Abstract
The radicular pain syndrome is a major problem in public health care that can lead to chronic back and leg pain in 30%. Ischalgia and back pain are the most prominent signs of dorsal root affection. Until now, no clinical or neurophysiological test procedure exists that evaluates the function of the dorsal root and predicts the prognosis of patients suffering from RPS. We have recently demonstrated that laser-evoked potentials (LEP) are able to demonstrate dorsal root damage. With this study, we investigated 54 patients with acute radicular symptoms and compared LEP parameters (side to side difference of latency and amplitude, transformed to a z-score) with their state of health after 3 months to calculate their predictive value for outcome prognosis. Most significantly, the latency difference between the LEP of the affected dermatome relative to the contralateral healthy dermatome was able to predict the prognosis. Latency z score above two demonstrates a 91% specificity (33% sensitivity) for a poor outcome at 3 months. A significant relation between amplitude changes and the main outcome measure could not be shown. Only extreme changes (z score >10) in amplitude show a high specificity for the persistence of ischialgia in particular (specificity 0.94; sensitivity 0.35). All other parameters, such as clinical scores or other LEP parameters, were not able to predict the outcome of patients. We propose that clinical testing using LEP with latency analysis is a useful tool for estimating the course of disease, so that patients with poor predictive parameters can be treated more invasively at early disease stages to avoid persistence of radiculopathy.
Keywords: Laser-evoked potential, Sciatica, Dorsal root impairment, Prognosis, Radiculopathy
Introduction
The radicular pain syndrome (RPS) is a frequent disease treated by a variety of medical specialists including orthopedic surgeons, neurosurgeons, neurologists and general practitioners. Whereas clinical examination and additional imaging are the usual basis for diagnosis of RPS, there is currently no method available to test the function of the dorsal root and its importance for prognosis of the disease. This is a relevant drawback because up to 30% of patients are expected to develop chronic RPS [10, 16]. Except for the cases with absolute and immediate indications for surgery, 3 months of conservative treatment generally pass before surgical intervention is considered, even if symptoms like ischialgia, a positive Lasègue sign or poor subjective health status have persisted longer than 3 months [19].
Early differentiation of the rather homogenous clinical symptomatology by an objective neurophysiologic test is necessary for undertaking early therapeutic interventions that could reduce the high rate of chronic RPS. Aside from the clear benefit to the patient, such a test would have enormous socio-economical impact because of the great prevalence of this disease among working populations in industrial countries.
Dermatomal laser-evoked potentials (LEP) elicited by slightly painful infrared laser stimuli proved to be a useful tool to examine pain pathways [2]. Previously, our group has shown that LEP yield a significantly higher sensitivity than the standard electrical SEP when probing the function of a single dorsal root [13, 18]. Others showed a clear correlation between functional loss of the dorsal root and the degree of mechanical and chemical irritation leading to dorsal root damage by disc herniations [23]. These pathophysiologic events are relevant factors for poor prognosis.
These findings raised the question of whether functional testing of the dorsal root by LEP obtained in the acute state of RPS can predict the outcome and course of disease. An evaluation of the prognostic value of early LEP changes appeared promising based on our experience that acute patients differ in the type of LEP changes as characterized by amplitude decrements or latency delays. This finding could indicate different severities or pathological processes at the dorsal root that are relevant criteria for predicting long-term outcome [17]. For this reason, we recorded LEP in 54 patients with acute radicular symptoms and performed a second measurement 3 months later. The outcome of radiculopathy was determined by clinical criteria.
Materials and methods
Patients
Fifty-four patients who suffered from acute sciatica due to the compression of a single nerve root by a herniated disc were included in the study. Clinical inclusion and exclusion criteria are summarized in Table 1. Morphological correlates of dorsal root impairment were proven by magnetic resonance imaging (MRI) in all patients. Conservative treatment was performed by analgesic drugs (non-steroidal drugs, metamizol, opioids if necessary) and physiotherapy (neurodynamic mobilization, isometric exercises). The last intake of analgesics was not shorter than 6 h before measurement. Treatment was not influenced or changed due to the study protocol. Demographic patients’ data and clinical appearance are summarized in Table 2.
Table 1.
Patients included had to fulfill all criteria of “minimal” and at least one criterion of “additional”
| Clinical inclusion and exclusion criteria | ||
|---|---|---|
| Minimal | Additional | Exclusion |
| Monodermatomal radicular pain down to the distal third of the lower leg | Positive sign of Lasègue | Any previous spine surgery or surgery of the central nervous system (CNS) |
| Pain intensity of 3–8 on the numerical rank scale (NRS from 0 = no pain to 10 = highest imaginable pain) | Monosegmental paresis (grade III° or less) | Infectious, inflammatory or neoplastic disease |
| First event | Monodermatomal dysesthesia or paresthesia | Epidural injections within 1 week before the LEP-application |
| Onset of symptoms not exceeding 10 weeks | Bilateral symptoms, bi- or multiradicular impairment | |
| Any metabolic disease affecting central or peripheral conduction velocity | ||
| Disease of the CNS or the peripheral nervous system | ||
Patients were excluded when one or more of the “exclusion” criteria were positive
Table 2.
Demographic and clinical data in patients with acute radiculopathy
| Patient | Gender | Age (years) | Length (cm) | Root affected | Motor paresis | Sharp/blunt discrimination | Temperature discrimination | Tactile vibration |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 33 | 161 | S1 right | 2/5 | Normal | Normal | 8/8 |
| 2 | Female | 74 | 162 | L5 right | 4/5 | Reduced | Normal | 6/8 |
| 3 | Male | 35 | 168 | S1 right | No | Reduced | Reduced | 6/8 |
| 4 | Male | 48 | 172 | L5 right | 4/5 | Reduced | Normal | 5/8 |
| 5 | Male | 50 | 168 | L3 right | No | Normal | Normal | 8/8 |
| 6 | Male | 65 | 178 | L5 right | 3/5 | Normal | Reduced | 4/8 |
| 7 | Male | 45 | 195 | S1 right | 3/5 | Reduced | Reduced | 6/8 |
| 8 | Male | 45 | 169 | S1 left | No | Normal | Reduced | 7/8 |
| 9 | Male | 60 | 167 | L5 left | 2/5 | Reduced | Reduced | 7/8 |
| 10 | Male | 25 | 164 | S1 right | No | Reduced | Reduced | 6/8 |
| 11 | Female | 68 | 163 | L5 left | No | Normal | Normal | 6/8 |
| 12 | Female | 66 | 158 | L5 left | 4/5 | Normal | Normal | 8/8 |
| 13 | Male | 39 | 186 | S1 left | No | Reduced | Reduced | 7/8 |
| 14 | Female | 46 | 170 | S1 left | No | Reduced | Reduced | 5/8 |
| 15 | Male | 21 | 179 | S1 left | No | Normal | Normal | 7/8 |
| 16 | Female | 24 | 165 | S1 left | 4/5 | Reduced | Normal | 6/8 |
| 17 | Male | 41 | 172 | L5 right | 4/5 | Reduced | Reduced | 6/8 |
| 18 | Male | 43 | 180 | S1 left | No | Reduced | Normal | 6/8 |
| 19 | Male | 68 | 180 | L5 left | No | Reduced | Normal | 4/8 |
| 20 | Male | 33 | 173 | S1 left | No | Reduced | Normal | 5/8 |
| 21 | Male | 56 | 172 | L4 left | No | Reduced | Reduced | 4/8 |
| 22 | Female | 51 | 167 | L4 left | No | Normal | Normal | 5/8 |
| 23 | Male | 49 | 173 | S1 right | 4/5 | Reduced | Reduced | 7/8 |
| 24 | Male | 59 | 181 | L5 left | No | Reduced | Reduced | 7/8 |
| 25 | Female | 64 | 167 | S1 right | No | Reduced | Normal | 7/8 |
| 26 | Male | 41 | 184 | L5 right | 4/5 | Normal | Normal | 7/8 |
| 27 | Male | 49 | 184 | S1 left | No | Reduced | Normal | 5/8 |
| 28 | Male | 43 | 183 | L5 right | 3/5 | Reduced | Normal | 4/8 |
| 29 | Male | 53 | 189 | L5 right | 4/5 | Reduced | Normal | 7/8 |
| 30 | Female | 45 | 166 | S1 right | No | Normal | Reduced | 6/8 |
| 31 | Male | 26 | 189 | S1 right | 4/5 | Reduced | Normal | 6/8 |
| 32 | Male | 27 | 183 | S1 left | No | Normal | Normal | 5/8 |
| 33 | Male | 23 | 186 | S1 left | No | Normal | Normal | 8/8 |
| 34 | Female | 39 | 175 | S1 left | No | Reduced | Reduced | 6/8 |
| 35 | Male | 44 | 178 | S1 left | 4/5 | Reduced | Reduced | 8/8 |
| 36 | Male | 67 | 176 | S1 right | No | Reduced | Reduced | 7/8 |
| 37 | Male | 27 | 186 | S1 left | No | Reduced | Reduced | 7/8 |
| 38 | Female | 24 | 163 | S1 right | 4/5 | Reduced | Reduced | 6/8 |
| 39 | Male | 56 | 170 | L5 right | No | Reduced | Reduced | 8/8 |
| 40 | Male | 44 | 188 | S1 left | 4/5 | Reduced | Reduced | 3/8 |
| 41 | Male | 66 | 178 | L4 right | No | Normal | Normal | 6/8 |
| 42 | Female | 60 | 168 | L5 left | No | Reduced | Reduced | 4/8 |
| 43 | Female | 77 | 168 | L5 left | 4/5 | Reduced | Normal | 2/8 |
| 44 | Female | 36 | 168 | L5 right | 4/5 | Reduced | Reduced | 6/8 |
| 45 | Male | 38 | 189 | S1 right | 4/5 | Reduced | Reduced | 5/8 |
| 46 | Male | 37 | 186 | L5 left | No | Normal | Normal | 4/8 |
| 47 | Male | 22 | 181 | L5 left | 0 | Normal | Normal | 4/8 |
| 48 | Female | 41 | 173 | S1 right | 4/5 | Reduced | Reduced | 5/8 |
| 49 | Male | 28 | 193 | S1 right | 4/5 | Reduced | Reduced | 7/8 |
| 50 | Female | 26 | 170 | S1 right | No | Normal | Reduced | 7/8 |
| 51 | Male | 45 | 178 | L5 left | 3/5 | Reduced | Reduced | 6/8 |
| 52 | Male | 45 | 183 | S1 right | No | Normal | Normal | 8/8 |
| 53 | Male | 28 | 170 | S1 left | No | Normal | Normal | 7/8 |
| 54 | Male | 36 | 185 | S1 left | No | Normal | Normal | 6/8 |
Patients were recruited consecutively into the study after providing written informed consent. Participation in the study was voluntary, and patients were assured in writing that refusal to participate would not affect their care in any way. The local ethics committee approved the protocol. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization (ICH), Good Clinical Practice (GCP) and Good Epidemiological Practice (GEP) guidelines.
Laser method
A commercial thulium YAG laser stimulator designed for neurologic applications was used (Neurolaser, wavelength 1,960 nm, pulse duration 1 ms, 5 mm diameter; StarMedTec GmbH, Starnberg, Germany). Stimulus intensity was set to 1.5 and 2 times pain threshold. The interstimulus intervals were randomized from 10 to 20 s (a mean of 15 s). Three-seconds after each stimulus, a tone prompted the subjects to rate their perceived sensations on a numerical rank scale (NRS) ranging from 0 to 10. Individual side differences were calculated as well (NRShealthy − NRSaffected).
Laser stimuli were applied to the affected and to the corresponding contralateral healthy dermatome. The laser was shifted after each stimulus within the dermatome to avoid receptor habituation. Each test side was examined twice by blocks of 40 stimuli (10 min) in a balanced sequence (control–affected–affected–control) to minimize a decrement of vigilance over the session [2]. The electroencephalogram (EEG) was recorded over Fz, Cz, Pz, T3 and T4 according to the 10–20 electrode positioning system and referenced against linked ear lobes using a Nicolet® amplifier with a CED 1401 AD converter. An electrooculugram (EOG) was recorded as well to control for eye artefacts. All electrode impedance was kept below 5 kΩ. Data was recorded with a band pass filter of 0.016–70 Hz (−12 dB/octave cut off) and a sampling rate of 200 Hz. Following the visual inspection and rejection of contaminated EEG artefacts, peristimulus EEG segments of 2.6 s (100 ms before and 1,000 ms after stimulus onset) were averaged offline over the stimulus blocks.
The resulting dermatomal LEP were evaluated for latency [mean latency of the large vertex negativity (N2) and the following positivity (P2)] and the corresponding amplitude (differences between N2 and P2) in channel Cz, where pain related LEP are known to be maximal [2]. A representative LEP curve is presented in Fig. 1. The mean N2–P2 latency difference in milliseconds between the healthy and affected side was calculated (healthy–affected). For the amplitude analysis, the percentual side to side differences for the LEP N2–P2 peak-to-peak amplitude were calculated to normalize the data for inter-individual differences according to the formula:
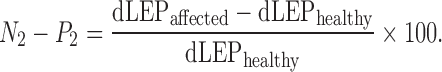 |
(dLEP = dermatomal LEP).
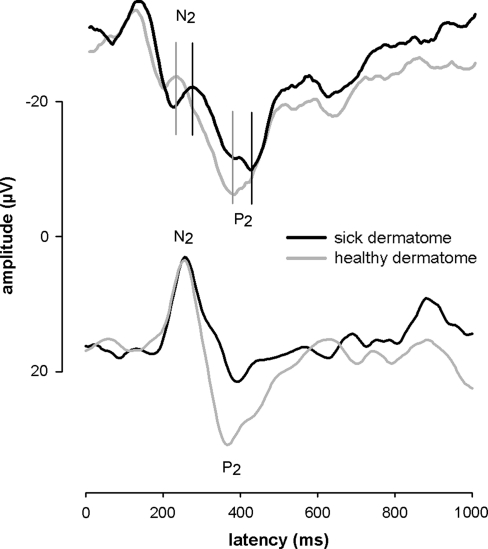
Fig. 1.
Typical LEP curves of two patients averaged after stimulation of an S1 dermatome on the healthy and affected side of the body. a The curve of the sick dermatome has remarkably longer N2–P2 latencies compared with the healthy dermatome. The vertical lines indicate latency delay between healthy and sick dermatome. b The curve of the sick dermatome has a remarkably lower N2–P2 amplitude and longer N2–P2 latencies compared with the healthy dermatome
To normalize the pathological side to side differences in latency and amplitude, we transformed the data to a z score. Therefore, we used the mean and standard deviation (latency = 19.3 ms, ±11.3 ms; amplitude = 10.8 ± 5.5%) values for latency and amplitude of a norm data collective already published, using the same stimulus and recording equipment. The z score was then calculated as follows using the mean values (mean) and standard deviations (SD) of the norm data:
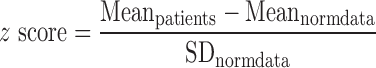 |
Timetable and outcome criteria
Clinical examinations and LEP were recorded during the first 8 weeks (T0) after the onset of symptoms and 3 months later (T1). To measure the clinical outcome after 3 months, we documented persisting leg pain (grade 1 = down to the middle of the thigh, grade 2 = down to the middle of the lower leg, grade 3 = down to the foot), persisting Lasègue-sign (grade 1 ≥60°, grade 2 = 30°–60°, grade 3 = below 30°) and the subjective health status on a scale from 0 (worse status) to 100 (best status). According to the age-related norm data of the VAS, a poor subjective health status was defined by values <75 (grade 1) and a normal subjective health status by values ≥75 (grade 0). To achieve a general parameter to classify the patients’ outcome, a poor outcome criterion (outbad) was calculated based on the clinical parameters, Lasègue-sign, leg pain and subjective health status, according to the formula:
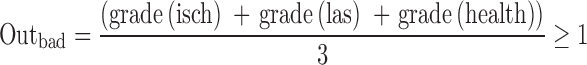 |
Statistical analysis
Statistical analysis was performed using SPSS© Version 9.0 (SPSS Inc.) and MATLAB© (The Mathworks, Inc.). Data were tested for normal distribution by a Kolmogorov–Smirnov test. To test the effects of pain stimulation, a 2 × 2 × 2 way ANOVA, with the variables of side (affected vs. healthy), block (first block vs. repetition) and intensity (high vs. low), was calculated for the pain ratings. The co-variables, gender, age, and therapy, were statistically evaluated as well. To increase the signal-to-noise ratio for the LEP amplitude detection, data within each subject were averaged over the variables block and intensity to analyze the differences between healthy and affected dermatomes using only a paired t test. Furthermore, for each clinical classification parameter of T1 (Lasègue-sign, ischialgia and subjective health status), we used all neurophysiological (latency and amplitude of LEP) and clinical parameters (rating, clinical neurological test procedure) of T0 for multiple stepwise Wilks’ Lambda discriminant analysis. Briefly, linear discriminant analysis is used to find a predictive model of group membership (good vs. poor outcome) based on the characteristics of each case (LEP and clinical T0 parameters). The resulting combinations may be used as a linear classifier. Furthermore, we conducted discriminant analysis for the pooled outcome score (outpoor, see above) and calculated a classifier function for the predictor N2–P2 latency, which fitted a multivariate normal density to each outcome group, with a pooled estimate of covariance.
To estimate the sensitivity and specificity of LEP changes concerning the prognostic impact the receiver operator curve (ROC) were calculated for those LEP parameters that showed a significant relationship to the main outcome measures. We used calculations from the statistical toolbox in Matlab (Matlab 7.4, The Mathworks Illinois). In a first step, LEP latencies and amplitude values were classified according to a linear discriminant function to plot the ROC curve fitting a multivariate normal density to each group (bad or good outcome), with a pooled estimate of covariance. Then, we applied a curve-fitting algorithm to calculate the function of the curve using the following formula:
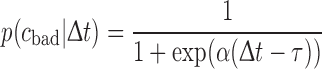 |
whereas Δt is the difference of the LEP parameter between affected and the normal side and α = 0.046 und τ = 25 are constants.
Results
Pain ratings
The ANOVA for the pain ratings revealed a significant side-specific effect (F(1,53) = 21.3, P < 0.001), indicating lower pain ratings for the affected side (NRS = 2.7, ±0.9) relative to the healthy side (NRS = 3.2, ±0.8). Furthermore, a significant block effect was found (F(1,53) = 43.2, P < 0.001), indicating lower pain ratings for the first block (NRS = 3.2, ±0.8) compared with the repetition (mean = 2.7, ±0.8). An effect of stimulus intensity was also detected (F(1,53) = 79.8, P < 0.001) indicating lower pain ratings for low-intensity laser stimuli (NRS = 2.7, ±0.9) compared with high-intensity stimuli (NRS = 3.2, ±0.8). No significant interactions were found between these variables. The discriminant analysis of the side differences of the pain ratings at T0 predicted neither a single criterion at T1 (positive Lasègue test Wilks’ Lambda = 0.97, F = 0.9, P = 0.41; ischialgia Wilks’ Lambda = 0.89, F = 2.1, P = 0.11; subjective health status Wilks’ Lambda = 0.97, F = 0.5, P = 0.68) nor the general outcome (Wilks’ Lambda = 1, 0, F = 0.005, P = 1.0).
Latency
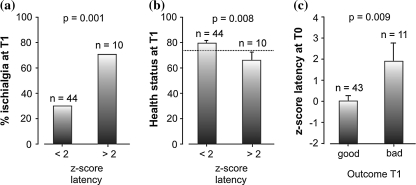
N2–P2 latency of all patients at T0 was significantly (T(53) = 4.0, P < 0.001) prolonged on the affected side (latency = 346 ms, ±74 ms) compared with the healthy side (latency = 331 ms, ±68 ms), which indicates a nerve conduction dysfunction at the affected side. Figure 1a illustrates a single patient LEP with a clear latency prolongation of 50.4 ms for the LEP N2–P2-wave. Z-transformation with the norm data collective results in a z score of 2.8 for this patient. For all 54 patients, 10 patients had a latency z score above 2, while 7 out of these 10 patients had a resisting ischialgia 3-month later at T1 (Fig. 2a). Furthermore, the 10 patients with a higher latency z score had significantly (T(52) = 2.8, P = 0.008) lower health status values 3 months later when compared with the patients without latency alterations (Fig. 2b). Interestingly, the patient outcome could be predicted by the LEP latency (Wilks’ Lamda = 0.86, F = 8.23, P < 0.001). Patients with a good outcome (n = 43) had significant (T(52) = 2.8, P = 0.009) lower latency z-scores (0.1 ± 1.8) compared with patients with a poor (n = 11) outcome (1.9 ± 3.0, Fig. 2c).
Fig. 2.
The LEP N2–P2 latency at the first measure (T0) predicts clinical outcome 3-month later (T1). In the study, collective of 10 out of 54 patients had latency z scores >2 in the LEP measurement at T0. Part a and b of the figure show the different outcome of these 10 patients compared with 44 patients with a z score <2. The columns indicate mean values, the error bars in figure b and c indicate standard errors. a Patients with longer N2–P2 latencies at T0 (z score >2) have a higher risk (P = 0.001) of persisting ischialgia at T1. b Patients with longer N2–P2 latencies at T0 (z score >2) significantly (P = 0.033) more often rate their subjective health status as poor (values below 75, indicated by dashed line) 3-month later. c Patients with longer N2–P2 latencies at T0 (z score >2) have a higher risk (P < 0.001) of bad outcome at T1
Amplitude
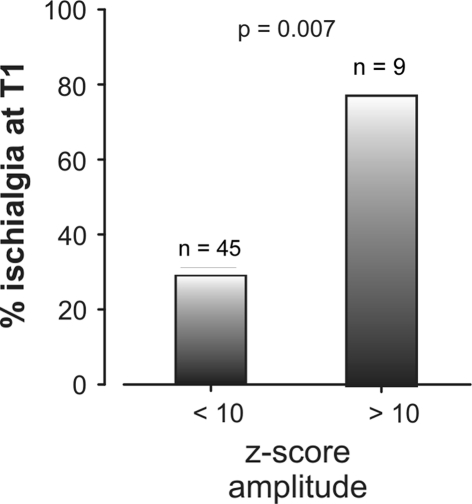
N2–P2 LEP amplitude at T0 was significantly (T(53) = −3.7, P < 0.01) reduced on the affected side (13.3, ±9.9 μV) compared with the healthy side (16.7, ±12.3 μV), which indicates a nerve dysfunction due to the less information transfer through the affected side for the mean of all 54 patients. Figure 1b illustrates a single patient LEP, which also shows besides the latency prolongation, a clear side difference of 31% (9.5 μV, z score 3.7) for the N2–P2 LEP amplitude. Interestingly, the LEP amplitude differences at T0 were able to predict a persistence of ischialgia at 3-month later (Wilks’ Lamda = 0.77, F = 4.54, P = 0.007). This relationship occurred at high z scores, indicating that only very high intra-individual amplitude side differences near to a complete loss of the LEP (z score >10, 9 patients out of 54) have prognostic relevance (Fig. 3). However, discriminant analysis revealed no predictive value of LEP amplitude differences at T0 with respect to “positive Lasègue sign” (Wilks’ Lamda = 0.98, F = 0.57, P = 0.56), “worse subjective health status” (Wilks’ Lamda = 0.93, F = 1.1, P = 0.33), or general outcome at T1.
Fig. 3.
Rate of persisting ischialgia (%) at T1 is dependent on LEP measurements 3 months earlier at T0. Patients with an amplitude z score ≥10 have a significantly higher risk of suffering from persistent ischialgia relative to patients with an amplitude z score <10. Z scores ≥10 indicate a significant increase in the risk of suffering from persistent ischialgia in a 3-month follow-up
Sensitivity and specificity
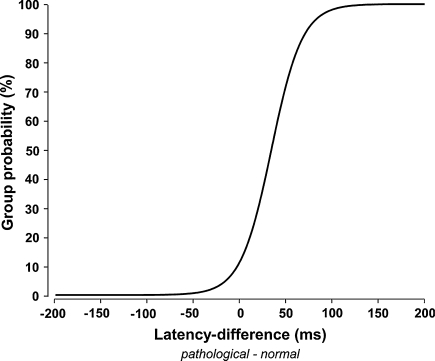
The ROC curve for the latencies (Fig. 4) show the probability of group membership (bad or good outcome) for an individual z latency score. Sensitivity and specificity were calculated for LEP latency changes that showed a significant relation to the main outcome measures. Latency z score above 2 separates good and bad outcome with a good specificity of 0.91 and a low sensitivity of 0.33. A significant relation between amplitude changes and the main outcome measure could not be shown. According to this finding, amplitude changes with a z score above two showed a very poor specificity of 0.36 and a poor sensitivity of 0.62. A significant relation between outcome and amplitude changes was only shown for very high amplitude changes and a corresponding persistence of ischialgia in particular. Only in these cases amplitude changes (z score >10) indicate the persistence of ischialgia or a recovery with a specificity of 0.94 and a sensitivity of 0.35. Thus, only a total or nearly total amplitude loss of the LEP at the affected side indicated the outcome.
Fig. 4.
Receiver operator curve for the probability to have a bad outcome according to the latency difference between the normal and the affected side. The threshold for the 95% niveau of a bad outcome is at 87 ms latency difference
Discussion
This study proved for the first time the prognostic relevance of dermatomal LEP as objective electrophysiological method in patients suffering from acute lumbar radiculopathy. Our results, furthermore, suggest that dermatomal LEP may be a useful tool for estimating the prognosis of recovery and potential development of chronic RPS. For clinical purposes, we evaluated disease progression by analyzing signs and symptoms typically regarded as relevant to decide surgical intervention. Notably, LEP latency differences at an early stage of disease predicted the outcome after a 3-month follow-up, and the probability of poor outcome increased nearly linearly for latency differences of z scores higher than 2. The high specificity of the LEP latency changes allow to identify patients with a good prognosis, whereas the sensitivity for both, latency and amplitude changes, is notably smaller. When compared with the process of separation between good and bad outcome by a “wait and see” strategy LEP measurements can significantly improve the early diagnostic procedure in radiculopathy. Except for one particular sub-population amplitude changes are not suitable to calculate the prognosis. Even a z score above ten as a functional correlate of an LEP loss indicates the persistence of ischialgia with a reliably high specificity.
The potential prognostic relevance of electrophysiological test methods has also been considered in earlier studies. Several trials were made to objectively document and grade dorsal root damage using somatosensory evoked potentials (SEP) [1, 5–7, 21, 26]. The results of these studies indicate that this technique has significant limitations in detecting monosegmental dorsal root dysfunction. Because neither the large nerve stems nor the cutaneous branches of the myelinated Aβ-fiber spectra represent a single dorsal root, afferent neuronal activation reaches the spinal cord and brain along several adjacent dorsal roots, thereby bypassing the lesion [4, 8, 9, 11, 24]. Therefore, electrophysiological testing using SEP on the Aβ-fiber system is ineffective for clinical questions concerning dorsal root function.
Until now, no convincing criteria existed to estimate the prognosis of acute radiculopathy, although such criteria are highly desirable given the great emotional strain of the patients, the enormous socio-economical impact of the disease and the potential to develop chronic pain and disability [10]. Some minimal prognostic data that is currently used regarding the course of radiculopathy after lumbar disc surgery is not transferable to the general group of patients with acute radiculopathy, since surgery is only applied in <20% of all patients with a 2–3-month anamnesis [16]. Other authors identified the degree of preoperative deficits in pain and motor and sensory systems as a predictor for unfavorable outcome after surgery [10, 16, 19]. The same seems to be true for the duration of symptoms. For instance, Nygaard et al. [14] showed that the duration of an attack of sciatica before surgery was a possible predictive factor of the overall result after surgery for lumbar disc herniation in 93 consecutive patients [14]. Another study confirmed this correlation between preoperative symptom duration and outcome after surgery [14, 25]. Therefore, it is important to estimate a prediction of clinical outcome to decide how invasively a patient should be treated to achieve optimal clinical recovery. However, the correlation between symptom duration and a worse outcome after surgery will not help to identify patients at a risk for poor outcome in early stages of radiculopathy, but instead merely indicates that a portion of patients, but not which patient would probably benefit from early intervention. The current clinical practice of providing surgical intervention for patients with persistent symptoms after a waiting period represents a negative selection bias towards patients with more severe and possibly irreversible root damage that might discredit a surgical technique, that is, otherwise effective if performed earlier. Therefore, the application of LEP and pain-evoked potentials offers the diagnostic access to a single dorsal root by which it is possible to predict subsequent clinical outcome on an individual case basis.
The results of our work raise two main issues that should be considered in future studies with dermatomal LEP. First, latency changes are superior to amplitude changes in predicting the outcome in radiculopathy. Most likely, this is due to the effect of the different sources of irritation on latency or amplitude. Its independence of individual differences in nerve conduction velocity and body height renders the intra-individual side to side variability of LEP latency very small. Furthermore, unlike LEP amplitude, latency is not affected by variations of attention and arousal, unless the patients are not in deeper sleep stages [22]. Therefore, changes in conduction velocity that occur unilaterally even in a small region of the pathway, such as the dorsal root yield a reliable and valid latency delay of LEP. In contrast, if changes appear bilaterally, e.g. in patients with central neuropathic syndromes, LEP latency is less sensitive due to the importance of inter-individual variability [7]. The fact that only extremely high amplitude changes (z scores >10) indicated persistent dorsal root impairment in our study likely reflects the well-known properties of long-latency-evoked potentials across all sensory modalities that they critically depend on a variety of factors, such as brain morphology, artifacts, averaging techniques, electrode impedance, vigilance, and habituation [2, 12]. The high amplitude changes are nearly comparable to an LEP loss indicating a severe conduction block.
The fact that LEP latency is superior to amplitude in the prediction of the outcome of radiculopathy might, however, also be based on distinct pathophysiological processes for amplitude or latency alterations. This topic is widely discussed in a previous paper of the authors [17]. Mechanical compression can lead to partial or total functional loss of the affected nerve fibers [20]. Experimental graded compression of nerves results in latency prolongation and can cause a complete conduction block, which results in amplitude loss [20]. Hence, a total or partial amplitude loss may be an indicator of fundamental mechanical compression. In addition, the local metabolic effect on nerve roots or root sleeves by substances leaking from the nucleus pulposus that possess inflammatory property as indicated by leukotaxis and increase in vascular permeability is an important factor that may contribute to latency changes. One major experimental finding of studies dealing with these inflammatory changes was a decrease in the conduction velocity of nerve roots that was antagonized by anti-inflammatory substances [3, 15]. It is, therefore, possible that relevant biochemical lesions may be detected by prolonged LEP latencies. In light of the role of the myelin sheath for the development of chronic pain, these changes are of high interest.
Conclusion
In conclusion, this study identified the LEP method as a clinically relevant tool to estimate the prognosis of acute radiculopathy. In principle, it can be applied to patients at the earliest stages of disease. Future studies could evaluate the validity of this method for the initiation of early interventions and, ideally, serve to differentiate the efficacy of different therapies (decompression plus additional aggressive anti-inflammatory vs. decompression alone vs. anti-inflammatory alone). Thus, supplementing clinical diagnostics and imaging techniques (e.g. magnetic resonance imaging) with LEP results would help physicians pursue an optimal and timely therapeutic plan of therapeutic interventions in patients suffering from nerve root compression.
Acknowledgments
This study was funded by the Deutsche Forschungsgesellschaft (DFG, Qu 156/1-1).
References
- 1.Aminoff MJ, Goodin DS. Dermatomal somatosensory evoked potentials in lumbosacral root compression. J Neurol Neurosurg Psychiatry. 1988;51:740–742. doi: 10.1136/jnnp.51.5.740-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bromm L. Neurophysiological evaluation of pain. Electroenzephalogr Clin Neuropysiol. 1998;107:227–253. doi: 10.1016/S0013-4694(98)00075-3. [DOI] [PubMed] [Google Scholar]
- 3.Cornefjord M, Olmarker K, Rydevik R, et al. Mechanical and biochemical injury of spinal nerve roots: a morphological and neurophysiological study. Eur Spine J. 1996;5:187–192. doi: 10.1007/BF00395512. [DOI] [PubMed] [Google Scholar]
- 4.Dvonch V, Scarff T, Bunch WH, et al. Dermatomal somatosensory evoked potentials: their use in lumbar radiculopathy. Spine. 1984;9:291–293. doi: 10.1097/00007632-198404000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Dvorak J. Neurophysiologic tests in diagnosis of nerve root compression caused by disc herniation. Spine. 1996;21:39S–44S. doi: 10.1097/00007632-199601010-00009. [DOI] [PubMed] [Google Scholar]
- 6.Eisen A. The use of somatosensory evoked potentials for the evaluation of the peripheral nervous system. Neurol Clin. 1988;6:825–838. [PubMed] [Google Scholar]
- 7.Fisher MA. Electrophysiology of radiculopathies. Clin Neurophysiol. 2002;113:317–335. doi: 10.1016/S1388-2457(02)00018-4. [DOI] [PubMed] [Google Scholar]
- 8.Foerster O. The dermatomes in man. Brain. 1933;56:139. doi: 10.1093/brain/56.1.1. [DOI] [Google Scholar]
- 9.Inouye Y, Buchtal F. Segmental sensory innervation determined by potentials recorded from cervical spinal nerves. Brain. 2003;100:731–748. doi: 10.1093/brain/100.4.731. [DOI] [PubMed] [Google Scholar]
- 10.Ito T, Takano Y, Yuasa N. Types of lumbar herniated disc and clinical course. Spine. 2001;26:648–651. doi: 10.1097/00007632-200103150-00017. [DOI] [PubMed] [Google Scholar]
- 11.Liguori R, Krarup C, Trojaborg W. Determination of the segmental sensory and motor innervation of the lumbosacral spinal nerves an electrophysiological study. Brain. 1992;115(Pt 3):915–934. doi: 10.1093/brain/115.3.915. [DOI] [PubMed] [Google Scholar]
- 12.Lorenz J, Garcia-Larrea L. Contribution of attentional and cognitive factors to laser evoked brain potentials. Neurophysiol Clin. 2003;33:293–301. doi: 10.1016/j.neucli.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Lorenz J, Hansen C, Kunze K, et al. Sensory deficits of a nerve root lesion can be objectively documented by somatosensory evoked potentials elicited by painful infrared laser stimulations: a case study. J Neurol Neurosurg Psychiatry. 1996;61:107–110. doi: 10.1136/jnnp.61.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nygaard OP, Kloster R, Solberg T. Duration of leg pain as a predictor of outcome after surgery for lumbar disc herniation: a prospective cohort study with 1-year follow up. J Neurosurg. 2000;92:131–134. doi: 10.3171/spi.2000.92.2.0131. [DOI] [PubMed] [Google Scholar]
- 15.Olmarker K, Rydevik B. Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced thrombus formation, intraneural edema, and reduction of nerve conduction velocity: possible implications for future pharmacologic treatment strategies of sciatica. Spine. 2001;26:863–869. doi: 10.1097/00007632-200104150-00007. [DOI] [PubMed] [Google Scholar]
- 16.Postacchini F. Lumbar disc herniation: a new equilibrium is needed between nonoperative and operative treatment. Spine. 2001;26:601. doi: 10.1097/00007632-200103150-00008. [DOI] [PubMed] [Google Scholar]
- 17.Quante M, Hauck M, Gromoll M, et al. Dermatomal laser-evoked potentials: a diagnostic approach to the dorsal root. Norm data in healthy volunteers and changes in patients with radiculopathy. Eur Spine J. 2007;16:943–952. doi: 10.1007/s00586-006-0253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quante M, Lampe F, Hauck M, et al. Laser-evoked potentials: diagnostic approach to the dorsal root. Orthopade. 2003;32:852–858. doi: 10.1007/s00132-003-0530-2. [DOI] [PubMed] [Google Scholar]
- 19.Rothoerl RD, Woertgen C, Brawanski A. When should conservative treatment for lumbar disc herniation be ceased and surgery considered? Neurosurg Rev. 2002;25:162–165. doi: 10.1007/s101430100184. [DOI] [PubMed] [Google Scholar]
- 20.Sato K, Konno S, Yabuki S, et al. A model for acute, chronic, and delayed graded compression of the dog Cauda equina. Neurophysiologic and histologic changes induced by acute, graded compression. Spine. 1995;20:2386–2391. doi: 10.1097/00007632-199511001-00003. [DOI] [PubMed] [Google Scholar]
- 21.Seyal M, Sandhu LS, Mack YP. Spinal segmental somatosensory evoked potentials in lumbosacral radiculopathies. Neurology. 1989;39:801–805. doi: 10.1212/wnl.39.6.801. [DOI] [PubMed] [Google Scholar]
- 22.Spiegel J, Hansen C, Treede RD. Clinical evaluation criteria for the assessment of impaired pain sensitivity by thulium-laser evoked potentials. Clin Neurophysiol. 2000;111:725–735. doi: 10.1016/S1388-2457(99)00297-7. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi N, Yabuki S, Aoki Y, et al. Pathomechanisms of nerve root injury caused by disc herniation: an experimental study of mechanical compression and chemical irritation. Spine. 2003;28:435–441. doi: 10.1097/00007632-200303010-00005. [DOI] [PubMed] [Google Scholar]
- 24.Tokuhashi Y, Satoh K, Funami S. A quantitative evaluation of sensory dysfunction in lumbosacral radiculopathy. Spine. 1991;16:1321–1328. doi: 10.1097/00007632-199111000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Vroomen PC, Krom MC, Knottnerus JA. Predicting the outcome of sciatica at short-term follow-up. Br J Gen Pract. 2002;52:119–123. [PMC free article] [PubMed] [Google Scholar]
- 26.Walk D, Fisher MA, Doundoulakis SH, et al. Somatosensory evoked potentials in the evaluation of lumbosacral radiculopathy. Neurology. 1992;42:1197–1202. doi: 10.1212/wnl.42.6.1197. [DOI] [PubMed] [Google Scholar]