Abstract
Body fluids such as urine potentially contain a wealth of information pertaining to age, sex, social and reproductive status, physiologic state, and genotype of the donor. To explore whether urine could encode information regarding environment, physiology, and development, we compared the volatile compositions of mouse urine using solid-phase microextraction and gas chromatography–mass spectrometry (SPME-GC/MS). Specifically, we identified volatile organic compounds (VOCs) in individual urine samples taken from inbred C57BL/6J-H-2b mice under several experimental conditions—maturation state, diet, stress, and diurnal rhythms, designed to mimic natural variations. Approximately 1000 peaks (i.e., variables) were identified per comparison and of these many were identified as potential differential biomarkers. Consistent with previous findings, we found groups of compounds that vary significantly and consistently rather than a single unique compound to provide a robust signature. We identified over 49 new predictive compounds, in addition to identifying several published compounds, for maturation state, diet, stress, and time-of-day. We found a considerable degree of overlap in the chemicals identified as (potential) biomarkers for each comparison. Chemometric methods indicate that the strong group-related patterns in VOCs provide sufficient information to identify several parameters of natural variations in this strain of mice including their maturation state, stress level, and diet.
Keywords: age, diet, solid-phase microextraction and gas chromatography–mass spectrometry, stress, urine, volatile organic compound
Introduction
Body fluids contain vast amounts of information that correlates with health, gender, age, stress level, and social status (Singer et al. 1997; Novotny 2003; Osada et al. 2003; Rock et al. 2006; Röck et al. 2007; Xu et al. 2007). Metabolic profiles of biological fluids have reflected differences in endocrine state, age, and genetic makeup of the donors (Rhodes et al. 1981; Holland et al. 1984; Singer et al. 1997; Bollard et al. 2001; Osada et al. 2003; Willse et al. 2005; Rock et al. 2006; Novotny et al. 2007). Moreover, these profiles are perturbed by disease-induced aberrations and thus provide the potential interpretation of differences between normal and pathological physiologic processes. For example, patients with diabetes mellitus display large differences in urinary volatile metabolites (Rhodes et al. 1981; Hollywood et al. 2006). Urinary profiling thus has the potential to lead to the discovery of novel disease linkages (Miyashita and Robinson 1980; Rhodes et al. 1981).
Urine, an excreted body fluid, is readily available and easily collected using noninvasive methods. Urine is rich in chemical information and is a potent source of pheromones and/or semiochemicals that play a significant role in reproduction and social interaction in rodents and other mammals (Singer 1991; Kayali-Sayadi et al. 2003; Burger 2006). Urine contains several thousands of components (Bollard et al. 2001) and at least 100 volatile compounds have been chemically identified in mouse urine. These volatile urinary compounds represent nearly all common chemical classes, including aldehydes, alcohols, ketones, esters, ethers, aromatics, and acids. Previous assays employed to understand these emitted chemical signals involve fractionation and/or targeting of specific components that in effect reduces the collective power of the emitted signal (metabolome).
We have utilized a global analysis approach to profile low molecular mass volatile and semivolatile (<290 Da) organic constituents in urine with a uniform experimental protocol. This approach to studying global urinary profiles in a system (i.e., organism) under a given set of conditions is particularly challenging (Rochfort 2005). These challenges include the wide range of abundances of volatile organic compounds (VOCs) within a single sample, diversity of chemical structures that are necessary to detect, the continuous nature of the data sets (information from a single compound is spread across many scans and may overlap/blend in with adjacent compounds) produced and the interdependence of individual VOC's. VOC profiling studies, using methodology borrowed from other global “omic” studies and new analysis methods, have the potential to link metabolites with their respective metabolic pathways and establish relationships with protein and gene expression levels.
In this study, we employed urine profiling methods to determine whether and how environmental, physiological, and developmental information is coded in the chemical signals present in urine. We generated mouse urinary profiles under conditions mimicking natural variations. The enormous volume of data generated and its multidimensional aspects required the use of novel predictive modeling methods (Brereton 2007, 2009; Dixon et al. 2007; Wongravee et al. 2009). Using these methods, we show strong group-related patterns in VOCs suggesting that there is enough information in these metabolites to code for the natural variations that mice experience. These chemometric methods permitted utilization of the full urinary profile for robust biomarker discovery. Specifically, we describe differences due to maturation state, diet, stress level, and diurnal variation to help further define “normal” urinary constituents. These data provide a discrete subset of compounds that can be further examined under additional conditions of environmental exposure and/or disease development. Furthermore, specific biochemical pathways and subsequent processes for producing and releasing the VOCs are implicated.
Materials and methods
Mice
Male mice, (C57BL/6J-H-2b), used in these studies were either ordered directly from a single vendor, Jackson Laboratory, Inc., (diet and stress studies) or generated from breeders obtained from Jackson Laboratory (maturation and diurnal rhythm studies) and maintained in the animal facility at Johns Hopkins University. All mice were housed under uniform conditions in the same room, 4 to a cage. Cages had corncob bedding and were encapsulated with air flowing into and out of the cage to reduce the odors/viruses/etc. Cages were changed once every 2 weeks. With the exception of mice in the diet study, mice were allowed free access to a standard diet (no. 2018S Harlan Teklad) and water. The animal room was maintained at 21–22 °C, 70% relative humidity, and 12:12 h light:dark lighting regime, with lights on at 0600 h.
Sample collection
Mouse urine was collected directly into cryogenic vials using gentle abdominal pressure. A minimum of 50 μL urine from a single collection was required for our solid-phase microextraction and gas chromatography–mass spectrometry (SPME-GC/MS) processing. The number of actual samples collected, processed, and analyzed (stated below) was lower than the theoretical number of possible samples because the mice either did not provide any urine sample or not enough to meet the 50 μL minimum criteria. After collection, vials were immediately capped and stored at −80 °C for up to 24 months. The animal handling procedures were approved by the Animal Care and Use Committee at the Johns Hopkins University.
Maturation state and diurnal rhythm studies
All animals derived from a common breeder population obtained from Jackson Laboratories. Urine was gathered from 2 panels of 10 mice every 4 weeks as they aged, beginning with 4 weeks and ending with 30 weeks. Urine was sampled for 5 days at an age point, both in the morning and in the late afternoon roughly 6 h apart (10 possible collections total per mouse per age point) resulted in 384 samples; the breakdown of the samples analyzed is as follows: 4 week (n = 48), 8 week (n = 144), AM (n = 93), and PM (n = 99).
Stress study
Urine was collected from 10 stressed and 10 control mice at 8–10 weeks of age. To introduce stress (Schaefer et al. 2000), 2 pieces of screen cut approximately to 15 × 15 cm and stapled around 3 edges was used to restrain individuals from the group of 10 mice for 30 min on 10 separate occasions. A mouse was placed in the screen enclosure and secured with binder clips. Urine was collected 90 min after onset of stress restraint. These samples were collected over the course of 2 weeks and resulted in 67 stress and 63 stress control samples.
Diet study
Urine was collected from 10 altered diet and 10 normal diet mice at 10–12 weeks of age. Altered diet mice were fed a high-fat diet for 2 weeks prior to urine collection and continued to be fed this diet until urine collection was complete. The standard diet fed to mice in the age, diurnal rhythm, and stress studies contains 18.2% protein and 5.8% fat by weight; the altered diet was composed of the same ingredients with the exception that it had additional cocoa butter, making it 16.4% protein and 15.3% fat by weight (Harlen Teklad). Urine was collected over a 2-week period on 10 separate days, totaling 10 possible separate urine collections per mouse (resulting in 59 altered diet and 60 control diet samples).
Sample preparation
To enhance volatile extraction from mouse urine, sodium chloride was added to headspace vials prior to the addition of urine. Two-milliliter crimp-cap top glass headspace vials (Supelco) were filled with 450 μL 25% sodium chloride (J.T. Baker, ACS Reagent) in distilled de-ionized water. Mouse urine was thawed and 50 μL of urine from individual mice (urine collections were not pooled) was then transferred into the prepared headspace vials. The vials were immediately sealed with magnetic crimp caps containing gastight silicon/polytetrafluoroethylene septum (Supelco). In general, vials were run on the GC/MS within 48 h of preparation. Controls were routinely run for air and stock NaCl solution.
Data generation using SPME-GC/MS
SPME of the headspace in vials containing mouse urine was performed with divinylbenzene/carboxen/polydimethylsiloxane 50/30 μm 1-cm-long metal fibers (Supelco) and Merlin microseals (Supelco). Fibers were preconditioned according to manufacturer recommendations. Sampling was accomplished using an automated headspace sampler (MPS2, Gerstel) connected to a GC/MS (6890/5973N, Agilent) outfitted with ChemStation D.01.02. Vials were placed onto a Peltier storage cooler held at 4 °C in order of analysis. Samples were randomized between time of collection, age groups, and individual mice. SPME fibers were replaced as needed, usually after running between 70 and 100 samples. The headspace sampling protocol was as follows: vial equilibration, 65 °C (2 min); extraction, 65 °C (35 min) with low-level agitation; desorption in inlet, 250 °C (2 min); 15 min bakeout of fiber in bakeout station. An SPME liner was used and changed as necessary (0.75 mm inner diameter [i.d.], Supelco). The GC column used was a DB-WAX column (0.25 mm i.d. × 30 m × 0.25 μm film thickness; Agilent Technologies). GC oven profile was as follows: 60 °C held for 2 min, ramped to 230 °C at 5.0 °C/min, and hold for 9 min. Helium was used as the carrier gas with a flow rate of 2 mL/min. The MS scanned from 41 to 400 m/z with a threshold of 10. The MS quad temperature was set to 150 °C and the MS source temperature to 230 °C.
Standards and chemical identification
Machine variability was monitored by running a VOC calibration standard (Sigma Aldrich) and running a daily DB-WAX test standard (Agilent Technologies). Chemicals which were confirmed by matching retention time (RT) and spectra to known chemical standards run in our laboratory under identical onditions are indicated in Table 1. The remaining chemicals were tentatively identified by Chemstation identification matches to the National Institute of Standards and Technology (NIST) database because the majority of chemicals in this group are not commercially available and include: 6-hydroxy-6-methyl-3-heptanone (HMH), dihydrofuran (DHF), 2-isopropyl-4,5-dihydrothiazole (IPT) and 2-sec-butyl-4,5-dihydrothiazole (SBT). Compounds that were not present in the NIST database were tentatively identified on the basis of comparison of spectra to chemicals reported in the literature (Liebich et al. 1977; Novotny et al. 1984; Novotny, Jemiolo, et al. 1999; Novotny, Ma, et al. 1999).
Table 1.
Details of number of samples and peaks for maturation state, stress, diet, and diurnal rhythm studies
| Study | Number of samples | Number of peaks | |
| Maturation state | 4 weeks | ≥ 8weeks | |
| 44 | 148 | 1039 | |
| Stress | Stressed mice | Control mice | |
| 67 | 63 | 1056 | |
| Diet | High fat | High fat control | |
| 59 | 60 | 996 | |
| Diurnal rhythms | AM | PM | |
| 93 | 99 | 1039 | |
Signal processing and data analysis
Data preprocessing, peak detection, and matching
The methods for data processing and analysis have been described in depth (Dixon et al. 2006). For GC/MS experiments, tunable parameters were fixed prior to performing preprocessing and were selected according to chromatogram scan rate, average peak width, noise levels, and retention time drift tolerance. All 3 steps (preprocessing, peak detection, and matching) were automated, and data were converted to compatible file formats and analyzed using Matlab and in-house algorithms.
Initial data smoothing and preparation were accomplished in 3 stages: 1) the mass channels are examined to determine which contain potential information—an m/z channel must contain 11 or more nonzero scans to be considered informative. An m/z channel with 10 or less nonzero scans is removed; 2) noise factors were calculated to confirm that selected peaks are real and informative; and 3) chromatographic peaks were smoothed using a wavelet filter to remove additional noise from the chromatograms that interferes with peak detection.
Subsequently, peaks were identified by 1) detection of peaks in each informative mass channel using the first derivative of segments along each single ion chromatogram; 2) peaks were validated by matching a set of predefined criteria including intensity and width (both minimum and maximum criteria were used); and 3) the mass channel peaks for the same compound were grouped—peaks in different mass channels will sometimes come from the same origin (and for that reason have similarly shaped peaks); they were grouped accordingly.
Finally, peak matching or alignment was performed to determine which peaks in different chromatograms correspond to the same compounds. This phase was also accomplished in 3 stages: 1) candidate target peaks that had sufficiently different retention times and mass spectra from one another to postulate they have originated from different compounds were identified; 2) groups of peaks associated with each target—these peaks found in different chromatograms are postulated to have the same chemical origin were defined; and 3) groups of peaks that had similar characteristics over all the chromatograms were merged. Rare peaks, occurring less than 5 times across the entire set of mouse samples, were removed as were 124 siloxane peaks that were from the SPME fiber. The data were used to create a “master peak table” using all samples from all studies, whose rows consist of 721 chromatograms and columns of 3401 unique compounds. For each study, a “local peak table” was obtained, consisting of the subset of peaks and samples found in each study; a further reduction was performed so that only peaks detected at least 5 times in the local subset of samples were retained. The number of samples and variables in each study are listed in Table 1. Further details of this approach are reported elsewhere (Wongravee et al. 2009). Peak areas for each peak in the chromatogram were square rooted to reduce the influence of very intense peaks; a further discussion rationale is discussed elsewhere (Dixon et al. 2007; Xu et al. 2007). These values were then normalized row scaled summed to 1 for each chromatogram because the total absolute intensity of each chromatogram could vary for several reasons and to provide greater reliability than rationing to an internal reference standard. Finally, each normalized square root peak area was standardized. This process gave each peak equal significance in the resultant analysis and does not assume that an intense peak has a more important influence over discrimination than a low intensity one. If the normalization was taken in the first step followed by the square root, the summation of each chromatogram would be unequal and incomparable because the normalization properties are destroyed.
Visualization method: principal components analysis
From Table 1, the data sets showed a very high number of variables (ca. 1000 variables in each study). Because it is difficult to visualize and determine the number of significant factors for data sets with high numbers of variables, data reduction was performed. Principal component analysis (PCA) is an orthogonal linear transformation method, which allows visualization of data in a new coordinate system where the first component represents the greatest variance of the data (principal component 1-PC1), the second greatest variance is projected to the second coordinate (PC2), and so on. PCA reduces dimensionality in multivariate data sets to those data characteristics that contribute most to the overall variance. PCA loadings identify components (peaks or combinations of peaks) that have the most influence over the difference between samples. More detailed information on PCA is discussed elsewhere (Brereton 2003, 2007). In this paper, we employed PCA solely for visualization and not modeling and as such were not concerned by how many components are significant (unlike for partial least squares [PLS] methods).
Determining potentially significant markers
Given that there were a large number of factors that influence the mouse urinary signal, for example, instrumental effects, analytical methods, environment (bedding etc.), food eaten, individuality, etc., and it is anticipated that only a very small number of compounds measured will be markers for the systematic factors that have been studied, a univariate method (T-statistic) and 2 multivariate methods based on PLS, PLS weights (PLSW), and PLS regression coefficients (PLSRC) (Dixon et al. 2007; Brereton 2009; Wongravee et al. 2009) were used to identify potential discriminatory markers in the GCMS peak tables. Thus in this manner, we endeavored to discriminate between 2 groups of samples, although there are alternative approaches such as the Fisher weight that can be employed when more than 2 groups of samples are part of the model.
Methods for determining relative significance of variables
t-Statistic
The t-statistic method is a common univariate indicator of significance of whether a variable differs significantly in distribution between 2 classes. However, the correlation between variables is not taken into account during the calculation. The differences between means of each variable relative to the pooled standard deviation are used for calculating the t-statistic. Variables with highest t-statistic values (extremely positive or negative) are suggested as potential discriminators for one or either of the classes according to the sign. Variables can be ranked according to the magnitude of the t-statistic; however, in this paper, we employed Monte Carlo permutations as an alternative as described below.
Partial least squares
PLS method is a common extension of multiple linear regression (Geladi and Kowalski 1986; Martens and Naes 1989; Brereton 2000). It is a multivariate data analysis technique that can be used to relate 2 or more blocks of information while identifying underlying relationships. PLS discriminant analysis (PLS-DA) (Ståhle and Wold 1987; Barker and Rayens 2003; Dixon et al. 2007) is an extension to discrimination, where one block contains the analytical data (in our case the peak table) and another the classifier (in our case a label +1 or −1 according to class). In the PLS method, it is necessary to determine the appropriate number of PLS components in the model and this was determined using bootstrap (Efron and Tibshirani 1993; Dixon et al. 2007) for each study. We have found that the bootstrap provides a stable solution although there are several alternative methods to determine the number of PLS components such as cross validation (Ståhle and Wold 1987) and permutation test (Wiklund et al. 2008).
PLS weights
This method used the weight matrix obtained from PLS to provide a statistical indicator as to which significant variables are most significant, in our case discrimination between 2 groups. If there was more than one significant component, the PLSW was calculated by the root mean square of the weight matrix for each variable over all PLS components in the model. Therefore, the PLSW were all positive, and the markers were ranked according to the size of PLSW.
PLS regression coefficients
This was used as an alternative approach for determining the relative significance of variables. In its simplest form, the regression model specifies the relationship between an experimental data matrix and a classifier. PLSRC were calculated by multiplying the weight matrix and the variable loading matrix obtained from the model. The magnitudes of the PLSRC were used to determine the relative significance of markers. The sign of PLSRC was used to determine which group the variable was a marker for, like the t-statistic, but is a multivariate rather than univariate indicator of significance.
Methods for determining how many variables are significant
The T-statistic, PLSW, and PLSRC described above were calculated for all variables in the data set. A simple approach was used to rank the variables according to the magnitude of the statistical indicator function. The variable with the highest magnitude was ranked number 1 as the best discriminator for the data set and so on. Whereas this approach can certainly help determine which variables are most likely to be discriminatory it does not necessarily demonstrate that they are discriminatory for a given data set. As previously shown, it is possible to generate a completely random data set (Brereton 2006, 2009), and yet, we can still order variables according to whether they appear significant or not. Because of the potential identification of random variables that have 99% significance, it was important to utilize additional criteria to identify the significance of the variables. A further problem is that the most variables are not normally distributed, so traditional statistical tests, for example, F test or analysis of variance, that assume normal distributions will not provide appropriate probabilities. Despite these drawbacks, statistics such as the t-statistic can still be used to assess the significance of variables providing they are not converted directly into a probability and other empirical approaches are employed to assess their significance. In this paper, we used both the Monte Carlo permutation method (Xu et al. 2007; Wongravee et al. 2009) and iterative reformulation of training set models (Wongravee et al. 2009) to determine how many and which variables were significant.
Monte Carlo method
The Monte Carlo permutation method allows us to assign an empirical probability of significance for each variable. The method works by randomly permuting the class membership of the samples and calculating the test statistics using each random permutation. This procedure was repeated many times (5000 times for the paper) to obtain a background null distribution of statistical indicators for each variable. Then, the values calculated from the data set were compared with this null distribution to determine their empirical significance. In this paper, we set the empirical significance threshold to 99%. If there are 5000 permutations, a threshold of 99% (0.99) represents the value of the indicator with true class information that exceeds the value from the null permutation 4950 times of 5000. A variable that gives test value ≥99% significance was suggested as a potential marker. Each marker had an empirical significance attached to it so that all markers with of ≥99% empirical probability were ranked in order of significance. This procedure was applied to all 3 statistical indicators (t-statistic, PLSW, and PLSRC) described in this paper.
Iterative reformulation of training sets
The traditional approach to variable selection is to perform this on the entire data set (autoprediction) and determine the significance of a marker compound from its test value. However, when we test models, we usually split the data into test and training sets (Brereton 2009), using the training set to establish the model and the test set to determine how well the model performs. It can be shown that if variables are selected in autopredictive mode (i.e., on the entire data set including both test and training sets; (Brereton 2006) there is a danger or overfitting. An alternative approach is to select potential markers only on the training set. To this end, we divided the data into test and training sets several times (100 in this paper), and each time the training set consisted of a different subset of samples. This reduced the danger of atypical training sets (e.g., including outliers) and also allowed for an average of several (=100 in this paper) models for assessment of performance. The difficulty with this approach is that each subset of data may result in different markers being selected, if these are chosen using the training set rather than using autopredictive model. If the data were random, we would expect wide variability in the markers chosen; however, if the same compound is selected many times as a marker for each split in the data, it is to be robust to the samples included in the training set and so likely to be a true marker. We call this method “iterative reformulation of training set models” (Wongravee et al. 2009). We used this method with the samples in the data set, which were divided into 2 independent data sets: training set (samples that are used to form the model) and test set (samples that are used to assess how good the model is—“validation set”). The test statistics (t-statistic, PLSW, and PLSRC) were performed on the training set to rank the variables. In each iteration, the most significant variables are recorded. In this paper, we report a modification of the previously published work as we chose the number of variables using Monte Carlo permutations on the autopredictive data set, at 99% significance level, so if these permutations resulted in 31 variables being found to be significant (e.g.), we counted the number of times that a variable is present in the top 31 in each iteration. The procedure was repeated 100 times, thus obtaining 100 lists of significant variables. If a variable was found to be in this list 70 times or more, the variable was suggested to be a real marker.
Results
VOCs and prediction of maturation state, stress level, diet, and diurnal rhythms
We compared the volatile compositions of urine using SPME-GC/MS to determine whether inbred mouse urine encodes information about the environment, physiology, and maturity of the animal. Specifically, we identified VOCs in individual urine samples taken under 4 experimental conditions—maturation state, diet, stress, and diurnal rhythms, designed to mimic natural variations. Supplementary Figure 1 shows a representative total ion chromatogram from the VOC profiles of the different experimental conditions that were performed. GC/MS profiles allowed identification of new differential compounds in addition to confirming the identity of previously known chemosignals. Table 1 describes the number of samples and peaks identified in the different experimental data sets. The 4 studies identified between 996 and 1056 unique GC/MS peaks in each experiment that were subsequently analyzed for distinguishing variables.
Identification of differentiating VOCs
Because visualization of each individual GC/MS data set (i.e., chromatogram and spectra) to identify unique features is impractical, chemometric approaches for data mining were employed to analyze and identify variables that were associated with the different experiments. An example of a chromatogram is shown in Supplementary Figure 1, which is a representative total ion chromatogram from the different experimental conditions with labeling of some of the identified peaks.
In Table 2, we list the number of markers suggested to be significant using chemometric methods, including PLSW, PLSRC, and T statistics as described above. In order to determine a subset of variables that have a high significance to the experimental conditions, we applied Monte Carlo methods with a threshold of 99% or iterative reformulation of the training sets with a 70% threshold. The number of times each candidate marker is found in the 100 iterative reformulation of training set models is illustrated in Supplementary Figure 2. These frequency plots illustrate that 41 of 79 possible variable counts (over all 3 statistics) are found in at least 99% of the iterations and only 13 between 70% and 80% of the time. Visualization of the graphs in Supplementary Figure 2 suggests that a 70% cutoff (after first using the number of variables that have an empirical probability of 99% using Monte Carlo methods) is a suitable choice—a few variables are found very occasionally but these are probably due to atypical selections of training set samples. In order to draw up a final list of markers, we select only those markers that are found to be significant using all 3 statistics and both Monte Carlo and iterative reformulation of training set models: both approaches select quite similar subsets of variables. Table 3 lists the variables and the corresponding compound names to the identified variables meeting the above criteria. Compounds were either identified using purified chemical standards as indicated or had tentative identifications that were of high confidence. As shown in Table 3, the maturation state comparison modeling results showed significant differences in 12 urinary components (IPT, 1-(1H-pyrrol-2-yl) ethanone, SBT, benzyl methyl ketone, 1-(1H-pyrrol-2-yl) ethanone, DHF 1.64 min (decomposition product of a pheromone HMH (at 13.1 min, a very broad peak, not in itself a differential marker) (Novotny et al. 1999), dimethyl disulphide, methylene chloride, DHF 2.60 min, 2-sec-butylthiazole, p-meth-1-en-8-ol, and 2-acetyl pyrroline; Novotny et al. 1999). Interestingly, the pheromones 2-heptanone (Novotny et al. 1986), alpha- and beta-farnesene (Novotny et al. 1984) appeared as initial significant variables but were not part of the final list of compounds that met the above threshold conditions. The abundance of 4-methyl phenol was greater in the older animals, consistent with previous studies (Osada et al. 2003), but failed to appear within our top ranking compounds. Several of the 12 biomarkers were also shared with the markers identified in subsequent conditions. Our results suggest that a novel group of previously unrecognized age-differential chemicals benzyl methyl ketone, 1-(1H-pyrrol-2-yl) ethanone, DHF 1.64 min, dimethyl disulphide, methylene chloride, DHF 2.60 min, 2-sec-butylthiazole, p-meth-1-en-8-ol, and 2-acetyl pyrroline) along with previously identified compounds (IPT and SBT) (Novotny et al. 1984, 1990; Osada et al. 2003) provide a robust signature of maturation state.
Table 2.
Number of variables deemed to be significant using variable selection
| Data set | Three methods | Two methods |
One method |
||||
| T, PLSW, and PLSRC | T and PLSW | T and PLSRC | PLSW and PLSRC | T | PLSW | PLSRC | |
| Monte Carlo; 99% empirical probability | |||||||
| Age | 11 | 146 | 11 | 11 | 166 | 148 | 11 |
| Stress | 15 | 79 | 15 | 17 | 97 | 83 | 17 |
| Diet | 7 | 86 | 7 | 7 | 110 | 90 | 7 |
| AM/PM | 8 | 53 | 9 | 8 | 68 | 57 | 9 |
| Iterative reformulation of training sets; found in 70% of training sets | |||||||
| Age | 5 | 5 | 5 | 5 | 7 | 5 | 5 |
| Stress | 10 | 11 | 10 | 10 | 11 | 13 | 11 |
| Diet | 3 | 4 | 4 | 3 | 5 | 4 | 4 |
| AM/PM | 4 | 5 | 4 | 4 | 5 | 5 | 4 |
T, T-statistic.
Table 3.
Listing of markers and compounds that were identified by variable selection methods as highly significant
| RT (min) | Sign | Compound name | Monte Carlo 99% empirical significance | Iterative reformulation of training set (70% threshold) | |
| Maturation study (4 wk/8+ wk) | 8.28 | −/+ | IPTa | ✓ | ✓ |
| 10.2 | −/+ | SBTa | ✓ | ✓ | |
| 16.21 | +/− | Benzyl methyl ketonea | ✓ | ✓ | |
| 21.42 | −/+ | 1-(1H-pyrrol-2-yl) Ethanonea | ✓ | ✓ | |
| 1.64 | −/+ | DHFa | ✓ | ✓ | |
| 2.70 | +/− | Dimethyl disulphidea | ✓ | ✓ | |
| 1.79 | +/− | Methylene chloridea | ✓ | ||
| 2.60 | +/− | DHFa | ✓ | ||
| 8.64 | −/+ | 2-Sec-butylthiazolea | ✓ | ||
| 16.19 | −/+ | ND | ✓ | ||
| 15.79 | +/− | p-Menth-1-en-8-ola | ✓ | ||
| 7.43 | −/+ | 2-Acetyl pyrrolinea | ✓ | ||
| Stress study (stress/control) | 5.36 | +/− | 6-Methyl-3-heptanonea | ✓ | ✓ |
| 22.68 | −/+ | Nerolidola | ✓ | ✓ | |
| 25.04 | −/+ | N-Phenyl formamidea | ✓ | ✓ | |
| 2.02 | +/− | Methyl isobutyl ketonea | ✓ | ||
| 10.2 | −/+ | SBTa | ✓ | ||
| 15.79 | −/+ | p-Menth-1-en-8-ola | ✓ | ✓ | |
| 12.35 | −/+ | Linaloola | ✓ | ✓ | |
| 15.28 | −/+ | Isovaleric acidbc | ✓ | ✓ | |
| 4.4 | +/− | 2-Heptanone | ✓ | ||
| 3.42 | +/− | ND | ✓ | ||
| 19.31 | −/+ | ND | ✓ | ✓ | |
| 10.41 | +/− | ND | ✓ | ✓ | |
| 6.88 | −/+ | Exo-brevicomina | ✓ | ✓ | |
| 11.68 | −/+ | Benzaldehyde | ✓ | ||
| 20.25 | −/+ | ND | ✓ | ✓ | |
| 16.19 | −/+ | ND*,a | ✓ | ||
| 21.42 | +/− | 1-(1H-pyrrol-2-yl)-Ethanonea | ✓ | ||
| 2.88 | −/+ | DHFa | ✓ | ||
| 14.67 | +/− | ND | ✓ | ||
| 0.98 | +/− | Trimethylaminea | ✓ | ||
| Diet study (high fat diet/standard diet) | 15.1 | +/− | 4,4,5-trimethyl-2-cyclohexenonea | ✓ | ✓ |
| 2.88 | +/− | DHFa | ✓ | ✓ | |
| 12.35 | +/− | Linaloola | ✓ | ||
| 23.75 | −/+ | 4-Methyl phenol (p-cresol)b | ✓ | ✓ | |
| 1 | +/− | Isopreneb | ✓ | ||
| 7.96 | +/− | ND | ✓ | ✓ | |
| 25.04 | −/+ | N-Phenyl formamidea | ✓ | ||
| 6.82 | −/+ | ND | ✓ | ||
| Diurnal rhythms (AM/PM) | 15.1 | −/+ | 4,4,5-Trimethyl-2-cyclohexenonea | ✓ | ✓ |
| 22.2 | −/+ | Phenola | ✓ | ||
| 2.43 | +/− | 2,3-Pentanedionead | ✓ | ✓ | |
| 16.21 | −/+ | Benzyl methyl ketonea | ✓ | ||
| 20.25 | −/+ | ND | ✓ | ✓ | |
| 14.58 | +/− | Acetophenoneb | ✓ | ||
| 2.88 | −/+ | DHFa | ✓ | ✓ | |
| 0.98 | +/− | Trimethylaminea | ✓ | ||
| 10.41 | +/− | ND | ✓ |
ND, identification not determined; −/+ or +/− indicates abundance relationship.
Tentative chemical identification from MS.
Identified using chemical standard.
Coeluting with methyl butyric acid.
Coeluting with 4-ethyl phenol.
We successfully identified 20 top ranked peaks in our stress comparison (restraint stress vs. control) (Table 3). The 20 chemicals showing differences are listed here (6-methyl-3-heptanone, nerolidol, N-phenyl formamide, methyl isobutyl ketone, SBT, p-menth-1-en-8-ol, linalool, isovaleric acid coeluting with 2-methyl-butyric acid, 2-heptanone, exo-brevicomin, benzaldehyde, peak at RT 20.25 min, 1-(1H-pyrrol-2-yl) ethanone, DHF 2.88 min, trimethylamine as well as 5 compounds whose structures could not be determined [ND 3.42, 19.31, 10.41, 16.19, 20.25, and 14.67 min]). SBT has been previously shown to be associated with aggression in male mice and is considered to be a pheromone (Novotny et al. 1985). In addition, 2-heptanone has also been identified as associated with adrenal gland activity in mice (Novotny et al. 1986). Of the 20 biomarkers, 9 were in common with at least one of the other 3 comparisons. These results identify a novel group of hitherto unidentified stress-differential chemicals in addition to identifying several compounds that have previously been associated with mouse adrenal and pheromone activity (Novotny et al. 1986).
A similar analysis in the diet comparison (high fat diet vs. regular diet) resulted in 8 significantly different VOCs (4,4,5-trimethyl-2-cyclohexenone, DHF 2.88 min, linalool, 4-methyl phenol, isoprene, N-phenyl formamide, along with 2 undetermined compounds [ND 7.96 and 6.82 min]). Two compounds, linalool and 4-methyl phenol were recently shown to differ significantly in mouse urine of mice on different diets and major histocompatibility complex (MHC) backgrounds (Kwak et al. 2008). Of these 8 biomarkers, 4 were in common with at least one of the other 3 comparisons. Additional identified and tentatively identified VOCs that differ in abundance but failed to score within the top 20 biomarkers included 2-methyl-3-buten-2-ol, 4-heptanone, 2-ethyl-6-methyl-pyridine, and N, N-dimethyl urea. We conclude that, the diet of an individual can selectively modulate metabolic functions and generate characteristic patterns of VOCs.
Maturation, diet, and stress all reflect reasonably long-term changes in metabolic state. We decided to examine whether short-term changes, those associated with diurnal rhythm (AM vs. PM), showed chemical differences. Nine chemicals differed in abundance with time-of-day (4,4,5-trimethyl-2-cyclohexenone, phenol, 2,3-pentanedione, benzyl methyl ketone, acetophenone, DHF 2.88 min, and trimethylamine along with 2 compounds that were not determined [ND 20.25 and 10.41 min]) (Table 3). Of the 9 biomarkers, 6 were in common with at least one of the other 3 comparisons, and 4 were in common with the stress comparison (DHF 2.88 min, trimethylamine, and 2 of the undetermined compounds [ND 20.25 and 10.41]). An additional chemically identified VOC (N, N-dimethyl urea) differed in abundance but only appeared in a few samples and failed to rank in the top 20 biomarkers. These data reveal a novel group of diurnal rhythm-differential chemicals provide a time-of-day signature.
A number of previously identified VOCs were detected in urine headspace but did not arise as highly differentiating compounds in our analysis. Sulfur-containing compounds, present in nearly all chromatograms, included tentatively identified methanethiol, dimethyl trisulphide, methyl(methylthio)methyl disulfide, (methylthio)methyl sulphoxide, and bis(2-sulfhydrylethyl)-disulfide. Two pheromones, HMH (Novotny et al. 1999) and dehydro-exo-brevicomin (Novotny et al. 1984) were also detected. Some compounds falling into the “nondifferentiating” class do so because they were not recognized in our analysis (they failed to meet certain criteria). For example, HMH had a very broad peak and as a result would not have met our algorithm's criteria and was eliminated from further analysis. Thus, we emphasize that some compounds, identified here as nondifferentiating, have the potential to be biomarkers with a different analytical approach.
Visualization of the overall representation of individual urine samples
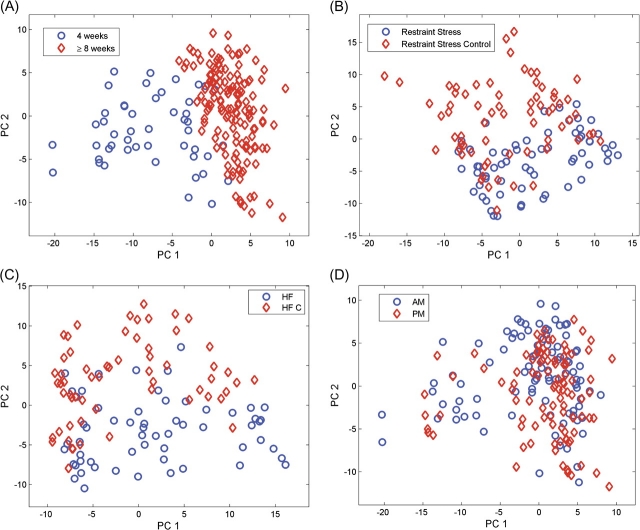
To obtain an independent visualization of the overall representation of individual urine samples in their respective classes, general clustering trends, and outliers among the observations the local peak tables were subjected to PCA (Brereton 2003, 2007) after preprocessing as discussed above. Figure 1 depicts PC scores plots, defined by the components of the PCA results, for the age, stress, diet, and diurnal rhythm studies in 2D space (PC2 vs. PC1). PCA projection demonstrates clear statistical distinctness between the 2 age groups (4 weeks vs. 8+ weeks). Further separation between 8 weeks and older groups is less clear and not illustrated. Good statistical separation in the PCA plots is demonstrated between the 2 classes in the conditions stressed versus control and altered diet versus control. We observe poor separation between the AM and PM classes using the first 2 PCs, however, this may be due to the nature of collection that was separated by 6 h rather than timed to be exactly at light and dark phases. Separation between AM and PM can be improved slightly by plotting other PCs but remains poor (data not shown).
Figure 1.
Graphs of the scores of the first 2 principal components of the preprocessed peak tables. (A) Maturation state comparison, 4 weeks versus 8+ weeks; (B) stress comparison, restraint stressed versus nonstressed control; (C) diet comparison, high fat (HF) diet versus control (HF C) diet; and (D) diurnal rhythm comparison, AM versus PM PC, principal components. This figure appears in color in the online version of Chemical Senses.
Interrelationships between the different experimental conditions
Many of the differential peaks identified in the modeling studies for individual comparisons, including some of the chemically identified VOCs, are shared across multiple conditions. Of the 49 compounds identified by MS from the high-ranking biomarker list in each condition, 19 (39%) were shared with at least one additional study. These higher ranking biomarkers have a greater tendency to show up in multiple comparisons. These biomarkers could arise because they are more stable and consistent within and between animals or alternatively reflect a common set of underlying biochemical and genetic pathways that are selectively and differentially modulated under the different situations.
Discussion
We have identified a large number of compounds in inbred male mouse urine, identified as peaks in GC/MS, that display a signature of variations in maturation state-, environmental-, and physiological-modulated patterns. Up to 39% of these compounds may be shared between the different conditions. The identity of the highest ranking biomarkers was determined. Using predictive modeling methods, we demonstrate strong group-related patterns of VOCs. Consistent with previous work, it is the characteristic groups of compounds that vary significantly that define these signatures rather than unique individual compounds (Singer et al. 1997; Willse et al. 2005). Our data support the hypothesis that differences in VOCs are sufficient to make robust predictions regarding the state of individuals within a population. It is interesting to speculate that we may have identified selective changes in small groups of chemicals that mice use to communicate variations in their natural experience to other animals.
VOC correlations in other studies
Some of the chemicals identified in this study were identified previously in specific individual conditions. The compound SBT that was shown to be elevated in older males was previously shown to be associated with age and was found in complex with the major urinary proteins (MUPs). (Osada et al. 2008). The upregulation of specific compounds with maturation may reflect the sexual maturation and increase sex-steroids that occur between 4½ and 6 weeks of age. For example, SBT and IPT (Table 3) that were elevated in older males are among those compounds upregulated or proposed to be upregulated by sex-steroids (Liebich et al. 1977; Novotny et al. 1984, 1990). Beta-farnesene appeared more abundant in older males in our analysis, although it was not one of the top 20 ranking biomarkers and was elevated in dominant male mouse urine (Novotny et al. 1990). We determined that the differential VOC, dimethyl disulphide, is present at higher levels in young males, consistent with an association with behavioral subordinance and upregulation in subordinate males (Keegans et al. 1993). In a different condition, we observed increased levels of 2-heptanone correlated with increased stress. Rodents that have been adrenalectomized (the surgical removal of one or both adrenal glands) and fail to induce/elicit classic hormonal stress responses contain lower levels of these compounds in urine (Novotny et al. 1986). Gutiérrez-García et al. (Gutiérrez-García et al. 2006, 2007) identified 2-heptanone and linalyl propionate, an ester of linalool that we identified as a stress biomarker (2-heptanone is increased and linalool is depressed following restraint stress), as associated with stress. Of the 9 biomarkers found in the diurnal rhythm comparison, 4 were in common with the stress condition (DHF 2.88 min, trimethylamine, and 2 chemicals not identified [ND 20.35 and 10.41 min]). Corticosterone, released from the adrenal glands during stress, is also released during natural diurnal rhythms. Peak concentrations occur at the beginning of the dark phase (activity period), with a decrease over the remainder of the 24-h period (Velasco et al. 1993). In our condition, the AM collection was made shortly after the light phase began, and the PM collection occurred just before the active dark phase or 6 h apart from the light phase. Thus, we would expect corticosterone levels to be lower in the PM collection (and more similar to the control nonstressed mice) than in the samples. The abundance intensity relationships for all the compounds are correlated between these 2 comparisons (when the abundance of these compounds is high in the AM, it is high in the stressed animals).
Source of VOCs
Some of the prominent VOCs identified in this study, including the previously identified male pheromones SBT, exo-brevicomin, and 2-heptanone are differentially bound and stabilized by MUPs (Humphries et al. 1999; Sharrow et al. 2002; Novotny 2003). The presence of these VOCs in multiple comparisons suggests that the stability of the VOC may contribute to its identification as a biomarker. Many of the compounds identified in this study can be traced to known metabolic pathways, including the degradation of amino acids, oxidative processes, hormone and/or steroid triggered production, and fatty acid degradation (Charlton and Roelofs 1991). Isoprene, a precursor of terpenes, is also a differential biomarker in our studies consistent with a role for the mevalonate biosynthesis pathway in VOC generation (Sacchettini and Poulter 1997; Kuzuyama 2002). Moreover, the elevated levels we observed for urinary isoprene in the high-fat diet group may be linked specifically to cholesterologenesis, as has been proposed for isoprene detected in breath (Legato 2000; Karl et al. 2001), and may be utilized as a noninvasive marker of blood cholesterol levels (Taucher et al. 1997; Karl et al. 2001).
Overlap of differential chemicals between studies
Why do many chemicals show up in multiple comparisons? The preferential identification of stable compounds, a natural merging of pathways, and interdependence between the different groups may underlie the existence of a common group of biomarkers. The stability of some compounds may be inherent in their structure, whereas others are stabilized by protein binding. The shared biomarkers 2-heptanone and SBT bind MUPs, making these compounds more stable and permitting their release as scent marks over time (Novotny et al. 1984; Humphries et al. 1999). Metabolic changes shared by several conditions produce a common subset of biomarkers because they are controlled by the same regulatory components. Diurnal rhythms, diet, and stress could exhibit the same chemicals because they each involve corticosteroid. This simple hormone can stimulate gluconeogenesis to ensure an adequate fuel supply; increase mobilization of free fatty acids, making them a more available energy source; conserve glucose for the brain by reducing glucose utilization in other tissues; stimulate protein catabolism to release amino acids for use in repair, enzyme synthesis, and energy production; act as an antiinflammatory agent; depress immune reactions; and increase the vasoconstriction caused by epinephrine (Munck and Naray-Fejes-Toth 1994; Sapolsky 2000).
Urinary profiling and biomarker discovery
Functional genomics, integrative and systems biology, pharmacogenomics, and biomarker discovery for disease prognoses, diagnoses, and therapy monitoring have all focused on metabolomics because many diseases are indeed a result of metabolic disorders (Hollywood et al. 2006). We identified biomarkers that may have the potential to lead to a better understanding of disease processes as specific metabolic pathways including degradation pathways of amino acids, oxidative pathways, production triggered by hormone and/or steroid levels (e.g., corticosteroids), and fatty acid metabolism have been highlighted as being important (Charlton and Roelofs 1991). These biomarkers, their specific metabolic pathways of origin, and an understanding of the interdependence between different pathways may serve as a hypothesis starting point for therapeutic intervention and drug discovery. Beyond disease, these data provide a discrete subset of compounds sufficient to predict several parameters of natural variations influencing mouse urinary profiles that can be further examined under additional conditions of environmental exposure. It is important to note that these studies were performed in a single inbred strain (C57BL/6J-H-2b), and further work in other strains or outbred strains will need to be done to confirm the predictive nature of these compounds for mice in general. However, our work highlights the potential for urinary profiling to provide a robust signature of the state of individuals.
Mice provide an ideal framework to better understand the mechanisms that produce and modulate VOCs in bodily emissions. In this study, we demonstrate that volatile signals are emitted by inbred mice that provide information on maturity, diet, stress, and diurnal rhythms; each important individual attributes with possible evolutionary influence. Our results also identify at least some of the components that create novel groups of hitherto unrecognized differential chemicals along with previously identified compounds that provide these robust signatures. We also showed a strong endocrine (especially sex-steroid effects) influence on urinary profiles. Additionally, components that appear repeatedly across comparisons are known to bind specific classes of carrier proteins, suggesting a critical mechanistic role for specific carrier proteins in determining unique urinary profiles. Finally, the presence of these shared chemicals that communicate individual status across multiple conditions suggests that a relatively restricted collection of chemicals may be used for biological communication.
Supplementary material
Supplementary material can be found at http://www.chemse.oxfordjournals.org/.
Funding
This work was sponsored by Defense Advanced Research Projects Agency under the Army Research Office Contract no. DAAD-19-03-0069 and the National Institutes of Health [R21DC008576].
Supplementary Material
Acknowledgments
The authors thank Drs Jae Kwak, George Preti, and Devandra Dubey for their opinions and advice. The authors acknowledge Alina D. Predescu, Christopher Doiron, Jon Hardy, and Tushar Patel for their technical assistance. Author contributions: M.L.S. and R.R.R. conceived and designed the experiments with input from J.E.Z. and N.M.H. M.L.S., M.E.H., and H.M.K. performed experiments. S.J.D., K.W., and R.G.B. analyzed the data. M.L.S. and R.R.R. wrote the paper. J.M.T. coordinated writing and teams. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the United States Government.
References
- Barker M, Rayens W. Partial least squares for discrimination. J Chemom. 2003;17:166–173. [Google Scholar]
- Bollard ME, Holmes E, Lindon JC, Mitchell SC, Branstetter D, Zhang W, Nicholson JK. Investigations into biochemical changes due to diurnal variation and estrus cycle in female rats using high-resolution (1)H NMR spectroscopy of urine and pattern recognition. Anal Biochem. 2001;295:194–202. doi: 10.1006/abio.2001.5211. [DOI] [PubMed] [Google Scholar]
- Brereton RG. Introduction to multivariate calibration in analytical chemistry. Analyst. 2000;125:2125–2154. [Google Scholar]
- Brereton RG. Chemometrics: data analysis for the laboratory and chemical plant. 1st ed. Chichester (UK): John Wiley & Sons; 2003. 503 p. [Google Scholar]
- Brereton RG. Consequences of sample sizes, variable selection model validation and optimization for predicting classification ability from analytical data. Trends Anal Chem. 2006;25:1103–1111. [Google Scholar]
- Brereton RG. Applied chemometrics for scientists. 1st ed. Chichester (England): John Wiley & Sons; 2007. 396 p. [Google Scholar]
- Brereton RG. Chemometrics for pattern recognition. 1st ed. Chichester (England): John Wiley & Sons; 2009. 522 p. [Google Scholar]
- Burger BV. Mammalian semiochemicals. Topics Curr Chem. 2006;240:231–278. [Google Scholar]
- Charlton RE, Roelofs WL. Biosynthesis of a volatile methyl-branched hydrocarbon sex pheromone from lucine by Arctiid moths. Arch Insect Biochem Physiol. 1991;18:81–97. [Google Scholar]
- Dixon SJ, Xu Y, Brereton RG, Soini A, Novotny MV, Oberzaucher E, Grammer K, Penn DJ. An automated method for peak detection and matching in large gas chromatography-mass spectrometry data sets. J Chemom. 2006;20:325–340. [Google Scholar]
- Dixon SJ, Xu Y, Brereton RG, Soini A, Novotny MV, Oberzaucher E, Grammer K, Penn DJ. Pattern recognition of gas chromatography mass spectrometry of human volatiles in sweat to distinguish the sex of subjects and determine potential discriminatory marker peaks. Chemom Intell Lab Syst. 2007;87:161–172. [Google Scholar]
- Efron B, Tibshirani R. An introduction to the bootstrap. London: Chapman and Hall; 1993. [Google Scholar]
- Geladi P, Kowalski BR. Partial least squares regression: a tutorial. Anal Chim Acta. 1986;185:1–17. [Google Scholar]
- Gutiérrez-García AG, Contreras CM, Mendoza-Lopez MR, Cruz-Sanchez JS, Garcia-Barradas O, Rodriguez-Landa JF, Bernal-Morales B. A single session of emotional stress produces anxiety in Wistar rats. Behav Brain Res. 2006;167:30–35. doi: 10.1016/j.bbr.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Garcia AG, Contreras CM, Mendoza-Lopez MR, Garcia-Barradas O, Cruz-Sanchez JS. Urine from stressed rats increases immobility in receptor rats forced to swim: role of 2-heptanone. Physiol Behav. 2007;91:166–172. doi: 10.1016/j.physbeh.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Holland ML, Rhodes GR, Wiesler D, Novotny M. Chromatographic profiling of urinary volatile and organic acid metabolites of normal and diabetic C57BL/Ks mice. J Chromatogr. 1984;306:23–37. doi: 10.1016/s0378-4347(00)80866-x. [DOI] [PubMed] [Google Scholar]
- Hollywood K, Brison DR, Goodacre R. Metabolomics: current technologies and future trends. Proteomics. 2006;6:4716–4723. doi: 10.1002/pmic.200600106. [DOI] [PubMed] [Google Scholar]
- Humphries RE, Robertson DH, Beynon RJ, Hurst JL. Unravelling the chemical basis of competitive scent marking in house mice. Anim Behav. 1999;58:1177–1190. doi: 10.1006/anbe.1999.1252. [DOI] [PubMed] [Google Scholar]
- Karl T, Prazeller P, Mayr D, Jordan A, Rieder J, Fall R, Lindinger W. Human breath isoprene and its relation to blood cholesterol levels: new measurements and modeling. J Appl Physiol. 2001;91:762–770. doi: 10.1152/jappl.2001.91.2.762. [DOI] [PubMed] [Google Scholar]
- Kayali-Sayadi MN, Bautista JM, Polo-Diez LM, Salazar I. Identification of pheromones in mouse urine by head-space solid phase microextraction followed by gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;796:55–62. doi: 10.1016/j.jchromb.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Keegans SJ, Morgan ED, Turillazi S, Jackson BD, Billen J. The dufour gland and the secretion placed on eggs of social wasps, Liostenogaster flavolineata and Parischnogaster jacoboni. J Chem Ecol. 1993;19:279–290. doi: 10.1007/BF00993695. [DOI] [PubMed] [Google Scholar]
- Kuzuyama T. Mevalonate and nonmevalonate pathways for the biosynthesis of isoprene units. Biosci Biotechnol Biochem. 2002;66:1619–1627. doi: 10.1271/bbb.66.1619. [DOI] [PubMed] [Google Scholar]
- Kwak J, Willse A, Matsumura K, Curran Opiekun M, Yi W, Preti G, Yamazaki K, Beauchamp GK. Genetically-based olfactory signatures persist despite dietary variation. PLoS one. 2008;3:e3591. doi: 10.1371/journal.pone.0003591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legato MJ. Dyslipidemia, gender, and the role of high-density lipoprotein cholesterol: implications for therapy. Am J Cardiol. 2000;86:15L–18L. doi: 10.1016/s0002-9149(00)01463-6. [DOI] [PubMed] [Google Scholar]
- Liebich HM, Zlatkis A, Bertsch W, Van Dahm R, Whitten WK. Identification of dihydrothiazoles in urine of male mice. Biomed Mass Spectrom. 1977;4:69–72. doi: 10.1002/bms.1200040202. [DOI] [PubMed] [Google Scholar]
- Martens, Naes T. PLS is a common extension of multiple linear regression. England: John Wiley & Sons Ltd; 1989. [Google Scholar]
- Miyashita K, Robinson AB. Identification of compounds in mouse urine vapor by gas chromatography and mass spectrometry. Mech Ageing Dev. 1980;13:177–184. doi: 10.1016/0047-6374(80)90060-3. [DOI] [PubMed] [Google Scholar]
- Munck A, Naray-Fejes-Toth A. Glucocorticoids and stress: permissive and suppressive actions. Ann N Y Acad Sci. 1994;746:115–130. doi: 10.1111/j.1749-6632.1994.tb39221.x. discussion 131–133. [DOI] [PubMed] [Google Scholar]
- Novotny MV. Pheromones, binding proteins and receptor responses in rodents. Biochem Soc Trans. 2003;31:117–122. doi: 10.1042/bst0310117. [DOI] [PubMed] [Google Scholar]
- Novotny MV, Harvey S, Jemiolo B. Chemistry of male dominance in the house mouse, Mus domesticus. Experientia. 1990;46:109–113. doi: 10.1007/BF01955433. [DOI] [PubMed] [Google Scholar]
- Novotny MV, Harvey S, Jemiolo B, Alberts J. Synthetic pheromones that promote inter-male aggression in mice. Proc Natl Acad Sci U S A. 1985;82:2059–2061. doi: 10.1073/pnas.82.7.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny MV, Jemiolo B, Harvey S, Wiesler D, Marchlewska-Koj A. Adrenal-mediated endogenous metabolites inhibit puberty in female mice. Science. 1986;231:722–725. doi: 10.1126/science.3945805. [DOI] [PubMed] [Google Scholar]
- Novotny MV, Jemiolo B, Wiesler D, Ma W, Harvey S, Xu F, Xie TM, Carmack M. A unique urinary constituent, 6-hydroxy-6-methyl-3-heptanone, is a pheromone that accelerates puberty in female mice. Chem Biol. 1999;6:377–383. doi: 10.1016/S1074-5521(99)80049-0. [DOI] [PubMed] [Google Scholar]
- Novotny MV, Ma W, Wiesler D, Zidek L. Positive identification of the puberty-accelerating pheromone of the house mouse: the volatile ligands associating with the major urinary protein. Proc Biol Sci. 1999;266:2017–2022. doi: 10.1098/rspb.1999.0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny MV, Schwende FJ, Wiesler D, Jorgenson JW, Carmack M. Identification of a testosterone-dependent unique volatile constituent of male mouse urine: 7-exo-ethyl-5-methyl-6,8-dioxabicyclo[3.2.1]-3-octene. Cell Mol Life Sci. 1984;40:217–219. doi: 10.1007/BF01963608. [DOI] [PubMed] [Google Scholar]
- Novotny MV, Soini HA, Koyama S, Wiesler D, Bruce KE, Penn DJ. Chemical identification of MHC-influenced volatile compounds in mouse urine. I: quantitative proportions of major chemosignals. J Chem Ecol. 2007;33:417–434. doi: 10.1007/s10886-006-9230-9. [DOI] [PubMed] [Google Scholar]
- Osada K, Tashiro T, Mori K, Izumi H. The identification of attractive volatiles in aged male mouse urine. Chem Senses. 2008;33:815–823. doi: 10.1093/chemse/bjn045. [DOI] [PubMed] [Google Scholar]
- Osada K, Yamazaki K, Curran M, Bard J, Smith BP, Beauchamp GK. The scent of age. Proc Biol Sci. 2003;270:929–933. doi: 10.1098/rspb.2002.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes G, Miller M, McConnell ML, Novotny MV. Metabolic abnormalities associated with diabetes mellitus, as investigated by gas chromatography and pattern-recognition analysis of profiles of volatile metabolites. Clin Chem. 1981;27:580–585. [PubMed] [Google Scholar]
- Rochfort S. Metabolomics reviewed: a new "omics" platform technology for systems biology and implications for natural products research. J Nat Prod. 2005;68:1813–1820. doi: 10.1021/np050255w. [DOI] [PubMed] [Google Scholar]
- Röck F, Hadeler K-P, Rammensee H-G, Overath P. Quantitative analysis of mouse urine volatiles: in search of MHC-dependent differences. PLoS ONE. 2007 doi: 10.1371/journal.pone.0000429. 2(5): e429. doi:10.1371/journal.pone.0000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock F, Mueller Weimar U, Rammensee H-G, Overath P. Comparative analysis of volatile constituents from mice and their urine. J Chem Ecol. 2006;32:1333–1346. doi: 10.1007/s10886-006-9091-2. [DOI] [PubMed] [Google Scholar]
- Sacchettini JC, Poulter CD. Creating isoprenoid diversity. Science. 1997;277:1788–1789. doi: 10.1126/science.277.5333.1788. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress hormones: good and bad. Neurobiol Dis. 2000;7:540–542. doi: 10.1006/nbdi.2000.0350. [DOI] [PubMed] [Google Scholar]
- Schaefer ML, Wong ST, Wozniak DF, Muglia LM, Liauw JA, Zhuo M, Nardi A, Hartman RE, Vogt SK, Luedke CE, et al. Altered stress-induced anxiety in adenylyl cyclase type VIII-deficient mice. J Neurosci. 2000;20:4809–4820. doi: 10.1523/JNEUROSCI.20-13-04809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrow SD, Vaughn JL, Zidek L, Novotny MV, Stone MJ. Pheromone binding by polymorphic mouse major urinary proteins. Protein Sci. 2002;11:2247–2256. doi: 10.1110/ps.0204202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AG. A chemistry of mammalian pheromones. J Steroid Biochem Mol Biol. 1991;39:627–632. doi: 10.1016/0960-0760(91)90261-3. [DOI] [PubMed] [Google Scholar]
- Singer AG, Beauchamp GK, Yamazaki K. Volatile signals of the major histocompatibility complex in male mouse urine. Proc Natl Acad Sci U S A. 1997;94:2210–2214. doi: 10.1073/pnas.94.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståhle L, Wold S. Partial least squares analysis with cross-validation for the two-class problem: a Monte Carlo study. J Chemom. 1987;1:185–196. [Google Scholar]
- Taucher J, Hansel A, Jordan A, Fall R, Futrell JH, Lindinger W. Detection of isoprene in expired air from human subjects using proton-transfer-reaction mass spectrometry. Rapid Commun Mass Spectrum. 1997;11:1230–1234. doi: 10.1002/(SICI)1097-0231(199707)11:11<1230::AID-RCM3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Velasco A, Huerta I, G-Granda T, Cachero TG, Menendez E, Marin B. Circadian rhythms of plasma corticosterone at different times after induction of diabetes. Responses to corticoadrenal stimulation in light and dark phases. Life Sci. 1993;52:965–974. doi: 10.1016/0024-3205(93)90532-8. [DOI] [PubMed] [Google Scholar]
- Wiklund S, Johansson E, Sjostrom L, Mellerowicz EJ, Edlund U, Shockcor JP, Gottfries J, Moritz T, Trygg J. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal Chem. 2008;80:115–122. doi: 10.1021/ac0713510. [DOI] [PubMed] [Google Scholar]
- Willse A, Belcher AM, Preti G, Wahl JH, Thresher M, Yang P, Yamazaki K, Beauchamp GK. Identification of major histocompatibility complex-regulated body odorants by statistical analysis of a comparative gas chromatography/mass spectrometry experiment. Anal Chem. 2005;77:2348–2361. doi: 10.1021/ac048711t. [DOI] [PubMed] [Google Scholar]
- Wongravee K, Heinrich N, Holmboe M, Schaefer ML, Reed RR, Trevejo J, Brereton RG. Variable selection using iterative reformulation of training set models for discrimination of samples: application to gas chromatography/mass spectrometry of mouse urinary metabolites. Anal Chem. 2009;81:5204–5217. doi: 10.1021/ac900251c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Dixon SJ, Brereton RG, Soini HA, Novotny MV, Trebesius K, Bergmaier I, Oberzaucher E, Grammer K, Penn DJ. Comparison of human axillary odour profiles obtained by gas chromatography mass spectrometry and skin microbial profiles obtained by gradient gel electrophoresis using multivariate pattern recognition. Metabolomics. 2007;3:427–437. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.