Abstract
Although Schmorl’s nodes (SNs) are a common phenomenon in the normal adult population, their prevalence is controversial and etiology still debatable. The objective was to establish the spatial distribution of SNs along the spine in order to reveal its pathophysiology. In this study, we examined 240 human skeleton spines (T4-L5) (from the Hamann–Todd Osteological Collection) for the presence and location of SNs. To determine the exact position of SNs, each vertebral body surface was divided into 13 zones and 3 areas (anterior, middle, posterior). Our results show that SNs appeared more frequently in the T7-L1 region. The total number of SNs found in our sample was 511: 193 (37.7%) were located on the superior surface and 318 (62.3%) on the inferior surface of the vertebral body. SNs were more commonly found in the middle part of the vertebral body (63.7%). No association was found between the SNs location along the spine and gender, ethnicity and age. This study suggests that the frequency distribution of SNs varies with vertebra location and surface. The results do not lend support to the traumatic or disease explanation of the phenomenon. SNs occurrences are probably associated with the vertebra development process during early life, the nucleus pulposus pressing the weakest part of the end plate in addition to the various strains on the vertebrae and the intervertebral disc along the spine during spinal movements (especially torsional movements).
Keywords: Spine, Spinal diseases, Intervertebral disc, Spine pathology, Disc herniation
Introduction
Schmorl’s nodes (SNs) have been previously studied and described as herniations of the intervertebral disc penetrating into the vertebral body [1–5]. SNs can appear on any spinal vertebra but tend to concentrate in the lower thoracic and lumbar regions. This is usually attributed to the load on the vertebrae, which increases as we descend along the spine [2, 4, 6–8].
Specific disorders which produce weakening of the vertebral end-plate are usually considered as predisposing factors for the manifestation of SNs. These include Scheuermann’s disease, metabolic and neoplastic diseases (osteoporosis, hyperparathyroidism), degenerative disk disease, and trauma [2].
To the best of our knowledge, no study has dealt with the localization of SNs along the spine (vertebra and surface) in a large skeletal sample with the aim of investigating and understanding the unique areas for the presence of SNs. The advantage of a skeletal study is that SNs can be easily identified on dried bones as they manifest characteristic deformation on the vertebra body surface [8].
The aims of the current study were to establish the spatial distribution of SNs along the spine and to reveal the preferred vertebral body surface in order to gain a better understanding of the factors associated with SNs manifestation.
Materials and methods
Macroscopic examination of SNs was performed on 240 skeleton spines (vertebrae T4 to L5) of complete skeletons from the Hamann–Todd Human (HTH) Osteological Collection, Cleveland Museum of Natural History, Cleveland, OH, USA. These individuals had died during the first half of the twentieth century with age at death, gender, ethnic origin, and cause of death documented. The sample studied was randomly taken from the collection and included 240 human skeletons (120 males and 120 females) divided into four groups by sex and ethnic origin (60 European-Americans males, 60 African-Americans males, 60 European-Americans females, and 60 African-Americans females). Each group was divided into 3 equal age cohorts ranging from 20 to 80 years (e.g., 20–39, 40–59, 60–80), with 20 individuals in each subgroup. Skeletons with documented general spinal disease pathologies (e.g., Spondyloarthropathy, Diffuse Idiopathic Skeletal Hyperostosis) were excluded from the study, leaving in the sample studied only individuals with no spinal pathology or those with only local degenerative changes (e.g., syndesmophytes).
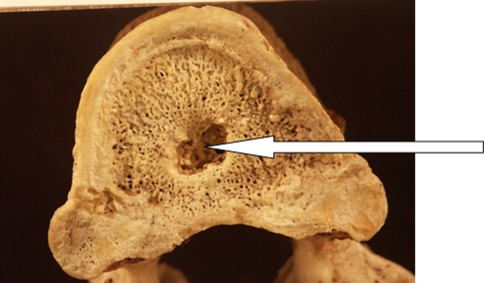
SNs were recorded as ‘present’ when the integrity of the vertebra body surface was disrupted, being excavated by any size of cup-shaped depression possessing sclerotic margins (Fig. 1). An individual was recorded as ‘positive’ for SNs if one of the vertebrae manifested evidence of SNs. SN intensity was calculated as the total number of SNs per spine. As SNs are rarely observed in the cervical and upper thorax vertebrae, in this study we examined vertebra T4 to L5.
Fig. 1.
Schmorl’s node: typical depression on a vertebral body surface
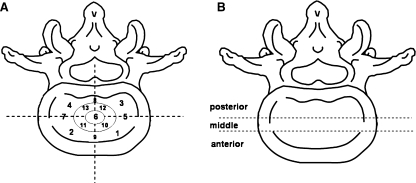
Vertebral body surface was divided into 13 zones (Fig. 2a). The center zone was labeled area 6. The outer peripheral area was divided into 4 zones, areas 1–4 (1 = left anterior, 2 = right anterior, 3 = left posterior and 4 = right posterior). Additionally, SNs locations were also described as ‘anterior’ (zones 1, 2), ‘middle’ (zones 5–7) and ‘posterior’ (zones 3, 4) parts of the body surface (Fig. 2b). The superior and inferior surfaces of each vertebra body along the spine (T4 to L5) were examined for the presence and location of SNs.
Fig. 2.
Left Vertebral body surface area divided into 13 zones. Right Vertebral body surface divided into ‘anterior’, ‘middle’ and ‘posterior’ parts
The association between gender, age, and ethnicity with the SNs location was examined using the Chi square test, Fisher exact test and Mann–Whitney test. The difference in SNs prevalence between the superior and the inferior vertebral body surfaces was measured by the McNemar test. Significant differences were defined as p < 0.05.
For intra-test reliability, three vertebrae of ten individuals were re-examined three times by one of the authors (G.D.) under similar experimental conditions. Measurements were taken every other day. For inter-test reliability, three vertebrae of ten individuals were re-examined by two investigators under similar experimental conditions.
Results
Intra and inter-observer reliability tests were found to be high (intra-class correlations coefficient >0.8). These values present substantial agreement according to Landis and Koch [9].
SNs location on vertebral body surface and along the spine
A total of 3,360 vertebrae of 240 different spines were examined (2,160 thoracic vertebrae and 1,200 lumbar vertebrae).
SNs and vertebral body surface
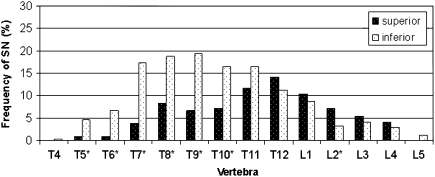
Our sample consisted of 511 nodes: 193 (37.7%) were located on the superior surface of the vertebra and 318 (62.3%) on the inferior surface (McNemar test, p < 0.05). SNs on the superior vertebral surface were most common on T12 (exhibited by 14.1% of the individuals) and on the inferior surface of T9 (19.5% of the individuals) (Fig. 3).
Fig. 3.
Prevalence of SNs by vertebra, superior and inferior surfaces. Asterisk Significant difference (p < 0.05) between surfaces
The frequency distribution of SNs along the spine significantly varied between the superior and inferior surface of the vertebral body. When examining the superior surface, the prevalence of SNs gradually increased from vertebrae T4 to T12 and subsequently decreasing towards L5. SNs were rarely seen on the superior surfaces of T4, T5, T6, and L5. When examining the inferior surface, the number of vertebrae with SNs gradually increased from T4 to T6 and subsequently considerably rose to T7, remaining high until T12. From T12, the number of vertebrae manifesting SNs gradually decreased to L5. The most affected vertebrae on the inferior surface were T7–T11 (Fig. 3).
In the thoracic region (except for T12), SNs were more commonly found on the inferior surface of the vertebra whereas in the lumbar region, the reverse was observed, i.e., SNs were more common on the superior surface of the vertebra (Fig. 3). Nevertheless, the discrepancies between the two surfaces as to SNs occurrences were more pronounced in the thoracic region.
SNs position on the vertebral body surface
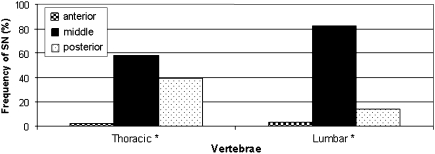
SNs were more commonly seen in the middle part of the vertebral body (63.7%); 33.7% of the nodes were located in the posterior part and only 2.6% in the anterior part of the vertebral surface. When analyzing the occurrences of SNs by spinal regions in the lumbar area, 82% were located in the central part of the vertebral surface compared to only 58.4% in the thoracic vertebrae. SNs in the posterior part of the vertebra were more common in thoracic vertebrae (39.3%) compared with 14.3% in the lumbar vertebra (Fig. 4).
Fig. 4.
Position of SNs in the thoracic and lumbar vertebrae. Asterisk Significant difference (p < 0.05)
When analyzed by zones, 115 nodes (22.8%) were located at zone 6 (center), 40 nodes (7.8%) at zone 8 (posterior to zone 6), 37 nodes (7.2%) at zone 12 and 48 nodes (9.4%) at zone 13, implying that most SNs were located at the center of the vertebra surface or slightly posterior to it.
No association was found between the SNs position on the vertebral body surface and gender, ethnicity and age.
Discussion
Prevalence of SNs in the human population
Prevalence depends on several factors: classification of SNs (i.e., minimal size of the concavity to be considered as a node); definition of “individuals with SNs” (one or multiple cases of SNs); methodology used (how many vertebrae were examined; which vertebrae and which vertebral surface was observed, superior, inferior or both?); demographics (sex ratio, ethnic origin, etc.) and socioeconomic characteristics (mainly daily activities) of the examined population.
It is therefore not surprising that the reported frequency of SNs in the literature varies (5–70%) [3, 5, 7, 10–12]. In our study, 116 individuals manifested one or more SNs along the spine (48.3%) (see also Dar et al. [13]). This prevalence is slightly lower than reported by Pfirrmann and Resnick [14] who studied 100 cadavers (58%). Yet, it is not possible to say whether this difference in SNs prevalence is due to the 100-year difference between these two North-Americans populations; to the differences in daily activities or to the fact that the researchers did not control for the parameters mentioned above.
The large range reported for SNs prevalence is not only due to the factors mentioned above but also due to the different methods applied for locating SNs [magnetic resonance imaging (MRI), computed tomography (CT) scans, roentgenograms, autopsies and skeletal material]. In the present study, we used skeletal material which permitted direct observation of the vertebral body surface without any imaging technique bias. Taking into consideration all of the above, the present and the previous paper on SNs [13] supply valid data to the prevalence of SNs.
Vertebra(e) most prone to SNs
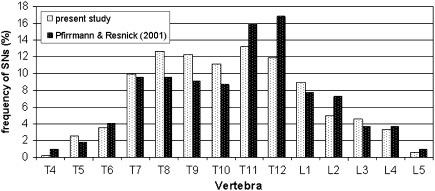
Our study showed that SNs appear more frequently in the T7–L1 region than in the higher thoracic vertebrae (T4–T6) or the lower lumbar vertebrae (L2–L5). This frequency distribution agrees with Pfirrmann and Resnick’s [14] study (Fig. 5) but not with Hilton et al. [7] and Williams et al. [15].
Fig. 5.
Prevalence of SNs in the thoraco-lumbar vertebrae in our study and Pfirrmann and Resnick [14] study: superior and inferior vertebral body surfaces combined
The majority of studies using archeological skeletal material also determined that SNs primarily concentrate in the thoracic region [6, 16] thus suggesting that SNs etiology is probably independent of the time dimension.
Why do SNs concentrate more in the thoracic region? And what does this imply?
Hilton et al. [7] suggested that since vertebral fractures most commonly occur at the T12 and L1 levels, this region is probably more susceptible to stress, the outcome of which is invagination of the nucleus into the vertebral body via the vertebral end-plate. The major obstacle to this theory, as the authors themselves note, is that vertebral fractures usually involve the superior vertebral body surface, whereas SNs mainly occur on the inferior vertebral body surface. It is therefore unlikely that the distribution of SNs can be explained solely by differences in the load magnitude along the spine. Additionally, if extra load would have been the sole cause for SNs development, we would expect increasing prevalence of SNs from T1 to L5 (maximum load), yet this is not the case. Therefore, the higher prevalence of SNs in the mid and lower thoracic region compared to the lumbar region suggests that other factors might be involved, to wit: (a) to tolerate increasing loading at the lower levels, the lumbar vertebral body cortex thickness is greater compared with the thoracic vertebrae [17]. Consequently, the lumbar vertebrae may have better resistance to herniation of the intervertebral disc into the body than the thoracic ones; (b) the thoracic vertebrae are more prone to rotational movement while in the lumbar area the torsion is minimal compared to movements on the sagittal plane [18]. As will be later discussed, the torsional movements and the following stress on the intervertebral disc are a major cause in the manifestation of SNs; and c. The lumbar vertebrae are much larger and therefore better resist stress. Mechanical stress in a vertebral body during axial loading is inversely proportional to the cross sectional area of the body [19]. This view gains indirect support from the fact the larger the lumbar vertebra, the lower the prevalence of SNs.
Is it possible that the different manifestation of SNs on the thoracic and lumbar vertebrae is due to different etiologies for SNs in these two regions? This possibility needs to be taken into consideration as there is evidence suggesting different etiologies causing vertebral deformities in the thoracic and lumbar spine [20].
Why are SNs more common on the inferior surface of the thoracic vertebrae (T4–T11) and on the superior surface of the lumbar vertebrae (L1–L5)?
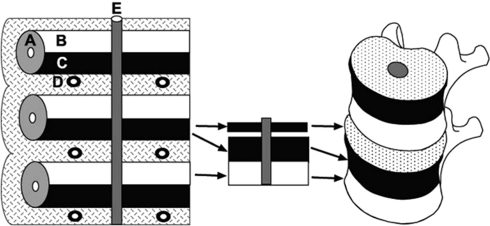
The relatively higher frequency of SNs on the inferior vertebral surface of the thoracic vertebrae compared to the superior surface is in concordance with several previous reports [2, 7, 15]. At present, no convincing explanation has been suggested for this phenomenon. We believe that the key could be in the developmental process of the vertebrae during early life. The formation of vertebral bodies begins approximately at the fourth week of embryonic life with a condensation of sclerotome cells around the notochord. At this stage, each sclerotome consists of loosely arranged cells cranially and densely packed cells caudally. The vertebral body develops from the fusion of the dense caudal portion of each sclerotome with the loose cranial portion of the adjacent sclerotome [21, 22]. Thus, each vertebral body is formed from the cranial and caudal halves of two successive sclerotome masses (Fig. 6). This developmental process may suggest that the inferior half of the vertebral body is mechanically weaker, at least in the early years of life, than the superior half.
Fig. 6.
Left A 4-week embryo. The celerotomes consist of loosely arranged cells cranially and densely packed cells caudally. Right The densely packed cells fuse with the loosely arranged cells of the caudal sclerotome to form the vertebral body. The intervertebral disc is form from cranial section of the densely arranged cells. A Sclerotomes, B loosely arranged cells cranially, C densely packed cells caudally, D inter-segmental arteries, E notochord
Why do not the lumbar vertebras show the same trend? We believe that the presence of SNs is multi-factorial, depending not just on the relative strength of the two segments of the vertebral body, but also on the amount of torsional movement in the various spinal segments and the stress resulting from these movements. The fact that the tensile strain at the superior vertebral body surfaces of the lumbar vertebrae is higher than in the inferior surface [23], may partially explain the pattern distribution of SNs in this area.
SNs positions on the vertebral body surface
We found that most SNs appear in the middle part of the vertebral body surface or slightly posterior to it. These findings are in contrast to Pfirrmann and Resnick [14] who found that almost two-third of the SNs are positioned in the posterior part of the vertebral body. They used their findings to support the notion that trauma is a major factor causing the appearance of SNs, as this area in the vertebral body is the most susceptible to injury. As previously mentioned, development of SNs cannot be explained solely on biomechanical grounds or traumatic events. Our findings of the SNs location in the middle-posterior part of the vertebral body surface corresponds with the location of the nucleus pulposus inside the intervertebral disc, the position of the notochord and the thinnest part of the endplate. These characteristics cause the center of the vertebral body to become the weakest part of the vertebral end-plate [24].
Current model for the pathophysiology of SNs
Based on the current findings and previous studies, we suggest a model explaining the pathophysiology of SNs. The model is not yet completed; however, it offers a reasonable explanation for many of the findings presented in this study. Following the adoption of erect posture and bipedal locomotion, the human spine had to cope with two contradicting requirements, i.e., the need for wide range of motion on one hand and stability on the other. Many of our daily activities require considerably rotational movements of the spine thus putting considerable stress on the intervertebral disc, especially the stretching of the annulus fibrosis. In the adult vertebra, when the epiphyseal ring is already complete and fused to the vertebral body, the stretching fibers of the annulus transmit the force via the ring from one vertebral body to another. Yet, the epiphyseal ring starts developing only around the age of 9 and is fully fused to the vertebral body only at the age of ca. 25, implying that in adolescent life the vertebral end-plate is the main structure anchoring the annulus fibrosus fibers. During adolescence, any movement will pose considerable stress on the endplate, especially twisting forces at its center. The outcome of these repetitive movements is small micro-fissures appearing in the central part of the cartilaginous endplate, enabling fluid to travel through and reach the bony surface. The fissures then expand and extrusion of nucleus pulposus material occurs and starts eroding into the vertebral body. Blood vessels may also penetrate through these fissures, becoming enlarged. With time, the local trabeculae degenerate and a small cavity is formed which eventually becomes encapsulated by a densely formed bony wall, preventing the process from continuing. Supporting evidence from Gracovetsky and Farfan’s [25] study showed that spinal inter-segmental movement of 20 (referring to axial rotation only) or more is associated with the development of local micro-trauma. Gregersen and Lucas [26] showed that the anatomical arrangement of the lumbar vertebrae allow only minimum axial rotation compared to the thoracic vertebrae. Kapandji [18] found that the center of the axial rotation of a typical thoracic vertebra lies within the intervertebral disc whereas for lumbar vertebra it is located posteriorly, suggesting that the thoracic vertebrae are exposed to a much greater twisting force.
Summarizing the above, it may well be that the combination of increased range of rotational movement, location of axis of rotation and low ratio of disc thickness to vertebral body height in the thoracic spine makes the endplate more vulnerable to micro-tear and to the development of SNs.
Certainly, other factors (e.g., congenital defects, trauma, and various spinal diseases) can contribute to the formation of SNs [2, 27], but considering the high prevalence of the phenomenon, they are not central. It is the normal loading regime and movement pattern of the spine which predisposes the individual to endplate rupture and subsequent SNs in adolescent life.
Conclusions
The distribution and location of SNs along the thoraco-lumbar spine do not tend to support the traumatic or disease explanation of the phenomenon. SNs occurrences are probably associated with the vertebra development process during early life, the nucleus pulposus pressure on the weakest part of the end plate and the various strains on the vertebrae and the intervertebral disc along the spine during spinal movements (especially torsional movements).
Acknowledgments
The author thanks Prof. Bruce Latimer, Mr. Lyman Jellema from the Cleveland Museum of Natural History, Cleveland, Ohio, for their support and assistance in using the invaluable Hamann-Todd osteological collection; to Mrs. Phyllis Curchack Kornspan for her editorial services; The Dan David Foundation, The Tassia and Dr. Joseph Meychan Chair of History and Philosophy of Medicine, and the Israel Science Foundation for their financial support.
References
- 1.McFadden KD, Taylor JR. End-plate lesions of the lumbar spine. Spine. 1989;14:867–869. doi: 10.1097/00007632-198908000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Resnick D, Niwayama G. Intervertebral disc herniations: cartilaginous (Schmorl’s) nodes. Radiology. 1978;126:57–65. doi: 10.1148/126.1.57. [DOI] [PubMed] [Google Scholar]
- 3.Hamanishi C, Kawabata T, Yosii T, Tanaka S. Schmorl’s nodes on magnetic resonance imaging. Their incidence and clinical relevance. Spine. 1994;19:450–453. doi: 10.1097/00007632-199402001-00012. [DOI] [PubMed] [Google Scholar]
- 4.Wu HT, Morrison WB, Schweitzer ME. Edematous Schmorl’s nodes on thoracolumbar MR imaging: characteristic patterns and changes over time. Skeletal Radiol. 2006;35:212–219. doi: 10.1007/s00256-005-0068-y. [DOI] [PubMed] [Google Scholar]
- 5.Stäbler A, Bellan M, Weiss M, Gärtner C, Brossmann J, Reiser MF. MR imaging of enhancing intraosseous disk herniation (Schmorl’s nodes) Am J Roentgenol. 1997;168:933–938. doi: 10.2214/ajr.168.4.9124143. [DOI] [PubMed] [Google Scholar]
- 6.Faccia KJ, Williams RC. Schmorl’s nodes: clinical significance and implications for the bioarcheological record. Int J Osteoarchaeol. 2008;18:28–44. doi: 10.1002/oa.924. [DOI] [Google Scholar]
- 7.Hilton RC, Ball J, Benn RT. Vertebral end-plate lesions (Schmorl’s nodes) in the dorsolumbar spine. Ann Rheum Dis. 1976;35:127–132. doi: 10.1136/ard.35.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saluja G, Fitzpatrick K, Bruce M, Cross J. Schmorl’s nodes (intervertebral herniations of intervertebral disc tissue) in two historic British populations. J Anat. 1986;145:87–96. [PMC free article] [PubMed] [Google Scholar]
- 9.Landis JR, Koch GG. The measurements of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 10.Schmorl G, Junghanns H. The Human Spine in Health and Disease (2nd American edition translated and edited by Besemann EF) New York: Grune and Stratton; 1971. [Google Scholar]
- 11.Hansson T, Roos B. The amount of bone mineral and Schmorl’s nodes in lumbar vertebrae. Spine. 1983;8:266–271. doi: 10.1097/00007632-198304000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Frymoyer JW, Newberg A, Pope MH, Wilder DG, Clements J, MacPherson B. Spine radiographs in patients with low-back pain. An epidemiological study in men. J Bone Joint Surg Am. 1984;66:1048–1055. [PubMed] [Google Scholar]
- 13.Dar G, Peleg S, Masharawi M, Steinberg N, May H, Hershkovitz I. Demographical aspects of Schmorl’s nodes—a skeletal study. Spine. 2009;34:E312–E315. doi: 10.1097/BRS.0b013e3181995fc5. [DOI] [PubMed] [Google Scholar]
- 14.Pfirrmann CW, Resnick D. Schmorl nodes of the thoracic and lumbar spine: radiographic-pathologic study of prevalence, characterization, and correlation with degenerative changes of 1, 650 spinal levels in 100 cadavers. Radiology. 2001;219:368–374. doi: 10.1148/radiology.219.2.r01ma21368. [DOI] [PubMed] [Google Scholar]
- 15.Williams FM, Manek NJ, Sambrook PN, Spector TD, Macgregor AJ. Schmorl’s nodes: common, highly heritable, and related to lumbar disc disease. Arthritis Rheum. 2007;57:855–860. doi: 10.1002/art.22789. [DOI] [PubMed] [Google Scholar]
- 16.Owsley DW, Orser CE, Jr, Mann RW, Moore-Jansen PH, Montgomery RL. Demography and pathology of an urban slave population from New Orleans. Am J Phys Anthropol. 1987;74:185–197. doi: 10.1002/ajpa.1330740207. [DOI] [PubMed] [Google Scholar]
- 17.Edwards WT, Zheng Y, Ferrara LA, Yuan HA. Structural features and thickness of the vertebral cortex in the thoracolumbar spine. Spine. 2001;26:218–225. doi: 10.1097/00007632-200101150-00019. [DOI] [PubMed] [Google Scholar]
- 18.Kapandji AI (2008) The Physiology of the Joints. vol III. The vertebral column, pelvic girdle and head, 6th edn. Churchill Livingstone, New York
- 19.Duan Y, Seeman E, Turner CH. The biomechanical basis of vertebral body fragility in men and women. J Bone Miner Res. 2001;16:2276–2283. doi: 10.1359/jbmr.2001.16.12.2276. [DOI] [PubMed] [Google Scholar]
- 20.Cvijetić S, McCloskey E, Korsić M. Vertebral osteophytosis and vertebral deformities in an elderly population sample. Wien Klin Wochenschr. 2000;112(9):407–412. [PubMed] [Google Scholar]
- 21.Gray H. Gray’s anatomy. 38. New York: Churchill Livingstone; 1995. [Google Scholar]
- 22.Moore K, Persuad T (2003) The developing human: clinically oriented embryology, 7th edn. WB Saunders, Philadelphia
- 23.Hongo M, Abe E, Shimada Y, Murai H, Ishikawa N, Sato K. Surface strain distribution on thoracic and lumbar vertebrae under axial compression. The role in burst fractures. Spine. 1994;24:1197–1202. doi: 10.1097/00007632-199906150-00005. [DOI] [PubMed] [Google Scholar]
- 24.Grant JP, Oxland TR, Dvorak MF. Mapping the structural properties of the lumbosacral vertebral endplates. Spine. 2001;26:889–896. doi: 10.1097/00007632-200104150-00012. [DOI] [PubMed] [Google Scholar]
- 25.Gracovetsky S, Farfan H. The optimum spine. Spine. 1986;11:543–573. doi: 10.1097/00007632-198607000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Gregersen GG, Lucas DB. An in vivo study of the axial rotation of the human thoracolumbar spine. J Bone Joint Surg Am. 1967;49:247–262. [PubMed] [Google Scholar]
- 27.Tribus CB. Scheuermann’s kyphosis in adolescents and adults: diagnosis and management. J Am Acad Orthop Surg. 1998;6:36–43. doi: 10.5435/00124635-199801000-00004. [DOI] [PubMed] [Google Scholar]