Abstract
Degenerative disc disease (DDD) causes gradual intervertebral space collapse, concurrent discogenic or facet-induced pain, and possible compression radiculopathy. A new minimal invasion procedure of percutaneous posterior-lateral lumbar interbody fusion (PPLIF) using a B-Twin stand-alone expandable spinal spacer (ESS) was designed to treat this disease and evaluated by follow-up more than 1 year. 12 cases with chronic low back pain and compressive radiculopathy due to DDD refractory were selected to conservative treatment. Under fluoroscopy in the posterior-lateral position, a K-wire was advanced into the intervertebral space and a dilator and working cannula were introduced into the disc space step by step. Discectomy and endplate scratching were performed through the cannula using pituitary forceps and endplate curettage. An ESS was inserted into the intervertebral space by a B-Twin expandable spinal delivery system after some bone graft chips implanted into the disc space. The ongoing study includes intraoperative difficulties, complications, radiologic evidence of fusion and clinical outcome as scored by pre- and postoperative questionnaires pertaining to pain intensity and degree of disability. The 12 procedures of lumbar interbody fusion using stand-alone expandable spinal system through percutaneous approach were successful. Radiologic study demonstrated fusion in a total of 11 cases and only 1 exception after more than 1 year visiting. The values of Visual Analog Scale (VAS) on movement and Oswestry Disability Index (ODI) dropped by more than 80 and 67.4%, respectively. Disk space heights averaging 9.0 mm before procedure were increased to 11.5 mm 1 month (a significant difference compared with preprocedure, P < 0.01) after surgery and stabilized at 10.8 mm upon final follow-up (a significant difference compared with preprocedure, P < 0.01). The results demonstrated that the percutaneous approach for posterior-lateral lumbar interbody fusion using expandable spinal system is a valuable micro-invasion method for the DDD patients and can achieve the same outcome as with other methods.
Keywords: Disk degenerative disease, Percutaneous, Lumbar interbody spinal fusion, Expandable spinal spacer, Stand-alone, B-Twin
Background
Advanced degenerative disc disease (DDD) is manifested by progressive collapse and consequent bulging of the redundant disc surface, the ligamenta flava, and the posterior longitudinal ligament. It makes the involved intervertebral space narrow and eventually causes arthrosis of the facet joints. These mechanical abnormalities tend to cause discogenic or facetogenic pain and may result in compressive radiculopathy. This kind of pain is refractory to conservative treatments, such as medication, physical therapy, and behavioral therapy. The structural solution of this problem requires reexpansion of the disc space to restore the intervertebral height and relieve foraminal stenosis. The conventional lumbar interbody fusion including the early posterior lumbar interbody fusion (PLIF) using autogenic bone graft or using conventional fusion cage (BAK or TFC) can provide great improvement but these surgeries require extensive tissue dissection and removal of lamina, ligaments and facet joint, which are posterior stabilizing structures. Those surgeries also need dural sac and nerve roots retraction to make way for the large cages which are introduced through the posterior aspect that increase the risk of dural laceration and neurologic damage [1–3].
With the development of the minimally invasive technique and the advanced medical equipment, a newly designed B-Twin ESS (B-Twin ESS) that is free of all the disadvantages enumerated was validated by a multicenter study [4]. The following study was carried out to evaluate the efficacy of our modification of minimally invasive unilateral percutaneous B-Twin stand-alone lumbar spinal fusion with one B-Twin ESS cage by posterior-lateral approach in 12 advanced DDD patients.
Methods
Patient selection
Inclusion criteria were disabling low back pain for more than 2 years, failed of conservative treatment or failed discectomy, distinct diagnosis of DDD on the basis of typical symptoms and diagnostic findings on radiologic imagings or discogram in one or two level between L2 and S1. Exclusion criteria included any disease that could affect bone quality (e.g., spine infection, tumor, metabolic bone disease), osteoporosis, metal allergies or spondylolisthesia up to type I.
Twelve patients with advanced DDD selected according to above criterias from December 2005 to June 2007 has received the procedure of unilateral percutaneous B-Twin stand-alone lumbar spinal fusion with one expandable implant at Shenzhen Nanshan hospital affiliated Guangdong medical college (Table 1). Four cases had histories of discectomy 1 or 2 years before. Seven patients with chronic low back pain and compressive radiculopathy were induced by collapse disc space. The study protocol was approved by the Human Ethics Review Committee of Guangdong medical school. The procedure and associated potential complications were explained to the patients, and informed consent was obtained before treatment.
Table 1.
Characteristics of patients with low back pain or radicular pain by DDD
| Case | Year | Gender | History (year) | Right/left | Disorder segment | Height of disc space | VAS (movement) | ODI |
|---|---|---|---|---|---|---|---|---|
| 1 | 52 | M | 10.0 | R | L5/S1 | 8.5 | 8.9 | 85 |
| 2 | 57 | M | 5.5 | L | L4/L5 | 9.6 | 7.8 | 81 |
| 3 | 36 | M | 2.8 | L | L4/L5 | 10.2 | 8.0 | 86 |
| 4 | 62 | M | 3.6 | L | L3/4 | 9.5 | 8.3 | 80 |
| 5 | 49 | M | 20.0 | R | L5/S1 | 9.8 | 8.7 | 82 |
| 6 | 52 | F | 10.0 | L | L4/5 | 7.5 | 9.1 | 82 |
| 7 | 63 | M | 3.5 | R | L2/3 | 8.1 | 7.5 | 78 |
| 8 | 45 | M | 8.5 | R | L5/S1 | 10. | 9.4 | 88 |
| 9 | 39 | F | 6.5 | L | L5/S1 | 9.7 | 6.9 | 75 |
| 10 | 43 | M | 7.8 | R | L4/5 | 8.5 | 8.5 | 83 |
| 11 | 51 | M | 5.0 | L | L5/S1 | 8.9 | 8.0 | 78 |
| 12 | 48 | M | 6.0 | L | L5S1 | 9.9 | 7.9 | 81 |
Device description
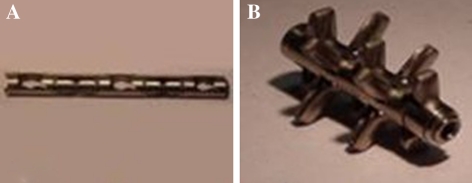
The device (Disc-O-Tech Medical Technologies Ltd, Herzliya, Israel) is made of titanium. When collapsed, five fins are enclosed within a cylinder of 5 mm in diameter (Fig. 1a). The implant is expanded fin by fin until it is 30 mm long and up to 15 mm in diameter (see IC) after it is inserted into the disc space by a single-use delivery system (see Fig. 1b). There are three available sizes in option: 9.5/11, 11.5/13 and 13.5/15. One of which is selected based on the preoperative X-rays and is adjusted intraoperatively as necessary. The device self-locks upon completion of the procedure.
Fig. 1.
a The cage in its reduced configuration. b The cage in its expanded configuration
Procedural technique
Pre-procedure
An intravenous antibiotic is given within 1 h before the procedure. Bone graft were prepared, including autograft bone obtained from anterior superior iliac spine or poster superior iliac spine, and allograft bone bought from a biochemical company.
Procedure
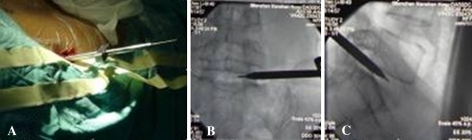
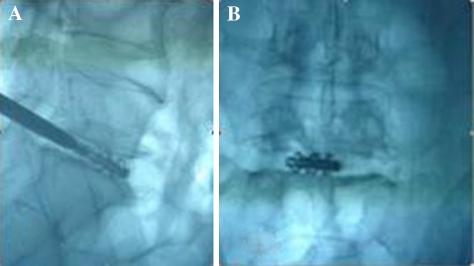
The patient was placed prone on the fluoroscopy table with a pillow under the abdomen. The disc puncture should be performed ipsilateral to the patient’s more symptomatic side. After locating the point of the puncture under fluroscopy according to the methods of discography [5], local anesthesia was given along the expected path of the needle tract. The skin entry points was enlarged using a blade after inserting the K-wire into the disc space. The dilator was introduced over the K-wire into the intervertebral space and then the 6 mm work cannula was inserted into the margin of the disc space over the dilator (Fig. 2a, b, c). When the K-wire and the dilator were removed, 1–2 ml of 2% lidocaine was injected into the disc through the working cannula in order to relive the pain. The routine procedure of spinal fusion consists of: (1) Disectomy, performed through the Cannula using adequate Pituitary Forceps; (2) Endplate curettage, to be completed meticulously in order to perform active cancellous bone bleeding to facilitate bone fusion (Fig. 3); (3) Implant diameter verification, to re-measure the space height by insertion of the trial implants into the intervertebral space (Fig. 4); (4) Bone graft introduction, to insert the bone chips of autograft bone from iliac creast or allograft bone from cadaver bone into the disc space through a sheath and (5) Implantation of the cage, to introduce the ESS into the intervertebral space by a single-use delivery system (Fig. 5a, b). Since the first fin is opened perpendicularly to the endplates, adjustments can be made at this stage by turning the delivery system 90° to reposition. All the stages were finished step by step and were monitored by C-arm fluoroscopy. The spacers for the 12 cases were installed “stand-alone”.
Fig. 2.
a The dilators and cannula were inserted into the intervertebral space, b AP plain of X-ray and c lateral plain of X-ray
Fig. 3.
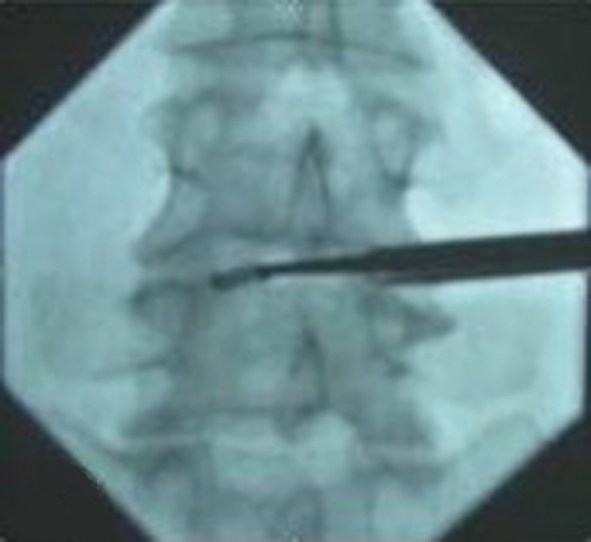
Eroding the endplates by a Caspar Curette
Fig. 4.
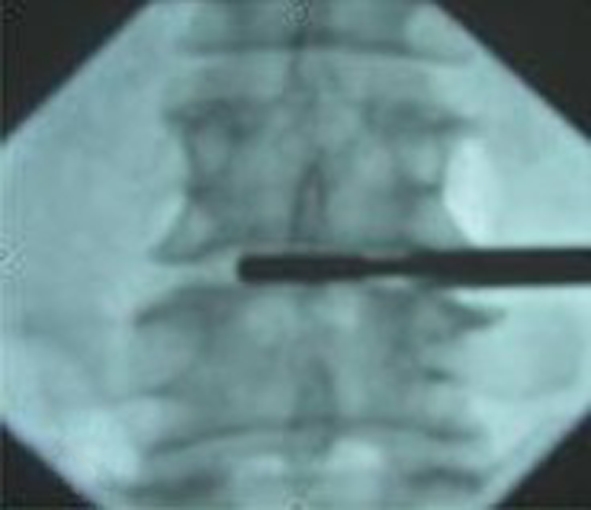
Verifying the implant position by a trial implant
Fig. 5.
a Implanting cage under C-arm. b Deliver system detached from the cage
Assessments
Preoperative patient information included their medical histories, socioeconomic status, physical examination, and results of imaging studies. Pain and disability were scored by a Visual Analog Scale (VAS) on movement and the Oswestry Index [6], respectively. The operative notes covered all difficulties and complications encountered. Data elicited at each follow-up visit (1, 6, 12 postoperatively and the latest time) included neurologic examination, X-ray studies, and repeat VAS and Oswestry Index scores. Upon termination of follow-up, the entire protocol on each patient was submitted to a clinical monitor for review.
Radiologic proof of spinal fusion required fulfillment of the following criteria: no radiolucent gap at the device-vertebral endplate interface, no evidence of mobility in flexion–extension X-ray film, and presence of bridging trabeculae across the area of arthrodesis. In equivocal cases, we added a computed tomography (CT) scan with sagittal reformation.
Statistical analysis
Discriptive statistics were applied to determine means and standard deviations. The scoring binomial test was applied to changes in the VAS score and the Oswestry Disability Index (ODI).
Results
The procedures of 12 cases were performed successfully, including: puncture of K-wire, insertion of cannel, discectomy, curettage of endplate and implantation of cage. The average time of procedure lasted 103 ± 42 min (range 60–170 min), and mean blood loss was 35 ± 18 mL (range 20–60 mL).
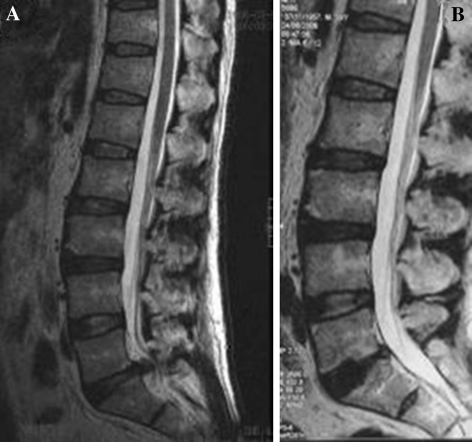
The iliac crest bone was used as autograft bone in the procedure of spinal fusion for four cases while the cadaver bone was used as allograft bone in the other eight patients. The shortest follow-up period was 15 months (mean 24.5 months, range from 15–36 months). No procedure-related complications had happened, such as implant malposition or migration. The seventh patients was complained of no change or worse recovery after 3 months follow-up visiting and then the implant had to be removed. There were no dural lacerations, infections or neurological damage. The disc space height averaged 9.0 ± 0.9 mm before surgery and increased to 11.5 ± 1.2 mm after surgery 1 month and stabilized at 10.8 ± 1.0 mm at 1 year follow-up for 11 cases and that also make the foraminal stenosis to be relived (Fig. 6a, b). Furthermore, the radicular pain was eradicated by the spinal fusion. The mean disability score of VAS decreased from 8.3 to 1.6, a 80% improvement (P < 0.01) and the score of ODI decreased from 81.6 to 26.4, a 67.6% improvement (P < 0.01) after a year follow-up visit (Table 2).
Fig. 6.
a Sagittal T2-weighted magnetic resonance image of the lumbar spine, showing degenerated, collapsed, and herniated disc at L5/S1 of a 52-year-old man with low back pain of 10 year duration with compressive radicular pain 2 year. b Follow-up radiograph 2 years postoperatively showing implanted B-Twin ESS. Disc height is partially restored
Table 2.
Results of postoperative follow-up visit
| Case | VAS (0–10) (movement) |
ODI | Disc space height (mm) | Visit period | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-operative | Post-operative (M) | Pre-operative | Postoperative (M) | Pre-operative | Postoperative (M) | |||||||||
| 1 | 6 | 12 | L | 1 | 6 | 12 | L | 1 | 12 | |||||
| 1 | 8.9 | 3.1 | 2.0 | 2 | 0 | 85 | 52 | 25 | 28 | 23 | 8.5 | 11.2 | 10.5 | 36 |
| 2 | 7.8 | 4.3 | 2.5 | 3.2 | 1 | 81 | 54 | 26 | 22 | 20 | 9.6 | 11.8 | 11.1 | 32 |
| 3 | 8.0 | 2.4 | 1.5 | 1 | 1 | 86 | 35 | 22 | 20 | 25 | 10.2 | 12.5 | 12.2 | 32 |
| 4 | 8.3 | 4.8 | 4.5 | 2.4 | 2 | 80 | 54 | 47 | 30 | 35 | 9.5 | 11.5 | 10.8 | 30 |
| 5 | 8.7 | 3.5 | 3.0 | 2 | 2 | 82 | 49 | 18 | 20 | 22 | 9.8 | 13.8 | 11.3 | 25 |
| 6 | 9.1 | 4.0 | 2.5 | 2.6 | 2.3 | 82 | 48 | 35 | 32 | 30 | 7.5 | 9.8 | 9. 0 | 23 |
| 7* | 7.5 | 6.7 | 78 | 58 | 8.1 | 10.5 | ||||||||
| 8 | 9.4 | 5.0 | 4.5 | 2.5 | 0 | 88 | 50 | 35 | 30 | 20 | 10. | 12.6 | 11.1 | 21 |
| 9 | 6.9 | 3.8 | 3 | 2.5 | 3 | 75 | 43 | 33 | 34 | 30 | 9.7 | 11.8 | 11.0 | 20 |
| 10 | 8.5 | 4.0 | 3.5 | 3 | 3 | 83 | 45 | 40 | 34 | 32 | 8.5 | 10.2 | 8.9 | 18 |
| 11 | 8.0 | 4.2 | 3.0 | 0 | 0 | 78 | 44 | 35 | 28 | 25 | 8.9 | 10.5 | 9.8 | 18 |
| 12 | 7.9 | 3.9 | 3.0 | 2.0 | 3.0 | 81 | 47 | 34 | 30 | 28 | 9.9 | 12.2 | 11.5 | 15 |
| Mean | 8.3 | 4.1 | 3 | 2.1 | 1.6 | 81.6 | 48.3 | 31.8 | 28 | 26.4 | 9.2 | 11.5* | 10.8* | 24.5 |
| S | 0.7 | 1.1 | 0.9 | 1.0 | 1.2 | 3.7 | 6.3 | 8.5 | 5.4 | 5.1 | 1.4 | 1.2 | 1.0 | 6.1 |
The implant was removed from the intervertebral space after 2 months late of spinal fusion because of worse efficacy
M month, MM micrometer, L the latest time follow-up visit
* A significant difference compared with preprocedure, P < 0.01
From the last follow-up visit to present, 11 patients (91%) considered the procedure to have been worthwhile and most of them return to work and the radiographic imagings showed that bridging trabeculae across the vertebral bodies and no radiolucent gap at the device vertebral end plate interface (Fig. 7a, b).
Fig. 7.
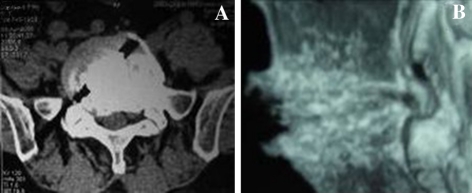
a Follow-up 1 year postoperatively CT scan with transverse plain showing the B-Twin ESS in the involved segment and well-incorporated implant. b CT scan with sagittal reformation showing bridging trabeculae across the vertebral bodies and no radiolucent gap
Discussion
The conventional lumbar interbody fusion including the early posterior lumbar interbody fusion (PLIF) is advocated as a main treatment of choice for chronic low back pain and compressive radicular pain due to the advanced disc degenerative disease because 70–90% of the patients were satisfied with the results [1, 2, 7] and 75–95% returned to work [7, 8]. Although PLIF with conventional cages has established itself as a surgical approach of choice, its record of major complications has been puzzling. A recent review reported complications in 95% of the cases and re-operation in 25–45% [2, 9]. Expandable spinal cages have the potential to achieve similar results with less invasive techniques. The biomechanical properties of the B-Twin ESS were designed to provide immediate mechanical constraint in all planes. The constraints of flexion and lateral bending are mediated by the annulus. This is attained by distraction of the disc space from the expansile spinal cages. This is only accomplished by a ‘Jacking up” mechanism. The stability in the axial plane is credited to the limited invasiveness of the surgical procedure which makes it possible to preserve the main stabilizers in the axial plane, namely the facet joints and the annulus fibrosus [10, 11].
Percutanous posterior-lateral lumbar interbody fusion (PPLLIF) is a new procedure developed in recent years with the novel B-Twin expandable spinal system emerging. The implant is typically proceeded bilaterally but our 12 cases were performed unilaterally and as a stand alone according to the chinese patients’ figure and economy level. The unilateral procedure of PPLLIF has more advantages compared with the PLIF using the ESS: firstly, the mean blood loss with 35 ± 18 mL (range 20–60 mL) was dramatically decreased compare to it of PLIF with 410 ± 330 ml (range 300–1,500 ml) [3]; Secondly, the poster-lateral puncture approach of PPLLIF was a minimal invasive procedure, especially the unilateral procedure, it did not interfered the construction of the spinal canal and did not require the sacrifice of the posterior stabilizing structure (such as ligament, lamina and facet joint). Therefore, it could better maintain the stability of the spine than the surgery of PLIF. Thirdly, it shortened the procedure time and reduced the risk of nerve damage. It is an alternative method for PLIF if the selective candidate was chosen wisely. The main concern was in regard to the penetration of the endplate by the fins and the possibility of implant migration. The averaged subsidence in the 12 cases was 0.35 mm per fin (0.7 mm per implant) after 1 year follow-up visit. It is deeper than 0.28 mm per fin (0.56 mm per implant) reported by Folman [4]. This might be in correlation with one-cage implanted into the spinal space for the 12 patients. This subsidence did not jeopardize the stability of the ESS, and the engagement of the fins into the vertebral endplate provided an element of resistance against migration. However, the quality of the bone is most essential in determining the anchorage of the fins into the endplate. This consideration may be of crucial importance when selecting candidates for PPLLIF using ESS. The 11 patients’ plain X-ray established that fusion was accomplished by 1 year follow-up. This favorable result may be ascribed to the relatively small implant-endplate contact area, and this will leave a large area free so that the bone graft is in contact with the bone; enabling bone to bone contiguity without having to depend on bone growth into and through the cage as in the cases of conventional cages. Moreover, meticulous curettage of the nucleus, rather than installation of the device within a reamed channel, was suggested to promote fusion [9]. In the 11 patients’ radiolucencies at the implant endplate, interface was not there, which means that fusion is already occurring. Stress views did not show any sign of instability. The length of the implant and its contouring may preclude radiological assessment of fusion. In this study, we found that the stability of one implant insertion into the intervertebral body is the same with that of two implants.
Bone graft for spinal fusion surgeries may either be harvested from the patient (autograft bone) or from a cadaver (allograft bone). In the final result, we did not find any difference of spinal fusion between patients using autograft bone or allograft bone. Wimmer et al. [12] followed 94 cases with the anterior interbody fusion and found that the resource of bone makes no difference in spinal fusion rate. The patients treated with the technique exhibited satisfactory results with improved neurological function. Eleven patients had good relief 1 month postoperatively. In the final analysis, VAS on movement and ODI (Oswestry disability index) dropped by more than 80 and 67.4%, respectively. The results was similar to the report of Kuslic et al. [13] who implanted the cage by anterior or posterior approach. Disk space heights averaging 9.0 mm before procedure was increased to 11.5 mm after surgery 1 mouth and stabilized at 10.8 mm (a significant difference compared with preprocedure, P < 0.01) upon after more than 1 year follow-up.
Conclusions
The percutaneous puncturing approach for poster-lateral lumbar interbody fusion using expandable spinal system is a valuable micro-invasion method for the DDD patients and our preliminary results are encouraging, but should also be confirmed by a multicentric study based on a large series, and the criteria of inclusion or exclusion must be strictly respected to obtain satisfactory clinical results.
Contributor Information
Lizu Xiao, FAX: +86-755-26565025, Email: xlz99@tom.com.
Deren Zhang, Email: nsyyjoe@yahoo.cn.
References
- 1.Agazzi S, Reverdin A, May D. Posterior lumbar interbody fusion with cages: an independent review of 71 cases. J Neurosurg. 1999;91:186–192. doi: 10.3171/spi.1999.91.2.0186. [DOI] [PubMed] [Google Scholar]
- 2.Brantigan JW, Steffee AD, Lewis ML, et al. Lumbar interbody fusion using the Brantigan I/F cage for PLIF and the variable pedicle screw placement system. Spine. 2000;25:1437–1446. doi: 10.1097/00007632-200006010-00017. [DOI] [PubMed] [Google Scholar]
- 3.Elias WJ, Simmons NE, Kaptain GJ. Complications of PLIF when using a titanium threaded cage device. J Neurosurg. 2000;93:45–52. doi: 10.3171/spi.2000.93.1.0045. [DOI] [PubMed] [Google Scholar]
- 4.Folman Y, Lee SH, Silvera JR, et al. Posterior lumbar interbody fusion for degenerative disc disease using a minimally invasive B-Twin expandable spinal spacer: a muticenter study [J] J Spinal Disord Tech. 2003;16(5):455–460. doi: 10.1097/00024720-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Fenton DS, Czervionke LF (2003) Image-guided Spine Intervention. Saunders: an imprint of Elserier. pp 227–255. ISBN 0-7216-0021-2
- 6.Fairbank J, Couper J, et al. Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66:271–273. [PubMed] [Google Scholar]
- 7.Lee CK, Vessa P, Lee JK. Chronic disabling low back pain syndrome caused by internal disc derangements. The result of disc excision and posterior lumbar interbody fusion. Spine. 1995;20:356–361. doi: 10.1097/00007632-199502000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Hacker RJ. Comparison of interbody fusion approaches of disabling low back pain. Spine. 1997;22:660–666. doi: 10.1097/00007632-199703150-00017. [DOI] [PubMed] [Google Scholar]
- 9.McAfee PC, Lee GA, Fedder IL, et al. Anterior BAK instrumentation and fusion: complete versus partial discectomy. Clin Orthop. 2002;394:55–63. doi: 10.1097/00003086-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Albumi K, Panjabi MM, Kramer K, et al. Biomechanical evaluation of lumbar spine stability after graded facetectomies. Spine. 1990;15:1142–1147. doi: 10.1097/00007632-199011010-00011. [DOI] [PubMed] [Google Scholar]
- 11.Krismer M, Haid C, Rabl W. The contribution of annulus fibers to torque resistance. Spine. 1996;21:2551–2557. doi: 10.1097/00007632-199611150-00004. [DOI] [PubMed] [Google Scholar]
- 12.Wimmer C, Kristner M, Cluch H, et al. Autogenoic versus allo-genic bone grafts in anterior lumbar interbody fusion. J Clin Orthop Relat Res. 1999;360:120–126. doi: 10.1097/00003086-199903000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Kuslich SD, Ulstrom CL, Griffit SH, et al. The Bagby and kuslich method of lumbar interbody fusion history techniques and 2-year follow-up result of a United States prospective multicenter trial. Spine. 1998;23:1267–1278. doi: 10.1097/00007632-199806010-00019. [DOI] [PubMed] [Google Scholar]