthesia and weakness, while reflex status was not significantly associated with any of the symptoms.
Table 3.
Estimated coefficients (β) with standard error (SE) from multiple linear regression models of sciatica symptoms by demographic and clinical characteristics
| Leg pain | Paresthesia | Weakness | ||||
|---|---|---|---|---|---|---|
| β | SE | β | SE | β | SE | |
| Age (+1 year) | −0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 |
| Sex (females vs. males) | 0.24 | 0.15 | 0.25 | 0.18 | 0.22 | 0.19 |
| Duration of current episode (+1 week) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Education (+1 year) | −0.04 | 0.03 | −0.01 | 0.03 | −0.01 | 0.03 |
| Working status (full time vs. not full time) | −0.28 | 0.18 | −0.52* | 0.22 | −0.39 | 0.24 |
| Smoking status (smoking vs. not smoking) | 0.22 | 0.14 | 0.19 | 0.18 | 0.03 | 0.19 |
| SF-36 General health (+1 unit) | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 |
| SF-36 Physical functioning (+1 unit) | −0.02** | 0.00 | −0.01* | 0.00 | −0.02** | 0.00 |
| HSCL-25 mean score (+1 unit) | 0.45* | 0.19 | 0.53* | 0.24 | 0.49 | 0.25 |
| SLR (abnormal vs. normal) | 0.63** | 0.15 | 0.42* | 0.19 | 0.07 | 0.20 |
| Sensibility (abnormal vs. normal) | −0.02 | 0.14 | 1.08** | 0.18 | 0.29 | 0.19 |
| Muscular strength (abnormal vs. normal) | −0.22 | 0.14 | 0.59** | 0.18 | 1.73** | 0.19 |
| Reflexes (abnormal vs. normal) | −0.22 | 0.14 | 0.02 | 0.17 | 0.17 | 0.18 |
Statistically significant β-coefficients are in bold
*P < 0.05; **P < 0.01
Muscular paresis explained 19% of the variability in self-reported weakness, sensory findings explained 10% of the variability in paresthesia, and SLR explained 9% of the variability in leg pain.
The correlation coefficients (Spearmans’ rho) were: 0.53 for weakness and paresthesia, 0.39 for paresthesia and leg pain and 0.26 for weakness and leg pain.
Discussion
To our knowledge, this study represents the first investigation of self-reported severity of paresthesia and weakness among patients with sciatica. Overall, leg pain was rated as the most bothersome symptom. Compared to leg pain, paresthesia was rated approximately 25% less bothersome, and weakness was rated about 40% less bothersome than leg pain. Women reported higher levels of bothersomeness for all three symptoms (i.e., leg pain, paresthesia and weakness), with mean scores approximately 10% higher than men. Regarding leg pain, our findings are in line with Peul et al. [28], and Hakkinen et al. [13]. Women’s higher symptom ratings can probably not be explained by patho-anatomical differences. An MRI study by Pfirrmann et al. [29] found nerve root compromise equally distributed between the sexes, and Jensen et al. [18] reported more nerve root compromise among men than in women. Our findings are in line with previous research showing that women show higher sensitivity to experimental pain [11, 31] and report higher pain intensity than men in various clinical conditions [32, 37]. Our results also indicate that women perceive paresthesia and weakness as more bothersome than men.
In the multivariate analyses, emotional distress was significantly associated with the bothersomeness of leg pain and paresthesia, but not formally significantly associated with weakness (P = 0.06). These results support previous evidence of illness and pain being associated with anxiety and depression [5, 6, 25, 38, 41]. Interestingly, the multivariate analyses indicated that the independent associations with emotional distress were almost equally strong for weakness and paresthesia as for leg pain.
Patients’ perceptions of their general health, assessed by the SF-36 general health item, were only modestly reduced compared to normative data from the general Norwegian population [23]. Female sciatica patients reported scores of about 15% lower and the male patients of 8% lower than the population reference. This finding supports Patrick’s suggestion [26] that patients may not consider a specific, circumscribed problem such as sciatica when making general health ratings. Furthermore, in the multivariate analyses, SF-36 general health was not significantly associated with any of the three symptoms.
It has previously been reported that sciatica patients who smoke have a higher risk of hospitalization [19] and undergo more operations for lumbar disc herniation than non-smokers [1]. The rate of daily smoking was 43% in the present study cohort compared with 25% in the adult Norwegian population at large in 2005 [30]. We did not find any independent role for smoking status on the bothersomeness of any of the symptoms in the multivariate models.
Our results indicate some uncertainty or ambiguity in patients’ interpretations of the meaning of numbness/tingling and weakness. Weakness and paresthesia correlated more strongly with each other than with leg pain. One possible explanation is that patients found it difficult to distinguish numbness/tingling from weakness. It is also possible that nerve fibers, which conduct weakness and numbness/tingling may be equally susceptible to disc-related injury. Both motor function and tactile/deep sensation are conducted by large myelinated fibers, which are known to be more vulnerable to compressive injury than smaller (“pain/thermal”) nerve fibers [35].
The strengths of this study are the large sample size, the strict inclusion criteria and the use of a validated questionnaire. Some limitations, however, should be taken into consideration when interpreting the results. First, our cohort was a sample of patients referred to the participating clinics and did not include all sciatica patients in need of secondary care in the target population. However, our patients closely resembled the Maine [3] and SPORT [40] studies with regard to demographic and clinical characteristics, but had somewhat less severe symptoms than in the randomized study by Peul et al. [27]. Second, because clinical tests were rated as normal or abnormal only, we were unable to fully assess whether patients with higher self-reported ratings of bothersomeness actually had more serious clinical findings. The sensory testing was performed by light touch, which may not provide the optimal clinical correlate of the sensation of numbness and tingling. To further explore the relations between subjective symptom ratings and raw clinical scores, a more detailed neurological examination, e.g., electrophysiological testing, should be performed. Third, this study used a cross-sectional design, which limits the ability to make causal inferences.
In conclusion, leg pain was rated as the most bothersome symptom, followed by paresthesia and weakness. Men reported lower symptom scores than women. Higher symptom scores were associated with higher emotional distress and lower physical function. In addition to leg pain, paresthesia and weakness should be assessed when measuring symptom severity in sciatica.
Acknowledgments
We thank Eli Molde Hagen, Dag Soldal, Knut Morten Huneide, Anett Bjørnødegård and Bjarte Justnæs for their help with the data collection and Leiv Sandvik for statistical advice.
Appendix: The Sciatica Bothersomeness Index
On a 0–6 point scale, please rate the following symptoms according to how bothersome they were in the past week.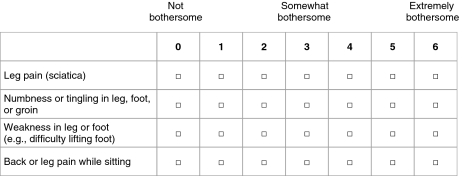
Footnotes
The study protocol was approved by the Regional Committee for Medical Research Ethics, Oslo (11 October 2004) and The Ombudsman for Privacy in Research at the Norwegian Social Science Data Services (2 March 2005).
References
- 1.An HS, Silveri CP, Simpson JM, File P, Simmons C, Simeone FA, Balderston RA. Comparison of smoking habits between patients with surgically confirmed herniated lumbar and cervical disc disease and controls. J Spinal Disord. 1994;7:369–373. doi: 10.1097/00002517-199410000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Atlas SJ, Gallagher PM, Wu YA, Singer DE, Gliklich RE, Metson RB, Fowler FJ., Jr Development and validation of a new health-related quality of life instrument for patients with sinusitis. Qual Life Res. 2005;14:1375–1386. doi: 10.1007/s11136-004-6674-7. [DOI] [PubMed] [Google Scholar]
- 3.Atlas SJ, Keller RB, Wu YA, Deyo RA, Singer DE. Long-term outcomes of surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: 10-year results from the Maine lumbar spine study. Spine. 2005;30:927–935. doi: 10.1097/01.brs.0000158954.68522.2a. [DOI] [PubMed] [Google Scholar]
- 4.Barry MJ, Fowler FJ, Jr, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT. The American urological association symptom index for benign prostatic hyperplasia. The measurement committee of the American urological association. J Urol. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 5.Buckelew SP, DeGood DE, Schwartz DP, Kerler RM. Cognitive and somatic item response pattern of pain patients, psychiatric patients, and hospital employees. J Clin Psychol. 1986;42:852–860. doi: 10.1002/1097-4679(198611)42:6<852::AID-JCLP2270420603>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 6.Cassem EH. Depression and anxiety secondary to medical illness. Psychiatr Clin North Am. 1990;13:597–612. [PubMed] [Google Scholar]
- 7.Cramer JA, Silberstein SD, Winner P. Development and validation of the Headache Needs Assessment (HANA) survey. Headache. 2001;41:402–409. doi: 10.1046/j.1526-4610.2001.111006402.x. [DOI] [PubMed] [Google Scholar]
- 8.Daltroy LH, Cats-Baril WL, Katz JN, Fossel AH, Liang MH. The North American Spine Society lumbar spine outcome assessment instrument: reliability and validity tests. Spine. 1996;21:741–749. doi: 10.1097/00007632-199603150-00017. [DOI] [PubMed] [Google Scholar]
- 9.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): a self-report symptom inventory. Behav Sci. 1974;19:1–15. doi: 10.1002/bs.3830190102. [DOI] [PubMed] [Google Scholar]
- 10.Dunn KM, Croft PR. Classification of low back pain in primary care: using “bothersomeness” to identify the most severe cases. Spine. 2005;30:1887–1892. doi: 10.1097/01.brs.0000173900.46863.02. [DOI] [PubMed] [Google Scholar]
- 11.Fillingim RB. Sex, gender, and pain: women and men really are different. Curr Rev Pain. 2000;4:24–30. doi: 10.1007/s11916-000-0006-6. [DOI] [PubMed] [Google Scholar]
- 12.Grovle L, Haugen AJ, Keller A, Natvig B, Brox JI, Grotle M. Reliability, validity, and responsiveness of the Norwegian versions of the Maine-Seattle back questionnaire and the sciatica bothersomeness and frequency indices. Spine. 2008;33:2347–2353. doi: 10.1097/BRS.0b013e31818047d6. [DOI] [PubMed] [Google Scholar]
- 13.Hakkinen A, Kautiainen H, Jarvenpaa S, Arkela-Kautiainen M, Ylinen J. Changes in the total Oswestry index and its ten items in females and males pre- and post-surgery for lumbar disc herniation: a 1-year follow-up. Eur Spine J. 2007;16:347–352. doi: 10.1007/s00586-006-0187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansson E, Hansson T. The cost-utility of lumbar disc herniation surgery. Eur Spine J. 2007;16:329–337. doi: 10.1007/s00586-006-0131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heliovaara M, Impivaara O, Sievers K, Melkas T, Knekt P, Korpi J, Aromaa A. Lumbar disc syndrome in Finland. J Epidemiol Commun Health. 1987;41:251–258. doi: 10.1136/jech.41.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hesbacher PT, Rickels K, Morris RJ, Newman H, Rosenfeld H. Psychiatric illness in family practice. J Clin Psychiatry. 1980;41:6–10. [PubMed] [Google Scholar]
- 17.Hill JC, Dunn KM, Lewis M, Mullis R, Main CJ, Foster NE, Hay EM. A primary care back pain screening tool: identifying patient subgroups for initial treatment. Arthritis Rheum. 2008;59:632–641. doi: 10.1002/art.23563. [DOI] [PubMed] [Google Scholar]
- 18.Jensen TS, Albert HB, Soerensen JS, Manniche C, Leboeuf-Yde C. Natural course of disc morphology in patients with sciatica: an MRI study using a standardized qualitative classification system. Spine. 2006;31:1605–1612. doi: 10.1097/01.brs.0000221992.77779.37. [DOI] [PubMed] [Google Scholar]
- 19.Kaila-Kangas L, Leino-Arjas P, Riihimaki H, Luukkonen R, Kirjonen J. Smoking and overweight as predictors of hospitalization for back disorders. Spine. 2003;28:1860–1868. doi: 10.1097/01.BRS.0000083284.47176.80. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi S, Baba H, Uchida K, Kokubo Y, Kubota C, Yamada S, Suzuki Y, Yoshizawa H. Effect of mechanical compression on the lumbar nerve root: localization and changes of intraradicular inflammatory cytokines, nitric oxide, and cyclooxygenase. Spine. 2005;30:1699–1705. doi: 10.1097/01.brs.0000171910.97937.0e. [DOI] [PubMed] [Google Scholar]
- 21.Konstantinou K, Dunn KM. Sciatica: review of epidemiological studies and prevalence estimates. Spine. 2008;33:2464–2472. doi: 10.1097/BRS.0b013e318183a4a2. [DOI] [PubMed] [Google Scholar]
- 22.Lamping DL, Schroter S, Marquis P, Marrel A, Duprat-Lomon I, Sagnier PP. The community-acquired pneumonia symptom questionnaire: a new, patient-based outcome measure to evaluate symptoms in patients with community-acquired pneumonia. Chest. 2002;122:920–929. doi: 10.1378/chest.122.3.920. [DOI] [PubMed] [Google Scholar]
- 23.Loge JH, Kaasa S. Short form 36 (SF-36) health survey: normative data from the general Norwegian population. Scand J Soc Med. 1998;26:250–258. [PubMed] [Google Scholar]
- 24.Olmarker K, Blomquist J, Stromberg J, Nannmark U, Thomsen P, Rydevik B. Inflammatogenic properties of nucleus pulposus. Spine. 1995;20:665–669. doi: 10.1097/00007632-199503150-00006. [DOI] [PubMed] [Google Scholar]
- 25.Parker JC, Buckelew SP, Smarr KL, Buescher KL, Beck NC, Frank RG, Anderson SK, Walker SE. Psychological screening in rheumatoid arthritis. J Rheumatol. 1990;17:1016–1021. [PubMed] [Google Scholar]
- 26.Patrick DL, Deyo RA, Atlas SJ, Singer DE, Chapin A, Keller RB. Assessing health-related quality of life in patients with sciatica. Spine. 1995;20:1899–1908. doi: 10.1097/00007632-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Peul WC, Houwelingen HC, Hout WB, Brand R, Eekhof JA, Tans JT, Thomeer RT, Koes BW, Leiden: The Hague Spine Intervention Prognostic Study Group Surgery versus prolonged conservative treatment for sciatica. N Engl J Med. 2007;356:2245–2256. doi: 10.1056/NEJMoa064039. [DOI] [PubMed] [Google Scholar]
- 28.Peul WC, Brand R, Thomeer RT, Koes BW. Influence of gender and other prognostic factors on outcome of sciatica. Pain. 2008;138:180–191. doi: 10.1016/j.pain.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Pfirrmann CW, Dora C, Schmid MR, Zanetti M, Hodler J, Boos N. MR image-based grading of lumbar nerve root compromise due to disk herniation: reliability study with surgical correlation. Radiology. 2004;230:583–588. doi: 10.1148/radiol.2302021289. [DOI] [PubMed] [Google Scholar]
- 30.Retrieved (2008). http://www.ssb.no/english/subjects/03/01/royk_en/
- 31.Riley JL, 3rd, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74:181–187. doi: 10.1016/S0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 32.Robinson ME, Wise EA, Riley JL, Atchison JW. Sex differences in clinical pain: a multi-sample study. J Clin Psychol Med. 1998;5:413–424. doi: 10.1023/A:1026282210848. [DOI] [Google Scholar]
- 33.Rydevik B, Brown MD, Lundborg G. Pathoanatomy and pathophysiology of nerve root compression. Spine. 1984;9:7–15. doi: 10.1097/00007632-198401000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Rydevik BL, Pedowitz RA, Hargens AR, Swenson MR, Myers RR, Garfin SR. Effects of acute, graded compression on spinal nerve root function and structure. An experimental study of the pig cauda equina. Spine. 1991;16:487–493. doi: 10.1097/00007632-199105000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Sakamoto Y, Nakamura T, Takagi K. Functional and morphological changes of lumbar nerve roots induced by mechanical compression or the nucleus pulposus in contact with the root: analysis of fiber size-dependent vulnerability in rabbits. J Orthop Sci. 2004;9:598–604. doi: 10.1007/s00776-004-0837-9. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi N, Yabuki S, Aoki Y, Kikuchi S. Pathomechanisms of nerve root injury caused by disc herniation: an experimental study of mechanical compression and chemical irritation. Spine. 2003;28:435–441. doi: 10.1097/00007632-200303010-00005. [DOI] [PubMed] [Google Scholar]
- 37.Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 38.Wallis BJ, Lord SM, Bogduk N. Resolution of psychological distress of whiplash patients following treatment by radiofrequency neurotomy: a randomised, double-blind, placebo-controlled trial. Pain. 1997;73:15–22. doi: 10.1016/S0304-3959(97)00060-2. [DOI] [PubMed] [Google Scholar]
- 39.Ware JE., Jr SF-36 health survey update. Spine. 2000;25:3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 40.Weinstein JN, Tosteson TD, Lurie JD, Tosteson AN, Hanscom B, Skinner JS, Abdu WA, Hilibrand AS, Boden SD, Deyo RA. Surgical vs nonoperative treatment for lumbar disk herniation: the spine patient outcomes research trial (SPORT): a randomized trial. JAMA. 2006;296:2441–2450. doi: 10.1001/jama.296.20.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wells KB, Golding JM, Burnam MA. Psychiatric disorder in a sample of the general population with and without chronic medical conditions. Am J Psychiatry. 1988;145:976–981. doi: 10.1176/ajp.145.8.976. [DOI] [PubMed] [Google Scholar]
- 42.Winkelstein BA, Weinstein JN, DeLeo JA. The role of mechanical deformation in lumbar radiculopathy: an in vivo model. Spine. 2002;27:27–33. doi: 10.1097/00007632-200201010-00009. [DOI] [PubMed] [Google Scholar]
- 43.Younes M, Bejia I, Aguir Z, Letaief M, Hassen-Zrour S, Touzi M, Bergaoui N. Prevalence and risk factors of disk-related sciatica in an urban population in Tunisia. Joint Bone Spine. 2006;73:538–542. doi: 10.1016/j.jbspin.2005.10.022. [DOI] [PubMed] [Google Scholar]


