Abstract
The aim of this study is to determine the predictive values of laboratory indicators of pyogenic vertebral osteomyelitis (PVO) and a potential cure if the microorganism cannot be identified. Forty-five consecutive patients with PVO were enrolled. Antibiotic therapy with or without surgery was performed according to microorganism. In the negative-culture (NC) group, cefazolin was administered in cases of hematogenous PVO, and vancomycin was administered in cases of postoperative or procedure-related PVO. The clinical, laboratory, and radiological findings were followed up with regard to an appropriate response to antimicrobial therapy. Nine patients were treated with antibiotics alone. We were able to identify the microorganism in 34 cases (75.6%). Ten cases in NC group were cured without recurrence, but one was not. Identification of the microorganisms did not have any significant influence on the treatment outcome, duration of antibiotic administration or normalization of laboratory profiles. For erythrocyte sedimentation rate (ESR) values over 55 mm/h and C-reactive protein (CRP) values of 2.75 mg/dL at fourth week after antibiotic administration by means of ROC curve analysis, we expect significantly high rates of treatment failure by Pearson χ2 test (χ2 = 4.344, Odds ratio = 5.15, p = 0.037, 95% CI 1.004–26.597). Even in patients with negative culture findings, it is expected that a good outcome will be achieved by the administration of cefazolin or vancomycin for about 6 weeks. It is concluded that antibiotics selected according to the etiological setting can be initiated without the need to start empirical antibiotics. In every instance at fourth week after the initiation of antibiotic therapy, the values of CRP and ESR can provide meaningful information regarding whether clinicians need to reevaluate the effectiveness of antibiotics by performing follow-up imaging studies and monitoring the patient’s clinical manifestations.
Keywords: Pyogenic vertebral osteomyelitis, Negative culture study, ESR and CRP, Antibiotics
Introduction
Pyogenic vertebral osteomyelitis (PVO) with or without spinal epidural abscess is a rare clinical condition [1–3]. It usually presents insidiously and follows an inactive clinical course, making early diagnosis difficult. The incidence of PVO is currently on the rise due to the increase in the elderly population with chronic debilitating diseases such as diabetes mellitus and impaired immunocompetence, increases in spinal instrumentation and the use of epidural catheters for pain therapy and surgery, and the rise in the prevalence of intravenous drug abuse [4, 5]. PVO may produce acute neurological deterioration or moribund status resulting from septicemia. Clinicians should be aware of the clinical characteristics, natural course, neuroimaging parameters, laboratory findings and neurological changes associated with PVO. Such knowledge would aid in the early detection of PVO as well as the application of proper treatment. The mainstay of treatment for PVO has been prompt administration of appropriate antibiotics, and it is often combined with bracing or surgical intervention. Due to wide variation in the clinical presentation of PVO and the absence of a single laboratory finding indicating the degree of infection, it is often difficult to establish a therapeutic plan and remain confident in it. The situation is worse when the microorganism is not identified. It is well known that determination of microorganism may help clinicians choose a specific antibiotic and predict the treatment outcome. However, most reports showed a high rate of negative culture (NC), ranging from 10 to 30% [6–8], which indicates that it is still hard to determine the microorganism. It is difficult for clinicians to choose antibiotics in cases with NCs and to judge the antibiotic response in the absence of clinical improvement. Therefore, we conducted a retrospective review to investigate the outcome of culture-negative and culture-positive patients with PVO and to identify laboratory parameters that can predict the outcome of PVO.
Materials and methods
We retrospectively reviewed 45 cases admitted to our hospital with the clinical diagnosis of hematogenous or postoperative non-tuberculous PVO between May 2003 and December 2007. The diagnosis of PVO was made based on the fulfillment of the following three criteria: (a) compatible clinical symptoms and signs (pain or neurological deterioration with or without fever), (b) confirmation of initial MRI diagnosis (loss of disc height at the level of involvement with enhancement, end-plate erosions, vertebral destruction, epidural or paravertebral abscess, spinal cord compression) and (c) biological markers revealing infectious conditions and/or bacterial isolation from an image-guided discovertebral sample by blood culture or from the surgical field. At least two blood cultures were obtained from each patient before antibiotic therapy was initiated. In non-surgical cases, tissues for culture were obtained by computed tomography-guided percutaneous sampling. When the microorganism was not identified in the first culture, a second sampling was performed at a different site. Antibiotic administration started after the surgical sampling or last percutaneous sampling. Patients with NC studies were rechecked to confirm the fulfillment of the following conditions: (1) laboratory findings of inflammation, (2) imaging study evidence of PVO, (3) at least two negative blood cultures and negative percutaneous disc or vertebral body biopsy if performed, (4) no histological evidence of granulomatous inflammation or fungal infection, and (5) negative microbiology after prolonged cultures for Mycobacterium tuberculosis and PCR study of the Mycobacterium complex. All patients were treated by neurosurgeons (Kim HJ, Kim KJ, Chung SK). Decompression and reconstruction with instruments were performed if there was any intractable pain, failure of medical management, neurological impairment with or without an associated abscess, vertebral destruction causing spinal instability and/or segmental kyphosis, and operative treatments such as tissue debridement. Antibiotics were administered after consultation with an infection specialist from the internal medicine department. In cases with NC results, empirical antibiotics were chosen after considering the possible portal of entry. The same protocol was followed in patients referred from other hospitals.
The clinical, laboratory, and radiological findings were followed to assess the response to antimicrobial therapy. Data on age, sex, site of infection, concomitant diseases, risk factors, and preceding events were extracted from the patients’ electronic medical records. Inflammatory markers such as full blood cell count, including white blood cell count (WBC), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) level, were followed weekly during admission and monthly thereafter. We defined the normalization of values of WBC, ESR and CRP as beneath 10,000/μl, 20 mm/h and 0.5 mg/dL, respectively. All patients were followed up for at least 3 months after discharge.
Statistical analysis
Continuous data were described as means and standard deviations or range, and categorical variables were expressed as percentages. The χ2 test (or Fisher’s exact test if an expected value was less than 5) was used to compare categorical variables between the groups. Student’s t test and ANOVA were used to compare continuous variables. The presumption was performed by means of multiple logistic regression analysis and ROC curve analysis to identify parameters that could be used to predict clinical outcome using SPSS 12.0 software (Apache software foundation, Chicago, IL, USA).
Results
Patient characteristics and outcomes
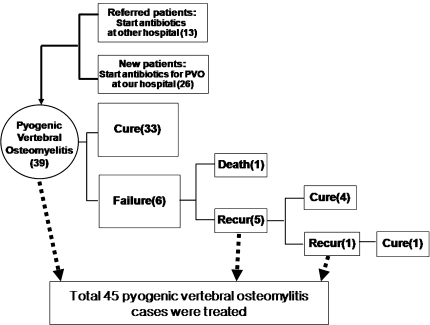
A total of 45 PVO cases were treated between May 2003 and December 2007. The study population included 24 men and 21 women who ranged in age from 30 to 81 years (mean age 58). Thirteen cases were treated at other hospitals, 26 were newly diagnosed with PVO at our institute and 6 were cases of recurrent PVO. A flowchart showing the clinical conditions of the patients from admission is presented in Fig. 1. Twenty-four patients had one or more comorbid conditions. The most frequent comorbidity was diabetes mellitus (n = 16), followed by cirrhosis or chronic alcohol liver disease (n = 7), other systemic infectious disease (n = 3), chronic renal failure or end stage renal disease (n = 2) and malignancy (n = 2). The most frequent presenting clinical manifestation was pain (n = 32), followed by fever (n = 20). The characteristics of the patients are listed in Table 1. The lumbar vertebrae were most frequently involved (26 patients), followed by the thoracic (6 patients) and cervical (4 patients) vertebrae. Nine patients had involvement of multiple vertebrae. In five patients (11.1%), PVO presented as a disease of a single vertebra, usually with a collapsed vertebral body. In others, it involved two or more contiguous vertebral bodies and intervening disk spaces (88.9%). Thirty-six patients underwent surgical management, such as curettage of abscess and infected bone with or without reconstruction. Seven of the 36 patients who underwent surgical management had NCs and 29 had positive cultures (PCs). A frank paravertebral mass was indentified in 5 cases, and an epidural abscess was identified in 14 cases. Nineteen patients had no definite causative procedure or surgery before visiting the hospital. The configuration of PVO and other characteristics are shown in Table 2. Severe complications developed in eight patients. Six patients experienced drug-induced fever or eosinophilia, and two had meningitis. One patient died during treatment and 26 (57.8%) recovered without residual abnormalities. The most frequent residual abnormalities at the final follow-up were pain (N = 8, 17.8%) and functional impairment (N = 11, 24.4%). The rates of treatment failure and complication were not affected by the extent of PVO, coexistence of an abscess, comorbidity, surgical management, route of entry, newly diagnosed, referral from another hospital, age and sex.
Fig. 1.
The flow chart of the initial conditions of patients with pyogenic vertebral osteomyelitis (PVO)
Table 1.
Demographics of pyogenic vertebral osteomyelitis (PVO) patients
| Variable | N |
|---|---|
| Sex | Male 24, Female 21 |
| Age (years) | 59.7 (30–81) |
| Mean symptom duration (days) | 53.9 (1–360) |
| Initial symptom presentation | |
| Pain | 32 |
| Fever | 20 |
| Weakness | 9 |
| Wound problem | 4 |
| Comorbidity | |
| Diabetes | 16 |
| Chronic liver disease | 7 |
| Systemic infection | 3 |
| Cancer | 2 |
| End stage renal disease | 2 |
| None | 21 |
Table 2.
The characteristics of PVO: configuration, treatment modality and route of entry
| Variable | N | % |
|---|---|---|
| Site of involvement | ||
| Cervical | 4 | |
| Thoracic | 6 | |
| Lumbar | 26 | |
| Mutifocal | 9 | |
| Involved vertebral body | ||
| 1 | 5 | |
| 2 | 26 | |
| 3 | 9 | |
| 4 | 3 | |
| 5 | 1 | |
| 6 | 1 | |
| Concomitant abscess with PVO | ||
| With abscess | 21 | 46.7 |
| Without abscess | 24 | 53.3 |
| Treatment modality | ||
| Surgery + antibiotics | 36 | 80 |
| Antibiotics only | 9 | 20 |
| Route of entry | ||
| Community acquired | 19 | 42.2 |
| Nosocomial | ||
| Procedure related | 5 | 11.1 |
| Postoperative | 21 | 46.7 |
PVO pyogenic vertebral osteomyelitis
Microorganism and antibiotics
We were able to identify the microorganism in 34 cases (75.6%). The most frequently identified organism was Staphylococcus aureus (N = 16, 35.6%). Nine cases of S. aureus infection were methicillin-resistant, and seven were methicillin-sensitive. The microorganisms were composed of 16 S. aureus followed by 4 methicillin-resistant S. epidermidis and 4 Streptococcus agalactiae each other. All microorganisms and susceptible antibiotics administered are listed in Table 3. The mean duration of antibiotic administration was 8.0 weeks (±4.1 weeks). Vancomycin was initiated in methicillin-resistant Staphylococcus and Streptococcus species following the identification of the causative microorganism. Nafcillin or cefazolin was prescribed for patients with MSSA. All MSSA cases showed improvement without recurrence. In the NC group, cefazolin was administered in cases of hematogenous PVO, and vancomycin was chosen for the treatment of postoperative or procedure-related PVO. There was one case of postoperative discitis at the L3-4 level, but the other ten cases were cured without recurrence. There was no significant difference in WBC and ESR value on the first day (the day on which the antibiotic was initiated) or the first, second, fourth, sixth, eighth week between the PC group and NC groups. On the other hand, there was a significant difference in CRP value on the first day (PC 12.9 g/dL, NC 5.1 g/dL, p = 0.007). There was no significant difference in the duration of normalization of values of WBC and ESR, but the duration of CRP normalization was significantly longer between the PC group and NC groups. In the NC group (PC 25.9 days vs. NC 53.6 days, p = 0.005), the rate of failure to identify the microorganism was 24.4% (n = 11) in the enrolled patients and 26.9% (n = 7) in the newly diagnosed patients. Identification of the causative microorganism did not have any significant effect on treatment outcome.
Table 3.
Identified microorganisms and administered antibiotics
| Causative organism | 1st line antibiotics | 2nd line antibiotics | N | % |
|---|---|---|---|---|
| MRSA | Vancomycin ± Ripamfin | Teicoplanin or Linezolid ± Ripamfin | 9 | 20 |
| MSSA | Nafcillin | Cefazolin | 7 | 15.6 |
| MRSE | Vancomycin | Teicoplanin or Linezolid | 4 | 8.9 |
| Streptococcus agalactiae (group B) | Penicillin | Cefazolin | 4 | 8.9 |
| E. coli | Cefotaxime → Ciprofloxacin(PO) | Piperacillin/Tazobactam → Ciprofloxacin(PO) | 3 | 6.7 |
| Streptococcus viridans | Penicillin | N | 2 | 4.4 |
| Klebsiella pneumoniae | Cefotaxime → Ciprofloxacin(PO) | N | 1 | 2.2 |
| Enterobacter cloacae | Cefotaxime + Amikacin | N | 1 | 2.2 |
| Acinetobacter baumannii | Ceftazidime → Piperacillin/Tazobactam | N | 1 | 2.2 |
| Beta-Streptococcus, Group C Streptococcus, MSSA | Ampicillin/sulbactam → Cefazolin | N | 1 | 2.2 |
| Bacillus species, MRSE, Enterotoccocus faecalis (group D) | Vancomycin + Fluconazole | N | 1 | 2.2 |
| Unidentified | Cefazolin ± Ripamfin, Vancomycin ± Ripamfin | N | 11 | 24.4 |
| 45 | 100 |
MRSA methicillin-resistant Staphylococcus aureus, MSSA methicillin-sensitive Staphylococcus aureus, MRSE methicillin-resistant Staphylococcus epidermidis, PO per os (Change IV antibiotics with PO antibiotics)
The laboratory markers reflecting clinical outcome
Retrospectively, the patients were categorized into either the controlled group or failure group in order to evaluate the treatment outcome. ‘Controlled’ was defined as completion of antibiotic therapy corresponding to improved clinical manifestations (no fever and no wound problem) and improved laboratory parameters or imaging findings without recurrence for 3 months. Treatment failure included disease progression and recurrence. Deaths related to PVO and newly detected abscess or infectious condition at another site within 3 months were considered indicative of disease progression. Clinical and laboratory deterioration with or without abnormal radiological findings 3 months after the completion of antibiotic treatment was classified as recurrence. Recurrence was disclosed in seven cases, and one case was treated for two consecutive recurrences. We compared the WBC, ESR and CRP values on the first day, as well as in the first, second, fourth, sixth, eighth week (Fig. 2a–c). There were no significant differences in the laboratory parameter values between the groups. The most significantly sensitive and specific values of ESR and CRP for predicting the likelihood of treatment failure were obtained by means of ROC curve analysis during the fourth week after antibiotic administration (Fig. 2d). If the ESR value was over 50 mm/h and the CRP value was over 2.75 mg/dL, we expected a significantly high risk of treatment failure by Pearson χ2 test (χ2 = 4.344, Odds ratio = 5.15, p = 0.037, 95% CI 1.004–26.597). There were no significant correlations between clinical outcome and variables such as sex, age, operative management, identification of microorganism, previous history of treatment failure and abscess formation. However, multiple logistic regression analysis revealed that comorbidity had a significant influence on treatment outcome (p = 0.017, 95% CI 1.751–333.333) (Table 4).
Fig. 2.
Comparison of laboratory markers; a white blood cell (WBC) count, b erythrocyte sedimentation rate (ESR) and c C-reactive protein (CRP) between the positive culture group and negative culture group. Both sensitive and specific values of ESR and CRP were pointed out by ROC curve analysis (d) at the fourth week after antibiotic treatment
Table 4.
Factors associated with relapse in patients with pyogenic vertebral osteomyelitis by means of multiple logistic regression analysis
| Risk factors | RR (treatment failure) | p value | 95% CI |
|---|---|---|---|
| Older age | 1.069 | 0.144 | 0.978–1.168 |
| Longer symptom duration | 0.994 | 0.357 | 0.981–1.007 |
| Male (vs. female) | 0.42 | 0.418 | 0.051–3.428 |
| Newly diagnosed (vs. referred patients) | 1.283 | 0.802 | 0.184–8.959 |
| With comorbidity | 22.727 | 0.017 | 1.751–333.333 |
| With abscess | 1.08 | 0.944 | 0.129–9.011 |
| Identified organism | 2.66 | 0.462 | 0.196–35.714 |
Discussion
Pyogenic vertebral osteomyelitis accounts for approximately 1–7% of all bone infections [9–11]. It encompasses a spectrum of pathological conditions, including discitis, spondylitis, and spondylodiscitis [1, 12]. The incidence of spinal infections appears to have increased in recent years, and this disease is now estimated to occur in approximately 1 per 100,000 persons annually [9, 13, 14]. This rise in the incidence of spinal infections can be attributed to the increasing prevalence of elderly and immunocompromised individuals in the population [12, 15]. In this study, the presence of comorbidity was the only significant factor determining the patients’ outcome using multiple logistic regression analysis. In this study, diabetes mellitus (35.6%) represented the most common comorbidity, followed by chronic liver disease (15.6%), systemic infectious condition (6.7%), end stage renal disease (4.4%) and cancer (4.4%). These rates differed somewhat from those previously reported, with diabetes mellitus occurring in 7–28% of patients [7, 16–21], cancer occurring in 3–15%, rheumatoid arthritis occurring in 2–8%, and corticosteroid use reported in 2–7% [8, 17–20, 22, 23]. There was no difference in treatment outcome between the referred patients and patients who were newly diagnosed in our hospital. The extent of the level of vertebra affected and presence of an abscess failed to influence the clinical outcomes of patients with PVO. In addition, there was no significant difference in patient outcome between those who underwent surgery and those who did not. However, we cannot say that surgery is not necessary in the treatment of PVO because the patients were not randomly allocated into surgery and non-surgery groups, as would be the case in a prospective study, and the surgery was performed when medical treatment alone was not sufficient. Despite the surgical advances and availability of antibiotics, there is a significant risk of mortality and relapse following PVO [17, 23]. Surgical intervention is reserved for specific indications, such as the identification of the microorganism via an open biopsy, controlling spinal infections, symptomatic spinal cord compression or radicular neurologic deficit, correction of deformity such as post-infection kyphosis, and, finally, the management of severe persistent pain [24, 25].
Staphylococcus aureus was the most commonly isolated organism in this study (16 cases, 35.6%). This finding is in agreement with most of the reported literature, with S. aureus accounting for 42–84% of the isolated organisms in some studies [21, 24, 26–28]. Especially, MRSA is found in 30–40% of nosocomial S. aureus strains, but in less than 1% of community-acquired strains [29, 30]. Two distinct differences in our study are the increased incidence of MRSA cases in community-acquired PVO infections (50%) and the relatively lower incidence of MRSA cases in nosocomial series (26.9%). Streptococcus species (N = 6 cases, 13.3%) were the second most commonly encountered organism in the current study. Multiple organisms, isolated in two cases in our current PVO series, have been shown by others to occur at a lower rate [26, 31]. Polymicrobial infections did not alter the patient outcome [1]. There were 11 cases (24.4%) of NC findings in the current patients, which is in agreement with the previous findings [1, 10, 24]. By means of administration of cefazolin or vancomycin, patients in whom the causative microorganism could not be identified achieved relatively favorable rates of cure (90.9%) in this series. These agents should be effective against Staphylococci, which account for 50% of all PVO cases [32, 33]. In addition, the etiological circumstances deserve careful consideration due to differences in the incidence of methicillin-resistant S. aureus between nosocomially acquired PVO and community-acquired PVO [10]. In the present study, 19 cases (42.2%) had been referred from another hospital. In these patients, antibiotic therapy was initiated by the referring primary hospitals. However, cultures were obtained before the initiation of antibiotic treatment. In all cases of PVO, a specific microbiologic diagnosis is desirable for susceptibility testing-guided antibiotic treatment. However, an etiological diagnosis is not always possible. Possible causes include procedure failure, lower virulence and early initiation of broad spectrum antibiotics before microbiologic diagnosis. There were 11 cases (24.4%) of NC study in this study, which is similar to the results of previous studies [1, 10, 24]. However, identification of the microorganism did not significantly influence the final outcome. Whatever the cause of NC, these patients were less likely to present with fever and to have relatively lower WBC counts, CRP and ESR when compared to the PC group between the first day and fourth week. They also have a relatively shorter duration of normalization of WBC, ESR and CRP. These results may be attributable to a more locally infective process without bacteremia [6], a less infective process caused by a low virulence pathogen or an advanced natural healing status of PVO [34, 35]. The evaluation of treatment effectiveness in the management of PVO is not simple. No single parameter is conclusive in revealing the status of the patient. Imaging abnormalities frequently persist despite clinical and biological cure of PVO, and they are not associated with a greater risk of relapse [29, 36, 37]. Therefore, clinical symptoms and signs with laboratory markers still deserve to be considered as important parameters when evaluating the clinical response and determining recurrence after treatment discontinuation. Some reports have recommended that patients should be followed up throughout the course of treatment and for 1 year after its completion in order to detect relapses [22]. This should include clinical assessment of pain and neurological features, laboratory assessment with serial monitoring of CRP and ESR, and radiological examination using plain radiographs if needed. Reduction in back pain and recovery of constitutional symptoms suggest successful treatment. However, clinicians cannot exclude the possibility of recurrence or treatment failure on the basis of clinical symptom relief alone [10]. Many investigations showed laboratory markers as tools for diagnosis and good predictors of clinical outcome. WBC count is an unreliable laboratory marker in the diagnosis of many spinal infectious processes [23, 24, 26, 31, 38]. It is often only slightly elevated, and it is often normal in elderly or immunocompromised patients. It has been reported that the WBC count is elevated in only 42.6% of PVO cases [38]. However, the ESR and CRP levels were more sensitive to the presence of a spinal infection (elevated ESR = 84% and CRP = 84%) [23, 24, 26, 31, 38]. In other literature, the ESR was found to be a more sensitive test of spinal infection because it is elevated in 70–100% of cases, as compared with only 13–60% of cases in which an elevated leukocyte count is identified [1, 24, 28, 31]. A decreasing ESR during the first month of conservative therapy is also a good prognostic sign, although a favorable outcome is also seen in 40% of cases with a persistently elevated ESR [8]. Increases in CRP levels occurring after the 4th postoperative day are likely to be caused by complications of a neurosurgical procedure [39]. CRP normalizes more quickly than ESR, and it may be more helpful in tracking recovery [14, 29, 37]. A 50% drop in the CRP level each week is indicative of a good response [29, 30].
In our series, the ESR and CRP values at the fourth week after the initiation of antibiotics were noteworthy as predictive markers for the effectiveness of treatment. However, the values of ESR and CRP (ESR = 50 mm/h, CRP = 2.75 mg/dL) during the fourth week cannot be represented as an absolute value for predictors of cure or recurrence. The value would be more reliable if obtained in a larger and more homogenous population. At the fourth week after initiation of antibiotic therapy, these parameters can provide meaningful information as to whether clinicians need to recheck the effectiveness of antibiotics by performing follow-up imaging studies and assessing the patient’s clinical manifestations. These imply that these parameters, when elevated, represent “red flags” for a more serious condition [1]. The follow-up of laboratory parameters may be more important when the microorganism is unknown.
Conclusion
Identification of microorganisms causing PVO will help neurosurgeons initiate susceptible antibiotics. However, the microorganism is still detected at very low rates. Even in patients with NC results, it is expected that a good outcome will be achieved by administration of empirical cefazolin or vancomycin for about 6 weeks. The final treatment outcomes of the patients in the PC and NC groups were similar. In every instance, at the fourth week after initiation of antibiotics therapy, the values of CRP and ESR can provide meaningful information as to whether clinicians need to recheck the effectiveness of the treatment by performing follow-up imaging studies and evaluating the patient’s clinical manifestations.
References
- 1.Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine. 2000;25:1668–1679. doi: 10.1097/00007632-200007010-00010. [DOI] [PubMed] [Google Scholar]
- 2.Mann S, Schutze M, Sola S, Piek J. Nonspecific pyogenic spondylodiscitis: clinical manifestations, surgical treatment, and outcome in 24 patients. Neurosurg Focus. 2004;17:E3. doi: 10.3171/foc.2004.17.6.3. [DOI] [PubMed] [Google Scholar]
- 3.Osenbach RK, Zeidman SM. Infections in neurological surgery: diagnosis & management. Philadelphia: Lippincott-Raven; 1999. [Google Scholar]
- 4.Belzunegui J, Intxausti JJ, Dios JR, Del Val N, Rodriguez Valverde V, Gonzalez C, Queiro R, Figueroa M. Haematogenous vertebral osteomyelitis in the elderly. Clin Rheumatol. 2000;19:344–347. doi: 10.1007/PL00011175. [DOI] [PubMed] [Google Scholar]
- 5.Gasbarrini AL, Bertoldi E, Mazzetti M, Fini L, Terzi S, Gonella F, Mirabile L, Barbanti Brodano G, Furno A, Gasbarrini A, Boriani S. Clinical features, diagnostic and therapeutic approaches to haematogenous vertebral osteomyelitis. Eur Rev Med Pharmacol Sci. 2005;9:53–66. [PubMed] [Google Scholar]
- 6.Bhagat S, Mathieson C, Jandhyala R, Johnston R. Spondylodiscitis (disc space infection) associated with negative microbiological tests: comparison of outcome of suspected disc space infections to documented non-tuberculous pyogenic discitis. Br J Neurosurg. 2007;21:473–477. doi: 10.1080/02688690701546155. [DOI] [PubMed] [Google Scholar]
- 7.Bontoux D, Codello L, Debiais F, Lambert de Cursay G, Azais I, Alcalay M. Infectious spondylodiscitis. Analysis of a series of 105 cases. Rev Rhum Mal Osteoartic. 1992;59:401–407. [PubMed] [Google Scholar]
- 8.Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1997;79:874–880. doi: 10.1302/0301-620X.79B5.8078. [DOI] [PubMed] [Google Scholar]
- 9.Chelsom J, Solberg CO. Vertebral osteomyelitis at a Norwegian university hospital 1987–97: clinical features, laboratory findings and outcome. Scand J Infect Dis. 1998;30:147–151. doi: 10.1080/003655498750003537. [DOI] [PubMed] [Google Scholar]
- 10.Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A (2008) Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum. doi:S0049-0172(08)00070-X [pii] 10.1016/j.semarthrit.2008.03.002 [DOI] [PubMed]
- 11.Roblot F, Besnier JM, Juhel L, Vidal C, Ragot S, Bastides F, Le Moal G, Godet C, Mulleman D, Azais I, Becq-Giraudon B, Choutet P. Optimal duration of antibiotic therapy in vertebral osteomyelitis. Semin Arthritis Rheum. 2007;36:269–277. doi: 10.1016/j.semarthrit.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Acosta FL, Jr, Chin CT, Quinones-Hinojosa A, Ames CP, Weinstein PR, Chou D. Diagnosis and management of adult pyogenic osteomyelitis of the cervical spine. Neurosurg Focus. 2004;17:E2. doi: 10.3171/foc.2004.17.6.2. [DOI] [PubMed] [Google Scholar]
- 13.Hopkinson N, Stevenson J, Benjamin S. A case ascertainment study of septic discitis: clinical, microbiological and radiological features. QJM. 2001;94:465–470. doi: 10.1093/qjmed/94.9.465. [DOI] [PubMed] [Google Scholar]
- 14.Lam KS, Webb JK. Discitis. Hosp Med. 2004;65:280–286. doi: 10.12968/hosp.2004.65.5.13703. [DOI] [PubMed] [Google Scholar]
- 15.An HS. Principles and techniques of spine surgery. Baltimore: Williams & Wilkins; 1998. [Google Scholar]
- 16.Colmenero JD, Jimenez-Mejias ME, Sanchez-Lora FJ, Reguera JM, Palomino-Nicas J, Martos F, Garcia de las Heras J, Pachon J. Pyogenic, tuberculous, and brucellar vertebral osteomyelitis: a descriptive and comparative study of 219 cases. Ann Rheum Dis. 1997;56:709–715. doi: 10.1136/ard.56.12.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen AG, Espersen F, Skinhoj P, Frimodt-Moller N. Bacteremic Staphylococcus aureus spondylitis. Arch Intern Med. 1998;158:509–517. doi: 10.1001/archinte.158.5.509. [DOI] [PubMed] [Google Scholar]
- 18.Kowalski TJ, Berbari EF, Huddleston PM, Steckelberg JM, Osmon DR. Do follow-up imaging examinations provide useful prognostic information in patients with spine infection? Clin Infect Dis. 2006;43:172–179. doi: 10.1086/505118. [DOI] [PubMed] [Google Scholar]
- 19.Legrand E, Flipo RM, Guggenbuhl P, Masson C, Maillefert JF, Soubrier M, Noel E, Saraux A, Di Fazano CS, Sibilia J, Goupille P, Chevalie X, Cantagrel A, Conrozier T, Ravaud P, Liote F. Management of nontuberculous infectious discitis. Treatments used in 110 patients admitted to 12 teaching hospitals in France. Joint Bone Spine. 2001;68:504–509. doi: 10.1016/S1297-319X(01)00315-3. [DOI] [PubMed] [Google Scholar]
- 20.Nolla JM, Ariza J, Gomez-Vaquero C, Fiter J, Bermejo J, Valverde J, Escofet DR, Gudiol F. Spontaenous pyogenic vertebral osteomyelitis in nondrug users. Semin Arthritis Rheum. 2002;31:271–278. doi: 10.1053/sarh.2002.29492. [DOI] [PubMed] [Google Scholar]
- 21.Sapico FL, Montgomerie JZ. Vertebral osteomyelitis. Infect Dis Clin North Am. 1990;4:539–550. [PubMed] [Google Scholar]
- 22.McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34:1342–1350. doi: 10.1086/340102. [DOI] [PubMed] [Google Scholar]
- 23.Osenbach RK, Hitchon PW, Menezes AH. Diagnosis and management of pyogenic vertebral osteomyelitis in adults. Surg Neurol. 1990;33:266–275. doi: 10.1016/0090-3019(90)90047-S. [DOI] [PubMed] [Google Scholar]
- 24.Emery SE, Chan DP, Woodward HR. Treatment of hematogenous pyogenic vertebral osteomyelitis with anterior debridement and primary bone grafting. Spine. 1989;14:284–291. [PubMed] [Google Scholar]
- 25.Schimmer RC, Jeanneret C, Nunley PD, Jeanneret B. Osteomyelitis of the cervical spine: a potentially dramatic disease. J Spinal Disord Tech. 2002;15:110–117. doi: 10.1097/00024720-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Borowski AM, Crow WN, Hadjipavlou AG, Chaljub G, Mader J, Cesani F, vanSonnenberg E. Interventional radiology case conference: the University of Texas Medical Branch. Percutaneous management of pyogenic spondylodiskitis. AJR Am J Roentgenol. 1998;170:1587–1592. doi: 10.2214/ajr.170.6.9609179. [DOI] [PubMed] [Google Scholar]
- 27.Collert S. Osteomylelitis of the spine. Acta Orthop Scand. 1977;48:283–290. doi: 10.3109/17453677708988770. [DOI] [PubMed] [Google Scholar]
- 28.Rath SA, Neff U, Schneider O, Richter HP. Neurosurgical management of thoracic and lumbar vertebral osteomyelitis and discitis in adults: a review of 43 consecutive surgically treated patients. Neurosurgery. 1996;38:926–933. doi: 10.1097/00006123-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Cottle L, Riordan T. Infectious spondylodiscitis. J Infect. 2008;56:401–412. doi: 10.1016/j.jinf.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Grados F, Lescure FX, Senneville E, Flipo RM, Schmit JL, Fardellone P. Suggestions for managing pyogenic (non-tuberculous) discitis in adults. Joint Bone Spine. 2007;74:133–139. doi: 10.1016/j.jbspin.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Sapico FL, Montgomerie JZ. Pyogenic vertebral osteomyelitis: report of nine cases and review of the literature. Rev Infect Dis. 1979;1:754–776. doi: 10.1093/clinids/1.5.754. [DOI] [PubMed] [Google Scholar]
- 32.Khan IA, Vaccaro AR, Zlotolow DA. Management of vertebral diskitis and osteomyelitis. Orthopedics. 1999;22:758–765. doi: 10.3928/0147-7447-19990801-07. [DOI] [PubMed] [Google Scholar]
- 33.Tali ET. Spinal infections. Eur J Radiol. 2004;50:120–133. doi: 10.1016/j.ejrad.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 34.Fraser RD, Osti OL, Vernon-Roberts B. Iatrogenic discitis: the role of intravenous antibiotics in prevention and treatment. An experimental study. Spine. 1989;14:1025–1032. doi: 10.1097/00007632-198909000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Winn HR, Youmans JR. Youmans neurological surgery. Philadelphia: Saunders; 2004. [Google Scholar]
- 36.Zarrouk V, Feydy A, Salles F, Dufour V, Guigui P, Redondo A, Fantin B. Imaging does not predict the clinical outcome of bacterial vertebral osteomyelitis. Rheumatology (Oxford) 2007;46:292–295. doi: 10.1093/rheumatology/kel228. [DOI] [PubMed] [Google Scholar]
- 37.An HS, Seldomridge JA. Spinal infections: diagnostic tests and imaging studies. Clin Orthop Relat Res. 2006;444:27–33. doi: 10.1097/01.blo.0000203452.36522.97. [DOI] [PubMed] [Google Scholar]
- 38.Lee DG, Park KB, Kang DH, Hwang SH, Jung JM, Han JW. A clinical analysis of surgical treatment for spontaneous spinal infection. J Korean Neurosurg S. 2007;42:317–325. doi: 10.3340/jkns.2007.42.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bengzon J, Grubb A, Bune A, Hellstrom K, Lindstrom V, Brandt L. C-reactive protein levels following standard neurosurgical procedures. Acta Neurochir (Wien) 2003;145:667–670. doi: 10.1007/s00701-003-0083-5. [DOI] [PubMed] [Google Scholar]