Abstract
The aetiology of idiopathic scoliosis (IS) remains unknown; however, there is a growing body of evidence suggesting that the spine deformity could be the expression of a subclinical nervous system disorder. A defective sensory input or an anomalous sensorimotor integration may lead to an abnormal postural tone and therefore the development of a spine deformity. Inhibition of the motor cortico-cortical excitability is abnormal in dystonia. Therefore, the study of cortico-cortical inhibition may shed some insight into the dystonia hypothesis regarding the pathophysiology of IS. Paired pulse transcranial magnetic stimulation was used to study cortico-cortical inhibition and facilitation in nine adolescents with IS, five teenagers with congenital scoliosis (CS) and eight healthy age-matched controls. The effect of a previous conditioning stimulus (80% intensity of resting motor threshold) on the amplitude of the motor-evoked potential induced by the test stimulus (120% of resting motor threshold) was examined at various interstimulus intervals (ISIs) in both abductor pollicis brevis muscles. The results of healthy adolescents and those with CS showed a marked inhibitory effect of the conditioning stimulus on the response to the test stimulus at interstimulus intervals shorter than 6 ms. These findings do not differ from those reported for normal adults. However, children with IS revealed an abnormally reduced cortico-cortical inhibition at the short ISIs. Cortico-cortical inhibition was practically normal on the side of the scoliotic convexity while it was significantly reduced on the side of the scoliotic concavity. In conclusion, these findings support the hypothesis that a dystonic dysfunction underlies in IS. Asymmetrical cortical hyperexcitability may play an important role in the pathogenesis of IS and represents an objective neurophysiological finding that could be used clinically.
Keywords: Adolescent idiopathic scoliosis, Dystonia, Cortico-cortical inhibition, Cortical hyperexcitability, Transcranial magnetic stimulation
Introduction
The aetiology of idiopathic scoliosis (IS) remains unknown; however, there is a growing body of evidence suggesting that spine deformity could be the musculoskeletal expression of a subclinical nervous system disorder. Some clinical studies have shown abnormalities in the balance control and proprioception in scoliotic patients as compared with age- and gender-matched healthy controls. Patients with IS have been found to have abnormal postural perception [1–4], impaired dynamic balance control [5], as well as angle joint reproduction asymmetries [6, 7]. Vibratory sensitivity has also been found to be abnormal, increased [8, 9] or decreased [10] in IS patients. Scoliotic curves have been experimentally induced in animals by damaging the posterior horn and Clarke column [11], posterior column [12], dorsal nerve roots [13, 14] and brain stem nuclei related to postural equilibrium [15]. All these clinical and experimental observations support the hypothesis that either a defective sensory input or an anomalous sensory-motor integration might lead to an abnormal modulation of the postural tone, which progressively induces the development of a spinal deformity. Thus, we propose an aetiopathogenic relation between dystonic disorder and IS.
Several sensory system alterations have been found in dystonic patients, suggesting that some dystonias could be primarily due to a sensory disorder [16, 17].
The use of electric or magnetic paired pulse stimulation of the cerebral motor cortex has been found to be an excellent tool for the study of intracortical excitability [18]. An electric or a magneto-electric stimulus is capable of inducing sufficient current within the brain to depolarize neurons and evoke a muscular contraction by induction of cortico-spinal volleys. The neural response to the cortical stimuli can be modulated by other previous cortical stimuli acting as a conditioning factor. Using transcranial magnetic stimulation (TMS) for both conditioning and test stimuli, a physiological inhibiting effect has been described on the evoked motor responses at short (2–3 ms) interstimulus intervals (ISIs) between the conditioning and the test stimuli. At longer intervals (around 10–12 ms), the response to the test stimulus is facilitated by the preceding conditioning stimulus reflecting neuronal hyperexcitability [19, 20]. These phenomena of interstimulus interactions have been related to the activation of intracortical neuronal circuits [21, 22].
Several studies using paired pulse TMS have revealed alterations of motor cortical excitability in some forms of focal dystonia. Patients with idiopathic dystonia exhibit an abnormal decrease in cortico-cortical inhibition [22–24]. This lower excitability of intracortical inhibitory circuits has been therefore suggested to be in part responsible of the inadequate motor control seen in dystonic patients. In addition, proof-of-principle data reveal that repetitive TMS, at parameters of frequency and intensity that enhance intracortical inhibition, can transiently ameliorate symptoms in dystonia [25].
The study of cortical excitability using paired pulse TMS in IS patients could strengthen the hypothesis relating IS with an underlying dystonic disorder. The occurrence of this motor control abnormality during the period of spine growth could result in scoliotic deformity. If so, these findings could lead to novel therapeutic interventions.
Patients and methods
Twenty-two subjects were included in this study: nine adolescents with IS, five with congenital scoliosis (CS) and eight age-matched healthy controls. The three groups had a similar mean age: 14.3 years (SD 1.6) for the IS group; 14. 2 years (SD 1.2) for the congenital scoliosis group; and 14.0 years (SD 0.7) for the control group. All scoliotic curves were right thoracic in the IS group with an average deformity of 47º Cobb (43º–68º). The Risser sign was less than four in all cases. The age of detection of the deformity was 11 years or later. At the time of study, seven IS patients were under brace-wearing treatment and were in waiting list for surgical correction. One participant had already been operated on. In cases with CS, curves were caused by mixed defects in vertebral formation and segmentation [26]. One CS patient had right thoracic curve, one left thoracic, one double thoracic and two patients right thoracic and left lumbar. The mean magnitude of the curves was 42º Cobb (37º–50º). Patients had no other pathologic antecedents, and the medical and neurological exams (other than for the spine deformity) were completely normal in all participants. In two IS patients with rapid progression, a brain and spine magnetic resonance imaging (MRI) exam did not reveal any abnormality. All five CS patients underwent MRI exams (including brain imaging) that did not revealed medular and brain stem pathology.
Healthy teenagers were recruited as healthy controls. Prior to inclusion in the study, an orthopaedic exam failed to detect any spine deformities in any of these adolescents. Medical and neurological exams were similarly unremarkable and none had a history of significant illnesses or previous neurological disease.
All subjects gave their written informed consent (or assent) to the study and their parents did too. The study had been approved by the local ethics committee.
Paired pulse TMS was used to study cortico-cortical inhibition as described by Ridding et al. [22]. Stimuli were applied using a Dantec Twintop (Medtronic, Minneappolis, MN) equipped with a focal eight-shaped coil. Subjects sat comfortably on an armchair and were requested to be relaxed. TMS was applied to the motor cortex and evoked motor potentials were recorded from the contralateral abductor pollicis brevis (APB) muscle using pairs of surface electrodes. A comparison of right and left hemispheres was performed in each group and among groups. The duration of the TMS study was around 60 min per patient.
Threshold level
We followed the guidelines endorsed by the International Federation of Clinical Neurophysiology. The stimulation coil was moved over the scalp around the motor cortex in order to identify the optimal scalp position over which TMS induced motor-evoked potentials (MEPs) of maximal amplitude in the contralateral APB. The coil was held tangentially to the scalp with the handle pointing posteriorly and oriented 45º laterally from the mid-sagittal plane. In this position, the induced current is approximately perpendicular to the central sulcus and flows posterior to anterior thus inducing MEPs of maximal amplitude. Threshold intensity was determined by progressively decreasing the intensity of the TMS discharge, expressed as percentage of the maximal capacity of the stimulator. Motor threshold intensity was considered as the minimum intensity required to induce a motor response higher than 50 μv peak-to-peak amplitude in at least five out of ten trials.
Intracortical excitability: paired pulse curve determination
Paired pulse TMS was performed as follows: First, we recorded ten MEPs with the test stimulus alone in order to define a baseline. The average MEP peak-to-peak amplitude of these baseline responses was used to quantify the effect of the conditioning stimuli on the test stimuli. Then, we recorded responses to pairs of stimuli with a variable ISI, with a subthreshold TMS pulse (conditioning stimulus) followed by a second supra-threshold discharge (test stimulus). The conditioning stimulus intensity was set at 80% of the intensity of the test stimulus and thus, the conditioning stimulus alone failed to evoke measurable MEPs. The test stimulus was set at approximately 120% of resting motor threshold and calibrated to induce MEPs with a peak-to-peak amplitude of approximately 1.0 mV. The effect of the conditioning stimulus on the test stimulus was examined at various ISIs (1, 2, 3, 4, 6, 8, 10, 15 and 20 ms). At each interestimulus interval, ten MEPs were recorded and averaged off-line. Pairs of stimuli were applied separated by at least 7 s to avoid carry-over effects. The order of the pairs with various ISIs was random. Similarly, the order of the hemispheres tested was randomly assigned and counterbalanced across subjects.
MEPs were recorded using an AD conversion tablet (digitization rate at 5 Hz) and the Maclab program (ADInstruments Ltd, Hastings, UK). For the analysis of data, peak-to-peak amplitudes and area under the curve for each MEP were measured, and measurements then expressed as percentage of the change from the average of the ten baseline MEPs were recorded in response to the test stimulus alone. Latency of the MEPs was also measured.
From the paired pulse curve, it is possible to identify two distinct phases that are referred to as short-interval intracortical inhibition (SICI) and intracortical facilitation (ICF) [20–24]. The SICI represents an inhibition phase with ISIs from 1 to 6 ms, maximally around 3 ms. ICF refers to a facilitation phase with ISIs greater than 6 ms, generally maximal around 10–12 ms. We calculated SICI and ICF for each subject and hemisphere and compared them across groups.
The sample size of the study was calculated using the SISA program [27]. Considering results in the inhibitory phase previously reported by Ridding [22] in dystonic patients compared with normal controls [dystonic: average supression 80 (SD 17), control 50 (SD 15)], for an alpha value of 0.05 and beta power of 0.8, we determined that five subjects per group were needed.
Results were statistically analysed with the SPSS 10.0 package. Overall, multivariate ANOVA test with Tukey′s post hoc test was performed in order to compare groups for the effect of the conditioning stimulus on cortical excitability. This analysis was carried out using three different approaches: first, the evoked potentials induced by paired stimulation were analysed separately for each ISI; second, the average MEP magnitude of all combined intervals was compared; and third, the magnitude of SICI and ICF were compared across study groups. For comparisons between hemispheres within groups, Student t-test was used. P values lower than 0.05 were considered as reflecting statistical significance.
Results
Thresholds, single stimulation and latencies
There were no differences between patients with IS, patients with CS and healthy controls in motor threshold, and no differences in motor threshold across hemispheres in any of the subject groups. The amplitude of the MEPs as well as their latency was not significantly different across groups.
Paired pulse testing
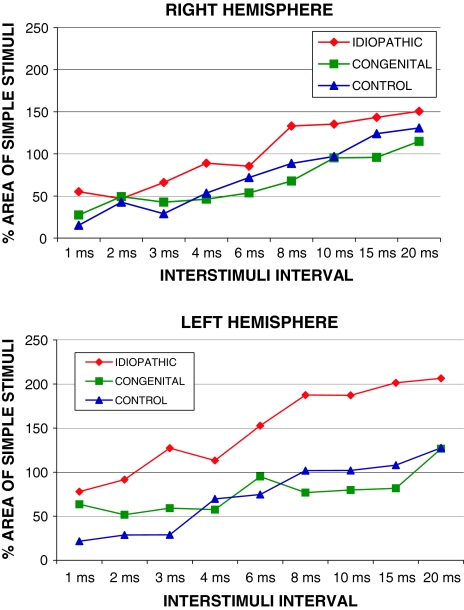
When the amplitude of the evoked potentials were studied along the whole ISIs (from 1 to 20 ms), there was a statistically significant difference at the left hemisphere between patients with IS and both group of patients with congenital scoliosis and healthy controls (Table 1). Patients with IS showed a significantly greater average amplitude of the MEPs elicited by left hemispheric stimulation (average whole ISI left hemisphere: IS 149.50, SD 48.87; CS 76.89, SD 23.25; control 73.67, SD 39.40; F 10.90 P = 0.001; post hoc: IS-CS P = 0.001, IS-control P = 0.02, CS-control P = 0.982). In right hemisphere, IS showed a slight increase in the average of all ISI potentials but without statistical significance (average whole ISI right hemisphere: IS 100.54, SD 40.53; CS 65.88 SD 29.54; control 72.52, SD 40.84; F 3.279, P = 0.062). Figure 1 shows the progression of the facilitation–inhibition curve at each ISI applied.
Table 1.
Summary of the results on MEPs induced by paired pulse TMS at different ISIs in all three groups of subjects
| ISI (ms) | Hemisphere | Idiopatic (SD) | Congenital (SD) | Control (SD) | Sig | |
|---|---|---|---|---|---|---|
| F | P | |||||
| 1 | Right hem | 55.1 (44.2) | 27.6 (7.2) | 15.3 (5.8) | 3.68 | 0.05 |
| Left hem | 77.9 (43.8) | 63.5 (24.4) | 21.6 (9.9) | 6.81 | 0.007 | |
| 2 | Right hem | 46.8 (29.0) | 49.2 (50.7) | 42.63 (24.3) | 0.64 | 0.94 |
| Left hem | 91.6 (43.6) | 51.7 (39.3) | 28.8 (15.2) | 7.22 | 0.005 | |
| 3 | Right hem | 66.0 (39.1) | 42.6 (22.2) | 29.1 (20.9) | 3.074 | 0.73 |
| Left hem | 127.3 (75.2) | 59.2 (40.6) | 28.9 (12.2) | 7.24 | 0.005 | |
| 4 | Right hem | 89.0 (70.6) | 46.1 (43.7) | 53.4 (25.1) | 1.24 | 0.31 |
| Left hem | 113.3 (54.7) | 57.5 (48.1) | 69.7 (49.0) | 1.98 | 0.17 | |
| 6 | Right hem | 85.4 (33.9) | 53.7 (12.7) | 71.9 (29.1) | 1.33 | 0.29 |
| Left hem | 152.8 (39.9) | 95.1 (28. 6) | 74.6 (42.0) | 8.58 | 0.003 | |
| 8 | Right hem | 133.2 (63.4) | 67.9 (54.6) | 88.7 (33.1) | 2.49 | 0.11 |
| Left hem | 187.6 (70.0) | 76.9 (79.7) | 101.8 (56.9) | 4.70 | 0.03 | |
| 10 | Right hem | 135.3 (33.9) | 95.2 (78.1) | 96.9 (36.0) | 1.91 | 0.18 |
| Left hem | 187.2 (58.0) | 79.8 (91.5) | 102.0 (51.0) | 5.82 | 0.013 | |
| 15 | Right hem | 143.5 (34.3) | 95.9 (95.1) | 124.0 (60.0) | 0.87 | 0.44 |
| Left hem | 201.3 (56.5) | 81.8 (82.8) | 108.0 (54.6) | 7.05 | 0.006 | |
| 20 | Right hem | 150.7 (50.1) | 114.8 (81.7) | 130.9 (68.4) | 0.45 | 0.65 |
| Left hem | 206.5 (63.1) | 126.5 (19.3) | 127.6 (46.3) | 5.04 | 0.02 | |
Values represent the percentage area under the curve as compared with the MEPs to the test TMS pulses alone
ISI interstimuli interval
Fig. 1.
MEPs during paired pulse TMS according to ISI between conditioning and test stimuli. The graph displays average area under the curve for each subject group as percentage of baseline results for the MEPs evoked by the test stimuli alone
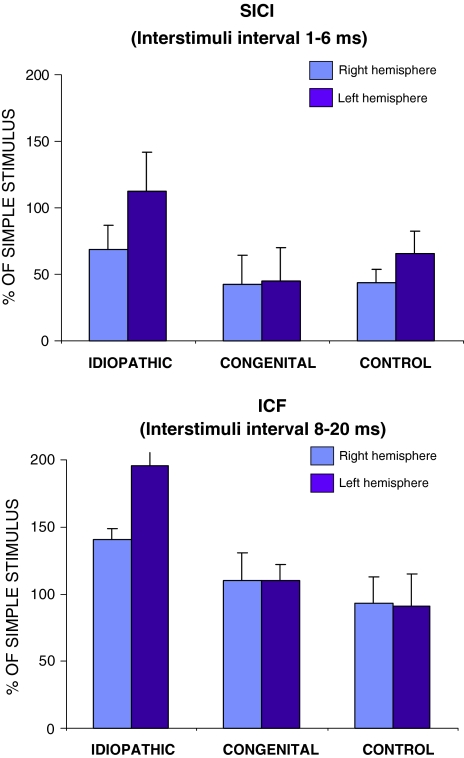
The paired pulse curves for each group are shown in Fig. 1 for right and left hemispheres (Figs. 1, 2). Patients with congenital scoliosis and healthy controls showed a similar amount of SICI in the right and left hemispheres. Comparison of SICI and ICF across hemispheres failed to reveal significant difference in these two groups (Fig. 2; P > 0.05). However, patients with IS showed a significant interhemispheric asymmetry. First, MEP amplitude, collapsing across all ISIs, was significantly greater in the left than the right hemisphere (Fig. 1; P = 0.01). In addition, SICI was significantly lesser in the left than the right hemisphere in IS patients (Fig. 2; P = 0.006). Finally, ICF was significantly greater in the left hemisphere as compared to the right (Fig. 2; P = 0.01).
Fig. 2.
Short-latency intracortical inhibition (SICI) and ICF in right and left hemispheres in the three groups of subjects. Bars average area under the curve of the MEPs normalized to the responses to the test stimuli alone. The clear hemispheric asymmetry in the idiopathic scoliosis patients for both SICI (P = 0.006) and ICF (P = 0.01)
Comparing across groups, the amount of SICI in the left hemisphere was significantly greater for the patients with IS than the patients with congenital scoliosis or the healthy controls (Fig. 2; average IS 112.6% ± 29.5; controls 44.7% ± 25.3; congenital 65.4% ± 17.1; F 7.200 P = 0.006, post-hoc IS-IC P = 0.018; IS-control P = 0.001; CS-control P = 0.576). The amount of SICI in the right hemisphere also tended to be greater in patients with IS than in the other groups, but this did not show statistical significance (Fig. 2; average IS 68.5% SD 18.5; controls 42.4% SD 21.8; congenital 43.8% SD 10.0, F 2.961, P = 0.079).
ICF was also significantly larger in the left hemisphere in patients with IS as compared to both healthy controls and patients with congenital scoliosis (Fig. 2; average IS 195.7% ± 9.8; control 109.8% ± 12.2; congenital 91.2% ± 23.6; F 7.119, P = 0.006; post hoc IS-CS P = 0.01; IS-control P = 0.05; CS-control P = 0.686). In the right hemisphere, ICF also tended to be greater in IS patients but this did not reach statistical significance (Figs. 2, 3; average IS 140.6% ± 8.0; control 110.1% ± 20.5; congenital 93.4% ± 19.3; F 1.397, P = 0.274).
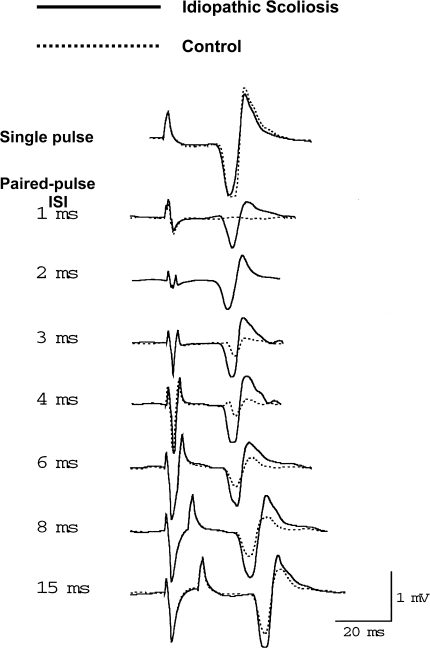
Fig. 3.
Example of MEPs obtained with single pulse TMS and at various ISI in a healthy control and a idiopathic scoliosis patient
Discussion
This study demonstrates that patients with IS show a significant hemispheric asymmetry in cortical excitability as characterized by short-latency cortico-cortical inhibition (SICI) and ICF after paired pulse stimulation of the motor cortex. The reduction of SICI reflects a relative decrease of the intracortical inhibition circuits at the motor cortex. A similar abnormal motor cortex excitability has previously been described in patients with dystonia using the same paired pulse TMS technique [22–25]. The inappropriately increased motor activity, which is characteristic of dystonic patients, has been related to an unbalanced cortico-cortical modulation with decreased intracortical motor inhibition. Our results support the hypothesis that a dystonic disorder might underlay the pathogenesis of IS. A deregulation with hemispheric asymmetry in the modulation of the motor activity controlling spine posture at intracortical level could be the cause of progressive scoliotic deformity.
In the last decades, several lines of evidence have revealed a sensory disorder at various CNS levels in patients with IS. Several authors have found alterations of proprioception and equilibrium control in scoliotic patients [1, 3–10, 28]. Yamada [29] suggested that any disruption in the postural reflex system might induce scoliosis. Dysfunction of the posterior columns, reflected by a disorder in the vibratory and postural perceptions has been proposed by several authors [8, 9] as a primary etiologic factor in IS. Some clinical studies in patients with IS using somato-sensory evoked potentials, which primarily monitor the function of the posterior columns, failed to find any asymmetry in IS patients or difference as compared to healthy controls [30, 31] while other studies have found abnormalities [5, 32]. Postural tone, equilibrium and proprioception are mediated by the posterior columns, vestibular nuclei and visual afferences. All these functions require a correct integration at cortical level. In fact, the alterations of the postural and ocular motor control found in IS have been suggested to be due to abnormal processing of sensory afferences at cerebral cortex, and not to conduction disturbances [2, 30, 32, 33]. Ultimately, a sensory alteration is likely to impact on motor control, given the necessary tight coupling of sensorimotor integration.
Indeed, similar to IS, disturbances of sensory control and sensorimotor integration have been described in dystonia as well, and a number of reports argue for the presence of a primary subclinical sensory deficit in this disease [34, 35]. Abnormal processing or integration of proprioceptive input at central level, which can lead to or be expressed by inadequate control of motor output, has been proposed as a possible pathogenic mechanism of dystonia [16, 17, 36].
The relationship between scoliosis and dystonia seems to be reinforced by their association in human clinic. Patients with idiopathic cervical dystonia, a most frequent focal dystonia, develop scoliosis in 39% of cases [37]. Furthermore, scoliosis develops in late childhood or early puberty more frequently among patients with cervical dystonia [38]. Scoliosis is a constant finding in severe forms of dystonia such as dystonia musculorum deformans, and also in other forms of generalized dystonia [39, 40]. Sometimes, scoliosis is the first sign of a dystonia and the deformity progression can be controlled after treating the dystonia, for example, with l-dopa [41, 42].
A similar alteration in cortical motor excitability to that found in patients with IS has been also described in patients with Parkinson’s disease [43, 44]. The incidence of scoliosis in Parkinson patients is higher than in the normal population varying from 33 to 90% [45–47]. The scoliotic deformity in patients with Parkinsonism is not related to age, disease stage, duration of symptoms, response to l-dopa or the presence of dyscinesia [47].
Normal adults do not normally show significant differences in paired pulse responses to TMS between left and right hemispheres [48]. In our study, healthy adolescents and congenital scoliosis patients also did not show asymmetry in SICI or ICF. The asymmetric intracortical modulation found in IS patients after paired pulse TMS is a new finding not previously described. Left hemisphere showed a lower cortico-cortical inhibition after short ISIs (<6 ms), and a greater facilitation after long intervals (from 8 to 20 ms). In a study with six dystonic patients, all of them showed decreased cortico-cortical inhibition in the affected hemisphere but in four patients there was a normal intracortical inhibition in the nondystonic hand area [49]. Earlier studies on patients with idiopathic dystonia using paired pulse TMS demonstrated the same cortico-cortical inhibition abnormality but failed to encounter hemispheric asymmetry, even though the dystonic symptoms were clearly lateralized [22]. These findings were thought to reflect a bilateral subclinical neurophysiologic disorder. However, using supra-threshold conditioning and test stimuli, the inhibitory effect was reduced more in the symptomatic than in the asymptomatic hemisphere [50]. Maybe the degree of hemispheric asymmetry in intracortical modulation is greater in IS. This is consistent with studies that have shown asymmetries in perceptual and cognitive processes suggestive of a greater degree of left–right asymmetry throughout the cortical organization of IS patients [32]. Lao et al. [5] found asymmetries in the gait parameters associated with the direction of the curve, but only in IS patients with abnormal tibial somatocortical evoked potentials. This suggests that impairment of the somatosensory pathway may lead to poorer balance control under dynamic situations.
Kimiskidis [51] used single pulse TMS in IS patients as compared with normal controls and failed to find asymmetries in the threshold, amplitude or latency of upper limb motor potentials amplitude. Kimiskidis also examined the TMS-induced silent period, a measure of GABA-B modulation, and found that IS patients showed a slightly shorter silent period in left hemisphere than controls. The findings did not reach statistical significance, but point to a similar hemispheric asymmetry as we found in our study. Paired pulse TMS at short ISI and single pulse TMS-induced silent periods explore slightly different aspects of cortical inhibition. Short ISI-paired pulse TMS inhibition is thought to reflect cortical GABA-A inhibitory circuits [52] while silent period inhibition has a spinal component in its first part and a cortical in its later part which reflects GABA-B inhibitory circuits [53, 54]. Thus, put together, the findings by Kimiskidis and us, suggest that while there is a hemispheric asymmetry in intracortical inhibition in IS, GABA-A circuits are primarily affected over GABA-B circuits. Some studies in focal hand dystonia have revealed a similar dissociation in TMS-induced silent period and SICI [24, 55, 56], as well as shown task-dependent alteration of intracortical inhibition [25, 57].
A methodological objection that could be raised against our work is that motor cortical inhibition and facilitation were investigated on muscles that are not implicated in spine function. The major reasons for recording evoked potentials at the APB were the proven reliability of the technique, the fact that these cortical inhibition–facilitation phenomena after TMS have been previously well characterized in this muscle, and the fact that spinal segmental circuits play a minor role in the cortica-spinal projection to the hand, so that cortical excitability modulation provides a more direct reflection of motor output. Chen et al. [50] have shown that the paired pulse modulation of cortical excitability is a generalized phenomenon, being present in multiple assessed muscles. Therefore, it seems reasonable to assume that our findings can be extrapolated to other muscle groups and that the findings in the APB ultimately reflect a more widespread disturbance.
An important question that should be raised is whether our findings are cause or consequence of the spinal deformity. One interpretation could be to relate the abnormal hyperexcitability with the acquired skeletal deformity, which is assumed that neurophysiologic findings are induced by the spinal curve. The abnormal posture might provoke asymmetric proprioceptive afferences, which might induce changes in the cortical excitability. In our study, to control for this possibility, we included a group of patients with congenital scoliosis caused by different well-defined vertebral malformations. The reduced cortico-cortical inhibition pattern found in IS patients was not present in patients with congenital scoliosis despite the fact that the spine curvature was similar. In fact, the neurophysiologic findings obtained in patients with congenital scoliosis were similar to those in healthy controls. Therefore, the cortical hyperexcitability found in our series of patients with IS cannot be attributed to spinal deformity.
The results of this study represent an objective neurophysiologic finding of a neurologic dysfunction in IS that could help understand its aetiology. We only studied patients with severe curves and quite typical clinical manifestations, e.g. right thoracic curves. Thus, it is attractive to entertain the notion that our findings of asymmetric intracortical excitability could serve as a progression marker for IS. Future studies will examine whether paired pulse TMS can be used as a predictor of progression of spinal deformity in IS.
References
- 1.Sahlstrand T, Örtengren R, Nachemson A. Postural equilibrium in adolescent idiopathic scoliosis. Acta Orthop Scand. 1978;49:54–65. doi: 10.3109/17453677809050088. [DOI] [PubMed] [Google Scholar]
- 2.Herman R, Mixon J, Fisher A, Maulucci R, Stuyck J. Idiopathic scoliosis and the central nervous system: a motor control problem. Spine. 1985;10:1–14. doi: 10.1097/00007632-198501000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Adler N, Bleck EE, Rinsky IA, Young W. Balance reactions and eye hand coordination in idiopathic scoliosis. J Orthop Res. 1986;4:102–107. doi: 10.1002/jor.1100040113. [DOI] [PubMed] [Google Scholar]
- 4.Byl NN, Gray JM. Complex balance reactions in different sensory conditions: adolescents with and without idiopathic scoliosis. J Orthop Res. 1993;11:215–227. doi: 10.1002/jor.1100110209. [DOI] [PubMed] [Google Scholar]
- 5.Lao MLM, Chow DHK, Guo X, Cheng JCY, Holmes AD. Impaired dynamic balance in adolescent with idiopathic scoliosis and abnormal somatosensory evoked potential. J Pediatr Orthop. 2008;28:846–849. doi: 10.1097/BPO.0b013e31818e1bc9. [DOI] [PubMed] [Google Scholar]
- 6.Barrack RL, Withecloud TS, Burke SW, Cook SD, Harding AS. Propiocepcion in idiopathic scoliosis. Spine. 1984;9:681–685. doi: 10.1097/00007632-198410000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Cook SD, Harding Af, Burke SW, Whitecloud TS, Barrack RL, Leinhardt TM. Upper extremity propioception in idiopathic scoliosis. Clin Orthop. 1986;213:118–124. [PubMed] [Google Scholar]
- 8.Barrack RL, Wyatt MP, Whitecloud TS, Whitecloud TS, Barrack RL, Leinhardt TM. Vibratory hipersensivity in idiopathic scoliosis. J Pediatr Orthop. 1988;8:389–395. doi: 10.1097/01241398-198807000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Wyatt MP, Barrack RL, Mubarack SJ, Whitecloud TS, Burke SW. Vibratory response in idiopathic scoliosis. J Bone Joint Surg. 1986;68B:714–718. doi: 10.1302/0301-620X.68B5.3782230. [DOI] [PubMed] [Google Scholar]
- 10.McInnes E, Hill DL, Raso VJ, Chetner B, Greenhill BJ, Moreau MJ. Vibratory response in adolescents who have idiopathic scoliosis. J Bone Joint Surg. 1991;73A:1208–1212. [PubMed] [Google Scholar]
- 11.Pincott JR, Taffs LF. Experimental scoliosis in primates. J Bone Joint Surg. 1982;64B:503–507. doi: 10.1302/0301-620X.64B4.6284765. [DOI] [PubMed] [Google Scholar]
- 12.Barrios C, Tunon MT, Salis JA, Beguiristain JL, Cañadell J. Scoliosis induced by medullary damage: an experimental study in rabbits. Spine. 1987;12:433–439. doi: 10.1097/00007632-198706000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Pincott JR, Davies JS, Taffs LF. Scoliosis caused by section of dorsal spinal nerve roots. J Bone Joint Surg. 1984;66B:27–29. doi: 10.1302/0301-620X.66B1.6693473. [DOI] [PubMed] [Google Scholar]
- 14.Suk SI, Song HS, Lee CK. Scoliosis induced by anterior and posterior rhizotomy. Spine. 1988;14:692–697. doi: 10.1097/00007632-198907000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Barrios C, Arrotegui JI. Experimental kyphoescoliosis induced in rats by selective brain stem damage. Int Orthop. 1992;16:146–151. doi: 10.1007/BF00180206. [DOI] [PubMed] [Google Scholar]
- 16.Hallett M. Is dystonia a sensory disorder? Ann Neurol. 1995;38:139–140. doi: 10.1002/ana.410380203. [DOI] [PubMed] [Google Scholar]
- 17.Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Mov Disord. 2003;18:231–240. doi: 10.1002/mds.10327. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. The Lancet neurol. 2003;2:145–156. doi: 10.1016/S1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- 19.Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD. Stimulation of the human motor cortex through the scalp. Exp Physiol. 1991;76:159–200. doi: 10.1113/expphysiol.1991.sp003485. [DOI] [PubMed] [Google Scholar]
- 20.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Cortico cortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziemann U, Rothwell JC, Ridding MC. Interactions between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ridding MC, Sheean G, Rothwell JC, Inzelberg R, Kujiray T, Inzelberg R, Kujiray T. Changes in the balance between motor cortical excitation and inhibition in focal task specific dystonia. J Neurol Neurosurg Psych. 1995;59:493–498. doi: 10.1136/jnnp.59.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mavroudakis N, Caroyer JM, Brunko E, Zegers de Beyl D. Abnormal motor evoked responses to transcranial magnetic stimulation in focal dystonia. Neurology. 1995;45:1671–1677. doi: 10.1212/wnl.45.9.1671. [DOI] [PubMed] [Google Scholar]
- 24.Ikoma K, Samii A, Mercuri B, Wassermann EM, Hallett M. Abnormal cortical motor excitability in dystonia. Neurology. 1996;46:1371–1376. doi: 10.1212/wnl.46.5.1371. [DOI] [PubMed] [Google Scholar]
- 25.Siebner HR, Tormos JM, Ceballos-Baumann AO, Auer C, Catala MD, Conrad B, Pascual-Leone A. Low-frequency repetitive transcranial magnetic stimulation of the motor cortex in writer’s cramp. Neurology. 1999;52:529–537. doi: 10.1212/wnl.52.3.529. [DOI] [PubMed] [Google Scholar]
- 26.Winter RB. Congenital spinal deformity. In: Lonstein JE, Bradford DS, Winter RB, Ogilvie JW, editors. Moe′s textbook of scoliosis and other spinal deformities. Philadelphia: WB Saunders; 1995. [Google Scholar]
- 27.Uitenbroek DG (1997) SISA-Binomial. http://www.quantitativeskills.com/sisa/distributions/binomial.htm. Accessed 1 June 2008
- 28.Yamada H, Yamamoto H, Ikada T, et al. A neurological approach to the etiology and therapy of scoliosis. J Bone Joint Surg. 1971;53A:197–198. [Google Scholar]
- 29.Yamada K, Yamamoyo H, Nakagawa Y, Tezuka A, Tamura T, Kawata S. Etiology of idiopathic scoliosis. Clin Orthop. 1984;184:50–57. [PubMed] [Google Scholar]
- 30.Brinker MR, Willis JK, Cook SD, Whitecloud TS, Bennett JT, Barrck RL, Ellman MG. Neurologic testing with somatosensory evoked potentials in idiopathic scoliosis. Spine. 1992;17:277–279. doi: 10.1097/00007632-199203000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Fernández-Bermejo E, García-Jimenez MA, Fernandez-Palomeque C, Munuera L. Adolescent idiopathic scoliosis and joint laxity. Spine. 1993;18:918–922. doi: 10.1097/00007632-199306000-00018. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg CJ, Dowling FE, Fogarty EE, Moore DP. Adolescent idiopathic scoliosis and cerebral asymmetry: an examination of a nonspinal perceptual system. Spine. 1995;20:1685–1691. doi: 10.1097/00007632-199508000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Machida M, Dubousset J, Imamura Y, Iwaya T, Yamada T, Kimura J, Toriyama S. Pathogenesis of idiophatic scoliosis: SEPs in chicken with experimentally induced scoliosis and in patients with idiopathic scoliosis. J Pediatr Orthop. 1994;14:329–335. doi: 10.1097/01241398-199405000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Tinazzi M, Rosso T, Fiaschi A. Role of the somatosensory system in primary dystonia. Mov Disord. 2003;18:605–622. doi: 10.1002/mds.10398. [DOI] [PubMed] [Google Scholar]
- 35.Hallet M. The neurophysiology of dystonia. Arch Neurol. 1998;55:601–603. doi: 10.1001/archneur.55.5.601. [DOI] [PubMed] [Google Scholar]
- 36.Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia. Brain. 1998;121:1195–1212. doi: 10.1093/brain/121.7.1195. [DOI] [PubMed] [Google Scholar]
- 37.Jankovic J, Leder S, Warner D, Schwartz K. Cervical dystonia: clinical findings and associated movement disorders. Neurology. 1991;41:1088–1091. doi: 10.1212/wnl.41.7.1088. [DOI] [PubMed] [Google Scholar]
- 38.Defazio G, Abbruzzese G, Girlanda P, Buccafusca M, Curra A, Marchese R, Martino D, Masi G, Mazzella L, Vacca L, Livrea P, Berardelli A. Primary cervical dystonia and scoliosis: a multicenter case-control study. Neurology. 2003;60:1012–1015. doi: 10.1212/01.wnl.0000049932.22065.60. [DOI] [PubMed] [Google Scholar]
- 39.Duane DD. Familiar cervical dystonia, head tremor and scoliosis: a case report. Adv Neurol. 1998;78:117–120. [PubMed] [Google Scholar]
- 40.Furukawa Y, Kish SJ, Lang AE. Scoliosis in a Dopa responsive dystonia family with mutation of of the GTP cyclohydrolase I gene. Neurology. 2000;54:2187. doi: 10.1212/wnl.54.11.2187. [DOI] [PubMed] [Google Scholar]
- 41.Micheli F, Pardal MF, Gatto E, Paradiso G. Dopa responsive dystonia masquerading as idiopathic kyphoscoliosis. Clin Neuropharmacol. 1991;14:367–371. doi: 10.1097/00002826-199108000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Narayan RK, Loubser PG, Jankovic J, Donovan WH, Bontke CF. Intrathecal baclofen for intractable axial dystonia. Neurology. 1991;41:1141–1142. doi: 10.1212/wnl.41.7.1141. [DOI] [PubMed] [Google Scholar]
- 43.Berardelli A, Rona S, Inghilleri M, Manfredi M. Cortical inhibition in Parkinson’s disease: a study with paired magnetic stimulation. Brain. 1996;119:71–77. doi: 10.1093/brain/119.1.71. [DOI] [PubMed] [Google Scholar]
- 44.Ridding MC, Inzelberg R, Rothwell JC. Changes in excitability of motor cortical circuitry in patients with Parkinson disease. Ann Neurol. 1995;37:181–188. doi: 10.1002/ana.410370208. [DOI] [PubMed] [Google Scholar]
- 45.Duvoisin RC, Marsden CD. Note in the scoliosis of Parkinsonism. J Neurol Neurosurg Psychiatr. 1975;38:787–793. doi: 10.1136/jnnp.38.8.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baik JS, Kim JS, Park HP, Han SW, Park JH, Lee MS. Scoliosis in patients with Parkinson’s disease. J Clin Neurol. 2009;5:91–94. doi: 10.3988/jcn.2009.5.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grimes JD, Hassan MN, Trent G, Halle D, Armstrong G. Clinical and radiographic features of scoliosis in Parkinson disease. Adv Neurol. 1986;45:353–355. [PubMed] [Google Scholar]
- 48.Cahn S, Herzog AG, Pascual-Leone A. Paired-pulse transcranial magnetic stimulation: effects of hemispheric laterality, gender, and handedness in normal controls. J Clin Neurophys. 2003;20:371–374. doi: 10.1097/00004691-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Takeuchi N, Chuma T, Ikoma Y, Matsuo Y, Mano Y. The intracortical inhibition on the motor cortex contralateral to normal hands in patients with dystonia: a study using paired transcranial magnetic stimulation. Int Congress Series. 2005;1278:257–259. doi: 10.1016/j.ics.2004.11.120. [DOI] [Google Scholar]
- 50.Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, Cohen LG. Intracortical inhibition and facilitation in different representation of the human motor cortex. J Neurophysiol. 1998;80:2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- 51.Kimiskidis VK, Potoupnis M, Papagiannopoulos SK, Dimopoulos G, Kazis DA, Markou K, Zara F, Kapetanos G, Kazis AD. Idiopathic scoliosis: a transcranial magnetic stimulation study. J Muskuloskelet Neuronal Interact. 2007;7:155–160. [PubMed] [Google Scholar]
- 52.Ziemann U, Lonecker S, Steihoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability: a transcranial magnetic stimulation study. Ann Neurol. 1996;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- 53.Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after transcranial brain stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-L. [DOI] [PubMed] [Google Scholar]
- 54.Inghilleri A, Beradelli A, Marchetti P, Manfredi M. Effects of diazepam, baclofen and thiopental on the silent period evoked by transcranial magnetic stimulation in humans. Exp Brain Res. 1996;109:467–472. doi: 10.1007/BF00229631. [DOI] [PubMed] [Google Scholar]
- 55.Mavroudakis N, Caroyer JM, Brunko E, Zegers de Beyl D. Abnormal motor evoked responses to transcranial magnetic stimulation in focal dystonia. Neurology. 1995;45:1671–1677. doi: 10.1212/wnl.45.9.1671. [DOI] [PubMed] [Google Scholar]
- 56.Rona S, Berardelli A, Vacca L, Inghilleri M, Manfredi M. Alterations of motor cortical inhibition in patients with dystonia. Mov Disord. 1998;13:118–124. doi: 10.1002/mds.870130123. [DOI] [PubMed] [Google Scholar]
- 57.Stinear CM, Byblow WD. Task dependent modulation of silent period duration in focal hand dystonia. Mov Disord. 2005;20:1143–1151. doi: 10.1002/mds.20514. [DOI] [PubMed] [Google Scholar]