Abstract
Major biological processes occur at the biological membrane. One of the great challenges is to understand the function of chemical or biological molecules inside the membrane; as well of those involved in membrane trafficking. This requires obtaining a complete picture of the in situ structure and dynamics as well as the topology and orientation of these molecules in the membrane lipid bilayer. These led to the creation of several innovative models of biological membranes in order to investigate the structure and dynamics of amphiphilic molecules, as well as integral membrane proteins having single or multiple transmembrane segments. Because the determination of the structure, dynamics and topology of molecules in membranes requires a macroscopic alignment of the system, a new membrane model called ‘bicelles’ that represents a crossover between lipid vesicles and classical micelles has become very popular due to its property of spontaneous self-orientation in magnetic fields. In addition, crucial factors involved in mimicking natural membranes, such as sample hydration, pH and salinity limits, are easy to control in bicelle systems. Bicelles are composed of mixtures of long chain (14–18 carbons) and short chain phospholipids (6–8 carbons) hydrated up to 98% with buffers and may adopt various morphologies depending on lipid composition, temperature and hydration. We have been developing bicelle systems under the form of nano-discs made of lipids with saturated or biphenyl-containing fatty acyl chains. Depending on the lipid nature, these membranous nano-discs may be macroscopically oriented with their normal perpendicular or parallel to the magnetic field, providing a natural ‘molecular goniometer’ for structural and topological studies, especially in the field of NMR. Bicelles can also be spun at the magic angle and lead to the 3D structural determination of molecules in membranes.
Keywords: Bicelle, Biphenyl and saturated lipids, Magnetic field orientation, Phase diagrams, Model membrane, NMR, Membrane protein structure
1. Introduction
In the course of time, several models have been specially designed to investigate the structure and dynamics of integral membrane proteins in their natural membrane environment such as micelles [1,2], multilamellar vesicles [3], unilamellar vesicles [4] or mechanically oriented bilayers between glass plates [5,6]. Within the last 25 years a new membrane model system became very popular, representing an intermediate between the previously used lipid vesicles and classical micelles. These model systems are based on the pioneering work on ‘bilayered micelles’ [7]. Further improvement of this model and the resemblance to bile salt phosphatidylcholine, entailed a change in the terminology of these systems in 1995 to ‘bicelles’ [8]. Due to remarkable features such as their self-alignment in the magnetic field, they became a valuable tool especially for applications in the NMR field [9].
Bicelles are a mixture of aliphatic long chain lipids (between 12 and 18 carbons) and short chain lipids (6–8 carbons). Their morphology is fairly versatile depending on composition, temperature and hydration. The most recognized organization is a nano-disc with the long chain lipids present in majority in the disc plane and the short chain lipids mainly distributed in the torus of the disc. In this review we will focus on bicelles that are disc-shaped and that adapt a uniform and spontaneous alignment under static conditions in the magnetic field, B0. Phase diagrams will be discussed, together with examples of molecules inserted into the membrane for either doping the membrane with electric charges or for structural and dynamical determination.
2. Morphology of bicelles
The morphology of bilayered lipid mixtures is fairly versatile and changes upon lipid composition, hydration and temperature. In the literature, several models for bicelles have been reported: disc-shaped, cylindrical ‘wormlike’ micelles or perforated lamellae [10-12]. For the latter, the structure would be composed of multilamellar sheets, oriented by the magnetic field, containing holes formed by short chain phospholipids. Neutron diffraction experiments have also been performed on such a lipid mixture in macroscopically confined sample geometry, suggesting that bilayered micelles can form two distinct oriented domains of perforated lamellae seemingly littered with defects [13-18]. By electron microscopy, disc dimensions of 30–100 nm diameter and 4–5 nm thickness have been measured for TBBPC/DCPC and DMPC/DCPC, DPPC/DHPC, DiOMPC/DiOHPC bicelles [11,19]. In this morphology, the long chain lipids are mainly present in the disc and the short chain lipids are present on the half torus. Devaux and Warschawski pointed out that the short chain amphiphile DCPC undergoes a rapid exchange between toroidal and planar regions, and hence proposed their “mixed bicelle model” [20]. The detergent-like short chain lipids play the role of stabilizing the edges of the bicelle nano-disc. In contrast to liposomes, bicelles do not have an aqueous inside. The properties are close to a liquid crystal phase with one or two-dimensional ordering.
Fig.1 presents the characteristic 31P and 14N NMR spectra as well as the images obtained by transmission electron microscopy for DMPC/DCPC systems (X = 78%, h = 80%, 100 mM NaCl) and TBBPC/DCPC systems (X = 85.7%, h = 80%, 100 mM NaCl). A reasonable monodispersity of discoidal nano-objects is observed for both systems; moreover, the sizes measured on the TEM images are in very good agreement with those obtained from 31P NMR (14N NMR) by directly integrating the area under the two (four) sharp peaks, assigned to the lipids in the plane and in the half-torus of the disc [11,19,21]. Among the advantages of the bicelle, the fact that the sample hydration can be varied almost at will (in the range 60–98%) is of particular interest. It must be mentioned here that magnetic alignment is a cooperative effect that is critically dependent on the viscosity/hydration of the sample. A good orientation can be obtained with moderate hydrations (70–90%). In comparison, parameters such as sample hydration, pH and salinity proved to be difficult to control with mechanically oriented samples between glass plates. In addition, the same well-hydrated bicelle sample can be used to perform MAS and wide-line NMR, to improve spectral NMR resolution and to determine the orientation of membrane proteins [22,23], vide infra.
Fig. 1.
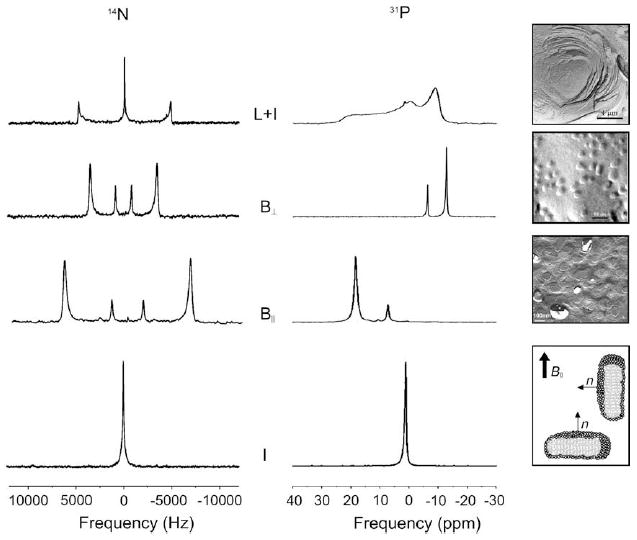
Proton-decoupled 31P and 14N NMR spectra characterizing bicellar (B), lamellar (L) or isotropic (I) structures and freeze-fracture electron microscopy pictures (from top to bottom) of DMPC liposomes (h = 80%), DMPC/DCPC systems (X = 78%; h = 80%, 100 mM KCl) and TBBPC/DCPC systems (X = 78%; h = 80%, 100 mM NaCl). At the bottom right, sketches of bicellar discs oriented with their normal parallel (B∥) or perpendicular (B⊥) to the magnetic field direction B0. Adapted from [19,45].
3. Magnetic field orientation of discs
In earlier studies, CHAPSO, a mild zwitterionic bile salt derivative, has been used to form CHAPSO–DMPC mixtures [24]. They exhibit a magnetic alignment with the membrane normal perpendicular to the static magnetic field, B0, over a wide range of compositions, pH, ionic strength and temperature. This magnetic orientation can be 90° flipped by adding amphiphilic aromatic hydrocarbons. Discoidal bicelles also show an alignment in high magnetic fields [25]. This is due to the anisotropic diamagnetic susceptibility of phospholipids, Δχ (difference between the parallel (χ∥) and the perpendicular (χ⊥) magnetic susceptibility to the long lipid axis: Δχ = χ∥ − χ⊥). Due to their small but measurable negative magnetic susceptibility, dialkanoylphospholipids are found to weakly align with their membrane normal perpendicular to the magnetic field. As a consequence, DMPC/DCPC bicelles align with their bilayer normal oriented perpendicular to B0. Addition of unsaturated lipids such as POPC does not change the bicelle orientation [26].
Bicelle disc orientation can undergo a 90° flip by adding aromatic amphiphiles or paramagnetic lanthanide ions that have a positive Δχ and bind to the phosphatidylcholine headgroups [27-29]. Among lanthanide ions, Tm3+ has the largest positive Δχ, allowing optimal alignment at a lower concentration compared to Eu3+, Er3+ and Yb3+ [30,31]. The disadvantage of this approach is the uncontrolled interaction of lanthanides with molecules inserted into the membrane. Moreover, it is necessary to keep the amount of lanthanide ions to a minimum as they can interfere with spectroscopic experiments (line broadening) [27,30-32].
Changing the alignment of the bicelle normal from perpendicular to parallel is also possible by adding molecules with a large positive Δχ, such as peptides [21] or amphiphilic aromatic compounds [33], taking advantage of the fact that phenyl rings have a strong positive Δχ [34,35]. Another approach has been to change the long chain phospholipid itself, by designing a modified phosphatidylcholine dodecanoyl-2-(4-(4-biphenyl)butanoyl)-sn-glycero-3-phosphocholine (DBBPC), containing a biphenyl unit in one of its acyl chains [36,37]. Mixtures of this newlipid DBBPC with DCPC can form bicelles, under specific conditions of temperature, hydration, and lipid composition, which align with their membrane normal parallel to the magnetic field; this is due to the large positive Δχ of the biphenyl unit. DBBPC/DCPC bicelles are stable for a ratio q [DBBPC/DHPC] ~6 and a temperature range from 10 to 54 °C [36]. More recently, the C12 aliphatic chain of DBBPC has been replaced by a C14 to mimic the chain length of natural membrane lipids closer, resulting in the phospholipid TBBPC (tetradecanoyl-2-(4-(4-biphenyl)butanoyl)-sn-glycero-3-phosphocholine) [19]. Freeze-fracture transmission electron microscopy images confirm that TBBPC/DCPC mixtures form discoidal nano-objects with an average diameter of 800 Å. The existence domains of TBBPC/DCPC have been established by 31P and 14N solid-state NMR (Figs.1 and 2) and their specific alignment in the magnetic field has been characterized by small angle X-ray scattering (SAXS) [19]. It has also been shown that TBBPC/DCPC bicelles keep their orientation outside the magnetic field for several days.
Fig. 2.
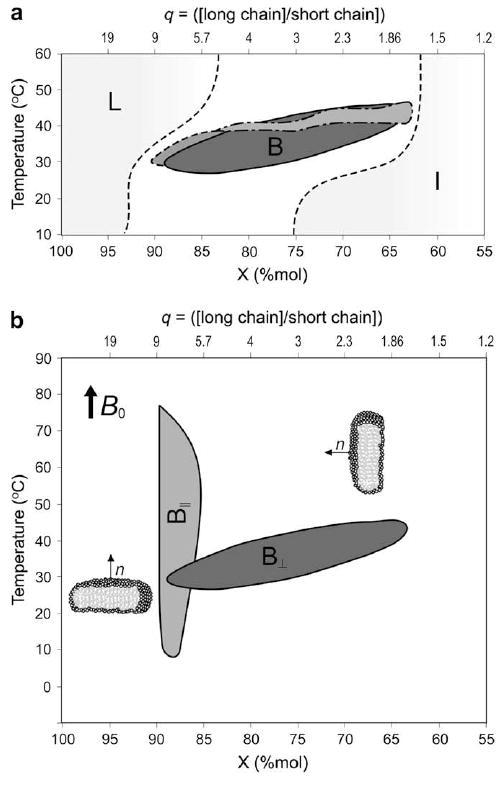
(a) Temperature–composition diagrams of bicelle systems containing saturated fatty acyl chains: ester lipids DMPC/DCPC (solid line —, dark gray), DPPC/DHPC (dashed line - - -, gray) and ether lipids DiOMPC/DiOHPC (dashed line -.-.-, gray), in 80% (w/w) D2O, as determined from orientational properties by phosphorus NMR. Shaded areas indicate the lamellar (L) and the isotropic phase (I, mixed micelles, isotropic bicelles). Lines are drawn as eye guides to delineate domains. Adapted from [10,38]. (b) Temperature–composition diagrams of DMPC/DMPC (solid line, light gray area) and TBBPC/DCPC (dashed line, dark gray area) in 80% (w/w) D2O. These lipid mixtures are macroscopically oriented by magnetic fields (B0), either with their bilayer normal, n, perpendicular (B⊥) to the field (DMPC/DCPC) or parallel (B∥, TBBPC/DCPC). Mole fractions X are expressed in percent. q represents the long chain-to-short chain molar ratio. Lines are drawn as eye guides to delineate domains. Adapted from [19].
4. Phase diagrams of magnetically oriented discs
Temperature–composition–hydration diagrams have been established to map out the different regions where bicelle discs are oriented by magnetic fields. 31P solid-state NMR was proven to be a straightforward and powerful technique to characterize lipid phases (Fig. 1). The 31P NMR line shapes could also be used to monitor the degree of bicelle orientation in the magnetic field.
In Fig. 2a, temperature–composition diagrams of DMPC/DCPC; DiOMPC/DiOHPC and DPPC/DHPC systems are presented for 80% hydration [10,19,38]. The different lipid domains that these mixtures can form, under specific temperature–composition–hydration conditions, are the following: self-orienting bicelles into the magnetic field, self-orienting bicelles coexisting with isotropic or unoriented phases, unoriented plus isotropic phases, isotropic phases (micelles or small unoriented bicelles) and lamellar phases. For clarity, only the bicellar domain (B) is presented in Fig. 2 with an indication of the area of the isotropic (I) and lamellar phases (L). The different bicellar domains are found for lipid compositions between 63% and 90% of long chain lipid, for a temperature range of 25–45 °C and for hydrations from 40% to 95% (w/w) (data not shown). Increasing the chain length of both short chain and long chain lipids, i.e., going from DMPC/DCPC to DPPC/DHPC, results in shrinkage of the zone where the oriented discs are observed. The bicelle existence domain is also smaller for ether lipids, which are very important for biological applications, as they are more stable in pH and over time than ester lipids (hydrolysis of the ester bond) [38]. Bicelles doped with ether lipids help extending the pH range of bicelles and permit structural investigation of proteins at low pH values. The presence of salts is not essential for bicelle formation, however, it allows enlarging the bicelle existence domain and in some cases improving the bicelle orientation in the magnetic field [10]. Dehydration can also stabilize the bicelle structure due to a tighter packing (lesser swelling); they appear to be more stable over a wider temperature range, keeping their alignment in the field more easily [10].
A comparison of the temperature–composition diagrams of DMPC/DCPC and TBBPC/DCPC systems is presented in Fig. 2b. TBBPC/DCPC bicelles (B∥) exist over a wide temperature scale (10–70 °C) and a narrow composition range, which can only be varied by 3%. On the contrary, chain-saturated lipid bicelles (B⊥) are obtained for a large compositional range (22%) and a narrow temperature scale (25–45 °C) [19,39].
5. Doping bicelles with charged lipids or with sterols
One step to mimic closer biological membranes is to embed in the zwitterionic DMPC/DCPC bicelle 10 mole % acidic phospholipids such as DMPS (dimyristoyl phosphatidylserine), DMPG dimyristoyl phosphatidylglycerol (DMPG) or zwitterionic DMPE (dimyristoyl phosphatidylethanolamine) [40]. This allows altering the charge characteristics of the membrane without any interference with the overall bicellar ordering [41]. For instance, it is more valuable to study the structure and orientation of the N-terminal myristoyl group of the positively charged 14-residue peptide μ-GSSKSKPKDPSQRR from the tyrosine kinase pp60v-src [41] or the neuropeptide methionin enkephalin [40] with negatively charged lipid membranes. By doping the bicelle solutions with small amounts of charged amphiphiles, such as hexadecyl(cetyl)-trimethyl-ammonium bromide (CTAB), it has been shown that the degree of alignment and the stability in temperature could be improved. Moreover, the presence of charged lipids can increase the sample lifetime and reduces the destructive effects of high salt concentrations [42]. It is also possible to embed up to 50% of positively charged lipids such as Gemini surfactants (Oda and Dufourc, unpublished) that stabilize the bicelle phase.
Saturated and biphenyl bicelles can be doped with very hydrophobic molecules such as sterols. Ring-deuterated sterol (10 mole %) has been inserted into both types of bicelles and well-resolved 2H NMR spectra are obtained (Fig. 3). A factor of ≈2 between the quadrupolar splittings of DMPC/DCPC and TBBPC/DCPC bicelles is observed; this is due to the 90° difference in orientation of the two systems. Indeed, the quadrupolar interaction is bound to the (3cos2β − 1)/2 scaling factor, where β is the angle between the bicelle normal and the magnetic field.
Fig. 3.
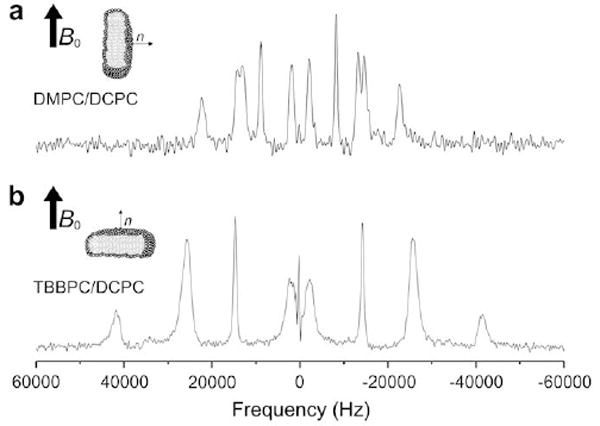
Solid-state deuterium NMR spectra of 10 mole % deuterium labeled sterol (analogous to cholesterol, containing 5 deuterons in the steroid moiety) embedded in DMPC/DCPC (a) and TBBPC/DCPC (b) bicelles at ambient temperature. Note that the bottom spectrum is broader than the upper in relation with the (3cos2β − 1)/2 scaling in T2.
6. Bicelles for probing membrane dynamics
Solid-state deuterium NMR provides an excellent tool to study membrane dynamics [43-46]. With the help of deuterated lipids incorporated into bicelles, one can obtain a clear view of the different dynamics across the lipid structure. 2H NMR spectra provide a direct measurement of carbon–deuterium order parameters, SCD, whereas motional correlation times, τc, can be obtained from measurement of NMR relaxation times, T1Z, T2E. An example of 2H NMR spectra of DMPC/DCPC bicelles where DMPC is selectively labelled on the choline headgroup (DMPC–2H13), on the glycerol backbone (DMPC–2H5), on the aliphatic chain (DMPC–2H54) and uniformly (DMPC–2H72), is presented in Fig. 4.
Fig. 4.
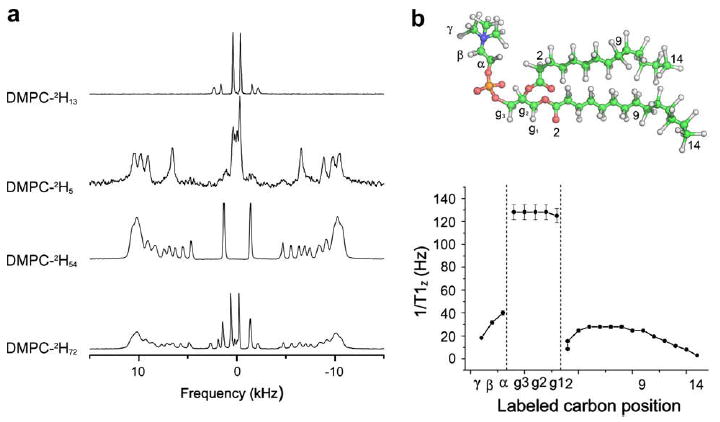
(a) 2H NMR spectra of selectively deuterated and perdeuterated DMPC phospholipids embedded in a DMPC/DCPC bicelle system (X = 78%, 100 mM KCl, 307 K). Labelled positions are indicated on spectra. (b) Molecular structure of DMPC and spin lattice relaxation rates 1/T1Z, as a function of labelled carbon position, for perdeuterated DMPC incorporated in bicelles (Adapted from [47]).
The order parameter SCD can be directly calculated by measuring the individual quadrupolar splitting ΔνQ and contains information on intramolecular, molecular and collective motions [44]. A dynamic profile of each labelled position of the DMPC molecule inserted into bicelles can thus be obtained. The rate of spin lattice relaxation (1/T1Z) reports on motional modes occurring at the nanosecond timescale (bond rotations, gauche-trans isomerisation, molecular rotation, wobbling, etc.). The dynamics of DMPC molecules in bicelles is separated into three parts: the choline head-group, the glycerol backbone, and the aliphatic chains. The glycerol backbone shows a high relaxation rate value compared to the choline polar headgroup or the aliphatic chains, revealing the fact that the glycerol backbone is more rigid than the phosphocholine headgroup facing the water, and the acyl chains constituting the oily bilayer interior. The different contributions to the DMPC dynamics are the wobbling of the molecule around its principal axis of motion and the intramolecular motions (bond rotations, etc.). The rigidity of the glycerol backbone, in contrast to the highly mobile acyl chain and phospholipid headgroup, has been used to determine both the location of the principal axis of motion and the molecular order parameter, Smol, of DMPC [47]. This was the first time that the exhaustive dynamics of the lipids, from head to tail, had been described. This completed the work of pioneers in the field who already reported on specific labelled positions using liposomes [43,46] and offered an entire view of lipid dynamics, which was rendered possible only from the increase in spectral resolution due to bicelle alignment. In conclusion, partially or uniformly deuterated phospholipids embedded in lipid bilayers are a powerful tool to study the dynamics of membranes [47].
7. Bicelles for membrane protein topology
One of the main applications of bicelles is to study the structure and orientation of membrane proteins inserted into these membrane models. The integral membrane protein, diacylglycerolkinase, has been inserted into bicelles while keeping its biological activity, which proves that bicelles can be effectively used as model membrane for structural studies by NMR [8]. Other studies have been performed on surface associated membrane proteins, like the cytochrome c and the leucine enkephalin [8]. High-resolution solid-state NMR studies have been performed on a number of membrane proteins incorporated in aligned bicelles including a GPCR [48], MerF [49], OmpX [50], and Vpu [51].
More recently, the membrane-bound form of the major coat protein of Pf1 bacteriophage, which is a well-characterized membrane protein with a single transmembrane helix [52-54], has been successfully reconstituted in TBBPC bicelles [55]. The sample aligns magnetically over a wide range of temperatures (20–60 °C), and yields well-resolved one- and two-dimensional solid-state NMR spectra (Fig. 5).
Fig. 5.
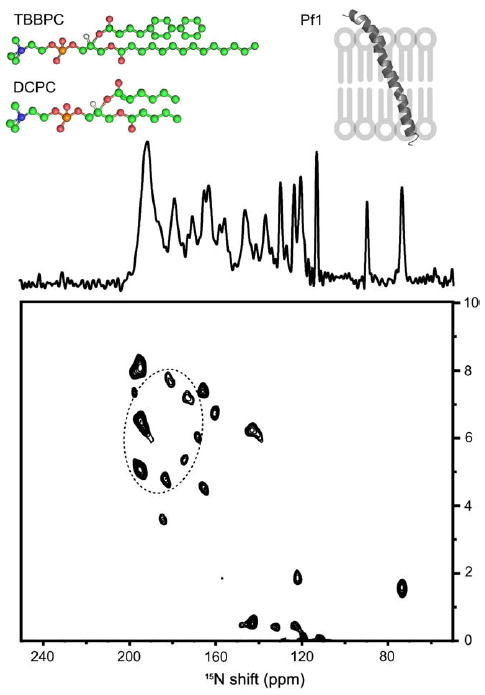
One-dimensional 15N chemical shift (centre) and two-dimensional 15N chemical shift/1H–15N dipolar coupling SLF (bottom) solid-state NMR spectra of uniformly 15N labelled Pf1 coat protein aligned with its bilayer normal parallel to the applied magnetic field in TBBPC:DCPC bicelles (X = 89% (q = 8), h = 80%, 40 °C). Top: structures of TBBPC and DCPC together with a sketch representing the helices’ orientations as deducted from spectra. (Adapted from [55]).
The one-dimensional NMR spectrum exhibits many individual resonances distinguishable within the 70–220 ppm span of the 15N chemical shift interaction. However, most of the signals are within the range associated with tilted transmembrane helices (160–220 pm) [56]. On the two-dimensional separated local field (SLF) experiment, based on the correlation between the 15N chemical shift anisotropy and the 15N–1H dipolar coupling of each amide site, the characteristic PISA wheel pattern is observed; this one corresponds to helical wheel projections formed by resonances from residues in the transmembrane helix. The orientation of the helix relative to the membrane plane normal can be estimated by superimposing ideal PISA wheels on the experimental data. In this case, the ideal PISA wheel (dashed circle) corresponds to a α-helix with uniform dihedral angles (phi = −61° and psi = −45°) and a tilt angle of 21° with an order parameter of the lipid bilayer of 0.83 [55].
8. Sample spinning of bicelles for 3D structure of membrane-associated peptides
The bicelle sample can be rapidly spun at the magic angle (54.7°) to resolve individual isotropic chemical shifts [22] and then use multidimensional liquid state NMR for structural determination. Comparing with liposomes, the bicelle structure allows attaining sharper lines due to slightly greater membrane dynamics. Fast magic angle sample spinning of DMPS-doped bicelles containing the methionin enkephalin neuropeptide (Menk) allows obtaining well-resolved 1H NMR spectrum of the lipid and the peptide (Fig. 6b). It is interesting to note that resonances are broader and show different chemical shifts in oriented bicelles (upper non-spinning spectrum) on comparing to MAS spectrum. In fact line positions and width in the upper part correspond to an anisotropic spectrum whereas an isotropic spectrum is observed under MAS conditions.
Fig. 6.
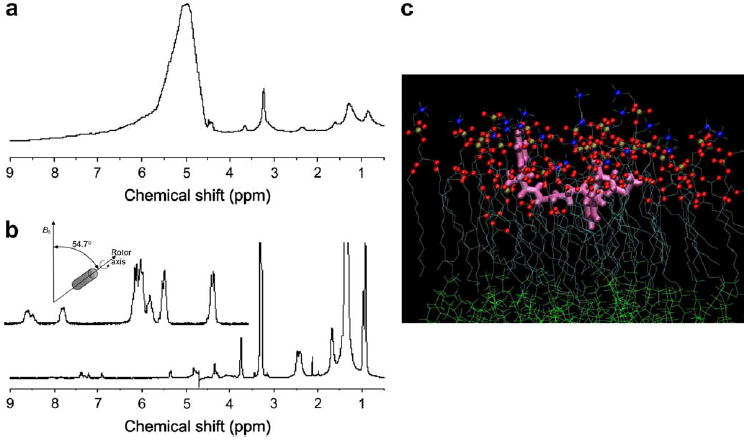
(a) Static 1H NMR spectrum of DMPC/DCPC bicelles containing the neuropeptide Menk (lipid-to-peptide molar ratio of 25), at 25 °C. (b) Same as (a) with a magic angle sample spinning speed of 10 kHz. A sketch of the MAS set up together with an expansion of the amide resonances are also shown in (b). (c) Position of Menk at the bilayer interface as reconstructed from 2D-NMR constrains and using molecular dynamics. Color code: peptide in pink; lipids: oxygen atoms of fatty acid chains in red, phosphorus in gold, nitrogen in blue, carbon skeleton in light blue, water molecules are not represented for clarity.
The use of classical multidimensional liquid state NMR sequences (TOCSY, NOESY) combined with molecular modeling, allows finding both the 3D structure of Menk and its localization at the membrane interface (Fig. 6c). This structure confirms earlier works that determined the structure of a parent enkephalin, Leuenkephalin, using transferred NOE experiments, which are more difficult to set up [57].
DMPC/DHPC bicelles have also been studied under variable-angle sample spinning (VAS). Because the slow spinning of the sample can influence the liquid-crystalline director, this technique allows modifying the alignment otherwise provided by the field [58-60]. In such experiments the angle between the sample spinning axis and the magnetic field direction has been varied. With respect to the magic angle used in solid-state NMR, it has been observed that the liquid-crystalline director of the bicelles, n, is perpendicular to the rotation axis for a sample spinning at an angle smaller than the magic angle and parallel to the rotation axis for a spinning angle larger than 54.7°. Since the technique proved to be very sensitive to the orientation of the bicelle, it can become a valuable tool to study membrane-bound peptides and proteins.
9. Weak alignment of soluble proteins for structure determination
The use of bicelles as a weak orienting medium to obtain residual alignment of soluble proteins has opened the field for studying the solution structure of numerous other types of compounds, including carbohydrates, peptides, and natural products [61]. Magnetic interactions such as residual dipolar coupling and chemical shift anisotropy can be measured and interpreted as inter-atom distances and dihedral angle values [61]. In this respect discs of molar ratio of 1:2.9 DCPC/DMPC corresponding to a thickness of ~40 Å and a diameter of ~400 Å have been used for residual dipolar coupling measurements to resolve structural constrains for determination of the structure and dynamics of human ubiquitin [62]
10. Other applications
Lorigan and co-workers reported nitroxide spin label data of a bicelle system that orients in the lower magnetic field of a conventional EPR spectrometer [63,64]. This opens up not only the way for bicelles as a medium for EPR spectroscopy, but also pointed out that in the future both NMR and EPR can be performed on the same sample to gain complementary dynamic information in two different time scales [65]. In this respect the effect of a fatty acid and of cholesterol in DMPC/DCPC magnetically aligned phospholipid bilayer membranes has been investigated by using EPR and solid-state NMR spectroscopy [63,64]. Both techniques well complement each other to define the structural and dynamical properties of the corresponding molecules in the membrane.
The transmembrane part of the tyrosine kinase receptor neu/erbB-2, NeuTM35, has been inserted into DMPC/DCPC bicelles to perform topological and structural studies of hydrophobic peptides using circular dichroism [39]. This membrane protein fragment has been shown to be a α–π–α helix [66] that may form dimers in the bilayer, in cancer-related processes. Because DMPC/DCPC lipid systems undergo a micelle-to-bicelle transition from 10 °C to 40 °C, circular dichroism was used to study the state of association of hydrophobic helices within the membrane. The data obtained suggest that the transmembrane fragment of the neu/erbB-2 receptor is monomeric in micellar medium and dimeric/multimeric in bicelle membranes [39,67].
Bicelles have also been studied by neutron diffraction to analyze their morphology [13,68] and the addition of a bicellar solution has been used to crystallize the bacteriorhodopsin from Halobacterium salinarum, opening the way to crystallize membrane proteins from lipid/detergent mixtures [69].
11. Conclusion
Bicelles have opened a new field for the study of membrane associated or embedded molecules. The versatility of the system is remarkable and makes the bicellar discs a molecular goniometer that can be oriented in magnetic fields almost at will, with a full control of hydration, pH and ionic strength. The new biphenyl bicelles that remain oriented outside the magnetic field for very long time, open the field to structural investigations by non-magnetic methods such as electronic or vibrational spectroscopies, neutrons or X-ray crystallography.
Acknowledgments
Grants from CNRS and French Ministry of Research. The Aquitaine Government is thanked for equipment funding.
Abbreviations
- DMPC
1,2-dimyristoyl-sn-glycero-3-phosphocholine (14:0/14:0)
- DPPC
1,2-dipalmitoyl-sn-glycero-3-phosphocholine (16:0/16:0)
- DCPC
1,2-dicaproyl-sn-glycero-3-phosphocholine (6:0/6:0)
- DHPC
1,2-diheptanoyl-sn-glycero-3-phosphocholine (7:0/7:0)
- DiOMPC
1,2-di-O-tetradecyl-sn-glycero-3-phosphocholine (ether lipid) (14:0/14:0)
- DiOHPC
1,2-di-O-hexyl-sn-glycero-3-phosphocholine (ether lipid) (6:0/6:0)
- TBBPC
1-tetradecanoyl-2-(4-(4-biphenyl)butanoyl)-sn-glycero-3-phosphocholine (14:0/BB)
- DBBPC
1-dodecanoyl-2-(4-(4-biphenyl)butanoyl)-sn-glycero-3-phosphocholine (12:0/BB)
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (16:0/18:1)
- Menk
Tyr–Gly–Gly–Phe–Met (methionin enkephalin)
- X
lipid composition of long chain lipid = long chain lipid/total lipids (mole percent)
- q
long chain-to-short chain lipid (mole ratio)
- h
sample hydration = mass of water/total sample mass
- SAXS
small angle X-ray scattering
- NMR
nuclear magnetic resonance spectroscopy
- MAS
magic angle sample spinning
- SCD
carbon deuterium bond order parameter
- Smol
molecular order parameter
- EPR
electron paramagnetic resonance spectroscopy
References
- 1.Arora A, Abildgaard F, Bushweller JH, Tamm LK. Structure of outer membrane protein A transmembrane domain by NMR spectroscopy. Nature Structural Biology. 2001;8:334–338. doi: 10.1038/86214. [DOI] [PubMed] [Google Scholar]
- 2.Fernández C, Adeishvili K, Wüthrich K. Transverse relaxation-optimized NMR spectroscopy with the outer membrane protein OmpX in dihexanoyl phosphatidylcholine micelles. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2358–2363. doi: 10.1073/pnas.051629298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones DH, Barber KR, Grant CWM. Sequence-related behaviour of transmembrane domains from class I receptor tyrosine kinases. Biochimica et Biophysica Acta. 1998;1371:199–212. doi: 10.1016/s0005-2736(98)00015-7. [DOI] [PubMed] [Google Scholar]
- 4.Da Costa G, Chevance S, Le Rumeur E, Bondon A. Proton NMR detection of porphyrins and cytochrome c in small unilamellar vesicles: role of the dissociation kinetic constant. Biophysical Journal. 2006;90:L55–57. doi: 10.1529/biophysj.106.081521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marassi FM, Opella SJ. Simultaneous assignment and structure determination of a membrane protein from NMR orientational restraints. Protein Science. 2003;12:403–411. doi: 10.1110/ps.0211503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marassi FM, Ramamoorthy A, Opella SJ. Complete resolution of the solid-state NMR spectrum of a uniformly 15N-labeled membrane protein in phospholipids bilayers. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:8551–8556. doi: 10.1073/pnas.94.16.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabriel NE, Roberts MF. Spontaneous formation of stable unilamellar vesicles. Biochemistry. 1984;23:4011–4015. doi: 10.1021/bi00313a001. [DOI] [PubMed] [Google Scholar]
- 8.Sanders CR, Landis GC. Reconstitution of membrane proteins into lipid-rich bilayered mixed micelles for NMR studies. Biochemistry. 1995;34:4030–4040. doi: 10.1021/bi00012a022. [DOI] [PubMed] [Google Scholar]
- 9.Sanders CR, Prosser RS. Bicelles: a model membrane system for all seasons? Structure. 1998;6:1227–1234. doi: 10.1016/s0969-2126(98)00123-3. [DOI] [PubMed] [Google Scholar]
- 10.Raffard G, Steinbruckner S, Arnold A, Davis JH, Dufourc EJ. Temperature–composition diagram of dimyristoylphosphatidylcholine–dicaproylphos-phatidylcholine “bicelles” self-orienting in the magnetic field. A solid state H-2 and P-31 NMR study. Langmuir. 2000;16:7655–7662. [Google Scholar]
- 11.Arnold A, Labrot T, Oda R, Dufourc EJ. Cation modulation of bicelle size and magnetic alignment as revealed by solid-state NMR and electron microscopy. Biophysical Journal. 2002;83:2667–2680. doi: 10.1016/S0006-3495(02)75276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harroun TA, Koslowsky M, Nieh MP, de Lannoy CF, Raghunathan VA, Katsaras J. Comprehensive examination of mesophases formed by DMPC and DHPC mixtures. Langmuir. 2005;21:5356–5361. doi: 10.1021/la050018t. [DOI] [PubMed] [Google Scholar]
- 13.Nieh MP, Raghunathan VA, Wang H, Katsaras J. Highly aligned lamellar lipid domains induced by macroscopic confinement. Langmuir. 2003;19:6936–6941. [Google Scholar]
- 14.Gaemers S, Bax A. Morphology of three lyotropic liquid crystalline biological NMR media studied by translational diffusion anisotropy. Journal of the American Chemical Society. 2001;123:12343–12352. doi: 10.1021/ja011967l. [DOI] [PubMed] [Google Scholar]
- 15.Nieh MP, Glinka CJ, Krueger S, Prosser RS, Katsaras J. SANS study of the structural phases of magnetically alignable lanthanide-doped phospholipid mixtures. Langmuir. 2001;17:2629–2638. [Google Scholar]
- 16.Nieh MP, Raghunathan VA, Glinka CJ, Harroun TA, Pabst G, Katsaras J. Magnetically alignable phase of phospholipid “bicelle” mixtures is a chiral nematic made up of wormlike micelles. Langmuir. 2004;20:7893–7897. doi: 10.1021/la048641l. [DOI] [PubMed] [Google Scholar]
- 17.Soong R, Macdonald PM. Lateral diffusion of PEG-lipid in magnetically aligned bicelles measured using stimulated echo pulsed field gradient H-1 NMR. Biophysical Journal. 2005;88:255–268. doi: 10.1529/biophysj.104.043620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Dam L, Karlsson G, Edwards K. Direct observation and characterization of DMPC/DHPC aggregates under conditions relevant for biological solution NMR. Biochimica et Biophysica Acta. 2004;1664:241–256. doi: 10.1016/j.bbamem.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Loudet C, Manet S, Gineste S, Oda R, Achard MF, Dufourc EJ. Biphenyl bicelle disks align perpendicular to magnetic fields on large temperature scales: a study combining synthesis, solid-state NMR, TEM, and SAXS. Biophysical Journal. 2007;92:3949–3959. doi: 10.1529/biophysj.106.097758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Triba MN, Warschawski DE, Devaux PF. Reinvestigation by phosphorus NMR of lipid distribution in bicelles. Biophysical Journal. 2005;88:1887–1901. doi: 10.1529/biophysj.104.055061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Picard F, Paquet MJ, Levesque J, Belanger A, Auger M. P-31 NMR first spectral moment study of the partial magnetic orientation of phospholipid membranes. Biophysical Journal. 1999;77:888–902. doi: 10.1016/S0006-3495(99)76940-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlotti C, Aussenac F, Dufourc EJ. Towards high-resolution 1H-NMR in biological membranes: magic angle spinning of bicelles. Biochimica et Biophysica Acta. 2002;1564:156–164. doi: 10.1016/s0005-2736(02)00446-7. [DOI] [PubMed] [Google Scholar]
- 23.Sizun C, Aussenac F, Grelard A, Dufourc EJ. NMR methods for studying the structure and dynamics of oncogenic and antihistaminic peptides in biomembranes. Magnetic Resononance in Chemistry. 2004;42:180–186. doi: 10.1002/mrc.1336. [DOI] [PubMed] [Google Scholar]
- 24.Sanders CR, Prestegard JH. Magnetically orientable phospholipid bilayers containing small amounts of a bile salt analogue, CHAPSO. Biophysical Journal. 1990;58:447–460. doi: 10.1016/S0006-3495(90)82390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ram P, Prestegard JH. Magnetic field induced ordering of bile salt/phospholipid micelles: new media for NMR structural investigations. Biochimica et Biophysica Acta. 1988;940:289–294. doi: 10.1016/0005-2736(88)90203-9. [DOI] [PubMed] [Google Scholar]
- 26.Triba MN, Devaux PF, Warschawski DE. Effects of lipid chain length and unsaturation on bicelles stability. A phosphorus NMR study. Biophysical Journal. 2006;91:1357–1367. doi: 10.1529/biophysj.106.085118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prosser RS, Bryant H, Bryant RG, Vold RR. Lanthanide chelates as bilayer alignment tools in NMR studies of membrane-associated peptides. Journal of Magnetic Resonance. 1999;141:256–260. doi: 10.1006/jmre.1999.1855. [DOI] [PubMed] [Google Scholar]
- 28.Crowell KJ, Macdonald PM. Europium III binding and the reorientation of magnetically aligned bicelles: insights from deuterium NMR spectroscopy. Biophysical Journal. 2001;81:255–265. doi: 10.1016/S0006-3495(01)75696-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiburu EK, Moton DM, Lorigan GA. Development of magnetically aligned phospholipid bilayers in mixtures of palmitoylstearoylphosphatidylcholine and dihexanoylphosphatidylcholine by solid-state NMR spectroscopy. Biochimica et Biophysica Acta. 2001;1512:206–214. doi: 10.1016/s0005-2736(01)00320-0. [DOI] [PubMed] [Google Scholar]
- 30.Prosser RS, Hunt SA, DiNatale JA, Vold RR. Magnetically aligned membrane model systems with positive order parameter: switching the sign of Szz with paramagnetic ions. Journal of the American Chemical Society. 1996;118:269–270. [Google Scholar]
- 31.Prosser RS, Hwang JS, Vold RR. Magnetically aligned phospholipid bilayers with positive ordering: a new model membrane system. Biophysical Journal. 1998;74:2405–2418. doi: 10.1016/S0006-3495(98)77949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katsaras J. Highly aligned lipid membrane systems in the physiologically relevant “excess water” condition. Biophysical Journal. 1997;73:2924–2929. doi: 10.1016/S0006-3495(97)78320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanders CR, Schaff JE, Prestegard JH. Orientational behavior of phosphatidylcholine bilayers in the presence of aromatic amphiphiles and a magnetic field. Biophysical Journal. 1993;64:1069–1080. doi: 10.1016/S0006-3495(93)81473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakurai I, Kawamura Y, Ikegami A, Iwayanagi S. Magneto-orientation of lecithin crystals. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:7232–7236. doi: 10.1073/pnas.77.12.7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Visscher I, Stuart MCA, Engberts J. The influence of phenyl and phenoxy modification in the hydrophobic tails of di-n-alkyl phosphate amphiphiles on aggregate morphology. Organic and Biomolecular Chemistry. 2006;4:707–712. doi: 10.1039/b514285g. [DOI] [PubMed] [Google Scholar]
- 36.Cho GJ, Fung BM, Reddy VB. Phospholipid bicelles with positive anisotropy of the magnetic susceptibility. Journal of the American Chemical Society. 2001;123:1537–1538. doi: 10.1021/ja005605+. [DOI] [PubMed] [Google Scholar]
- 37.Tan C, Fung BM, Cho G. Phospholipid bicelles that align with their normals parallel to the magnetic field. Journal of the American Chemical Society. 2002;124:11827–11832. doi: 10.1021/ja027079n. [DOI] [PubMed] [Google Scholar]
- 38.Aussenac F, Lavigne B, Dufourc EJ. Toward bicelle stability with ether-linked phospholipids: temperature composition, and hydration diagrams by H-2 and P-31 solid-state NMR. Langmuir. 2005;21:7129–7135. doi: 10.1021/la050243a. [DOI] [PubMed] [Google Scholar]
- 39.Loudet C, Khemtemourian L, Aussenac F, Gineste S, Achard MF, Dufourc EJ. Bicelle membranes and their use for hydrophobic peptide studies by circular dichroism and solid state NMR. Biochimica et Biophysica Acta. 2005;1724:315–323. doi: 10.1016/j.bbagen.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 40.Marcotte I, Dufourc EJ, Ouellet M, Auger M. Interaction of the neuropeptide Met-enkephalin with zwitterionic and negatively charged bicelles as viewed by P-31 and H-2 solid-state NMR. Biophysical Journal. 2003;85:328–339. doi: 10.1016/S0006-3495(03)74477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Struppe J, Whiles JA, Vold RR. Acidic phospholipid bicelles: a versatile model membrane system. Biophysical Journal. 2000;78:281–289. doi: 10.1016/S0006-3495(00)76591-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Losonczi JA, Prestegard JH. Improved dilute bicelle solutions for high-resolution NMR of biological macromolecules. Journal of Biomolecular NMR. 1998;12:447–451. doi: 10.1023/a:1008302110884. [DOI] [PubMed] [Google Scholar]
- 43.Davis JH. The description of membrane lipid conformation, order and dynamics by H-2-Nmr. Biochimica et Biophysica Acta. 1983;737:117–171. doi: 10.1016/0304-4157(83)90015-1. [DOI] [PubMed] [Google Scholar]
- 44.Douliez JP, Leonard A, Dufourc EJ. Restatement of order parameters in biomembranes: calculation of C–C bond order parameters from C–D quadrupolar splittings. Biophysical Journal. 1995;68:1727–1739. doi: 10.1016/S0006-3495(95)80350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dufourc EJ. Solid state NMR in biomembranes. In: Larijani B, Woscholski R, Rosser CA, editors. Chemical Biology J. Wiley & Sons, Ltd.; London: 2006. pp. 113–131. [Google Scholar]
- 46.Seelig J. Deuterium magnetic resonance: theory and application to lipid membranes. Quarterly Reviews of Biophysics. 1977;10:353–418. doi: 10.1017/s0033583500002948. [DOI] [PubMed] [Google Scholar]
- 47.Aussenac F, Laguerre M, Schmitter JM, Dufourc EJ. Detailed structure and dynamics of bicelle phospholipids using selectively deuterated and perdeuterated labels. 2-H NMR and molecular mechanics study. Langmuir. 2003;19:10468–10479. [Google Scholar]
- 48.Park SH, Prytulla S, De Angelis AA, Brown JM, Kiefer H, Opella SJ. High-resolution NMR spectroscopy of a GPCR in aligned bicelles. Journal of the American Chemical Society. 2006;128:7402–7403. doi: 10.1021/ja0606632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Angelis AA, Howell SC, Nevzorov AA, Opella SJ. Structure determination of a membrane protein with two trans-membrane helices in aligned phospholipid bicelles by solid-state NMR spectroscopy. Journal of the American Chemical Society. 2006;128:12256–12267. doi: 10.1021/ja063640w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahalakshmi R, Marassi FM. Orientation of the Escherichia coli outer membrane protein OmpX in phospholipid bilayer membranes determined by solid-state NMR. Biochemistry. 2008;47:6531–6538. doi: 10.1021/bi800362b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park SH, De Angelis AA, Nevzorov AA, Wu CH, Opella SJ. Three-dimensional structure of the transmembrane domain of Vpu from HIV-1 in aligned phospholipid bicelles. Biophysical Journal. 2006;91:3032–3042. doi: 10.1529/biophysj.106.087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee S, Mesleh MF, Opella SJ. Structure and dynamics of a membrane protein in micelles from three solution NMR experiments. Journal of Biomolecular NMR. 2003:327–334. doi: 10.1023/a:1024047805043. [DOI] [PubMed] [Google Scholar]
- 53.Opella SJ, Zeri AC, Park SH. Structure, dynamics and assembly of filamentous bacteriophages by nuclear magnetic resonances spectrocopy. Annual Review of Physical Chemistry. 2008;59:635–657. doi: 10.1146/annurev.physchem.58.032806.104640. [DOI] [PubMed] [Google Scholar]
- 54.Shon KJ, Kim Y, Colnago LA, Opella SJ. NMR studies of the structure and dynamics of membrane-bound bacteriophage Pf1 coat protein. Science. 1991;252:1303–1305. doi: 10.1126/science.1925542. [DOI] [PubMed] [Google Scholar]
- 55.Park SH, Loudet C, Marassi FM, Dufourc EJ, Opella SJ. Solid-state NMR spectroscopy of a membrane protein in biphenyl phospholipid bicelles with the bilayer normal parallel to the magnetic field. Journal of Magnetic Resonance. 2008;193:133–138. doi: 10.1016/j.jmr.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bechinger B, Sizun C. The alignment and structural analysis of membrane polypeptides by 15N and 31P solid-state NMR spectroscopy. Concepts in Magnetic Resonance. 2003;18A:130–145. [Google Scholar]
- 57.Milon A, Miyazawa T, Higashijima T. Transferred nuclear overhauser effect analyses of membrane-bound enkephalin analogs by H-1 nuclear magnetic resonance – correlation between activities and membrane-bound conformations. Biochemistry. 1990;29:65–75. doi: 10.1021/bi00453a009. [DOI] [PubMed] [Google Scholar]
- 58.Zandomeneghi G, Tomaselli M, van Beek JD, Meier BH. Manipulation of the director in bicellar mesophases by sample spinning: a new tool for NMR spectroscopy. Journal of the American Chemical Society. 2001;123:910–913. doi: 10.1021/ja0019326. [DOI] [PubMed] [Google Scholar]
- 59.Lancelot N, Elbayed K, Bianco A, Piotto M. Measurement of scaled residual dipolar couplings in proteins using variable-angle sample spinning. Journal of Biomolecular NMR. 2004;29:259–269. doi: 10.1023/B:JNMR.0000032548.60663.1f. [DOI] [PubMed] [Google Scholar]
- 60.Lancelot N, Elbayed K, Piotto M. Applications of variable-angle sample spinning experiments to the measurement of scaled residual dipolar couplings and N-15 CSA in soluble proteins. Journal of Biomolecular NMR. 2005;33:153–161. doi: 10.1007/s10858-005-3210-1. [DOI] [PubMed] [Google Scholar]
- 61.Tjandra N, Bax A. Direct measurement of distances and angles in biomolecules by NMR in a dilute liquid crystalline medium. Science. 1997;278:1111–1114. doi: 10.1126/science.278.5340.1111. [DOI] [PubMed] [Google Scholar]
- 62.Bax A, Tjandra N. High-resolution heteronuclear NMR of human ubiquitin in an aqueous liquid crystalline medium. Journal of Biomolecular NMR. 1997;10:289–292. doi: 10.1023/a:1018308717741. [DOI] [PubMed] [Google Scholar]
- 63.Nusair NA, Tiburu EK, Dave PC, Lorigan GA. Investigating fatty acids inserted into magnetically aligned phospholipid bilayers using EPR and solid-state NMR spectroscopy. Journal of Magnetic Resonance. 2004;168:228–237. doi: 10.1016/j.jmr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 64.Lu JX, Caporini MA, Lorigan GA. The effects of cholesterol on magnetically aligned phospholipid bilayers: a solid-state NMR and EPR spectroscopy study. Journal of Magnetic Resonance. 2004;168:18–30. doi: 10.1016/j.jmr.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 65.Garber SM, Lorigan GA, Howard KP. Magnetically oriented phospholipid bilayers for spin label EPR studies. Journal of the American Chemical Society. 1999;121:3240–3241. doi: 10.1021/ja005574i. [DOI] [PubMed] [Google Scholar]
- 66.Goetz M, Carlotti C, Bontems F, Dufourc EJ. Evidence for an alpha-helix -> pi-bulge helicity modulation for the neu/erbB-2 membrane-spanning segment. A H-1 NMR and circular dichroism study. Biochemistry. 2001;40:6534–6540. doi: 10.1021/bi0027938. [DOI] [PubMed] [Google Scholar]
- 67.Khemtemourian L, Buchoux S, Aussenac F, Dufourc EJ. Dimerization of Neu/Erb2 transmembrane domain is controlled by membrane curvature. European Biophysics Journal with Biophysics Letters. 2007;36:107–112. doi: 10.1007/s00249-006-0111-5. [DOI] [PubMed] [Google Scholar]
- 68.Nieh MP, Glinka CJ, Krueger S, Prosser RS, Katsaras J. SANS study on the effect of lanthanide ions and charged lipids on the morphology of phospholipid mixtures. Biophysical Journal. 2002;82:2487–2498. doi: 10.1016/s0006-3495(02)75591-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Faham S, Bowie JU. Bicelle crystallization. A new method for crystallizing membrane proteins yields a monomeric bacteriorhodopsin structure. Journal of Molecular Biology. 2002;316:1–6. doi: 10.1006/jmbi.2001.5295. [DOI] [PubMed] [Google Scholar]


