Abstract
The insula is a brain structure implicated in disparate cognitive, affective, and regulatory functions, including interoceptive awareness, emotional responses, and empathic processes. While classically considered a limbic region, recent evidence from network analysis suggests a critical role for the insula, particularly the anterior division, in high-level cognitive control and attentional processes. The crucial insight and view we present here is of the anterior insula as an integral hub in mediating dynamic interactions between other large-scale brain networks involved in externally oriented attention and internally oriented or self-related cognition. The model we present postulates that the insula is sensitive to salient events, and that its core function is to mark such events for additional processing and initiate appropriate control signals. The anterior insula and the anterior cingulate cortex form a “salience network” that functions to segregate the most relevant among internal and extrapersonal stimuli in order to guide behavior. Within the framework of our network model, the disparate functions ascribed to the insula can be conceptualized by a few basic mechanisms: (1) bottom–up detection of salient events, (2) switching between other large-scale networks to facilitate access to attention and working memory resources when a salient event is detected, (3) interaction of the anterior and posterior insula to modulate autonomic reactivity to salient stimuli, and (4) strong functional coupling with the anterior cingulate cortex that facilitates rapid access to the motor system. In this manner, with the insula as its integral hub, the salience network assists target brain regions in the generation of appropriate behavioral responses to salient stimuli. We suggest that this framework provides a parsimonious account of insula function in neurotypical adults, and may provide novel insights into the neural basis of disorders of affective and social cognition.
Keywords: Functional connectivity, Brain networks, Resting-state fMRI, Granger causality, Anterior insula, Diffusing tensor imaging
Introduction and overview
The aim of our review was to describe a new framework for the study of human insula function. We argue for a network approach and present a model that synthesizes a wide range of cognitive and affective operations that have been ascribed to the insula in recent literature. We believe that such a perspective is needed because of the explosive range of brain imaging studies that have implicated the insula in multiple, often disparate, cognitive, and affective processes. Underlying the plethora of activation studies (Kurth et al. 2010) is, we believe, a simple and parsimonious model that can help to illuminate key aspects of insula function. Our model describes how the insula facilitates bottom–up access to the brain’s attentional and working memory resources. The model postulates that the insula is specifically sensitive to salient environmental events, and that its core function is to mark such events in time and space for additional processing.
The insula is unique in that it is situated at the interface of the cognitive, homeostatic, and affective systems of the human brain, providing a link between stimulus-driven processing and brain regions involved in monitoring the internal milieu (Craig 2009). We suggest that in order to comprehend insula function it is important to not only understand cognitive and affective tasks which modulate activity in this region, but to also identify the functional circuits that are associated with it. As we seek to show, this approach can provide novel insights into the input–output relations of the insula and the functions it serves.
The organization of this review is as follows. We begin by defining and providing examples of what we mean by a “network perspective”, then continue to briefly review the complex functions that the insula has been implicated in, with special emphasis on the anterior insula (AI), an area activated across multiple sensory and cognitive domains (Kurth et al. 2010). We next review functional and structural circuits associated with the insula, and then discuss why the AI can be considered the hub of a “salience network” (SN). We describe the core dynamical functions of this network and show how the AI functions as an “integral hub”, whose main role is to mediate information flow across other brain networks involved in attentional processing and cognition. We conclude by briefly discussing how our model can be used to better understand psychopathology in disorders such as autism, anxiety disorders, and schizophrenia.
A network perspective
A network approach to brain function provides a principled approach to predicting core functions and deficits associated with specific brain systems. It is becoming increasingly apparent that cognitive neuroscience needs to go beyond the mapping of complex cognitive and psychological constructs onto individual brain areas. Nowhere is this more apparent than the study of insular cortex function. In this article, we take a systems neuroscience view that considers cognitive function to arise from the interactions of brain areas in large-scale distributed networks (Bressler 1995; Dosenbach et al. 2008; Goldman-Rakic 1995; McIntosh 2000; Mesulam 1998). The network paradigm is becoming increasingly useful for understanding the neural underpinnings of cognition (Fuster 2006). Furthermore, a consensus is now emerging that the key to understanding the functions of any specific brain region lies in understanding how its connectivity differs from the pattern of connections of other functionally related brain areas (Passingham et al. 2002).
Diffusion tensor imaging (DTI) and resting-state functional magnetic resonance imaging (fMRI) are the two most widely used methods for studying structural and functional brain connectivity, respectively, in vivo. Postmortem methods of studying structural brain connectivity have yielded some knowledge; however, human brain samples required for such studies are often difficult to obtain. Furthermore, postmortem studies are labor intensive, and investigators can often only study two or three brain regions at a time (Mesulam 2005). On the other hand, neuroimaging methods for demarcating brain networks in vivo are not unambiguous and have thus far lacked adequate spatial resolution to clearly demarcate the connectivity of the human insula. Resting state MRI assesses inter-regional functional connectivity based on physiological coupling that changes on a moment-by-moment basis, rather than anatomical connectivity. Examination of such physiological connectivity offers an alternate approach for studying intrinsic connectivity of the human brain. This approach has led to important new insights about insula connectivity, as we discuss below.
To date, functional brain imaging has primarily focused on localization of function, revealing activation in specific brain regions during performance of particular cognitive tasks. More recently, however, interest has shifted toward developing a deeper understanding of intrinsic brain architecture that influences cognitive and affective information processing (Fox and Raichle 2007; Greicius et al. 2003). This research suggests that the human brain is intrinsically organized into distinct functional networks that support complex mental processes. Brain imaging studies have found that cognitively demanding tasks consistently decrease activation in the same set of brain regions. Raichle et al. (2001) argue that these regions constitute a default mode of brain function. By seeding the PCC during a resting-state fMRI scan and demonstrating functional connectivity with other default mode network (DMN) regions, we showed that these regions constitute a tonically coupled network (Greicius et al. 2003). Resting-state fMRI connectivity analyses detect functional connectivity during several minutes of task-free fMRI scanning. Analysis of restingstate functional connectivity, using both model-based and model-free approaches, has proved to be a useful technique for investigating functionally coupled networks in the human brain (Fig. 1). While the method relies on analysis of low-frequency signals in fMRI data, electrophysiological studies point to a neuronal basis for these signals (He et al. 2008; Nir et al. 2008).
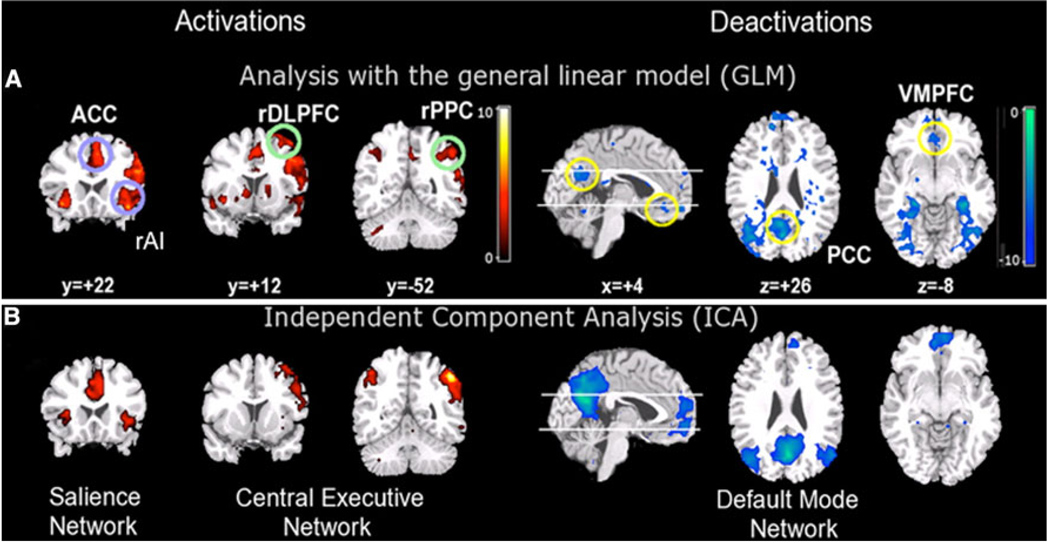
Fig. 1.
Three major brain networks identified during cognition. Activations in the central executive and salience networks and deactivations in the default mode network during auditory event segmentation. a Analysis with the general linear model revealed regional activations (left) in the right AI and ACC (blue circles); DLPFC and PPC (green circles) and deactivations (right) in the VMPFC and PCC. b Independent component analysis provided converging evidence for spatially distinct networks. From left to right: salience network (rAI and ACC), central executive network (rDLPFC and rPPC), and default mode network (VMPFC and PCC) (adapted from Sridharan et al. 2008)
Recent work in systems neuroscience has characterized several major brain networks that are identifiable in both the resting (Damoiseaux et al. 2006; Seeley et al. 2007) and the active brain (Toro et al. 2008). The two most prominent networks could be identified easily by looking at the profile of activation and deactivation typically observed during cognitive tasks. They are the central executive network (CEN), whose key nodes include the dorsolateral prefrontal cortex (DLPFC), and posterior parietal cortex (PPC) and the default mode network (DMN), which includes the ventromedial prefrontal cortex (VMPFC) and posterior cingulate cortex (PCC). During the performance of cognitively demanding tasks, the CEN typically shows increases in activation, whereas the DMN shows decreases in activation (Greicius et al. 2003; Greicius and Menon 2004; Raichle et al. 2001) (Fig. 1).
Importantly, these regions are typically activated and deactivated together, suggesting that they serve different cognitive operations. CEN nodes that show strong intrinsic functional coupling also show strong coactivation during cognitively challenging tasks. In particular, the CEN is critical for active maintenance and manipulation of information in working memory, and for judgment and decision-making in the context of goal directed behavior (Koechlin and Summerfield 2007; Miller and Cohen 2001; Muller and Knight 2006; Petrides 2005). The DMN includes the medial temporal lobes (MTL) and the angular gyrus, in addition to the PCC and the VMPFC. The PCC is activated during tasks that involve autobiographical memory and self-referential processes (Buckner and Carroll 2007); the VMPFC is associated with social cognitive processes related to self and others (Amodio and Frith 2006); the MTL is engaged in episodic and autobiographical memory (Cabeza et al. 2004), and the angular gyrus is implicated in semantic processing (Binder et al. 2009). The precise functions collectively subserved by the DMN are still largely unknown, but the individual brain regions comprising it are hypothesized to be involved in integration of autobiographical, self-monitoring, and related social cognitive functions (Spreng et al. 2009). Regardless of the specific functions subserved by each region of the DMN, it is noteworthy that dynamic suppression of this network during cognitively demanding tasks is associated with accurate behavioral performance (Kelly et al. 2008; Polli et al. 2005; Weissman et al. 2006). Over the past few years, a wide range of studies have taken advantage of this notion of the PCC and the VMPFC as core nodes of a coherent network to constrain the interpretation of activations and deactivation during cognition.
In summary, analysis of resting-state fMRI has allowed us to discover the organization and connectivity of several major brain networks that cannot be easily captured by other existing techniques. Conceptualizing the brain as comprised of multiple, distinct, and interacting networks provides a systematic framework for understanding fundamental aspects of human brain function.
Multiple functions of the insula: toward a network model
The insula is a functionally heterogeneous brain region that participates in visceral sensory and somatic sensory processes, autonomic regulation of the gastrointestinal tract and heart, as well as a functioning as a motor association area (Augustine 1996). This early viewpoint has given rise to a much more complex and multifaceted view of insular cortex function, as reviewed extensively in this special issue. Most of the early work on insula function was based on animal models; yet, it is worth noting that the majority of the human insula likely has no equivalent in the rat or monkey (Craig 2009).
Much of the recent critical thinking of insula function in humans has focused on its key role in the experience of emotion derived from information about bodily states. Critchley et al. (2001) found that patients with pure autonomic failure (an idiopathic disorder in which peripheral denervation disrupts autonomic responses) show reduced activation in the right insula during performance of “stressor” tasks (e.g. mental arithmetic) compared to controls. These patients also exhibited subtle impairments in emotional responses, and identified with statements such as “I have lost my ability to feel emotional”. These data are in line with the idea that signals from the autonomic nervous system shape emotional experience (Damasio 1996), and that the insula is a key brain region involved in this process. Critchley and colleagues have also reported that activity in the right AI predicts participants’ accuracy in a task requiring detection of one’s own heartbeat, and that gray matter volume in the AI correlates with interoceptive accuracy and subjective ratings of visceral awareness. Additionally, emotional experience correlated with interoceptive accuracy in the participants studied (Critchley et al. 2004). This study thus provides evidence for the claim that there are strong links between the right AI, perception of one’s own bodily state, and the experience of emotion. Intriguingly, smokers with brain damage involving the insula found it easier to quit smoking than smokers with brain damage involving other areas, demonstrating a role for the insula in the representation of conscious bodily urges (Naqvi et al. 2007). A recent PET study of blinking corroborates this finding, identifying the insula as a region involved in the control and suppression of natural urges (Lerner et al. 2009).
Functional neuroimaging studies have shown that the right AI is active during a wide variety of tasks involving the subjective awareness of both positive and negative feelings, including studies of anger, disgust, judgments of trustworthiness, and sexual arousal (see Craig 2002 for review). The AI has also been shown to be implicated in high-level social cognitive processes such as deception. It was recently demonstrated that the breach of a promise can be predicted by brain activity patterns including activations in the AI, ACC, and inferior frontal gyrus, implicating the AI and associated circuits in the representation of malevolent intentions before dishonest or deceitful acts are actually committed (Baumgartner et al. 2009). The AI is also implicated in empathy, or the “capacity to understand emotions of others by sharing their affective states” (Singer 2006). A study by Singer and colleagues showed that while the posterior insula was activated when subjects received painful stimulation, AI and ACC was activated both during pain perception and when the subject witnessed a loved one receiving painful stimulation. Activation in the AI was positively correlated with participants’ scores on an empathy scale. The results of this experiment led the authors to conclude that AI activation reflects emotional experience that may constitute the neural basis of empathy (Singer et al. 2004). A more recent study shows a role for the AI and ACC in complex social emotions including admiration for virtue, admiration for skill, compassion for social or psychological pain, and compassion for physical pain (Immordino-Yang et al. 2009). The insula has also been implicated in emotional judgment. A recent study shows that activity in bilateral AI underlies emotional interference resolution in working memory (Levens and Phelps 2010). Another group reported that insular activity during decision-making related to gambling predicted the extent of risky decisions. The authors suggest that the posterior insula plays a role in activating representations of homeostatic states associated with the experience of risk, which exerts an influence on subsequent decisions (Xue et al. 2010). Singer and colleagues have recently proposed an integrative model of the role of the AI in feelings, empathy, and uncertainty and risk in decision-making. According to this model, the insular cortex is purported to integrate external sensory and internal physiological signals with computations about their uncertainty. This integration is expressed as a dominant feeling state that modulates social and motivational behavior in conjunction with bodily homeostasis (Singer et al. 2009).
While these studies suggest an important role for the insula in social, affective, and higher-order mental processes guiding behavior, a more parsimonious account of its core functions is suggested by paradigms that have used far more simple stimuli and experimental manipulations. Specifically, we note that across visual, tactile, and auditory modalities, the insula responds strongly to deviant stimuli embedded in a stream of continuous stimuli (Crottaz-Herbette and Menon 2006; Downar et al. 2001; Linden et al. 1999). Furthermore, once unfamiliar deviants replace familiar deviants, the latter engages the AI to a greater extent in each one of these modalities (Downar et al. 2000, 2002). Studies such as these suggest that the insula plays a major role in detection of novel salient stimuli across multiple modalities. In our view, this represents a useful starting for point for synthesizing the wide range of complex functions that have been ascribed to the insula.
For reasons discussed in the previous section, we take the perspective that the contributions of the insula are best understood in the context of its extrinsic inputs and outputs, and knowledge of its connectivity is crucial for understanding the functions that the insula can perform. On the one hand, the extant literature suggests that the functions of the posterior insula can be segregated from those of the AI, and that the posterior insula plays a greater role in regulating physiological reactivity and homeostatic states. On the other hand, given the expansion that the human AI has undergone, animal models have proven less useful in identifying core functions of the human AI (Craig 2009). To address this question, we suggest that it is important to take a closer look at what is known regarding the structural and functional connectivity of the human insular cortex. From this perspective, we suggest that the disparate functions ascribed to the insula can be conceptualized by a few basic mechanisms: (1) bottom–up saliency detection, (2) switching between other large-scale networks to facilitate access to attention and working memory when a salient event occurs, (3) interaction of the anterior and posterior insula to modulate physiological reactivity to salient stimuli, and (4) access to the motor system via strongly coupling with the anterior cingulate cortex (ACC).
Structural connectivity of the insula
Knowledge of structural connectivity between the insula and other brain regions can inform theories of its functional role and help to gain insight into the configuration of associated networks. At present, however, the anatomical connections of the human insula are poorly understood. Our understanding of structural connections of the insula has primarily been obtained from careful tracer studies in animals. Located deep within the lateral sulcus of the brain, the insular cortex has been considered to be paralimbic (Mesulam and Mufson 1982) or “limbic integration cortex” (Augustine 1996). This characterization is largely based on the patterns of anatomical connectivity of this region, which has efferent projections to the amygdala, lateral orbital cortex, olfactory cortex, ACC, and superior temporal sulcus (STS), and receives input from orbito-frontal, olfactory cortex, ACC, and STS (Mesulam and Mufson 1982; Mufson and Mesulam 1982). After this early pioneering work in the monkey, additional crucial insights into insula-related circuitry came from Bud Craig and colleagues working in cat and monkey models. Specifically, their research has identified ascending pathways from the spinal cord, which target the insula and ACC, a “homeostatic afferent pathway” (Craig 2002, 2003). This pathway carries information about the physiological status of tissues in the body, and originates from lamina I neurons in the spinal cord that give rise to the lateral spinothalamic tract. This tract first synapses in the ventromedial nucleus of the thalamus. These thalamic nuclei project topographically to the mid/posterior dorsal insula, which in turn projects to AI. Descending pathways from the dorsal posterior insula have been described which terminate in the brainstem parabrachial nuclei, constituting a mechanism for cortical control of brainstem homeostatic systems (Craig 2002).
Inferring structural connectivity of the human insula from these studies is not straightforward because this region has undergone significant expansion in humans (Craig 2009). Furthermore, anatomical parcellation of this region is not straightforward, and is further complicated by findings, suggesting that morphometry of the human insula is much more complex than in non-human primates (Afif and Mertens 2010), and the recent recognition that even the gross morphology of the human insula is highly variable across individuals (Naidich et al. 2004). Insights into insula function in humans have been gained from lesion studies (see Ibañez et al. 2010, this special issue); however, this classic neuropsychological approach may not provide the most accurate view of a region which has functions that emerge as properties of the network in which it participates, as we argue is the case for the insula.
In humans, to our knowledge, there have been no postmortem tracer studies of insula connectivity. DTI studies of white matter microstructure and pathways are now beginning to provide new information about the structural connectivity of the human insula. A recent study used K-means clustering for connectivity-based segmentation of the insula using DTI. They found that the proportion of voxels that can be attributed to a cluster is relatively low in the insula, suggesting a high level of heterogeneity in this cortical region compared to the supplementary motor area (Nanetti et al. 2009). Anatomically, the insula is characterized by a transitional cytoarchitecture (Mesulam and Mufson 1982; Mufson and Mesulam 1982) which makes parcellation of the human insula considerably more difficult, although cytoarchitectonic boundaries of the human posterior insula have recently been documented using ten postmortem brains (Kurth et al. 2010).
Progress in non-invasive mapping of white matter pathways around the insular cortex using DTI has been limited due to the problem of resolving crossing fibers; however, rapid progress is being made in this area using better data acquisition techniques and improved analytic methods for fiber tracking (Wedeen et al. 2008). For example, white matter pathways between the insula and the posterior parietal cortex (Uddin et al. 2010) and the ACC have recently been demonstrated (van den Heuvel et al. 2009). Beyond this, the structural connectivity of the human insular cortex remains largely unknown.
Functional connectivity of the human insula and the salience network
The complex, and as yet only partially characterized, pattern of structural connectivity of the human insula reviewed above and in other articles in this special issue highlights the need for a more principled understanding of its functional links. In task-based functional imaging, it has been difficult to isolate insula responses because it is often co-activated with the ACC, the dorsolateral and ventrolateral prefrontal cortex, and the posterior parietal cortex. To circumvent this problem, Dosenbach et al. (2007) used resting-state functional connectivity to show that these regions can be grouped into distinct ‘fronto-parietal’ and ‘cingulo-opercular’ components. Seeley et al. (2007) used region-of-interest (ROI) and independent component analyses (ICA) of resting-state fMRI data to demonstrate the existence of an independent brain network comprised of the AI, dorsal ACC, along with subcortical structures including the amygdala, substantia nigra/ventral tegmental area, and thalamus. This network is distinct from the two other well-characterized large-scale brain networks previously discussed [the central executive network (CEN) and the default mode network (DMN)] (Fig. 2). We refer to this AI- and ACC-based network as a “salience network” that functions to identify the most relevant among several internal and extrapersonal stimuli in order to guide behavior (Seeley et al. 2007). Converging evidence from a number of brain imaging studies across several task domains suggest that the AI and ACC nodes of the SN respond to the degree of subjective salience, whether cognitive, homeostatic, or emotional (Craig 2009; Craig 2002). As described in the following section, the identification of such a network is relevant for understanding the role of the AI in cognition and behavior.
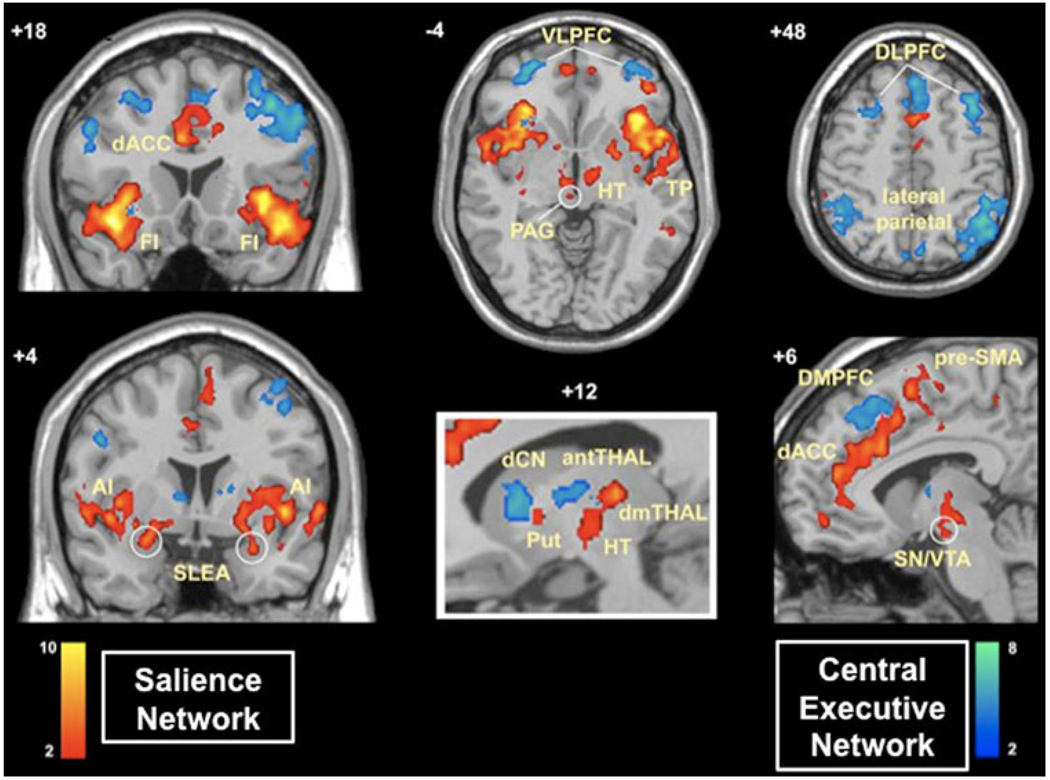
Fig. 2.
Two independent control networks identified using intrinsic physiological coupling in fMRI data. The salience network (shown in red) is important for monitoring the saliency of external inputs and internal brain events, and the central executive network (shown in blue) is engaged in higher-order cognitive and attentional control. The salience network is anchored in anterior insular and anterior cingulate cortices and features extensive connectivity with subcortical and limbic structures involved in reward and motivation. Central executive network links the dorsolateral frontal and parietal neocortices, with subcortical coupling that is distinct from that of the salience network (adapted from Seeley et al. 2007)
Resting-state fMRI studies have also shed light on differences between functional circuits associated with the anterior and posterior insula. Taylor et al. (2009) examined functional connectivity of the left and right anterior, mid, and posterior insula. They found that the AI was functionally connected with the pregenual ACC, anterior mid-cingulate cortex, and posterior mid-cingulate cortex, while the mid/posterior insula was only connected with the posterior mid-cingulate cortex. This study suggests nuanced differences in connectivity between anterior and posterior insular regions, which may have important functional implications. Thus, while AI and ACC often co-activate in task-based studies, there may be subtle differences between functional subdivisions within each of these regions, which can provide insights into how this SN can participate in so many diverse functions.
The salience network and dynamic switching
Identification of the AI as an integral node in a strongly coupled brain network that is distinct from other major networks such as the CEN and the DMN facilitates a more principled understanding of the functions of the insula. Critically, the observation that the AI and the ACC are co-activated during a wide range of cognitive tasks provides a starting point for investigating its core functions. A crucial question is: How does the SN, identified by resting-state fMRI, modulate other core networks involved in cognitive information processing? One approach here is to examine how the salience network and its two major cortical nodes, the insula and the anterior cingulate cortex, influence other core networks that have a different intrinsic organization.
Sridharan and colleagues showed, across three independent datasets, that the right AI plays a critical and causal role in switching between two other major networks (the CEN and the DMN) known to demonstrate competitive interactions during cognitive information processing (Fox et al. 2005; Greicius et al. 2003). They used Granger causality analyses to examine the directionality of influence of the AI and ACC nodes of the SN on other brain regions. Granger causal analyses (GCA) enable the detection of causal interactions between brain regions by assessing the extent to which signal changes in one brain region can predict signal changes in another brain region (Goebel et al. 2003). Across stimulus modalities, the right AI plays a critical and causal role in activating the CEN and deactivating the DMN (Sridharan et al. 2008). This study also shows that the right AI is involved in switching between brain networks across task paradigms and stimulus modalities, and thus acts as a “causal outflow hub” coordinating two major large-scale networks. Latency analysis, including measures of the time to peak, further confirmed that right AI activity temporally precedes activity in the other nodes of the CEN and DMN (Fig. 3). This new understanding of the right AI as a critical node for initiating network switching provides key insight into the core functions of the AI (Fig. 4).
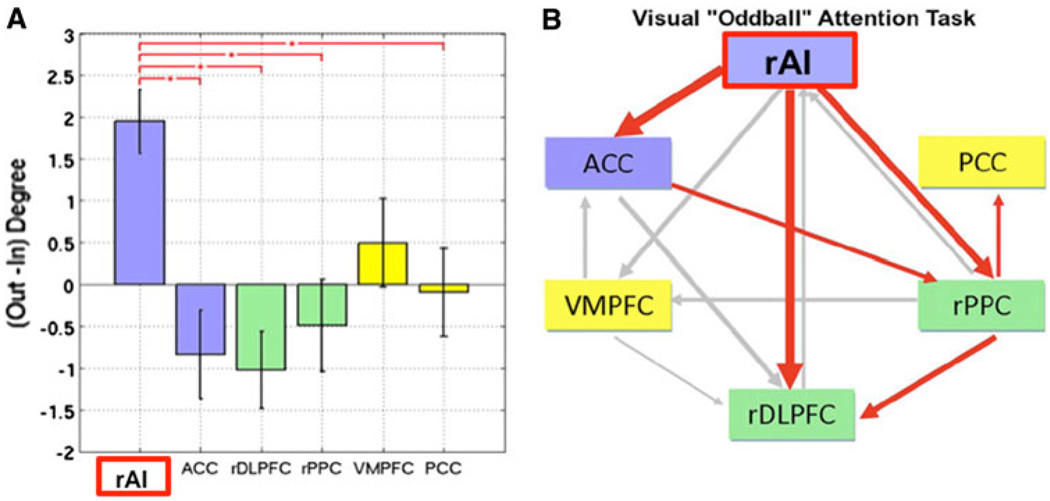
Fig. 3.
Net causal outflow of major nodes of the salience, central executive, and default mode networks. a Dynamical systems analysis revealed that the right anterior insula (rAI) has a significantly higher net causal outflow than any of the nodes of the central executive or default mode networks. b Granger causal analysis of connectivity showed significant causal outflow from the rAI to major nodes of the two other networks. These analyses, together with latency analyses, suggest that the rAI may function as a causal outflow hub for salient events (adapted from Sridharan et al. 2008)
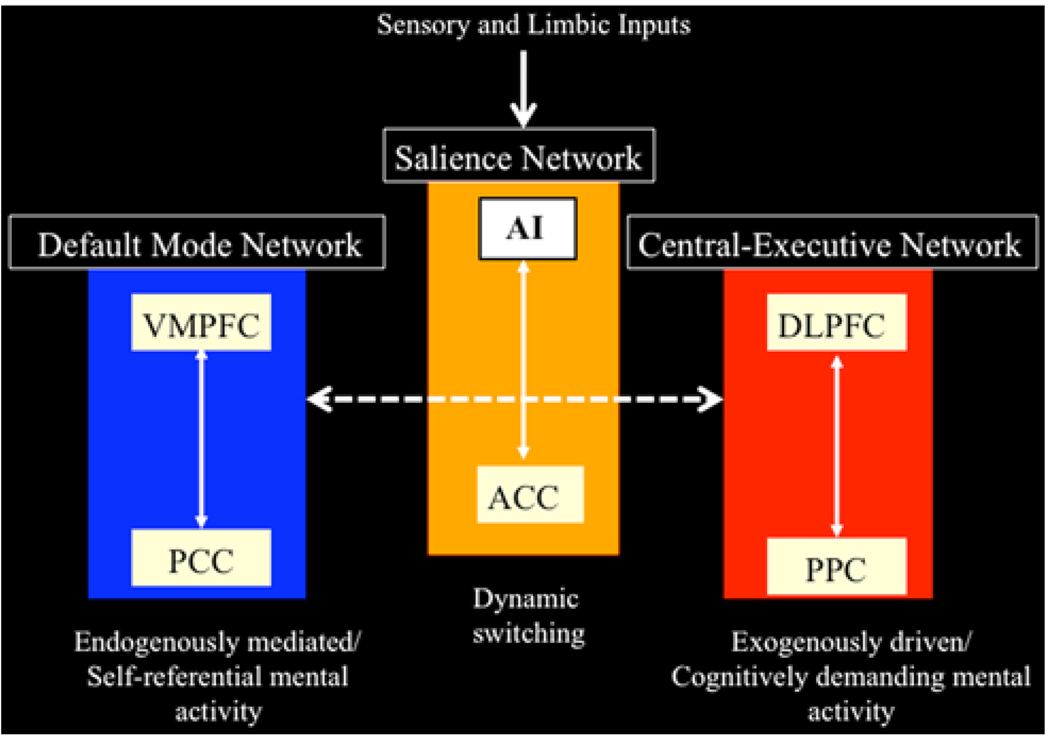
Fig. 4.
Network model of anterior insula function. The anterior insula is part of a salience network, which serves to initiate dynamic switching between the central executive and default mode networks. In our model, sensory and limbic inputs are processed by the anterior insula (AI), which detects salient events and initiates appropriate control signals to regulate behavior and homeostatic state (adapted from Uddin and Menon 2009)
As Allman et al. 2010 describe in this special issue, the AI and ACC share a unique feature at the neuronal level. In the human brain, the AI and ACC contain a specialized class of neurons with distinctive anatomical and functional features: the von Economo neurons (VENs) (Nimchinsky et al. 1999). The VENs have large axons which facilitate rapid relay of AI and ACC signals to other cortical regions (Allman et al. 2005) since conduction speeds are proportional to axonal diameter. Note that the presence or absence of VENs in a particular brain region can only be determined by analysis of postmortem brain tissue; thus, one cannot ascertain from neuroimaging work alone the potential functional significance of this cell type. However, we speculate that VENs may constitute the neuronal basis of fast control signals generated by the AI and ACC. In summary, the AI and ACC, anchored within the SN, are uniquely positioned to initiate control signals that activate the CEN and deactivate the DMN.
Saliency, attention, and cognitive control
Previous studies have suggested that the inferior frontal gyrus and ACC are involved in a variety of monitoring, decision-making, and cognitive control processes (Cole and Schneider 2007; Crottaz-Herbette and Menon 2006; Dosenbach et al. 2008; Eichele et al. 2008; Johnston et al. 2007; Posner and Rothbart 2007), but the AI has not been a particular focus of most of these studies. There are two notable exceptions. Dosenbach et al. (2007), who used functional connectivity analysis of resting-state fMRI epochs, argue that the AI helps to generate control signals involved in “stable maintenance of task mode and strategy”. We differ in suggesting that the AI is involved in transient detection of salient stimuli and initiating attentional control signals which are then sustained by the ACC and the ventrolateral and dorsolateral PFC. In contrast, Sridharan and colleagues used auditory and visual attention tasks to suggest that the AI generates a transient control signal in response to salient events.
Our model posits that the core function of the proposed SN and the AI, in particular, is to first identify stimuli from the vast and continuous stream of sensory stimuli that impact the senses. Once such a stimulus is detected, the AI facilitates task-related information processing by initiating appropriate transient control signals to engage brain areas mediating attentional, working memory, and higher order cognitive processes while disengaging the DMN via mechanisms that have been described in the previous section. Critically, these switching mechanisms help focus attention on external stimuli, as a result of which they take on added significance or saliency.
There are several processes fundamental to attention: automatic bottom–up filtering of stimuli, competitive selection, working memory, and top–down sensitivity control (Knudsen 2007). Filtering and amplification of specific stimuli can in principle occur at multiple levels in the hierarchy of ascending neural pathways that bring stimuli from the external world to the primary and secondary sensory cortex. At each level, filters enhance responses to stimuli that are infrequent in space or time or are of learned or instinctive biological importance. For example, neurons in the superior colliculus can amplify responses to specific visual stimuli based on stimulus-driven representations in local salience maps (Fecteau et al. 2004). The neural mechanisms for this can range from adaptation in individual neurons to center surround properties of local neural circuits. The large-scale network switching mechanisms we have described here can be thought of as the culmination of a hierarchy of saliency filters in which each successive stage helps to differentially amplify a stimulus sufficiently to engage the AI. The precise pathways and filters underlying the transformation of external stimuli and the manner in which the AI is activated remain to be investigated. Critically, our model suggests that once a stimulus activates the AI, it will have preferential access to the brain’s attentional and working memory resources.
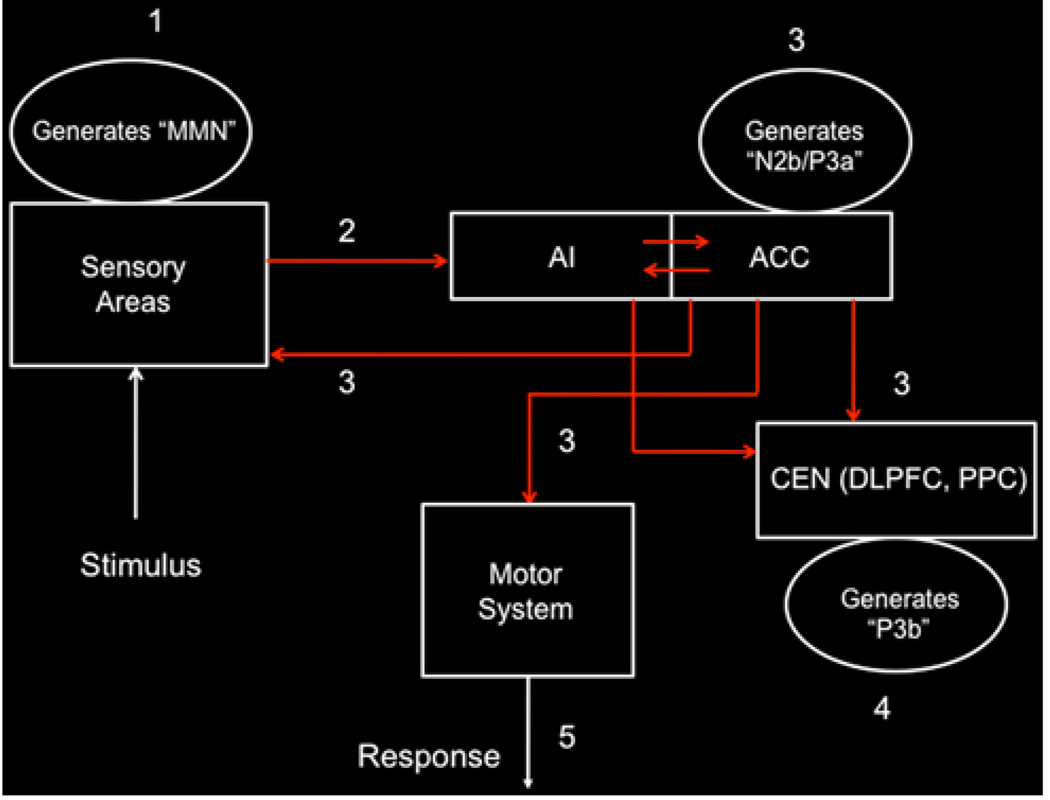
Although dynamical systems analysis of fMRI data can help capture aspects of causal interactions between distributed brain areas, a more complete characterization of bottom–up and top–down attentional control requires access to temporal dynamics on the 30–70-ms time scale. Analysis of combined EEG and fMRI data provides additional insights into how the SN plays an important role in attentional control (Crottaz-Herbette and Menon 2006). A schematic model of bottom–up and top–down interactions underlying attentional control suggested by the relative timing of responses in the AI and ACC versus other cortical regions based on our dynamic source imaging study and by lesion studies of the P3a complex (Soltani and Knight 2000) is shown in Fig. 5. The spatio-temporal dynamics underlying this process has four distinct stages. Stage 1 About 150 ms post-stimulus primary sensory areas detect a deviant stimulus as indexed by the mismatch negativity (MMN) component of the evoked potential. Stage 2 This “bottom–up” MMN signal is transmitted to other brain regions, notably the AI and the ACC. Stage 3 About 200–300 ms post-stimulus, the AI and ACC generate a “top–down” control signal as indexed by the N2b/P3a component of the evoked potential. This signal is simultaneously transmitted to primary sensory areas, as well as other neocortical regions. Stage 4 About 300–400 ms post-stimulus, neocortical regions, notably the premotor cortex and temporo-parietal areas, respond to the attentional shift with a signal that is indexed by the time-average P3b-evoked potential. Stage 5 The ACC facilitates response selection and motor response via its links to the midcin-gulate cortex, supplementary motor cortex, and other motor areas (Rudebeck et al. 2008; Vogt 2009).
Fig. 5.
Schematic model of dynamic bottom–up and top–down interactions underlying attentional control. Stage 1 About 150 ms post-stimulus primary sensory areas detect a deviant stimulus as indexed by the mismatch negativity (MMN) component of the evoked potential. Stage 2 This “bottom–up” MMN signal is transmitted to other brain regions, including the anterior insula (AI). The anterior insula provides selective amplification of salient events and triggers a strong response in the anterior cingulate cortex (ACC). Stage 3 About 200–300 ms post-stimulus, the ACC generates a “top–down” control signal as indexed by the N2b/P3a component of the evoked potential. This signal is simultaneously transmitted to primary sensory and association cortex, as well the central executive network. Stage 4 About 300–400 ms post-stimulus, neocortical regions, notably the premotor cortex and temporo-parietal areas, respond to the attentional shift with a signal that is indexed by the time-average P3b evoked potential. Stage 5 The ACC also facilitates response selection and motor response via its links to the midcingulate cortex, supplementary motor cortex, and other motor areas (adapted from Crottaz-Herbette and Menon 2006)
Within the framework of the network model described above, we suggest that the AI plays a more prominent role in detection of salient stimuli, whereas the ACC plays a more prominent role in modulating responses in the sensory, motor, and association cortices. A wide range of functional imaging studies and theoretical models have suggested that the ACC plays a prominent role in action selection (Rushworth 2008). Taken together, as part of a functionally coupled network, the AI and ACC help to integrate bottom–up attention switching with top–down control and biasing of sensory input. This dynamic process enables an organism to sift through many different incoming sensory stimuli and adjust gain for task-relevant stimuli, processes central to attention (Yantis 2008).
An examination of the differential pattern of input–output connectivity of the AI and the ACC provides further insights into the functions of the AI and SN. While the AI receives multimodal sensory input, the ACC and associated dorsomedial prefrontal cortex receive very little sensory input (Averbeck and Seo 2008). Conversely, while the ACC and associated dorsomedial prefrontal cortex send strong motor output, there is very little direct motor input to or output from the AI. Furthermore, the ACC and dorsomedial prefrontal cortex have direct connections to the spinal cord and sub-cortical oculomotor areas (Fries 1984) giving them direct control over action. With these differential anatomical pathways and von Economo neurons that facilitate rapid signaling between the AI and the ACC, the SN is well positioned to influence not only attention but also motor responses to salient sensory stimuli. In this manner, the AI plays both a direct and indirect role in attention, cognitive, and behavioral control. Critically, in the context of our model, this input–output pattern of input and output pattern suggests that the AI may generate the signals to trigger hierarchical control. Consistent with this view, among patients with frontal lobe damage, those with lesions in the AI were the most impaired in altering their behavior in accordance with the changing rules of an occulomotor-switching task (Hodgson et al. 2007). Our model further suggests that when the ACC is dysfunctional, the AI is well positioned to trigger alternate cognitive control signals via other lateral cortical regions such as the VLPFC and the DLPFC (Johnston et al. 2007). Thus, our model helps to clarify an important controversy regarding the primacy and uniqueness of control signals in the prefrontal cortex (Fellows and Farah 2005; Baird et al. 2006).
Saliency and awareness of physiological change: anterior–posterior insula interaction
We have primarily focused on AI function as this region has received the most attention in the human functional neuroimaging literature. However, an important aspect of insula function pertains to integration of homeostatic signals from the body. As reviewed by Craig (2010) in this speical issue and earlier in “Structural connectivity of the insula”, the anterior and posterior insula have differential connectivity and input–output relations. This anatomical pattern forms the basis for exploring how salience detection and awareness of physiological change are linked along the axis of the insula.
In order to gain access to the AI and associated SN, stimulus-related inputs may originate from sensory or association cortex. For example, in the visual domain, we have shown recently that insula can be accessed via a set of links along the dorsal visual pathway: primary visual cortex → posterior intraparietal sulcus → anterior intraparietal sulcus → AI (Uddin et al. 2010) (Fig. 6). Rather than representing secondary salience signals that primarily reflect body sensations via the medial orbitofrontal ‘vis-ceromotor’ network that helps control internal body states (Barrett and Bar 2009), however, our data suggest that the orsal visual system has direct anatomical projections into the AI. This pathway may help to generate signals reflecting saliency of visual stimuli in extrapersonal space. Alternatively, under specific conditions, and in particular stimulus modalities, inputs might reach the AI via the middle and posterior insula, a subdivision which is the preferential cortical target of the homeostatic afferent pathway. As previously discussed in “Structural connectivity of the insula”, and extensively reviewed in this special issue, the posterior, mid, and anterior insular cortices have different patterns of connectivity with other brain regions. In the model proposed by Craig, the subjective awareness of salient events is represented more anteriorly, whereas more sensory attributes are thought to be represented posteriorly (Craig 2002). However, we currently do not have a good understanding of how the posterior and anterior subdivisions of the insula interact, and the differential timing of autonomic response with respect to salient event detection in the human insular cortex remains largely unknown. Additional anatomical and functional studies that examine the relationship between autonomic reactivity and salient event detection are needed in order to further address the question of sensory access into specific subdivisions of the human insular cortex.
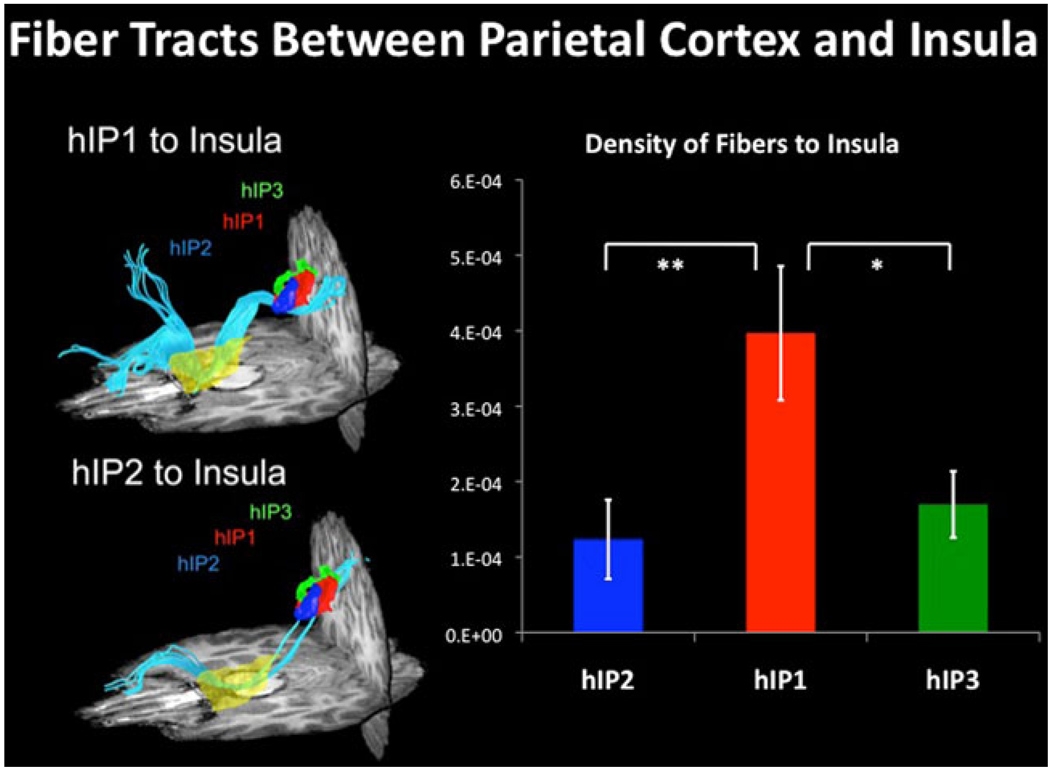
Fig. 6.
DTI tractography between parietal cortex and insula. DTI tractography and density of fibers between three subdivisions in human intraparietal sulcus (hIP2, hIP1, and hIP3) and insula. hIP1 shows greater structural connectivity than hIP2 and hIP3 with insula (*p < 0.05, **p < 0.01) (adapted from Uddin et al. 2010)
Implications for psychopathology
This new understanding of the right AI as a critical node for initiating network switching provides key insight into the potential for profound deficits in cognitive functioning should AI integrity or connectivity be compromised. For example, AI hyperactivity has been implicated in anxiety disorders, suggesting that when the SN goes into overdrive, pathology subsequently results (Paulus and Stein 2006; Stein et al. 2007). Individuals scoring high on the trait neuroticism, defined as the tendency to experience negative emotional states, demonstrate greater right AI activation during decision-making, even when the outcome of the decision is certain (Feinstein et al. 2006). A recent study found that during times when patients with schizophrenia reported experiencing auditory verbal hallucinations, increased activation of the right insula was observed (Sommer et al. 2008). In the previous examples, hyperactivity of the AI may be the basis for pathologically enhanced salience detection. Increased anxiety or neuroticism, for example, may be the consequence of the AI misattributing emotional salience to mundane events.
Pathology can also result from compromised salience detection mechanisms, resulting from decreased AI activity. In a comprehensive meta-analysis of functional neuroimaging studies of social processing in autism, Di Martino et al. (2009) demonstrated that across a of group of studies examining various aspects of social processing, one of the regions consistently showing significant hypoactivity in autism was the right anterior insula. It may be the case that reduced attention to social stimuli, a hallmark of ASD, is the result of ineffective salience processing in the AI. Taken together, these clinical findings suggest that an appropriate level of AI activity is necessary to provide an alert signal to initiate brain responses to salient stimuli, but this signal can be overactive, in the case of anxiety, or underactive, as may be the case in autism (Uddin and Menon 2009). Our model potentially provides a parsimonious account that may explain various clinical symptoms as a function of enhanced, reduced, or otherwise altered salience detection and subsequent attentional allocation.
Conclusions
In the model proposed here, the AI and ACC form the core of a SN that facilitates the detection of important environmental stimuli. Although salience filters likely exist at multiple levels of ascending pathways that bring sensory stimuli into the neocortex, what makes the SN special is that it triggers a cascade of cognitive control signals that have a major impact on how such a stimulus is subsequently processed.
Chronometric techniques and dynamical systems analysis suggest that the SN, and the AI in particular, plays a critical and causal role in switching between the frontoparietal CEN and the DMN across task paradigms and stimulus modalities. Our network model helps to synthesize disparate findings in the literature into a common framework and suggests a causal, and potentially critical, role for the AI in cognitive control. We propose that one fundamental mechanism underlying such control is a transient signal from the AI, which engages the brain’s attentional, working memory and higher-order control processes while disengaging other systems that are not immediately task relevant.
The AI, which forms part of the SN, is distinct from the functional circuits of the mid-posterior insula. These two relatively segregated streams nevertheless interact, presumably along the axis of the insula, in order to integrate salient stimuli and events with visceral and autonomic information. Taken together, they help to generate a state of heightened physiological awareness of salient stimuli. In closing, we note that examination of potential disruptions to these processes may help us to better understand brain mechanisms underlying psychopathology in several neurological and psychiatric disorders, including schizophrenia, autism, and anxiety disorders.
Acknowledgments
We thank Dr. Bud Craig and two anonymous reviewers for their insightful feedback and suggestions for improving the manuscript, and Dr. Miriam Rosenberg-Lee for useful comments on an early draft. This work was supported by grants from the National Institutes of Health (NS058899, HD047520, HD059205, HD057610 to VM), the National Science Foundation (BCS/DRL 0449927 to VM), and by a Mosbacher Postdoctoral Fellowship and Award Number K01MH092288 from the National Institute of Mental Health to LQU.
Contributor Information
Vinod Menon, Email: menon@stanford.edu, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, 780 Welch Road, Room 201, Stanford, CA 94304, USA; Department of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, CA 94304, USA; Program in Neuroscience, Stanford University School of Medicine, Stanford, CA 94304, USA.
Lucina Q. Uddin, Email: lucina@stanford.edu, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, 780 Welch Road, Room 201, Stanford, CA 94304, USA; Program in Neuroscience, Stanford University School of Medicine, Stanford, CA 94304, USA.
References
- Afif A, Mertens P. Description of sulcal organization of the insular cortex. Surg Radiol Anat. 2010 doi: 10.1007/s00276-009-0598-4. (in press) [DOI] [PubMed] [Google Scholar]
- Allman JM, Watson KK, Tetreault NA, Hakeem AY. Intuition and autism: a possible role for Von Economo neurons. Trends Cogn Sci. 2005;9(8):367–373. doi: 10.1016/j.tics.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, Park S, Goubert V, Hof PR. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct Funct. 2010;214(5–6) doi: 10.1007/s00429-010-0254-0. doi: 10.1007/s00429-010-0254-0. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22(3):229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Averbeck BB, Seo M. The statistical neuroanatomy of frontal networks in the macaque. PLoS Comput Biol. 2008;4(4):e1000050. doi: 10.1371/journal.pcbi.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird A, Dewar BK, Critchley H, Gilbert SJ, Dolan RJ, Cipolotti L. Cognitive functioning after medial frontal lobe damage including the anterior cingulate cortex: a preliminary investigation. Brain Cogn. 2006;60(2):166–175. doi: 10.1016/j.bandc.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Bar M. See it with feeling: affective predictions during object perception. Philos Trans R Soc Lond B Biol Sci. 2009;364(1521):1325–1334. doi: 10.1098/rstb.2008.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T, Fischbacher U, Feierabend A, Lutz K, Fehr E. The neural circuitry of a broken promise. Neuron. 2009;64(5):756–770. doi: 10.1016/j.neuron.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL. Large-scale cortical networks and cognition. Brain Res Brain Res Rev. 1995;20(3):288–304. doi: 10.1016/0165-0173(94)00016-i. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11(2):49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, et al. Brain activity during episodic retrieval of autobiographical and laboratory events: an fMRI study using a novel photo paradigm. J Cogn Neurosci. 2004;16(9):1583–1594. doi: 10.1162/0898929042568578. [DOI] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage. 2007;37(1):343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13(4):500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD. The sentient self. Brain Struct Funct. 2010;214(5–6) doi: 10.1007/s00429-010-0248-y. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neuroanatomical basis for first- and second-order representations of bodily states. Nat Neurosci. 2001;4(2):207–212. doi: 10.1038/84048. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neuro-sci. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Crottaz-Herbette S, Menon V. Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. J Cogn Neurosci. 2006;18(5):766–780. doi: 10.1162/jocn.2006.18.5.766. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2009;65(1):63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci. 2000;3(3):277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. The effect of task relevance on the cortical response to changes in visual and auditory stimuli: an event-related fMRI study. Neuroimage. 2001;14(6):1256–1267. doi: 10.1006/nimg.2001.0946. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J Neurophysiol. 2002;87(1):615–620. doi: 10.1152/jn.00636.2001. [DOI] [PubMed] [Google Scholar]
- Eichele T, Debener S, Calhoun VD, Specht K, Engel AK, Hugdahl K, et al. Prediction of human errors by maladaptive changes in event-related brain networks. Proc Natl Acad Sci USA. 2008;105(16):6173–6178. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau JH, Bell AH, Munoz DP. Neural correlates of the automatic and goal-driven biases in orienting spatial attention. J Neurophysiol. 2004;92(3):1728–1737. doi: 10.1152/jn.00184.2004. [DOI] [PubMed] [Google Scholar]
- Feinstein JS, Stein MB, Paulus MP. Anterior insula reactivity during certain decisions is associated with neuroticism. Soc Cogn Affect Neurosci. 2006;1(2):136–142. doi: 10.1093/scan/nsl016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Is anterior cingulate cortex necessary for cognitive control? Brain. 2005;128(Pt 4):788–796. doi: 10.1093/brain/awh405. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries W. Cortical projections to the superior colliculus in the macaque monkey: a retrograde study using horseradish perox-idase. J Comp Neurol. 1984;230(1):55–76. doi: 10.1002/cne.902300106. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The cognit: a network model of cortical representation. Int J Psychophysiol. 2006;60(2):125–132. doi: 10.1016/j.ijpsycho.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Goebel R, Roebroeck A, Kim DS, Formisano E. Investigating directed cortical interactions in time-resolved fMRI data using vector autoregressive modeling and Granger causality mapping. Magn Reson Imaging. 2003;21(10):1251–1261. doi: 10.1016/j.mri.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Architecture of the prefrontal cortex and the central executive. Ann N Y Acad Sci. 1995;769:71–83. doi: 10.1111/j.1749-6632.1995.tb38132.x. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004;16(9):1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. Proc Natl Acad Sci USA. 2008;105(41):16039–16044. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson T, Chamberlain M, Parris B, James M, Gutowski N, Husain M, et al. The role of the ventrolateral frontal cortex in inhibitory oculomotor control. Brain. 2007;130(Pt 6):1525–1537. doi: 10.1093/brain/awm064. [DOI] [PubMed] [Google Scholar]
- Ibañez A, Gleichgerrcht E, Manes F. Clinical effects of insular damage in humans. Brain Struct Funct. 2010 doi: 10.1007/s00429-010-0256-y. doi: 10.1007/s00429-010-0256-y. [DOI] [PubMed] [Google Scholar]
- Immordino-Yang MH, McColl A, Damasio H, Damasio A. Neural correlates of admiration and compassion. Proc Natl Acad Sci USA. 2009;106(19):8021–8026. doi: 10.1073/pnas.0810363106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K, Levin HM, Koval MJ, Everling S. Top-down control-signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron. 2007;53(3):453–462. doi: 10.1016/j.neuron.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39(1):527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends Cogn Sci. 2007;11(6):229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Kurth F, Eickhoff SB, Schleicher A, Hoemke L, Zilles K, Amunts K. Cytoarchitecture and Probabilistic Maps of the Human Posterior Insular Cortex. Cereb Cortex. 2010 doi: 10.1093/cercor/bhp208. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010 doi: 10.1007/s00429-010-0255-z. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A, Bagic A, Hanakawa T, Boudreau EA, Pagan F, Mari Z, Bara-Jimenez W, Aksu M, Sato S, Murphy DL, Hallett M. Involvement of Insula and Cingulate Cortices in Control and Suppression of Natural Urges. Cereb Cortex. 2009;19(1):218–223. doi: 10.1093/cercor/bhn074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens SM, Phelps EA. Insula and orbital frontal cortex activity underlying emotion interference resolution in working memory. J Cogn Neurosci. 2010 doi: 10.1162/jocn.2010.21428. (in press) [DOI] [PubMed] [Google Scholar]
- Linden DE, Prvulovic D, Formisano E, Vollinger M, Zanella FE, Goebel R, et al. The functional neuroanatomy of target detection: an fMRI study of visual and auditory oddball tasks. Cereb Cortex. 1999;9(8):815–823. doi: 10.1093/cercor/9.8.815. [DOI] [PubMed] [Google Scholar]
- McIntosh AR. Towards a network theory of cognition. Neural Netw. 2000;13(8–9):861–870. doi: 10.1016/s0893-6080(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Mesulam M. Imaging connectivity in the human cerebral cortex: the next frontier? Ann Neurol. 2005;57(1):5–7. doi: 10.1002/ana.20368. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey III: Efferent cortical output and comments on function. J Comp Neurol. 1982;212(1):38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. J Comp Neurol. 1982;212(1):23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- Muller NG, Knight RT. The functional neuroanatomy of working memory: contributions of human brain lesion studies. Neuroscience. 2006;139(1):51–58. doi: 10.1016/j.neuroscience.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Naidich TP, Kang E, Fatterpekar GM, Delman BN, Gultekin SH, Wolfe D, et al. The insula: anatomic study and MR imaging display at 1.5 T. Am J Neuroradiol. 2004;25(2):222–232. [PMC free article] [PubMed] [Google Scholar]
- Nanetti L, Cerliani L, Gazzola V, Renken R, Keysers C. Group analyses of connectivity-based cortical parcellation using repeated k-means clustering. Neuroimage. 2009;47(4):1666–1677. doi: 10.1016/j.neuroimage.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky EA, Gilissen E, Allman JM, Perl DP, Erwin JM, Hof PR. A neuronal morphologic type unique to humans and great apes. Proc Natl Acad Sci USA. 1999;96(9):5268–5273. doi: 10.1073/pnas.96.9.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Mukamel R, Dinstein I, Privman E, Harel M, Fisch L, et al. Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nat Neurosci. 2008;11(9):1100–1108. doi: 10.1038/nn.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham RE, Stephan KE, Kotter R. The anatomical basis of functional localization in the cortex. Nat Rev Neurosci. 2002;3(8):606–616. doi: 10.1038/nrn893. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60(4):383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360(1456):781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polli FE, Barton JJ, Cain MS, Thakkar KN, Rauch SL, Manoach DS. Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commission. Proc Natl Acad Sci USA. 2005;102(43):15700–15705. doi: 10.1073/pnas.0503657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annu Rev Psychol. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Behrens TE, Kennerley SW, Baxter MG, Buckley MJ, Walton ME, et al. Frontal cortex subregions play distinct roles in choices between actions and stimuli. J Neurosci. 2008;28(51):13775–13785. doi: 10.1523/JNEUROSCI.3541-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF. Intention, choice, and the medial frontal cortex. Ann N Y Acad Sci. 2008;1124:181–207. doi: 10.1196/annals.1440.014. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T. The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neurosci Biobehav Rev. 2006;30(6):855–863. doi: 10.1016/j.neubiorev.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13(8):334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Soltani M, Knight RT. Neural origins of the P300. Crit Rev Neurobiol. 2000;14(3–4):199–224. [PubMed] [Google Scholar]
- Sommer IE, Diederen KM, Blom JD, Willems A, Kushan L, Slotema K, et al. Auditory verbal hallucinations predominantly activate the right inferior frontal area. Brain. 2008;131(Pt 12):3169–3177. doi: 10.1093/brain/awn251. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21(3):489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164(2):318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulated cortex. Hum Brain Mapp. 2009;30(9):2731–2745. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cereb Cortex. 2008;18(11):2553–2559. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Menon V. The anterior insula in autism: under-connected and under-examined. Neurosci Biobehav Rev. 2009;33(8):1198–1203. doi: 10.1016/j.neubiorev.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen DA, Greicius MD, Menon V. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq011. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30(10):3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA. Cingulate neurobiology and disease. Oxford: Oxford University Press; 2009. [Google Scholar]
- Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, et al. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 2008;41(4):1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin IP, Bechara A. The impact of prior risk experiences on subsequent risky decision-making: The role of the insula. Neuroimage. 2010;50(2):709–716. doi: 10.1016/j.neuroimage.2009.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S. The neural basis of selective attention: cortical sources and targets of attentional modulation. Curr Dir Psychol Sci. 2008;17(2):86–90. doi: 10.1111/j.1467-8721.2008.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]