Abstract
A number of interspinous process devices have recently been introduced to the lumbar spinal market as an alternative to conventional surgical procedures in the treatment of symptomatic lumbar stenosis. One of those “dynamic” devices is the Coflex™ device which has been already implanted worldwide more than 14,000 times. The aim of implanting this interspinous device is to unload the facet joints, restore foraminal height and provide stability in order to improve the clinical outcome of surgery. Published information is limited, and there are so far no data of comparison between the implant and traditional surgical approaches such as laminotomy. The purpose of our prospective study is to evaluate the surgical outcome of decompressive surgery in comparison to decompressive surgery and additional implantation of the Coflex™ interspinous Device. 60 patients who were all treated in the Spine Center of Klinikum Neustadt, Germany for a one or two level symptomatic LSS with decompressive surgery were included. Two groups were built. In Group one (UD) we treated 30 patients with decompression surgery alone and group two (CO) in 30 patients a Coflex™ device was additional implanted. Pre- and postoperatively disability and pain scores were measured using the Oswestry disability index (ODI), the Roland–Morris score (RMS), the visual analogue scale (VAS) and the pain-free walking distance (WD). Patients underwent postoperative assessments 3, 6 and 12 month including the above-mentioned scores as well as patient satisfaction. In both groups we could see a significant improve (p < 0.001) in the clinical outcome assessed in the ODI, in the RMS for evaluation of back pain, in the VAS and in the pain-free WD at all times of reinvestigation compared to base line. At 1-year follow up there were no statistically differences between both groups in all ascertained parameters including patient satisfaction and subjective operation decision. Because there is no current evidence of the efficacy of the Coflex™ device we need further data from randomized controlled studies for defining the indications for theses procedures. To the best of our knowledge this is the first prospective controlled study which compares surgical decompression of lumbar spinal stenosis with additional implanting of an interspinous Coflex™ device in the treatment of symptomatic LSS.
Keywords: Interspinous process device, Coflex™ device, Lumbar dynamic stabilization, Lumbar spinal stenosis, Decompression surgery
Introduction
Lumbar spinal stenosis (LSS) due to degenerative changes is a disabling disease common in the elderly, a population that has been estimated to double by 2025 [18]. Surgery is an accepted, commonly performed treatment of symptomatic lumbar spinal stenosis and the fastest growing reason for spinal surgery in adults over 65 years of age [7, 21].
Decompressive surgery was shown to be a successful treatment in relieving symptoms of LSS and being superior to conservative treatment in long-term examinations also [1, 2, 15, 19, 21]. There is no clear evidence about the most effective technique of decompression or the extent of that decompression [7].
Interspinous-based dynamic stabilization after decompression surgery is currently being investigated as a good additional procedure which might improve the clinical outcome.
Therefore a growing number of interspinous process devices have been introduced to the lumbar spine implant market. There clinical goals range from treatment of degenerative spinal stenosis, dicogenic low back pain, facet syndrome, disc herniations and instability [4]. Those spacers can be divided into static and dynamic implants. One of the dynamic interspinous implants is the Coflex™ device (Paradigm Spine, LCC, New York, NY), formerly Interspinous ‘U’. It is a compressible U-shaped titan device that is interposed between the spinous process after decompressive surgery. It was first invented in 1994 by the French orthopaedic surgeon Jacques Samani as an alternative to arthrodesis, in order to protect adjacent levels after spinal surgery and for the protection of degenerative segments following decompressive surgery [17]. Based on the company’s indication the aim of this interspinous device is to unload the facet joints, restore foraminal height and provide stability after decompressive surgery in order to improve the clinical outcome.
Published information is limited, and there are no data of comparison between this implant and traditional surgical approaches such as laminotomy. To our knowledge, this is the first prospective case control study that compares decompressive surgery alone to additional implantation of the Coflex™ interspinous device in order to assess the safety and the efficacy of the implant.
Materials and methods
Patients presenting in the Spine Center of Klinikum Neustadt, Germany with the signs, symptoms and MRI findings of a lumbar spinal stenosis and a period of minimum 3 months of frustrating conservative treatment were eligible for microsurgical decompressive surgery. During October 2006 to June 2007 we prospectively followed a cohort of 150 patients where decompressive surgery due to spinal stenosis was carried out. For this study in- and exclusion criteria were defined as shown in Table 1. Only patients at the age of 40–80 with one or two level stenosis were included and no previous surgery at the lumbar spine took place. Patients with a stable degenerative spondylolisthesis grade one were included. We defined segmental instability on the standing lateral radiographs with a degenerative spondylolisthesis greater than grade one or a slip greater 3 mm in inclination.
Table 1.
In- and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Clinical und radiographic criteria of a symptomatic lumbar spinal stenosis | Isthmic spondylolisthesis |
| Failed conservative treatment >3 month | De novo scoliosis >15° |
| One or two level stenosis | Previous surgery lumbar spine |
| No signs of segmental instability | Signs of instability > Meyerding I |
| Age between 45 and 80 | Stenosis >2 Level |
The operation was performed under general anaesthesia and the patients were placed in a prone position. All of the subjects underwent posterior decompression surgery through a midline approach and microsurgical bilateral decompression. Decompression involved a partial laminotomy, removal of ligamentum flavum and undercutting facetectomy. Up to the surgeon the midline structures were preserved or resected and the Coflex™ interspinous device was implanted in one or two levels. The implanting technique of the Coflex™ device is simple. After resection of the interspinous ligaments the device size is chosen using templates and the device is inserted with tightened clips around the spinous process. We controlled the effect of the chosen template by radiographs to see the effect of distraction and segmental kyphosis.
No randomization took place.
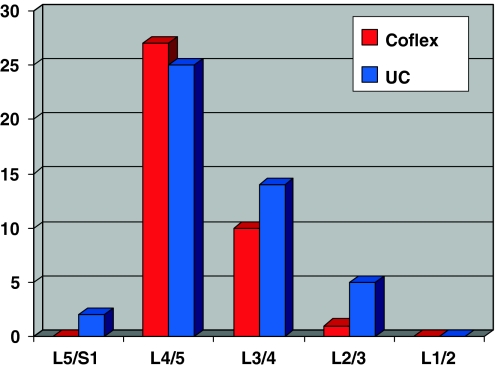
We were able to include a total of 60 patients in this study and two groups were built. In group one (undercutting-group UD) we treated 30 patients with decompression surgery alone and in group two (Coflex-Group CO) in 30 patients an interspinous Coflex™ device was additionally implanted. In group one (UD) there were 18 male and 12 female patients who ranged in age from 52 to 79 years (mean 68 years). In the Coflex group there were 16 male and 14 female patients who ranged in age from 49 to 79 years (mean 68.3 years). No significant difference was shown in the demographic data between the two groups. In the UC group there were 14 two-level interventions and 14 stable spondylolisthesis compared to 8 two-level interventions with 3 two-level Coflex™ implantation and 15 spondylolisthesis in the CO group. The distribution of the treated level in both groups is shown in Fig. 1.
Fig. 1.
The distribution of the treated level in the Coflex-group and the undercutting-group (UC)
The outcome was measured pre- and postoperatively with disability and pain scores using the Oswestry disability index (ODI), the Roland–Morris disability questionnaire (RMS) to evaluate the quality of back pain, the visual analogue scale (VAS) and the pain-free walking distance (WD). The WD was estimated by the patient, an unlimited WD was defined with the value of 5,000 m. The patients underwent postoperative assessments 3, 6 and 12 months including the above-mentioned scores as well as the survey of the patient satisfaction and operative decision.
Dynamic and static radiographs were obtained presurgery and postsurgery at first follow up. The mean total sagittal ROM (from full extension to full flexion) for the segmental intervertebral angles at the operated levels were measured and compared on flexion–extension radiographs in the two groups using Cobb’s method.
Statistical analysis
To test the differences between both operation groups and the characteristics of the scores and questionnaires over time, a repeated measures ANOVA was performed, using SPSS version 9.0 (SPSS®, Chicago, USA). For the dependent variables Oswestry score, Roland–Morris disability questionnaire, WD and VAS, a five-way ANOVA with the within subject variable ‘operation method’ was applied. For the dependent variable subjective operation decision and satisfaction of operation, a three-way ANOVA with the within subject variable ‘operation method’ was applied. Significant main and interaction effects were followed by post-hoc independent t tests.
Results
Oswestry score
The repeated measure ANOVA showed a significant main effect for the variable ‘time’ (F(1) = 63.9; p < 0.001), which means that all patients increased in function and therefore developed a lower Oswestry score over time. Furthermore, a significant interaction was found between the variables ‘time’ and ‘operation method’ (F(1) = 5.7, p < 0.05), which means that the Coflex operation method showed higher scores before the operation, revealed by independent t test (t(1) = 2.1, p < 0.05), which however equalled for the scores after the operation. Interestingly, no significant main difference between the operation method was found (F(1) = 1.5, p = 0.22) regarding the Oswestry score (Fig. 2).
Fig. 2.
Oswestry disability index preoperatively and at each follow up in the two treatment groups. All follow up scores are significant improved (p < 0.001) compared to base line with no significant differences between the treatments
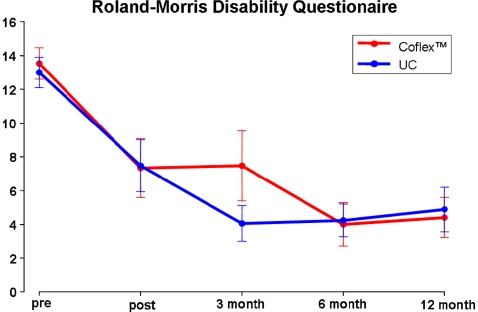
Roland–Morris disability questionnaire
The repeated measure ANOVA showed a significant main effect for the variable ‘time’ (F(1) = 24.2; p < 0.001), which means that all patients increased in function and therefore developed a lower Roland–Morris score (RMS) over time (Fig. 3). Furthermore, no significant difference between the operation method was found (F(1) = 0.2, p = 0.77) regarding the RMS.
Fig. 3.
Roland–Morris disability questionnaire preoperatively and at each follow up in the two treatment groups. All follow up scores are significant improved (p < 0.001) compared to base line with no significant differences between the treatments
VAS
The repeated measure ANOVA showed a significant main effect for the variable ‘time’ (F(1) = 50,5; p < 0.001), which means that all patients had less pain and therefore lower VAS values over time (Fig. 4). The strongest reduction of pain was direct after the operation, and did not further decrease over time. Again, no significant difference between the operation method was found (F(1) = 0.2, p = 0.66) regarding the VAS values.
Fig. 4.
Visual analogue scale preoperatively and at each follow up in the two treatment groups. All follow up scores show a significant reduction of pain (p < 0.001) compared to base line with no significant differences between the treatments
Walking distance
The repeated measure ANOVA showed a significant main effect for the variable ‘time’ (F(1) = 33.1; p < 0.001), which means that all patients had a prolonged WD over time (Fig. 5). Interestingly, a significant interaction was found between the variables ‘time’ and ‘operation method’ (F(1) = 2.5, p < 0.05). However, post-hoc t tests did not reveal any significant difference of WD between both the groups (Table 2) for each timepoint, which is also reflected in the ANOVA results, where no significant main difference between the operation method was found (F(1) = 1.7, p = 0.2) regarding the WD.
Fig. 5.
Walking distance over time. All patients had a significant prolonged walking distance (p < 0.001) with no significant main difference between the operation method was found
Table 2.
No significant difference in walking distance was found between the two groups, as revealed by independent post-hoc t tests
| Walking distance | t | p | stdr | 95% low | 95% high |
|---|---|---|---|---|---|
| Pre | −0.4 | 0.66 | 244.7 | −597.6 | 381.1 |
| Post | −0.1 | 0.945 | 462.77 | −957.9 | 893.4 |
| 3 Month | 1.7 | 0.1 | 493.3 | −162.7 | 1,812.5 |
| 6 Month | 0.5 | 0.625 | 507.5 | −766.8 | 1,265.1 |
| 12 Month | 1.8 | 0.075 | 527.2 | −100.5 | 2,016.6 |
tt value of the independent t test, p p value, stdr standard error and the last two columns show the confidence intervals
Subjective operation decision
The repeated measure ANOVA showed neither significant main effects nor any significant interactions for the subjective operation decision (Fig. 6).
Fig. 6.
Subjective operation decision and subjective satisfaction
Subjective satisfaction of the operation
The repeated measure ANOVA showed neither significant main effects nor any significant interactions for the subjective satisfaction of the operation (Fig. 6).
Complication
In the Coflex group we saw one implant-related complication with dislocation of the implant due to fracture of the spinous process. In the Coflex™ group two revisions with pedicle screw fusion of the segment were necessary, in the undercutting group one patient had to be instrumented and fused. In both groups we saw one cerebral spinal fluid leak.
Radiological analysis
Dynamic and static radiographs were obtained before surgery and postsurgery at first follow up. Segmental intervertebral angles (forced by lines drawn on the upper and lower endplates of adjacent vertebras) at the operated levels were measured and compared on standing lateral flexion–extension radiographs in the two groups. In the Coflex™ group we estimated a decreased of the range of motion for flexion and extension in the functional spinal unit of an average of 1.5° while in the undercutting group the range of motion increased at an average of 0.8° (Fig. 7).
Fig. 7.

Postoperative flexion–extension radiographs after decompressive surgery and Coflex implantation in L4/5 showing the range of motion for the functional spinal unit
Discussion
Lumbar spinal stenosis is an increasingly common diagnosis in ageing individuals and the rates of surgery have risen all over the world. In meta-analysis decompressive surgery was shown to be a successful treatment in relieving symptoms of lumbar spinal stenosis [15, 19]. Although decompressive surgery is an accepted commonly performed method, there is still controversy of the long-term benefit of surgical versus non-surgical treatment. In long-term outcomes surgically treated patients reported greater improvement in leg symptoms and back-related functional status than non-surgically treated patients [2]. Next to the expected improvement of leg pain it is well known that there is also a significant improvement in back pain in the short and midterm results [1].
Surgery typically means the microsurgical decompression of the neural structures by resection of the ligamentum flavum, partially laminotomy and undercutting facettectomy. Furthermore, spinal stenosis is often accompanied with spondylolisthesis or instability in the affected segment. Especially, in these patients, a nonrandomized controlled study [20] could show substantially greater improvement in pain and function during a period of 2 years in surgically treated patients with spinal stenosis and degenerative spondylolisthesis compared to nonsurgical treatment. Instability may also be a result of radical decompressive procedures and might lead to poor outcome [8]. For those reasons and with the introduction of pedicle screws and cages lumbar fusions with instrumentations became a common procedure after laminectomy and decompression for LSS [7]. However, it is also known that there is a higher rate of complications in instrumented fusions in the elderly patients, such as pseudarthrosis, implant failure due to loosening and complications because of the co-morbidity of the patients. That is one of the reasons why there is a need for less invasive strategies that provide a balance between safety and effectiveness [14]. A number of interspinous process devices have recently been introduced to the lumbar spinal market [4]. One of those “dynamic” devices is the Coflex™ device (formerly Interspinous ‘U’), which was originally invented by the orthopaedic surgeon Jacques Samani in 1994 as an alternative to arthrodesis, as a so-called “topping off” in order to protect adjacent levels after rigid spinal instrumentations or as a protection of degenerative motion segments following decompressive surgery [9]. The aim of implanting this interspinous device is to unload the facet joints, restore foraminal height and provide stability in order to improve the clinical outcome of surgery. Based on the company’s recommendation, the main indication for this device is a symptomatic moderate to severe stenosis in the region of L1 to L5 with or without concomitant low back pain including conditions such as stable grade I spondylolisthesis.
In an unpublished report by Samani, he saw in his 80 patients where the device was implanted with “no arthrodesis” good results and followed that the major indication for this device is to treat instability, lumbar spinal stenosis and recurrent disc herniations in the level L4/5. Kaech [9] used the implant in 18 patients with a variety of indications for surgery and followed that it appears to be a minimal invasive restabilization device for patients undergoing microsurgical decompressive procedures and who have signs of minor instability or the risk of a potentially increasing postoperative instability. The biomechanical effect of this interspinous device is now well investigated. Wilke et al. [22] and Kettler et al. [10] could show that the implant stabilized and overcompensate the instability caused by a decompression defect up to 50% of the range of motion of the intact state, but only for extension. There is almost no stabilization effect in flexion, lateral bending and axial rotation. More published information is limited. Initial experiences are only available in abstracts from papers presented in scientific meetings [3, 6, 12, 16]. All investigations deal with a retrospective analysis of different indications for implanting this device without a control group. The authors followed that the additional implantation of the Coflex™ device is safe, simple and gives good and excellent results in decompressive surgery of LSS. Kong et al. [13] made a comparison analysis between decompression with concomitant surgical placement of a Coflex™ Device and PLIF-instrumentation and found at 1-year follow up a comparable clinical outcome in the VAS and Oswestry score.
Taken all these results together, one must conclude that the evidence of the safety and efficacy of the Coflex™ interspinous implant must be still considered unknown. So far no comparison can be made between the implant and traditional surgical approaches such as microsurgical decompression for the surgical treatment of LSS [5].
Our current study reviewed the outcome of two comparable groups of patients from whom clinical data had been collected prior to and following a decompression surgery in one or two lumbar levels which fulfil critical inclusion criteria. Those included a stable degenerative spondylolisthesis grade one. In both groups, 30 patients could be included. In group one, decompressive surgery was carried out, in group two, an interspinous Coflex™ device was additionally implanted. However, no randomization took place and the decision for implanting the device after decompressive surgery was up to the surgeon. On the other side, the post-hoc test revealed no differences concerning the groups, only the pre-operative ODI showed higher values for the coflex group. In both groups we could see a significant improvement (p < 0.001) in the clinical outcome assessed in the ODI, in the RMS for the evaluation of back pain, in the VAS and in the pain-free WD at all times of reinvestigation compared to base line. At 1-year follow up, there were no statistical differences between both the groups in all ascertained parameters including the patient satisfaction and subjective operation decision. We could find a longer WD (mean value) for the Coflex group after 1 year, but this difference is not significant. As expected, the radiological results show an increase of the range of motion of the operated segment in the decompression group. With additional implanted interspinous device the range of motion in the operated segment decreased at an average of 1.5° while in the Undercutting group the range of motion increased at an average of 0.8°.
In conclusion, our data show a statistically significant (p < 0.001) improvement of all measured parameters after decompressive surgery of LSS with no additional clinical benefit of implanting an interspinous Coflex™ device in the short-term follow up of 1 year. This study underlines the missing evidence of the efficacy of the Coflex™ interspinous implant in surgical treatment of LSS in the short-term follow up. Comparable results were found by Kim [11] for the Diam™ interspinous device (Medtronic Sofamor Danek, Memphis, TN). The authors could not find, in a case control study, any differences in the clinical outcome (VAS and McNAb Score) between the two groups treated with lumbar surgery or lumbar surgery with placement of an interspinous Diam™ device after 1-year follow up. In our study, we were able to show no difference in clinical and subjective outcome parameters between both the methods. However, long-term follow up measured should show if this effect holds true after a more prolonged period.
Conclusion
After decompressive surgery for lumbar spinal stenosis, all measured parameters improved significantly (p < 0.001) compared to base line, independent of the operation method. This includes the back pain estimated in the Roland–Morris disability questionnaire. The additional placement of a Coflex™ interspinous device seems to be a safe procedure but did not improve the clinical outcome at the 12-month follow up interval. No difference in the patient satisfaction and the subjective operative decision was noted between the groups treated with or without the Coflex™ implant. This study has a limitation in the short-term follow up period of 12 month, the missing randomization and the number of patient being included. While there is no current evidence of the efficacy of the Coflex device, we have to collect more data in a longer follow up including further investigations for the WD such as a treadmill test to objectivist the accuracy of statements. Furthermore, in Germany a multicenter randomized controlled study was started in 2008 and we hope to get further data from this study for defining the indications for an interspinous process device.
References
- 1.Anjarwalla NK, Brown LC, McGregor AH. The outcome of spinal decompression surgery 5 years on. Eur Spine J. 2007;16:1842–1847. doi: 10.1007/s00586-007-0393-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atlas SJ, Keller RB, Wu YA, et al. Long-term outcomes of surgical and nonsurgical management of lumbar spinal stenosis: 8 to 10 year results from the maine lumbar spine study. Spine. 2005;30:936–943. doi: 10.1097/01.brs.0000158953.57966.c0. [DOI] [PubMed] [Google Scholar]
- 3.Bertagnoli R (2007) Coflex interspinous implant : motion preserving treatment in lumbar degenerative stenosis patients-min. 1-Y. Results. Berlin, SAS. SAS Global Symposium on Motion Preservation Technology 2007 Ref Type: report
- 4.Bono CM, Vaccaro AR. Interspinous process devices in the lumbar spine. J Spinal Disord Tech. 2007;20:255–261. doi: 10.1097/BSD.0b013e3180331352. [DOI] [PubMed] [Google Scholar]
- 5.Christie SD, Song JK, Fessler RG. Dynamic interspinous process technology. Spine. 2005;30:S73–S78. doi: 10.1097/01.brs.0000174532.58468.6c. [DOI] [PubMed] [Google Scholar]
- 6.Eif M, Schenke H (2005) The Interspinous U-indications, experience and results. SAS, New York, USA. SAS Global Symposium on Motion Preservation Technology. Ref Type: report
- 7.Gibson JN, Waddell G. Surgery for degenerative lumbar spondylosis: updated Cochrane Review. Spine. 2005;30:2312–2320. doi: 10.1097/01.brs.0000182315.88558.9c. [DOI] [PubMed] [Google Scholar]
- 8.Guigui P, Dessarts I, Morvan G, et al. Fractures of the ischium after laminoarthrectomy. Retrospective study of a series of 31 patients. Rev Chir Orthop Reparatrice Appar Mot. 1998;84:247–257. [PubMed] [Google Scholar]
- 9.Kaech DL, Fernandez C, Lombardi-Weber D. The Interspinous ‘U’: a new restabilization device for the lumbar spine. In: Kaech DL, Jinkins JR, editors. Spinal restabilization procedures. Amsterdam: Elsevier; 2002. pp. 355–362. [Google Scholar]
- 10.Kettler A, Drumm J, Heuer F, et al. Can a modified interspinous spacer prevent instability in axial rotation and lateral bending? A biomechanical in vitro study resulting in a new idea. Clin Biomech. 2008;23:242–247. doi: 10.1016/j.clinbiomech.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Kim KA, McDonald M, Pik JH, et al. Dynamic intraspinous spacer technology for posterior stabilization: case–control study on the safety, sagittal angulation, and pain outcome at 1-year follow-up evaluation. Neurosurg Focus. 2007;22:E7. [PubMed] [Google Scholar]
- 12.Kim W-K, Lee S-G, Yoo C-J et al (2005) Our experience of Interspinous U device in degenerative lumbar disease. SAS, New York, USA. SAS Global Symposium on Motion Preservation Technology. Ref Type: report
- 13.Kong DS, Kim ES, Eoh W. One-year outcome evaluation after interspinous implantation for degenerative spinal stenosis with segmental instability. J Korean Med Sci. 2007;22:330–335. doi: 10.3346/jkms.2007.22.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J, Hida K, Seki T, et al. An interspinous process distractor (X STOP) for lumbar spinal stenosis in elderly patients: preliminary experiences in 10 consecutive cases. J Spinal Disord Tech. 2004;17:72–77. doi: 10.1097/00024720-200402000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Mardjetko SM, Connolly PJ, Shott S. Degenerative lumbar spondylolisthesis. A meta-analysis of literature 1970–1993. Spine. 1994;19:2256S–2265S. doi: 10.1097/00007632-199410151-00002. [DOI] [PubMed] [Google Scholar]
- 16.Poelstra KA, Adelt D, Samani J et al (2007) Spinal stenosis decompression and Coflex interspinous stabilization: 1. Clinical results from an international multicenter retrospective study. Berlin, SAS, SAS Global Symposium on Motion Preservation Technology 7th Annual Meeting. Ref Type: report
- 17.Samani J (2000) Study of a semi-rigid Interspinous ‘U’ Fixation system. 106 patients over six years Ref Type: unpublished work
- 18.Singer BH, Manton KG. The effects of health changes on projections of health service needs for the elderly population of the United States. Proc Natl Acad Sci USA. 1998;95:15618–15622. doi: 10.1073/pnas.95.26.15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner JA, Ersek M, Herron L, et al. Surgery for lumbar spinal stenosis. Attempted meta-analysis of the literature. Spine. 1992;17:1–8. doi: 10.1097/00007632-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356:2257–2270. doi: 10.1056/NEJMoa070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356:2257–2270. doi: 10.1056/NEJMoa070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilke HJ, Drumm J, Haussler K, et al. Biomechanical effect of different lumbar interspinous implants on flexibility and intradiscal pressure. Eur Spine J. 2008;17:1049–1056. doi: 10.1007/s00586-008-0657-2. [DOI] [PMC free article] [PubMed] [Google Scholar]