Abstract
Choosing the right operation for metastatic spinal tumours is often difficult, and depends on many factors, including life expectancy and the balance of the risk of surgery against the likelihood of improving quality of life. Several prognostic scores have been devised to help the clinician decide the most appropriate course of action, but there still remains controversy over how to choose the best option; more often the decision is influenced by habit, belief and subjective experience. The purpose of this article is to review the present systems available for classifying spinal metastases, how these classifications can be used to help surgical planning, discuss surgical outcomes, and make suggestions for future research. It is important for spinal surgeons to reach a consensus regarding the classification of spinal metastases and surgical strategies. The authors of this article constitute the Global Spine Tumour Study Group: an international group of spinal surgeons who are dedicated to studying the techniques and outcomes of surgery for spinal tumours, to build on the existing evidence base for the surgical treatment of spinal tumours.
Keywords: Spine, Tumour, Metastasis, Classification, Surgery, Outcome, GSTSG
Introduction
The spine is the commonest site for bone metastases, and the incidence of spinal metastases is increasing [1] and this is not surprising, with increasingly older populations, longer life expectancy, and improvements in medical treatment [2]. As many as 70% of cancer patients have spinal metastases, and up to 10% of cancer patients develop metastatic cord compression [3]. The commonest tumours that involve the spine are breast, lung, renal, prostate, thyroid, melanoma, myeloma, lymphoma and colorectal cancer [3, 4]. With improvements in chemotherapy, radiotherapy and hormonal therapies, survival times have increased over the years [5] and perhaps patients’ expectations also. Surgical techniques have also improved, which, together with advances in technology, now allow the surgeon to treat spinal metastases more effectively than before [6, 7].
The role of surgery for metastatic spinal tumours is again under the spot light: surgery can improve mechanical stability, cord compression, and pain, but what role does surgery play in extending life expectancy [4, 7–10]? Older techniques of decompression without stabilisation have resulted in a worse outcome, and this has misled many in the past to believe that radiotherapy is the preferred option to surgery [11–14]. More recent evidence has shown that modern surgery (including anterior and posterolateral approaches with stabilisation) does in fact result in a better outcome than radiotherapy alone, and that quality of life after surgery is often improved [4, 12, 15–17]. However, when deciding to operate, we must remember that most patients with metastatic spinal tumours have a life expectancy which is governed by the tumour type and staging, and is usually <1–2 years. Therefore, surgery must not detract from the remaining quality of life. The complication rate for surgery can be as high as 20–30%, and this must be weighed against the intended benefits [18–20]. This applies especially to the more extensive en bloc resections which are associated with increased complexity and morbidity when compared with simpler palliative debulking procedures [6, 7, 10, 21]. Although it is now accepted that surgery is commonly the preferred treatment for spinal metastases, more evidence is needed to define the role and indications of the various surgical techniques and new treatments available.
Generally, it is accepted that surgery might be considered when a patient has a life expectancy of more than 3 months [22]. This estimation is often made typically by oncologists, but it is the surgeon who more fully appreciates the potential risks and benefits of surgical options and, therefore, it is important for surgeons to understand how prognostic factors influence quality and duration of life. The purpose of this article is to review the present systems available for classifying spinal metastases, how these classifications can be used to help surgical planning, discuss surgical outcomes, and make suggestions for future research. It is important for spinal surgeons to use the same classification systems for the techniques of surgery, staging of tumours, and outcome, to reach meaningful comparisons between published series. The authors of this article constitute the Global Spine Tumour Study Group (GSTSG): an international group of spinal surgeons who are dedicated to studying the outcomes of surgery for spinal tumours [21]. The group is collecting data to answer specific questions which are further discussed below.
Classification of metastatic spinal tumours
Staging is mandatory and is often performed by oncologists unless surgery is urgent, for example, in patients with rapidly deteriorating neurological function. However, several surgeons have described methods of defining the extent of spinal involvement specifically to aid surgical planning and management. Some of these systems are based on the overall tumour load and functional status of the patient, whilst others focus on the anatomical extent of tumour involvement.
Scoring and classification systems
Surgeons need to be aware of the patient’s overall tumour load, life expectancy, quality of life, and other treatment options available, before deciding how ‘aggressive’ one should be with surgery. There will always be an element of risk when choosing to operate: if a complication occurs, this can quickly negate any intended benefit for a patient’s quality of life.
Several classification systems for surgical staging have been described in an attempt to inform surgical strategies [7, 10, 23]. Tomita et al. studied the numerous major and minor prognostic factors for spinal tumours to describe a system based on three factors: the rate of growth of the primary tumour, number of bone metastases and visceral metastases [7] (Table 1).
Table 1.
Tomita prognostic score [7]
| Score 1 | Score 2 | Score 4 | |
|---|---|---|---|
| Primary tumour | Slow growth | Moderate growth | Rapid growth |
| Visceral metastases | Treatable | Untreatable | |
| Bone metastases | Solitary | Multiple |
For each category (primary tumour, visceral and bone metastases) a score of 1, 2 or 4 is allocated according to the table above; these scores are added to provide a total score up to a maximum of 10
The scores of these three components were added together to produce a total score in the range 2–10 (from good to poor prognosis, respectively). This system was constructed from retrospective data of 67 patients between 1987 and 1991, and the prognostic factors were given weighted scores after assessment of their statistical hazard ratios. The histology of the primary tumour correlates well with survival in both surgical patients [7, 10, 24, 25] and medical cohorts [25–28], with longer survival times seen in patients with myeloma, breast, prostate and thyroid cancers. The primary tumour type was, therefore, given more weight in the scoring system of Tomita et al. [7].
However, Tokuhashi et al. described a scoring system based on six parameters, which they later revised to take account of the stronger influence of primary tumour type on survival [10, 23]. The system comprised individual scores for the primary site of cancer, presence or absence of paralysis, Karnofsky’s performance status, number of extraspinal bone metastases, vertebral body metastases and visceral metastases, producing a total score in the range 0–15 (from poor to good prognosis). Because the most important factor governing prognosis is the primary tumour type, the score gave more weight to the less aggressive tumours: five points for thyroid, breast prostate and carcinoid tumours; through to 0 points for lung, osteosarcoma, stomach, bladder, oesophageal and pancreatic tumours (Table 2). In the original paper of Tokuhashi et al. [23], it was interesting to note that there was no significant difference between survival times of different prognostic factors when analysed individually, whereas when grouped together to produce the score a significant difference became apparent. This suggests that for each prognostic factor the variation in survival is so large that one should not make judgements based on a single factor alone, for example, the primary tumour type, without taking into account the status of the whole patient. In their later paper [10], increasing the number of patients (and therefore the ability to detect smaller differences between groups) produced some statistically significant differences within individual categories, but of the six criteria evaluated no single group was able to demonstrate a consistent difference in survival.
Table 2.
Revised Tokuhashi prognostic score [10]
| Score 0 | Score 1 | Score 2 | Score 3 | Score 4 | Score 5 | |
|---|---|---|---|---|---|---|
| Karnofsky’s performance (%) | 10–40 | 50–70 | 80–100 | |||
| Extraspinal bone metastases | 3 or more | 1–2 | 0 | |||
| Vertebral metastases | 3 or more | 2 | 1 | |||
| Visceral metastases | Unremovable | Removable | None | |||
| Primary site (e.g.) | Lung | Liver | Other | Kidney | Rectum | Breast |
| Palsy | Frankel A, B | Frankel C, D | Frankel E |
Scores for the six individual criteria above are added to provide a total score up to a maximum of 15
It is interesting to note that Tokuhashi et al. found that paralysis was a prognostic factor in metastatic disease, whereas other studies of metastatic spinal cord compression and neurological symptoms did not show a direct correlation between neurological deficit and survival [29, 30]. It is, therefore, possible that paralysis is associated with an increased tumour load or rapid tumour growth, rather than being directly or independently related to poor survival. The significant influence of primary tumour type, neurological status and number of vertebral metastases was corroborated by other groups [29, 31, 32]. However, Enkaoua et al. found that patients with metastases from an unknown primary tumour had a worse prognosis than those with the identifiable tumours, unlike Tokuhashi’s original description, which was later revised [10, 23, 29]. Zou et al. found that the Tokuhashi score was better for predicting short-term survival, whereas the Tomita score was more useful for predictions of long-term survival [33].
The GSTSG recommend the use of the Tomita and Tokuhashi staging systems, which are relatively straightforward to use and interpret. However, assessing the validity of these scores has previously been confounded by the choice of operation; for example, patients with good prognostic scores have received en bloc resections, whereas poor prognostic groups have received palliative treatment, and, therefore, it is difficult to say to what extent survival is influenced by the prognostic score or the surgery itself. By collecting a large amount of prospective data, the GSTSG intend to analyse survival times in different prognostic groups, matched by operation, to eliminate bias and rigorously validate these scoring systems.
Other classification systems have been described by North et al. and Harrington [9, 34]. North et al. retrospectively, studied 61 patients and found that risk factors for the ability to walk include non-breast metastases, the inability to walk before surgery, and operations other than corpectomy [9]. They also found that risk factors for decreased survival include non-breast metastases, recurrence after primary radiotherapy, multilevel surgery, and cervical location of tumour. Harrington used a simpler 5-point classification system which was based on the degree of spinal instability and neurological compromise [34] (Table 3). He felt that surgery was indicated only in the presence of spinal instability or mechanical pain, and perhaps over-emphasised the advantages of radiotherapy over surgery, which has been clarified by more recent evidence of the benefits of surgical intervention [15]. The Harrington classification is perhaps an over-simplification, resulting in quite broad categories of patients who may have very different prognoses. For example, a patient with nerve root pain, but good function may be allocated into the same group as a patient with complete paralysis from a large tumour.
Table 3.
Harrington classification of spinal metastases [34]
| 1 | No neurological involvement |
| 2 | Bone involvement without collapse or instability |
| 3 | Significant neurological impairment without bone involvement |
| 4 | Vertebral collapse with pain or instability, but no neurological impairment |
| 5 | Vertebral collapse with pain or instability and neurological impairment |
Anatomical classifications
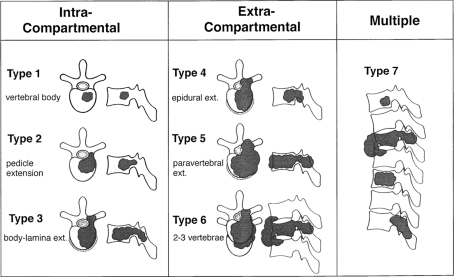
Anatomical classification systems can be useful for surgical planning, but are perhaps more suitable for the assessment of primary tumours rather than metastases. In general, to decide which type of operation to perform it is necessary to have more information than the anatomical context of the tumour alone. Tomita et al. devised a classification which comprises seven categories, depending on whether the metastasis is contained within the spinal bones (intracompartmental), out with the bones (extracompartmental), or multiple vertebral involvement (Fig. 1) [7]. This is a simple classification which is easy to remember and apply, and represents the natural stages of tumour progression from involvement of the vertebral body, to the pedicles and posterior elements, extradural and paravertebral spread, adjacent vertebrae and then multiple vertebrae. In practice, these stages do not necessarily occur in strict sequence, and usually types 4–7 are the levels of involvement which present to spinal surgeons.
Fig. 1.
Schematic diagram of the surgical classification of spinal tumours, from Tomita et al. [7] (with permission of Lippincott Williams and Wilkin)
Alternatively, McLain and Weinstein originally described the vertebral anatomy in terms of four zones and three concentric levels (Table 4) [35]. This scheme is very simple to use, but has the disadvantage that most spinal metastases would fall into the categories 3 and 4, resulting in a classification system that is not very discriminatory.
Table 4.
McLain and Weinstein classification [35]
| Zone 1 | The spinous process to the pars and inferior facet |
| Zone 2 | The superior facet, transverse process and pedicle |
| Zone 3 | Anterior three-fourth of the vertebral body |
| Zone 4 | Posterior one-fourth of the vertebral body |
| Level A | Intraosseous |
| Level B | Extraosseous |
| Level C | Distant tumour spread |
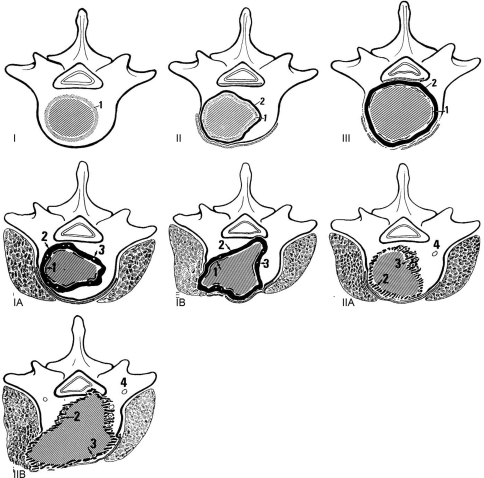
Enneking developed a classification system for primary long-bone tumours which has been adapted for use with spinal tumours. He described three stages of involvement of benign tumours, four stages for localised malignant tumours, and two further stages for metastatic high-grade tumours (Fig. 2) [36]. This system requires prior knowledge of the histology and degree of spread of the tumour throughout the body, which is not always available at the time of presentation. It may be applied to spinal tumours, but is not the most useful classification system, because it does not specifically document extradural spinal involvement and possible cord compression, and does not necessarily relate to prognosis.
Fig. 2.
The Enneking classification of primary tumour staging. Benign tumours are classified as stages I, II and III, depending on the tumour growth and aggressiveness (1 tumour capsule, 2 adjacent tissue reaction). Malignant tumours are classified as IA, IB, IIA and IIB depending on degree of spread (1 tumour capsule, 2 tissue reaction, 3 island of tumour within adjacent tissue reaction, 4 skip metastasis) [36] (with permission of Lippincott Williams and Wilkin)
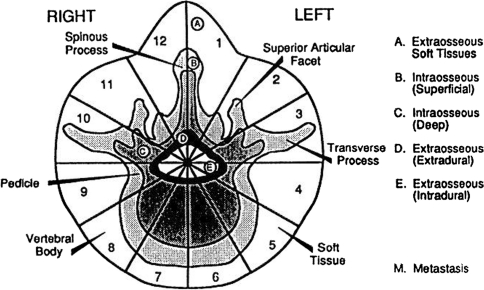
The shortcomings of the classification systems of McLain and Weinstein [35], and Enneking [36], were partly addressed by Boriani et al. who developed a new staging system for primary bone tumours [37] (Fig. 3). They designed a system for the anatomical staging of primary bone tumours of the spine to overcome the drawbacks of these other systems. Although originally intended for use with primary tumours, the system of Boriani et al. has sometimes been applied to spinal metastases also [37]. This system was designed to aid surgical planning, because en bloc resection usually involves the removal of wedges or sections of the vertebra from around the spinal cord, which must not be violated. However, it is not as useful for planning of metastatic tumour surgery, since en bloc resection is not the goal in the majority of such cases.
Fig. 3.
Weinstein, Boriani, Biagini (WBB) classification describes the vertebral involvement as sections of a clock face (“zones”) centred on the spinal cord, from zone 1 (left spinous process and lamina) through zone 6 (left anterior wedge of vertebral body) and back round to zone 12 (right spinous process and lamina). In addition, the prefixes A–E are used to denote radial levels (“layers”) of vertebral involvement, from extraosseous paraspinal tissues (layerA) through to extradural (layer D) and intradural (layer E) [37] (with permission of Lippincott Williams and Wilkin)
Although this and other anatomical classifications are useful, there is not much data to suggest a correlation between the use of these systems and clinical outcome, unlike the surgical staging systems of Tomita et al. [7] and Tokuhashi et al. [10]. The WBB system, for example, is very accurate in describing the axial tumour involvement, but the predictive relationship between WBB score and outcome is perhaps influenced more by the different types of surgery performed rather than the classification system itself.
Prognostic classification and surgical planning
Tomita et al. recommended that patients with very good prognostic scores (2–3) should undergo wide excision, whereas patients with intermediate scores should undergo marginal or intralesional excision (scores 4–5), and palliative surgery (scores 6–7), whilst non-surgical supportive care should be performed for the worst prognostic group (scores 8–10) [7]. Evaluation of this scoring system was performed prospectively in 61 patients from 1993 to 1996, in whom the score was used to determine the type of operation to be performed, and length of survival was documented. The mean survival was 38.2 months in those patients with a good prognostic score who underwent en bloc resection, 21.5 months in those patients with intermediate score who underwent intralesional debulking procedures, and 10.1 months in those patients who underwent palliative decompression and stabilisation only. These results suggest that their recommendations are reasonable and practical.
Tokuhashi et al. recommended excisional surgery for patients with a good prognosis (Tokuhashi score of 12–15), palliative surgery for most patients with an intermediate prognosis (score of 9–11), and conservative management for patients with a score of 8 or less [10]. They prospectively applied their scoring system to 118 patients to help determine the surgical strategy and found a good correlation between the prognostic score and the actual survival (r = 0.57, significant P < 0.0001), with a consistency rate between predicted and actual survival of 86.4%. This suggests that the scoring system of Tokuhashi et al. is also a useful tool for the assessment of prognosis in patients for whom surgery is being considered.
Recommendations of the GSTSG
Complications may occur in up to 25% of patients who undergo surgery for spinal metastases, the most common being wound infection [13, 17, 38]. Life expectancy is usually determined by the overall extent of the metastatic disease and, therefore, to be of benefit, surgery must improve quality of life. However, the incidence of complications increases with the complexity and extent of an operation, and, therefore, at some point there must be a trade-off between the benefits and risks of surgery [17]. Because surgery is palliative for the majority of patients with spinal metastases the assessment of overall quality of life is perhaps more relevant than physical scores and neurological outcome measures, and the GSTSG, therefore, advocates the use of quality of life measures for all patients undergoing surgery. Several studies have shown improvements in quality of life after surgery for metastases [4, 13, 39], with up to 80% of patients satisfied or very satisfied with the decision to operate [13, 39]. The greatest improvements are in the domains of pain, but also non-specific symptoms, such as tiredness, nausea, anxiety and appetite may improve after surgery [16]. The GSTSG uses the Euroquol EQ5D assessment tool for all patients with metastatic disease. This is a simple 5-point validated questionnaire that is simple for patients to complete and investigators to interpret [40].
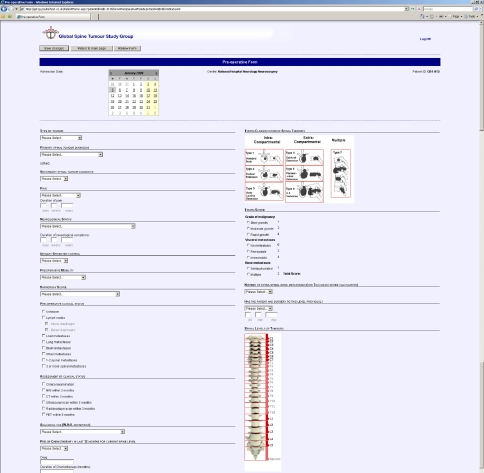
Owing to the heterogeneity of patients who are referred to spinal surgeons, the outcome of surgery for spinal metastases is variable and it is, therefore, difficult to come to generalised conclusions regarding the ideal management [3]. The strong beliefs that surgeons often hold regarding the ideal management create difficulties in performing a randomised study of surgical techniques. The best alternative is to prospectively collect a large amount of data from which the effect of confounding variables can be removed by patient matching [41]. It is necessary to acquire this data over a relatively short time span of a few years, to avoid time bias which might result from changes in technique or instrumentation. The GSTSG aims to acquire such data from multiple centres in a timely fashion, to compare the outcomes of different treatment groups and assess statistically significant differences in management, by collecting data on a secure internet database (Fig. 4).
Fig. 4.
Page snapshot of the Global Spine Tumour Study Group prospective database for metastatic tumour surgery. Preoperative data entry
One specific example is to compare the outcome of total en bloc spondylectomy (TES) with simpler debulking procedures, which are probably associated with fewer complications [42]. It has been suggested that TES should be reserved for patients with solitary spinal metastasis with otherwise good prognosis to justify taking this extra risk [6, 22, 43]. However, the true difference in outcome between TES and debulking surgery in a specific group of patients is not known, but this may be determined by analysing our database to control for confounding variables and obtain a more accurate estimation of the usefulness of these procedures, and whether the extra risk is justified by improved survival and quality of life.
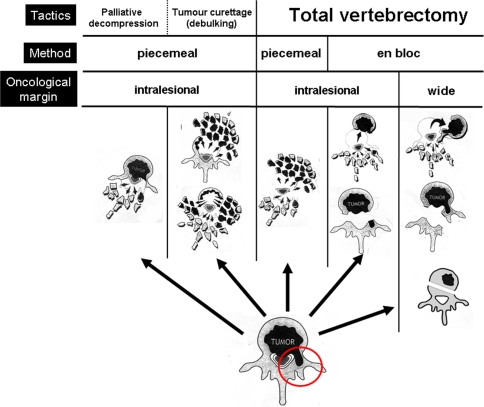
To collect data, it is important to be clear about how we define an operation, and to avoid using ambiguous terms which would invalidate the dataset. Boriani et al. stressed the importance of distinguishing between, and correctly using, the terms “radical, complete, extralesional and intralesional excision” [37]. The GSTSG has adopted a simple classification of surgical strategies, illustrated in Fig. 5, in which the excision of the involved vertebra is shown diagrammatically. The overall tactic of surgery may be for palliative decompression, tumour debulking, or total vertebrectomy (row 1, Fig. 5). The tactic chosen for a patient may then be achieved by piecemeal excision of tumour, or total vertebrectomy either by an en bloc or piecemeal method (row 2, Fig. 5). This method of excision will influence the tumour resection margins that may be intralesional, or wide/extralesional (row 3, Fig. 5). It is important for surgeons to be accurate and systematic in their description of surgical strategies and techniques, using a common surgical language, to allow meaningful comparisons of outcome. We present the first clear definitions for surgical methodology and strategy that may be applied to metastatic spinal tumours.
Fig. 5.
Classification of surgical strategies, as determined by the Global Spine Tumour Study Group
Other uses of the database include comparing the outcomes of different primary tumours, auditing complication rates, comparing surgical series to those of radiotherapy databases, and assessing quality of life. Presently, no published prognostic scoring system incorporates measures of quality of life beyond the Karnofsky’s score, and it may be important to incorporate these measures in future scores [44].
It is often difficult to acquire reliable evidence for the validity of surgical treatments. Unlike clinical drug trials, it is impossible or unethical to blind the surgeon and patient in a study of surgical treatments. Follow-up can also be more difficult when patients live long distances away, and loss to follow-up may be more common. However, as far as possible, it is still important to ensure that clinical practice of surgeons is influenced by a strong evidence base. Hosono et al. studied a large retrospective series of patients with spinal metastasis, and concluded “a large prospectively designed study of consecutive patients is essential to screen the possible prognostic factors in patients with spinal metastases” [25]: a viewpoint which we strongly advocate.
Acknowledgments
DePuy Spine funding for database design and IT support.
Footnotes
On behalf of the Global Spine Tumour Study Group.
References
- 1.Hatrick NC, Lucas JD, Timothy AR, et al. The surgical treatment of metastatic disease of the spine. Radiother Oncol. 2000;56:335–339. doi: 10.1016/S0167-8140(00)00199-7. [DOI] [PubMed] [Google Scholar]
- 2.Bailar JC, III, Gornik HL. Cancer undefeated. N Eng J Med. 1997;336:1569–1574. doi: 10.1056/NEJM199705293362206. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs WB, Perrin RG (2001) Evaluation and treatment of spinal metastases: an overview. Neurosurg Focus 11 (article 10) [DOI] [PubMed]
- 4.Ibrahim AG, Crockard HA, Antonetti P, et al. Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases? An international multi-centre prospective observational study of 223 patients. J Neurosurg Spine. 2005;8:271–278. doi: 10.3171/SPI/2008/8/3/271. [DOI] [PubMed] [Google Scholar]
- 5.Heary RF, Bono CM. Metastatic spinal tumors. Neurosurg Focus. 2001;11(6):e1. doi: 10.3171/foc.2001.11.6.2. [DOI] [PubMed] [Google Scholar]
- 6.Sakaura H, Hosono N, Mukai Y, et al. Outcome of total en bloc spondylectomy for solitary metastasis of the thoracolumbar spine. J Spinal Disord Tech. 2004;17:297–300. doi: 10.1097/01.bsd.0000096269.75373.9b. [DOI] [PubMed] [Google Scholar]
- 7.Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine. 2001;26:298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 8.Aebi M. Spinal metastasis in the elderly. Eur Spine J. 2003;12(Suppl 2):S202–S213. doi: 10.1007/s00586-003-0609-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.North RB, LaRocca VR, Schwartz J, et al. Surgical management of spinal metastases: analysis of prognostic factors during 10-year experience. J Neurosurg Spine. 2005;2:564–573. doi: 10.3171/spi.2005.2.5.0564. [DOI] [PubMed] [Google Scholar]
- 10.Tokuhashi Y, Matsuzaki H, Oda H, et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine. 2005;30:2186–2191. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 11.Findlay GF. Adverse effects of the management of malignant spinal cord compression. J Neurol Neurosurg Psychiatry. 1984;47:761–768. doi: 10.1136/jnnp.47.8.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinmetz MP, Mekhail A, Benzel EC. Management of metastatic tumors of the spine: strategies and operative indications. Neurosurg Focus. 2001;11(6):e2. doi: 10.3171/foc.2001.11.6.3. [DOI] [PubMed] [Google Scholar]
- 13.Weigel B, Maghsudi M, Neumann C, et al. Surgical management of symptomatic spinal metastases. Post-operative outcome and quality of life. Spine. 1999;24:2240–2246. doi: 10.1097/00007632-199911010-00012. [DOI] [PubMed] [Google Scholar]
- 14.Young RF, Post EM, King GA. Treatment of spinal epidural metastases. Randomized prospective comparison of laminectomy and radiotherapy. J Neurosurg. 1980;53:741–748. doi: 10.3171/jns.1980.53.6.0741. [DOI] [PubMed] [Google Scholar]
- 15.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 16.Wai EK, Finkelstein JA, Tangente RP, et al. Quality of life in surgical treatment of metastatic spine disease. Spine. 2003;28:508–512. doi: 10.1097/00007632-200303010-00018. [DOI] [PubMed] [Google Scholar]
- 17.Wise JJ, Fischgrund JS, Herkowitz HN, et al. Complication, survival rates, and risk factors of surgery for metastatic disease of the spine. Spine. 1999;24:1943–1951. doi: 10.1097/00007632-199909150-00014. [DOI] [PubMed] [Google Scholar]
- 18.Cooper PR, Errico TJ, Martin R, et al. A systematic approach to spinal reconstruction after anterior decompression for neoplastic disease of the thoracic and lumbar spine. Neurosurgery. 1993;32:1–8. doi: 10.1097/00006123-199301000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Gokaslan ZL, York JE, Walsh GL, et al. Transthoracic vertebrectomy for metastatic spinal tumours. J Neurosurg. 1998;89:599–609. doi: 10.3171/jns.1998.89.4.0599. [DOI] [PubMed] [Google Scholar]
- 20.Sundaresan N, Digiacinto GV, Hughes JE, et al. Treatment of neoplastic spinal cord compression: results of a prospective study. Neurosurgery. 1991;29:645–650. doi: 10.1097/00006123-199111000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Mazel C, Balabaud L, Bennis S, et al. Cervical and thoracic spine tumor management: surgical indications, techniques, and outcomes. Orthop Clin N Am. 2009;40:75–92. doi: 10.1016/j.ocl.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 22.National Collaborating Centre for Cancer (2008) Metastatic spinal cord compression. Diagnosis and management of adults at risk of and with metastatic spinal cord compression. NICE Guidelines CG75, TJ International Ltd, Cardiff, UK [PubMed]
- 23.Tokuhashi Y, Matsuzaki H, Toriyama S, et al. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine. 1990;15:1110–1113. doi: 10.1097/00007632-199011010-00005. [DOI] [PubMed] [Google Scholar]
- 24.Hirabayashi H, Ebara S, Kinoshita T, et al. Clinical outcome and survival after palliative surgery for spinal metastases. Cancer. 2003;97:476–484. doi: 10.1002/cncr.11039. [DOI] [PubMed] [Google Scholar]
- 25.Hosono N, Ueda T, Tamura D, et al. Prognostic relevance of clinical symptoms in patients with spinal metastases. Clin Orthop Relat Res. 2005;436:196–201. doi: 10.1097/01.blo.0000160003.70673.2a. [DOI] [PubMed] [Google Scholar]
- 26.Bartels RHMA, Feuth T, Maazen R, et al. Development of a model with which to predict the life expectancy of patients with spinal epidural metastasis. Cancer. 2007;110:2042–2049. doi: 10.1002/cncr.23002. [DOI] [PubMed] [Google Scholar]
- 27.Tatsui H, Onomura T, Morishita S, et al. Survival rates of patients with metastatic spinal cancer after scintigraphic detection of abnormal radioactive accumulation. Spine. 1996;21:2143–2148. doi: 10.1097/00007632-199609150-00017. [DOI] [PubMed] [Google Scholar]
- 28.Linden YM, Dijkstra SPDS, Vonk EJA, et al. Prediction of survival in patients with metastases in the spinal column. Cancer. 2005;103:320–328. doi: 10.1002/cncr.20756. [DOI] [PubMed] [Google Scholar]
- 29.Enkaoua EA, Doursounian L, Chatellier G, et al. Vertebral metastases: a critical appreciation of the preoperative prognostic Tokuhashi score in a series of 71 cases. Spine. 1997;22:2293–2298. doi: 10.1097/00007632-199710010-00020. [DOI] [PubMed] [Google Scholar]
- 30.Spiegel DA, Sampson JH, Richardson WJ, et al. Metastatic melanoma to the spine. Demographics, risk factors, and prognosis in 114 patients. Spine. 1995;20:2141–2146. doi: 10.1097/00007632-199510000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Sioutos PJ, Arbit E, Meshulam CF, et al. Spinal metastases from solid tumors. Analysis of factors affecting survival. Cancer. 1995;76:1453–1459. doi: 10.1002/1097-0142(19951015)76:8<1453::AID-CNCR2820760824>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 32.Zeng JC, Song YM, Liu H, et al. The predictive value of the Tokuhashi revised scoring system for the survival time of patients with spinal metastases (Chinese) Sichan Da Xue Xue Bao Yi Xue Ban. 2007;38:488–491. [PubMed] [Google Scholar]
- 33.Zou XN, Grejs A, Li HS, et al. Estimation of life expectancy for selecting surgical procedure and predicting prognosis of extradural spinal metastases. Ai Zheng. 2006;25:1406–1410. [PubMed] [Google Scholar]
- 34.Harrington KD. Metastatic disease of the spine. J Bone Joint Surg. 1986;68A:1110–1115. [PubMed] [Google Scholar]
- 35.McLain RF, Weinstein JN. Tumors of the spine. Semin Spine Surg. 1990;2:157–180. [Google Scholar]
- 36.Enneking WF, Spainer SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcomas. Clin Orthop. 1980;153:106–120. [PubMed] [Google Scholar]
- 37.Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine: terminology and surgical staging. Spine. 1997;22:1036–1044. doi: 10.1097/00007632-199705010-00020. [DOI] [PubMed] [Google Scholar]
- 38.Ghogawala Z, Mansfield FL, Borges LF. Spinal radiation before surgical decompression adversely affects outcomes of surgery for symptomatic metastatic spinal cord compression. Spine. 2001;26:818–824. doi: 10.1097/00007632-200104010-00025. [DOI] [PubMed] [Google Scholar]
- 39.Falicov A, Fisher CG, Sparkes J, et al. Impact of surgical intervention on quality of life in patients with spinal metastases. Spine. 2006;31:2849–2856. doi: 10.1097/01.brs.0000245838.37817.40. [DOI] [PubMed] [Google Scholar]
- 40.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 41.Choi D, Crockard HA. How and why should we benchmark clinical outcomes and quality of life for surgery in spinal metastases? Br J Neurosurg. 2009;23(1):3–4. doi: 10.1080/02688690802546872. [DOI] [PubMed] [Google Scholar]
- 42.Tomita K, Kawahara N, Baba H, et al. Total en bloc spondylectomy: a new surgical technique for primary malignant vertebral tumors of the spine. Spine. 1997;22:324–333. doi: 10.1097/00007632-199702010-00018. [DOI] [PubMed] [Google Scholar]
- 43.Liljenqvist U, Lerner T, Halm H, Buerger H, Gosheger G, Winkelmann W. En bloc spondylectomy in malignant tumors of the spine. Eur Spine J. 2008;17(4):600–609. doi: 10.1007/s00586-008-0599-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oberndorfer S, Grisold W. The management of malignant spinal cord compression. Spine. 2000;25:653–654. doi: 10.1097/00007632-200003010-00021. [DOI] [PubMed] [Google Scholar]