Abstract
To evaluate the impact of the longitudinal extension of intramedullary lesions on the neurological status and postoperative outcome. Forty-six patients operated in our Department between February 2004 and June 2007 have been included in this study. The patients were classified in two groups according to the longitudinal extension of the lesion over less than three vertebral segments (group A) and over exactly three or more vertebral segments (group B). The neurological status was assessed preoperatively, postoperatively and after 3 months and involved both the McCormick (McC) and Klekamp–Samii (KS) scales. The preoperative McC- and KS scores of the patients of group B were statistically significant lower (p < 0.038 and p < 0.027, respectively) than those of group A. Patients of both groups showed an initial postoperative clinical deterioration. The level of statistical significance was reached only in group B (group A McC p < 0.170, KS p < 0.105; group B McC p < 0.012, KS p < 0.020). The patients recovered well and no statistical difference was observed between the preoperative and the 3-month follow-up scores (group A McC p < 0.490, KS p < 0.705; group B McC p < 0.506, KS p < 0.709). Thus, patients with extended intramedullary lesions have a worse neurological status preoperatively, postoperatively and in the 3-month follow-up. The preoperative neurostatus is determinant for the outcome. Even in case of longitudinally extensive intramedullary lesions, early surgery is recommended since satisfactory results can be achieved.
Keywords: Spinal cord, Intramedullary lesion, Outcome
Introduction
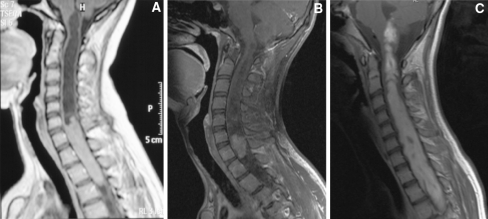
Intramedullary spinal cord lesions show different longitudinal involvement, varying from monosegmental to almost holocord extension (Figs. 1a–c). Predictors of a good functional outcome in intramedullary tumor surgery have been evaluated in several studies: the patient’s preoperative neurological status, stable values of the somatosensory-evoked potential (SSEP) and the motor-evoked potential (MEP) during intraoperative neurophysiological monitoring (IOM), the presence of intratumoral cysts, histology kind and a low histological tumor grade [4, 11–14, 16, 18]. As the longitudinal extension of intramedullary spinal cord tumors have not yet been investigated with regard to its impact on the postoperative functional outcome, we hypothesize that the size of intramedullary tumors is another factor that influences the clinical outcome.
Fig. 1.
a–c Sagittal T1-weighted MRI after gadolinium administration showing intramedullary neuroepithelial tumors with different longitudinal extension
Materials and methods
All patients undergoing microsurgery for intramedullary spinal cord lesions between February 2004 and November 2007 in the Department of Neurosurgery, University of Tuebingen, were retrospectively reviewed. Patients with neurofibromatosis type II as well as patients undergoing a multi-staged tumor resection were excluded from the study. All surgical interventions were performed employing a microsurgical technique, with a cavitron ultrasonic aspirator (CUSA) and continuous intraoperative neurophysiological monitoring (SSEP, MEP, D-Wave).
We investigated demographic information, clinical presentation, clinical and surgical reports, surgical DVD recordings, pre- and postoperative magnetic resonance imaging (MRI) and histological charts. All patients underwent preoperative, postoperative and 3-month follow-up functional assessments, which involved both the McCormick (McC) [10] and Klekamp–Samii (KS) [8] (Table 1) scales. The scoring scales were applied for both children and adults, taking into consideration the age-related abilities.
Table 1.
McCormick's clinical/functional classification scheme [10] and Klekamp–Samii clinical scoring system [8]
| Grade | Definition | ||||
|---|---|---|---|---|---|
| I | Neurologically normal; mild focal deficit not significantly affecting function of involved limb; mild spasticity or reflex abnormality; normal gait | ||||
| II | Presence of sensorimotor deficit affecting function of involved limb; mild to moderate gait difficulty; severe pain or dysesthetic syndrome impairing patient’s quality of live; still functions and ambulates independently | ||||
| III | More severe neurological deficit; requires cane/brace for ambulation or significant bilateral upper extremity impairment; may or may not function independently | ||||
| IV | Severe deficit; requires wheelchair or cane/brace with bilateral upper extremity impairment; usually not independent |
| Score | Sensory deficits, pain, dysesthesias | Motor weakness | Gait ataxia | Bladder function | Bowel function |
|---|---|---|---|---|---|
| 5 | No symptom | Full power | Normal | Normal | Normal |
| 4 | Present, not significant | Movement against resistance | Unsteady, no aid | Slight disturbance, no catheter | Slight disturbance, full control |
| 3 | Significant, function not restricted | Movement against gravity | Mobile with aid | Residual, no catheter | Laxatives, full control |
| 2 | Some restriction of function | Movement without gravity | Few steps with aid | Sometimes catheter | Sometimes loss of control |
| 1 | Severe restriction of function | Contraction without movement | Standing with aid | Often catheter | Often loss of control |
| 0 | Incapacitation of function | Plegia | Wheel chair | Permanent catheter | No control |
Two groups were classified regarding the longitudinal extension of the lesion: first, extension over less than three vertebral segments (group A) and, second, extension over exactly three or more vertebral segments (group B). Comparisons between groups have been carried out by means of the Fisher’s exact test for counted data, and Student’s t test or paired samples Student’s t test, respectively, for inter- or intraindividual comparisons with continuous data. Whenever an equally distribution of recorded values was not assumed, non-parametric test (Mann–Whitney for unpaired and Wilcoxon for paired data were performed. ANOVA for repeated measures was used to compare the segment and histology groups with respect to the three time-points pre, post and follow-up, including age at surgery as covariate. P < 0.05 was considered as statistically significant.
Results
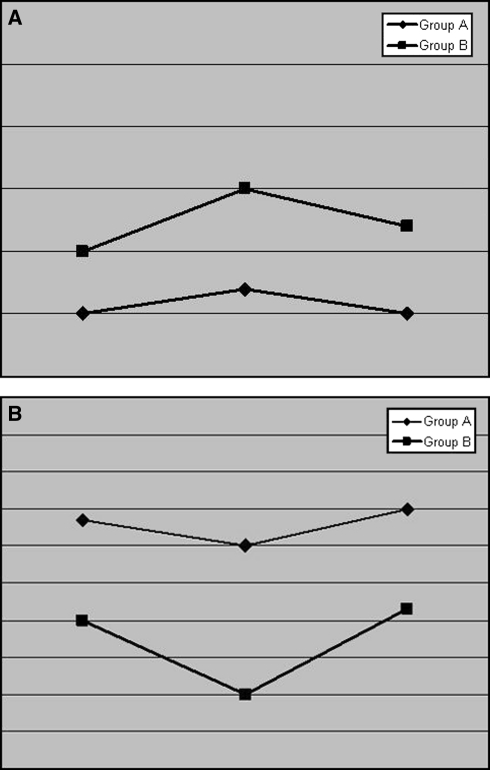
Forty-seven microsurgical interventions were performed in 46 patients, of whom 23 were women and 23 men (in group A 65% are women, in group B 33% are women). The patients’ age at the moment of surgery ranged from 2 to 81 years with a mean age of 46.5 ± 16.2 years in group A (n = 26) and 45.2 ± 24.9 years in group B (n = 21) (p = 0.841). The lesions extended over an average of 1.2 (group A) and 5.4 (group B) spinal segments; 53% of the lesions of group A and 57% of those of group B involved the thoracic segment. Histopathologic findings are summarized in Table 2. Tables 3 (KS) and 4 (McC) report the clinical scores and statistical p values of patients harboring lesions extending over less than three segments or over exactly or more than three segments preoperatively, postoperatively and 3 months after surgery. The course is graphically itemized in Figs. 2a, b.
Table 2.
Histologic findings according to intramedullary extension
| Group | Malformation | Neuroepithelial tumor | Vascular lesion | Other lesion |
|---|---|---|---|---|
| A | 23% (n = 6) | 31% (n = 8) | 46% (n = 12) | 0% |
| B | 0% | 86% (n = 18) | 0% | 14% (n = 3) |
Table 3.
Preoperative, early postoperative and 3-month follow-up scores according to the KS scale and respective p values
| Group | Preop | Postop | Follow-up |
|---|---|---|---|
| A | 21.7 ± 02.1 | 21 ± 2.3 | 22 ± 3.8 |
| B | 19 ± 5.5 | 17 ± 5.3 | 19.3 ± 4.6 |
| Group | Preop versus early postop | Preop versus 3 months follow-up | |
|---|---|---|---|
| A | p = 0.105 | p = 0.705 | |
| B | p = 0.020 | p = 0.709 |
Table 4.
Preoperative, early postoperative and 3-month follow-up scores according to the McC scale and respective p values
| Group | Preop | Postop | Follow-up |
|---|---|---|---|
| A | 1.5 ± 0.5 | 1.7 ± 0.6 | 1.5 ± 0.7 |
| B | 2 ± 0.9 | 2.5 ± 0.9 | 2.2 ± 1.1 |
| Group | Preop versus early postop | Preop versus 3 months follow-up | |
|---|---|---|---|
| A | p = 0.170 | p = 0.490 | |
| B | p = 0.012 | p = 0.506 |
Fig. 2.
a Course of the preoperative, early postoperative and 3-month follow-up neurostatus according to the McC scale b course of the preoperative, early postoperative and 3-month follow-up neurostatus according to the KS scale
There is a significant correlation between segment groups and histology (p < 0.001, χ2 test) as malformations and vascular lesions tend to have most in group A, whereas neuroepithelial tumors are the predominant lesions in group B (Table 2).
The preoperative McC- and KS scores of the patients of group B were statistically significant lower (p < 0.038 and p < 0.027, respectively, t test) than those of group A.
Eighty-one percent of the lesions in group A could be radically resected (1 biopsy, 3 subtotal resections), whereas in group B 67% of the resections were radical (3 biopsies, 2 subtotal resections) (p = 0.486, Fisher’s exact test). The degree of resection was determined, with an early postoperative MRI. One of the 26 cases in group A and four of the 21 lesions in group B were histologically classified as malignant. Patients of both groups showed an initial postoperative clinical deterioration.
ANOVA for repeated measures shows a significant influence of age at surgery with respect to pre, post and follow-up for KS and McC (p = 0.002 and p = 0.023, respectively). Furthermore, the effect of the segment group is statistically significant (KS p = 0.042, McC p = 0.014). In contrast, histology was not proven to have any significant influence (KS p = 0.656, McC p = 0.742). Using intra-individual comparisons, the level of statistical significance was reached only in group B (group A McC p = 0.170, KS p = 0.105; group B McC p = 0.012, KS p = 0.020, paired t test) (Fig. 2a, b) when comparing the preoperative with the immediate postoperative neurological status. In group A, the only statistically significant postoperative symptom which deteriorated transitorily was ataxia (p = 0.026). In contrast, in patients of group B, the statistically significant postoperative deterioration according to the KS scale was due to ataxia (p = 0.014), bladder (p = 0.024) and motor deficits (p = 0.026). Patients of both groups, however, recovered well and no statistical difference was observed between the preoperative and the 3-month follow-up scores (group A McC p = 0.490, KS p = 0.705; group B McC p = 0.506, KS p = 0.709) (Figs. 2a, b). The average length of in-patient stay was 6.1 ± 2.1 and 7.9 ± 3.1 days for patients of groups A and B, respectively (p = 0.266). The overall complication rate was 4.7%, with no statistically significant difference between the two groups.
Discussion
Several factors have been investigated to determine the predictors of the neurological outcome after the microsurgical treatment of intramedullary lesions. The most significant aspects are the preoperative neurostatus [1, 3, 4, 6, 7, 16, 17] and the histopathologic type of lesion and grading [2, 3, 6]. Further, the presence of syringomyelia or of a cystic component seems to be associated favorably with the neurological outcome [16]. The former may be explained by the fact that tumors that compress rather than invade the spinal cord are more likely to cause syringomyelia [16], the latter by a better-defined cleavage plane.
The deterioration of measurements at IOM is a strong predictor of the postoperative outcome [8, 14, 15]. Although SSEPs monitor dorsal myelotomy, MEPs acquire major importance during tumor dissection along the ventrolateral cleavage plane. SSEPs offer a high sensitivity, but poor specificity for intramedullary spinal cord surgery. They are helpful during dorsal myelotomy along the posterior median septum, but may be lost transitorily during this maneuver. A reliable correlation to postoperative proprioceptive or motor deficits, however, does not exist. Therefore, IOM has to be complemented with MEPs. When muscle and epidural MEPs are combined, an all-or-none muscle MEP criterion is justified [5]. Thus, complete tumor removal can be achieved with only transitory motor deficits [14]. An advantage of MEPs over SSEPs is that MEPs do not need averaging and thus offer prompt information about the monitored pathway status. In this context, the importance of the epidural MEP recording, the so-called D-wave, has to be stressed. A D-wave preserved for at least 50% of its original amplitude assures functional integrity of the corticospinal tracts’ fast neurons and thus a satisfactory long-term motor outcome to the patient [5, 13]. It represents a quantitative tool for predicting the postoperative motor status [15].
A more pronounced postoperative clinical deterioration with worse tendency of recovery has been linked to intramedullary spinal cord tumors involving the thoracic region [4, 17]. Moreover, the intraoperative finding of arachnoid scarring is considered to be a negative prognostic factor [16].
The histological type of lesion influences the longitudinal spinal cord involvement because malformations and cavernomas are mostly monosegmental, while glial tumors frequently extent over several spinal cord levels; especially, astrocytomas show an infiltrative growth pattern. Their microsurgical resection is often complicated by a lack of a cleavage plan. Particularly, in this situation, the above-mentioned IOM techniques acquire utmost significance. Histology kind and tumor extend are interconnected. The statistical results show a more important effect on the neurological status of the tumor’s extent than the tumor’s histology. An independent influence cannot be assumed, but while histology seems to play a secondary role, the extent of the lesion seems to play the prominent one.
In our series, the predictive value of the variable intramedullary extent was affected by lesions’ histology, tumor grade and degree of resection. However, the 3-month follow-up was positively influenced by the intraoperative use of continuous electrophysiological monitoring. Patients with long intramedullary spinal cord tumor extension have to be prepared for a transitory worsening during the immediately postoperative period, followed by a progressive clinical improvement. After 3 months, they usually regain the preoperative neurological status. Thus, even this patient group benefits from early surgery when neurological functions are not yet severely affected. Our results show that a significant recovery of preoperatively lost function does not occur. The temporary worsening is mostly due to the mechanical manipulation of the posterior columns during myelotomy, causing ataxia. The preoperative, postoperative and 3-month follow-up neurostatus, however, is lower in patients with multi-segmental lesions. The more severe preoperative neurological deficits in this group reflect the more grievous harm to the spinal cord tissue caused by the longitudinal tumor extension.
Conclusion
Patients with extended intramedullary lesions have a worse neurological status preoperatively, postoperatively and in the 3-month follow-up. The preoperative neurostatus is determinant for the outcome. Therefore, even in case of longitudinally extensive intramedullary lesions, early surgery is recommended since satisfactory results can be achieved in these cases.
References
- 1.Brotchi J, Dewitte O, Levivier M, Balériaux D, Vandesteene A, Raftopoulos C, Flament-Durand J, Noterman J. A survey of 65 tumors within the spinal cord: surgical results and the importance of preoperative magnetic resonance imaging. Neurosurgery. 1991;29:651–656. doi: 10.1097/00006123-199111000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Constantini S, Miller DC, Allen JC, Rorke LB, Freed D, Epstein FJ. Radical excision of intramedullary spinal cord tumors: surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg. 2000;93:183–193. doi: 10.3171/jns.2000.93.2.0183. [DOI] [PubMed] [Google Scholar]
- 3.Cooper PR, Epstein F. Radical resection of intramedullary spinal cord tumors in adults. Recent experience in 29 patients. J Neurosurg. 1985;63:492–499. doi: 10.3171/jns.1985.63.4.0492. [DOI] [PubMed] [Google Scholar]
- 4.Cristante L, Herrmann HD. Surgical management of intramedullary spinal cord tumors: functional outcome and sources of morbidity. Neurosurgery. 1994;35:69–74. doi: 10.1097/00006123-199407000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Deletis V, Sala F. Intraoperative neurophysiological monitoring of the spinal cord during spinal cord and spine surgery: a review focus on the corticospinal tracts. Clin Neurophysiol. 2008;119:248–264. doi: 10.1016/j.clinph.2007.09.135. [DOI] [PubMed] [Google Scholar]
- 6.Epstein FJ, Farmer JP, Freed D. Adult intramedullary astrocytomas of the spinal cord. J Neurosurg. 1992;77:355–359. doi: 10.3171/jns.1992.77.3.0355. [DOI] [PubMed] [Google Scholar]
- 7.Guidetti B, Mercuri S, Vagnozzi R. Long-term results of the surgical treatment of 129 intramedullary spinal gliomas. J Neurosurg. 1981;54:323–330. doi: 10.3171/jns.1981.54.3.0323. [DOI] [PubMed] [Google Scholar]
- 8.Klekamp J, Samii M (1993) Introduction of a score system for the clinical evaluation of patients with spinal processes. Acta Neurochir 123:221–223 [PubMed]
- 9.Kothbauer K, Deletis V, Epstein FJ. Intraoperative spinal cord monitoring for intramedullary surgery: an essential adjunct. Pediatr Neurosurg. 1997;26:247–254. doi: 10.1159/000121199. [DOI] [PubMed] [Google Scholar]
- 10.McCormick PC, Torres R, Post KD, Stein BM. Intramedullary ependymoma of the spinal cord. J Neurosurg. 1990;72:523–532. doi: 10.3171/jns.1990.72.4.0523. [DOI] [PubMed] [Google Scholar]
- 11.McGirt MJ, Chaichana KL, Atiba A, Attenello F, Woodworth GF, Jallo GI. Neurological outcome after resection of intramedullary spinal cord tumors in children. Childs Nerv Syst. 2008;24:93–97. doi: 10.1007/s00381-007-0446-y. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura M, Ishii K, Watanabe K, Tsuji T, Takaishi H, Matsumoto M, Toyama Y, Chiba K. Surgical treatment of intramedullary spinal cord tumors: prognosis and complications. Spinal Cord. 2008;46:282–286. doi: 10.1038/sj.sc.3102130. [DOI] [PubMed] [Google Scholar]
- 13.Raco A, Esposito V, Lenzi J, Piccirilli M, Delfini R, Cantore G. Long-term follow-up of intramedullary spinal cord tumors: a series of 202 cases. Neurosurgery. 2005;56:972–981. [PubMed] [Google Scholar]
- 14.Sala F, Bricolo A, Faccioli F, Lanteri P, Gerosa M. Surgery for intramedullary spinal cord tumors: the role of intraoperative (neurophysiological) monitoring. Eur Spine J. 2007;16:130–139. doi: 10.1007/s00586-007-0423-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sala F, Palandri G, Basso E, Lanteri P, Deletis V, Faccioli F, Bricolo A. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study. Neurosurgery. 2006;58:1129–1143. doi: 10.1227/01.NEU.0000215948.97195.58. [DOI] [PubMed] [Google Scholar]
- 16.Samii M, Klekamp J. Surgical results of 100 intramedullary tumors in relation to accompanying syringomyelia. Neurosurgery. 1994;35:865–873. doi: 10.1097/00006123-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Sandalcioglu IE, Gasser T, Asgari S, Lazorisak A, Engelhorn T, Egelhof T, Stolke D, Wiedemayer H. Functional outcome after surgical treatment of intramedullary spinal cord tumors: experience with 78 patients. Spinal Cord. 2005;43:34–41. doi: 10.1038/sj.sc.3101668. [DOI] [PubMed] [Google Scholar]
- 18.Tobias ME, McGirt MJ, Chaichana KL, Goldstein IM, Kothbauer KF, Epstein F, Jallo GI. Surgical management of long intramedullary spinal cord tumors. Childs Nerv Syst. 2008;24:219–223. doi: 10.1007/s00381-007-0405-7. [DOI] [PubMed] [Google Scholar]