Abstract
Chronic exposure to elevated free fatty acids, in particular long chain saturated fatty acids, provokes endoplasmic reticulum (ER) stress and cell death in a number of cell types. The perturbations to the ER that instigate ER stress and activation of the unfolded protein in response to fatty acids in hepatocytes have not been identified. The present study employed H4IIE liver cells and primary rat hepatocytes to examine the hypothesis that saturated fatty acids induce ER stress via effects on ER luminal calcium stores. Exposure of H4IIE liver cells and primary hepatocytes to palmitate and stearate reduced thapsigargin-sensitive calcium stores and biochemical markers of ER stress over similar time courses (6h). These changes preceded cell death, which was only observed at later time points (16h). Co-incubation with oleate prevented the reduction in calcium stores, induction of ER stress markers and cell death observed in response to palmitate. Inclusion of calcium chelators, BAPTA-AM or EGTA, reduced palmitate- and stearate-mediated enrichment of cytochrome c in post-mitochondrial supernatant fractions and cell death. These data suggest that redistribution of ER luminal calcium contributes to long chain saturated fatty acid-mediated ER stress and cell death.
Keywords: lipoapoptosis, unfolded protein response, hepatocyte, non-alcoholic fatty liver disease
Introduction
The endoplasmic reticulum (ER) is one of the largest cellular organelles, its membranes representing as much as half of the total membranes of the cell. An essential function of the ER is the proper assembly of proteins that are ultimately destined for intracellular organelles and the cell surface [1]. Cellular perturbations such as loss of the luminal oxidizing environment, imbalance in calcium homeostasis and aberrant N-linked glycosylation can disrupt ER homeostasis and lead to the accumulation of unfolded proteins and protein aggregates in the ER lumen. Disruption of ER homeostasis, termed ER stress, activates the UPR [2, 3]. In mammals, ER stress is sensed and the UPR activated by three ER transmembrane proteins, RNA-dependent protein kinase-like ER eukaryotic initiation factor-2α kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring ER-to-nucleus signaling protein 1 (IRE1). Failure of the UPR to mitigate ER stress leads to cell death.
Several studies have linked ER dysfunction and activation of the UPR to impairments in glucose homeostasis and diabetes [4, 5]. ER stress and activation of the UPR in the liver have also been observed in genetic and dietary murine models of obesity, dietary models of non-alcoholic fatty liver disease (NAFLD), and in humans with NAFLD [5–7]. A large portion of the elevated hepatic triglyceride stores in NAFLD appear to arise from re-esterification of circulating free fatty acids [8]. Elevated circulating free fatty acids are not only a characteristic feature of obesity and NAFLD, but are also positively correlated with liver disease severity in individuals with NAFLD [9]. Increased non-esterified fatty acids, in particular long chain saturated fatty acids, induce ER stress and activate the UPR in a number of cell types, including hepatocytes [6, 10–14]. Chemical chaperones, such as 4-phenyl butyric acid and taurine-conjugated ursodeoxycholic acid, reduce ER stress and activation of the UPR in the liver of obese mice and in hepatocytes exposed to elevated free fatty acids [14, 15]. Such data support the notion that excess lipids induce ER stress via mechanisms that involve impairments in protein folding and/or degradative capacity. It is presently unclear how lipids interfere with the functional capacity of the ER to process proteins in hepatocytes. The present study employed H4IIE liver cells and primary rat hepatocytes to examine the hypothesis that saturated fatty acids induce ER stress and cell death via perturbations to ER luminal calcium stores.
Methods
Materials and Reagents
Fatty acids (Sigma Chemical Company, St. Louis, MO) were complexed to bovine serum albumin at a 2:1 molar ratio [16, 17]. Thapsigargin (Sigma; 450 nM), a tumor-promoting sesquiterpene lactone that induces ER stress via inhibition of the ER-associated calcium ATPase [18], was used as a positive control. BAPTA-AM (1,2-bis-(o-aminopenoxy)-ethane-N,N,N’,N’-tetraacetic acid tetraacetoxymethyl ester, a calcium chelator) was purchased from Biomol (Plymouth Meeting, PA).
Cell Culture
H4IIE liver cells (American Type Culture Collection, Manassas, VI), a rat hepatoma cell line, were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM), supplemented with 10% fetal bovine serum, penicillin, and streptomycin sulfate [13, 17]. Control medium, referred to as LG, contained 8 mM glucose. Each independent experiment was performed in triplicate.
In preparation for primary cell culture, hepatocytes were isolated from male, Wistar rats (Charles River Laboratories, Wilmington, MA) by collagenase perfusion [19, 20]. All procedures involving rats were reviewed and approved by the Colorado State University Institutional Animal Care Committee. Cells were first incubated in RPMI (HyClone, Logan, UT) containing 11 mM glucose, 10−7 M dexamethasone, and 10−7 M insulin on Matrigel-coated plates (for RNA) or on collagen-coated plates containing 5% fetal bovine serum (for protein) for 4h (attachment period). The medium was then changed to one containing RPMI, 8 mM glucose, 10−7 M dexamethasone, and 10−8 M insulin. The following morning experimental treatments were performed using RPMI that contained 8 mM glucose and 10−7 M dexamethasone. Each independent experiment was performed in triplicate.
RNA Isolation and Analysis
Total RNA was extracted with TRIzol reagent using the manufacturer’s protocol (Invitrogen, Carlsbad, CA). Real Time PCR was performed following reverse transcription using 0.5 µg of DNAase-treated RNA, Superscript II RnaseH-, and random hexamers. PCR reactions were performed using transcribed cDNA and IQ-SYBR green master mix (Bio Rad, Hecula, CA). Primer sets can be found in a previous publication [13]. PCR efficiency was between 90% and 105% for all primer and probe sets and linear over 5 orders of magnitude. The specificity of products generated for each set of primers was examined for each fragment using a melting curve and gel electrophoresis. Reactions were run in triplicate and data calculated as the change in cycle threshold (ΔCT) for the target gene relative to the ΔCT for β2-microglobulin and cyclophilin (control genes) according to the procedures of Muller et al [21]. Results were similar regardless of the control gene used and data in the results section are reported using β2-microglobulin.
Western Blot Analysis
Western blot analysis was performed as described in detail previously [13, 22]. Membranes were incubated with antibodies against glucose regulated protein 78 (GRP78; Stressgen, Victoria BC, Canada), CCAAT/enhancer binding protein homologous protein (CHOP; Santa Cruz Biotechnology, Santa Cruz, CA), and actin (Sigma). Proteins were detected with horseradish peroxidase-conjugated secondary antibodies (Amersham Pharmacia Biotech, Piscataway, NJ) and an enhanced chemiluminescence reagent (Pierce, Rockford, IL). Density was quantified using a UVP Bioimaging system (Upland, CA).
Cytochrome c assay
Cells were harvested in PBS and collected by centrifugation at 600 × g for5 min. Cells were resuspended in a hypotonic buffer (10 mM Hepes, pH=7.8, 1 mM EGTA, 25 mM KCL) and incubated for 20 min on ice. Cell suspensions were centrifuged at 600 × g for 5min, the pellets resuspended in an isotonic buffer (hypotonic buffer containing 250 mM sucrose) and homogenized. Homogenates were centrifuged at 1000 × g for 10 min at 4C to remove cell debris, and the supernatants centrifuged at 16,000 × g for 20 min at 4C to pellet mitochondria and to obtain a post-mitochondrial supernatant fraction. Western blot analysis of cytochrome C oxidase IV and copper zinc superoxide dismutase (Abcam, Cambridge, MA) was used to confirm the purity of the post-mitochondrial supernatant fraction.
A cytochrome c ELISA kit (Invitrogen, Carlsbad, CA) was used to estimate cytochrome c protein content in the post-mitochondrial supernatant fraction. Measurements were performed in duplicate and cytochrome c content was analyzed at 450 nm and the optical density normalized to total protein content.
Cell Viability Analysis
Cell death was evaluated using the Cell Death Detection ELISA kit (Roche Diagnostics, Penzberg, Germany). This assay is based on the quantitative sandwich-enzyme immunoassay-principle using mouse monoclonal antibodies directed against DNA and histones. Cell survival was evaluated using 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays (Promega Inc., Madison, WI) based on the supplier’s protocol. Caspase-3 activity was determined with the Colorimetric Caspase-3 Activation Assay, which uses a caspase-specific peptide conjugated to the color reporter p-nitroanaline (R&D Systems, Minneapolis, MN).
Calcium Measurements
Lipid-induced depletion of thapsigargin-sensitive calcium stores was assessed using the Fluo-4 AM NW calcium assay kit (Molecular Probes, Eugene, OR). In brief, following incubation of cells with various fatty acids, cells were loaded with Fluo-4 AM in the presence of probenicid (4-dipropylamino-sulfonyl benzoic acid). Thapsigargin (450 nM) or vehicle (DMSO) was added and fluorescence (494 nm excitation; 516 nm emission) was measured over a 5 minute period. Data are expressed as fluorescence units in vehicle-treated cells – fluorescence units in thapsigargin-treated cells.
Data Analysis and Statistics
Statistical comparisons were calculated using analysis of variance and post-hoc comparisons among means using the Scheffe’s or Tukey’s test. Statistical significance was set at p<0.05. All data are reported as the means ± SDEV.
Results
Palmitate activates the UPR and induces cell death in liver cells
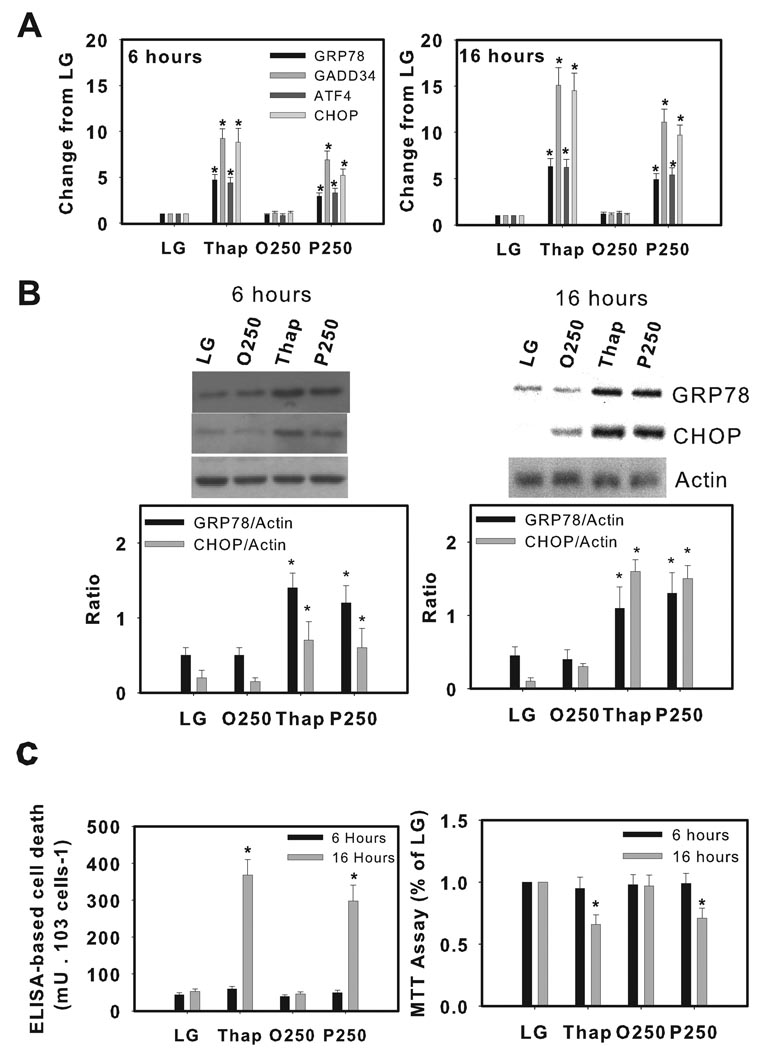
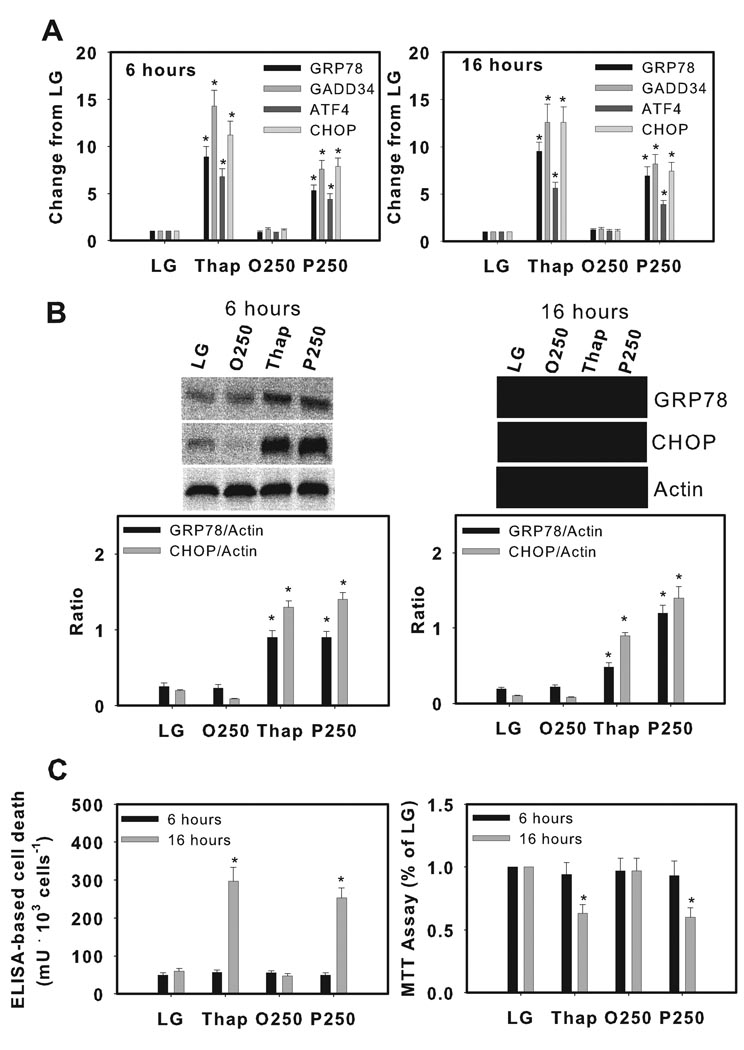
As previously demonstrated [13, 23], incubation of H4IIE liver cells with palmitate or thapsigargin for 6 or 16h increased biochemical markers of ER stress (Fig. 1A-B). In contrast, increased cell death (Fig. 1C) and reduced cell viability (Fig. 1C) were observed at 16h only. Oleate had no effect on any of these parameters (Fig. 1A–C). Palmitate or thapsigargin also increased biochemical markers of ER stress after 6 and 16h incubations in primary hepatocytes, however, cell death, reduced cell viability and increased caspase-3 activity were only observed after 16h incubations (Fig. 2A–C, Supplemental Fig. 1A).
Figure 1.
Induction of ER stress and cell death by thapsigargin and palmitate in H4IIE liver cells. H4IIE liver cells were incubated for 6 or 16 hours in control media (LG), or control media supplemented with thapsigargin (Thap, 450 nM), oleate (O, 250 µM), or palmitate (P, 250 µM).(A) Real Time PCR analysis of glucose regulated protein 78 (GRP78), growth arrest and DNA damage-inducible gene 34 (GADD34), activating transcription factor 4 (ATF4) and CCAAT/enhancer binding protein homologous protein (CHOP). Data from LG were set to 1. (B) Western blot analysis of GRP78, CHOP and actin (loading control) proteins. The gels shown are representative of five independent experiments and data in graphs are expressed as the ratio of the target protein to actin. (C) ELISA-based cell death and MTT viability assay. Data in graphs are reported as the mean ± SD of triplicate samples from 5 independent experiments. *, significantly (p<0.05) different from LG and O250.
Figure 2.
Induction of ER stress and cell death by thapsigargin and palmitate in primary hepatocytes. Rat primary hepatocytes were incubated for 6 or 16 hours in control media (LG), or control media supplemented with thapsigargin (Thap, 450 nM), oleate (O, 250 µM), or palmitate (P, 250 µM). (A) Real Time PCR analysis of glucose regulated protein 78 (GRP78), growth arrest and DNA damage-inducible gene 34 (GADD34), activating transcription factor 4 (ATF4) and CCAAT/enhancer binding protein homologous protein (CHOP). Data from LG were set to 1. (B) Western blot analysis of GRP78, CHOP and actin (loading control) proteins. The gels shown are representative of five independent experiments and data in graphs are expressed as the ratio of the target protein to actin. (C) ELISA-based cell death and MTT viability assay. Data in graphs are reported as the mean ± SD of triplicate samples from 5 independent experiments. *, significantly (p<0.05) different from LG and O250.
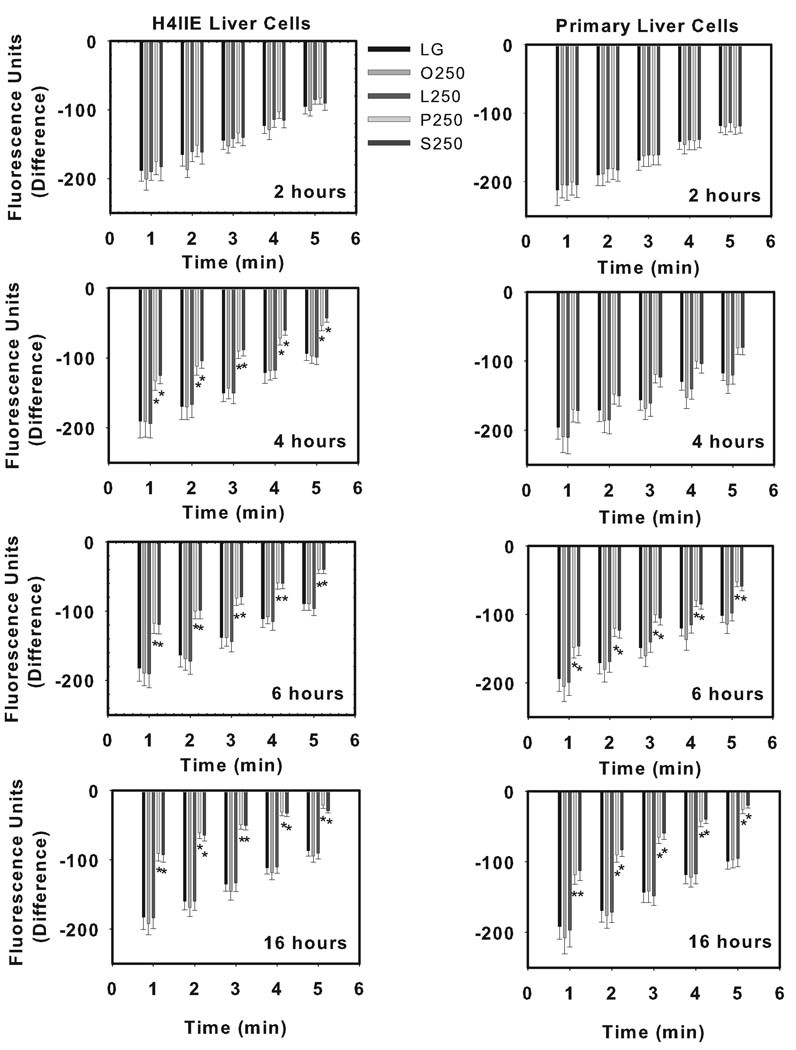
Long chain saturated fatty acids reduce thapsigargin-sensitive calcium stores
To examine whether fatty acids influence ER calcium stores, H4IIE liver cells and primary hepatocytes were incubated in the presence of various individual fatty acids for varying durations (2, 4, 6, or 16 h). In contrast to oleate and linoleate, which had no effect on thapsigargin-sensitive calcium stores, palmitate and stearate reduced thapsigargin-sensitive calcium stores in both H4IIE and primary liver cells (Fig. 3). A significant reduction in thapsigargin-sensitive calcium stores was observed following incubations lasting 4 h in H4IIE liver cells and 6 h in primary hepatocytes (Fig. 3).
Figure 3.
Fatty acid-mediated effects on thapsigargin-sensitive calcium stores. H4IIE liver cells (left side) or primary rat hepatocytes (right side) were incubated for 2, 4, 6 or 16 hours in control media (LG), or control media supplemented with oleate (O, 250 µM), linoleate (L, 250 µM), palmitate (P, 250 µM) or stearate (S, 250 µM). Following incorporation of Fluo-4 AM, cells were provided vehicle or thapsigargin (450 nM) and fluorescence was measured over a 5 minute period. Data are expressed as the difference in fluorescence units (fluorescence units in vehicle-treated cells – fluorescence units in thapsigargin-treated cells) and are reported as the mean ± SD of triplicate samples from 5–7 independent experiments. *, significantly (p<0.05) different from LG.
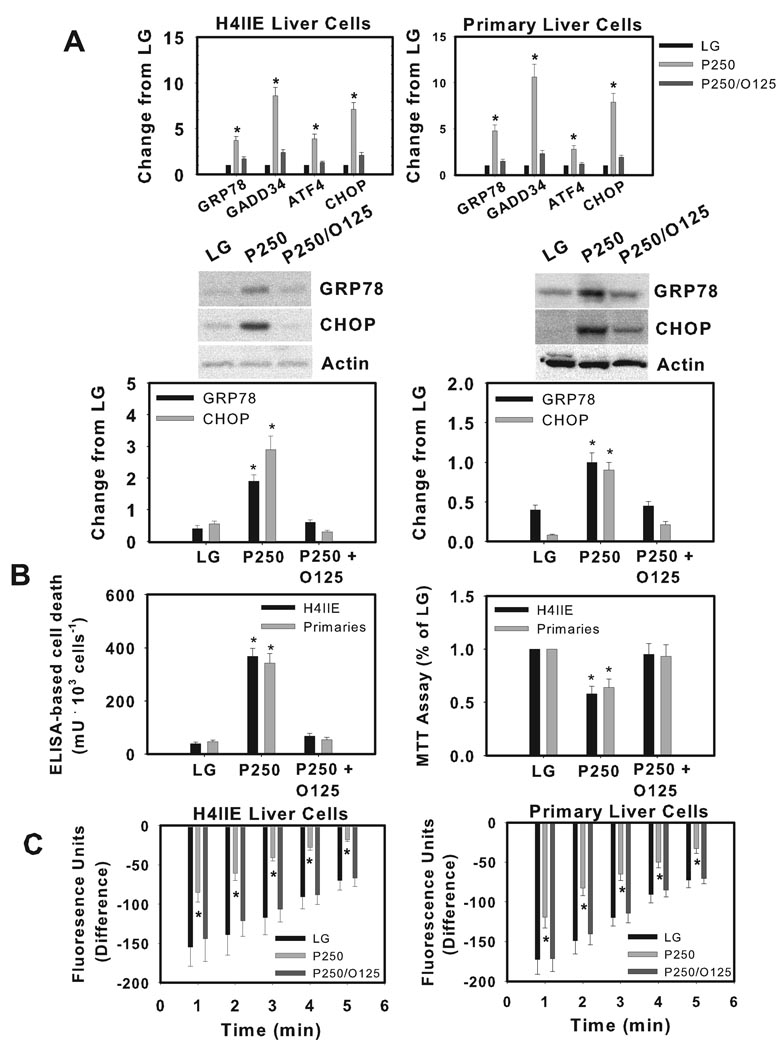
Co-incubation of palmitate with oleate prevents ER stress, cell death, and the reduction in thapsigargin-sensitive calcium stores
Co-incubation of palmitate (250 uM) and oleate (125 uM) prevented ER stress and activation of the UPR (Fig. 4A, including XBP1 splicing data not shown), prevented the increased cell death and reduced cell viability (Fig. 4B), and normalized thapsigargin-sensitive calcium stores (Fig. 4C) in both H4IIE and primary liver cells. In addition, co-incubation of palmitate and oleate reduced caspase-3 activity (Supplemental Fig. 1B).
Figure 4.
The effects of palmitate and oleate co-incubation on markers of ER stress, cell death and thapsigargin-sensitive calcium stores in H4IIE liver cells and primary hepatocytes. H4IIE liver cells or primary rat hepatocytes were incubated for 16 hours in control media (LG), or control media supplemented with palmitate (P, 250 µM) or palmitate (P, 250 µM) + oleate (O, 125 µM). (A) Real Time PCR analysis of GRP78, GADD34, ATF4 and CHOP mRNA. Data from LG were set to 1. Western blot analysis of GRP78, CHOP and actin (loading control) proteins. The gels shown are representative of five independent experiments and data in graphs are expressed as the ratio of the target protein to actin. (B) ELISA-based cell death and MTT viability assay. (C) Following incorporation of Fluo-4 AM, cells were provided vehicle or thapsigargin (450 nM) and fluorescence was measured over a 5 minute period. Data are expressed as the difference in fluorescence units (fluorescence units in vehicle-treated cells – fluorescence units in thapsigargin-treated cells). Data in graphs are reported as the mean ± SD of triplicate samples from 5–6 independent experiments. *, significantly (p<0.05) different from LG-.
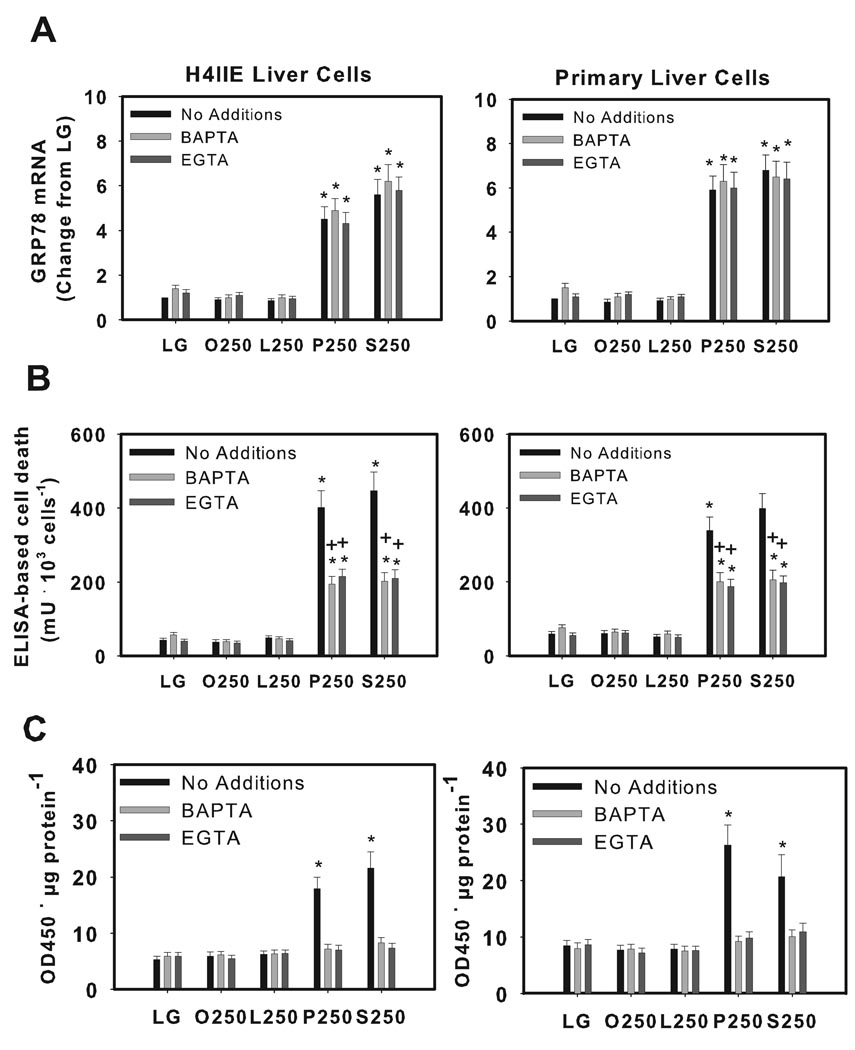
Redistribution of cellular calcium partially mediates palmitate-induced cell death via effects on mitochondria
BAPTA-AM or EGTA had no effect on biochemical markers of ER stress (Fig. 5A, supplemental Fig. 2A, including XBP1 splicing data not shown) but reduced cell death (Fig. 5B), increased cell viability (supplemental Fig. 2B), and prevented the appearance of cytochrome c in post-mitochondrial supernatant fractions (Fig. 5C). In addition, BAPTA-AM and EGTA reduced caspase-3 activity (Supplemental Fig. 1C).
Figure 5.
The effects of calcium chelators on fatty acid-mediated induction of ER stress, cell death and cytochrome release in H4IIE liver cells and primary hepatocytes. H4IIE liver cells or primary rat hepatocytes were incubated for 16 hours in control media (LG), or control media supplemented with oleate (O, 250 µM), linoleate (L, 250 µM), palmitate (P, 250 µM) or stearate (S, 250 µM) in the absence (no additions) or presence of BAPTA-AM (20 µM) or EGTA (1 mM). (A) Real time PCR analysis of GRP78 mRNA where LG (no additions) was set to 1. (B) ELISA-based cell death. (C) ELISA-based assay for cytochrome c protein in post-mitochondrial supernatant fractions. Data are reported as the mean ± SD for triplicate samples from 4–6 independent experiments. *, significantly (p<0.05) different from LG-. +, significantly (p<0.05) different from No Additions of the same treatment group.
Discussion
The delivery and accumulation of lipids in non-adipose tissues leads to cellular dysfunction and death. This phenomenon, termed lipotoxicity, has been implicated in the pathogenesis of diabetes, cardiac failure and NAFLD [5, 12, 24, 25]. Disruption of ER homeostasis and activation of the UPR has been observed in murine models of obesity, cardiac dysfunction and NAFLD [5, 6, 24]. Increased free fatty acids, in particular long chain saturated fatty acids, induce ER stress, activate the UPR and promote cell death in a number of cell types, including hepatocytes [11–13, 26, 27]. Thus, impairments in ER function appear to contribute to the pathogenesis of several diseases and to cellular impairments associated with lipotoxicity. The present study was undertaken to begin to examine the mechanisms that link saturated fatty acids to ER stress, UPR activation, and cell death in hepatocytes. Results demonstrate that 1) palmitate and stearate decrease thapsigargin-sensitive calcium stores with a time course coincident with UPR activation but that precedes cell death and 2) the presence of calcium chelators reduces, but does not prevent, palmitate- and stearate-mediated cell death.
Previous studies suggest that relatively high concentrations of palmitate perturb ER structure and function. Karaskov et al demonstrated, in INS-1 pancreatic β-cells, that palmitate (0.5 – 1 mM) altered the distribution of GRP78 from a reticular ER localization to a punctate/aggregated distribution, similar to that caused by thapsigargin [28]. Borradaile et al demonstrated, in CHO cells and H9c2 cardiomyocytes, that palmitate (0.5 mM) increased the saturation of ER membranes, induced ER dilatation, and reduced luminal calcium [12]. Recent work has suggested that palmitate, and to a lesser extent oleate, induce calcium release from the ER lumen with a time course that mirrors the appearance of ER stress and cell death in human β-cells and MIN6 cells [29]. The present study extends these observations by demonstrating that palmitate or stearate, at concentrations relevant to in vivo conditions [30], reduced thapsigargin-sensitive calcium stores in both immortalized and primary liver cells. Importantly, saturated fatty acid-mediated reductions in calcium stores occurred over a time course consistent with the upregulation of the UPR and prior to the induction of cell death [17]. Since both the oxidation state and concentration of calcium in the ER lumen are critical determinants of polypeptide folding and chaperone function [31, 32], these data are consistent with the notion that the ER is an early and proximal target of fatty acid overload in hepatocytes. It is hypothesized that the reduction ER luminal calcium stores provokes ER stress and activates the UPR via mechanisms that include a reduction in endogenous protein chaperone function. This hypothesis is consistent with previous studies in which supplementation with chemical chaperones reduced ER stress in both murine models of obesity and in liver cells exposed to increased lipid concentrations [14, 15].
The induction of ER stress and cell death by long-chain saturated fatty acids, such as stearate and palmitate, can be reduced or prevented by co-incubation with long chain mono- or poly-unsaturated fatty acids in several cell types [13, 33]. This may be due, in part, to alterations in the trafficking of saturated fatty acids away from the ER membrane in the presence of unsaturated fatty acids [33]. To further ascertain the functional role of redistribution of ER luminal calcium in hepatocytes we examined the effects of palmitate and oleate co-incubations of thapsigargin-sensitive calcium stores. The data demonstrate that co-incubation of palmitate and oleate not only reduces palmitate-mediated ER stress and cell death, but also prevents reductions in thapsigargin-sensitive calcium stores.
Elegant studies have demonstrated that saturated fatty acid-mediated cell death in hepatocytes involves activation of FoxO3a, c-Jun terminal kinase (JNK), and lysosome destabilization with subsequent activation of the intrinsic apoptotic pathway [34–36]. The intrinsic apoptotic pathway, involves mitochondrial permeabilization and release of cytochrome c from mitochondria, events that can be triggered by mitochondrial calcium influx [37, 38]. In the present study we sought to determine whether the reduction in thapsigargin-sensitive calcium stores contributed to saturated fatty acid-mediated cell death, and if so, whether this involved release of cytochrome c. We employed two calcium chelators, BAPTA-AM and EGTA, based on the assumption that these agents would effectively reduce cytosolic calcium levels elevated by saturated fatty acids. The presence of either BAPTA-AM or EGTA reduced, but did not prevent, palmitate- and stearate-mediated cell death. Moreover, the presence of either calcium chelator blocked the appearance of cytochrome c in post-mitochondrial supernatant factions observed following incubations with palmitate or stearate. Thus, these data suggest that long chain saturated fatty acids provoke cell death and reduce cell viability, in part, via calcium-mediated mitochondrial cytochrome c release and, presumably, activation of the intrinsic apoptotic pathway. The present data are consistent with a previous study, performed in rat neonatal cardiomyocytes, in which palmitate-induced apoptosis involved release of cytochrome c from the inner mitochondrial membrane [39]. It must, however, be emphasized that a) saturated fatty acid-mediated changes in cellular calcium stores is only one, among many factors (e.g. JNK, lysosomal destabilization), that contribute to cell death, and b) further work is necessary to define the precise role of ER calcium in lipid-mediated ER stress and cell death.
Lipotoxicity occurs in a number of cell types and tissues and appears to be an important mechanism for progression of a number of disease states [24, 34, 40]. The mechanisms that determine and regulate this process are complex and redundant. The present study has demonstrated that in liver cells, lipid-mediated disturbance of cellular calcium stores are linked to ER stress, UPR activation and cell death.
Supplementary Material
(A) Caspase-3 activity in H4IIE liver cells or primary hepatocytes incubated for 6 or 16 hours in control media (LG), or control media supplemented with thapsigargin (Thap, 450 nM), oleate (O, 250 µM), or palmitate (P, 250 µM). (B) Caspase-3 activity in H4IIE liver cells or primary rat hepatocytes incubated for 16 hours in control media (LG), or control media supplemented with palmitate (P, 250 µM) or palmitate (P, 250 µM) + oleate (O, 125 µM). (C) Caspase-3 activity in H4IIE liver cells or primary rat hepatocytes incubated for 16 hours in control media (LG), or control media supplemented with oleate (O, 250 µM), linoleate (L, 250 µM), palmitate (P, 250 µM) or stearate (S, 250 µM) in the absence (no additions) or presence of BAPTA-AM (20 µM) or EGTA (1 mM). Data are reported as the mean ± SD for triplicate samples from 4–6 independent experiments. *, significantly (p<0.05) different from LG-. +, significantly (p<0.05) different from No Additions of the same treatment group.
Acknowledgments
This work was supported by grants DK47416 and DK072017 from the National Institutes of Health and the Lillian Fountain Smith Foundation Endowment.
References
- 1.Pahl HL. Signal transduction from the endoplasmic reticulum to the cell nucleus. Physiol Rev. 1999;79:683–701. doi: 10.1152/physrev.1999.79.3.683. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum-stress mediated diabetes. J Clin Invest. 2002;109:525–532. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozcan U, Cao Q, Yilmaz E, Lee A-H, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun CZ, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147:943–951. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 7.Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, Sanyal AJ. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134:568–576. doi: 10.1053/j.gastro.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nehra V, Angulo P, Buchman AL, Lindor KD. Nutritional and metabolic considerations in the etiology of nonalcoholic steatohepatitis. Dig Dis Sci. 2001;46:2347–2352. doi: 10.1023/a:1012338828418. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Jhaveri R, Huang J, Qi Y, Diehl AM. Endoplasmic reticulum stress, hepatocytes CD1d and NKT cell abnormalities in murine fatty livers. Lab Invest. 2007;87:927–937. doi: 10.1038/labinvest.3700603. [DOI] [PubMed] [Google Scholar]
- 11.Kharroubi I, Ladriere L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic B-cell apoptosis by different mechanisms: Role of nuclear factor-KB and endoplasmic reticulum stress. Endocrinology. 2004;145:5087–5096. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- 12.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 14.Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest. 2008;118:316–332. doi: 10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem. 2001;276:14890–14895. doi: 10.1074/jbc.M010286200. [DOI] [PubMed] [Google Scholar]
- 17.Wei Y, Wang D, Pagliassotti MJ. Saturated fatty acid-mediated endoplasmic reticulum stress and apoptosis are augmented by trans-10, cis-12-conjugated linoleic acid in liver cells. Mol Cell Biochem. 2007;303:105–113. doi: 10.1007/s11010-007-9461-2. [DOI] [PubMed] [Google Scholar]
- 18.Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular calcium stores by specific inhibition of the endoplasmic reticulum calcium ATPase. Proc Natl Acad Sci U S A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berry M, Friend D. High-yield preparation of isolated rat liver parenchymal cells. A biochemical and fine structural study. J Cell Biol. 1969;43:506–519. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D, Wei Y, Schmoll D, Maclean KN, Pagliassotti MJ. Endoplasmic reticulum stress increases glucose-6-phosphatase and glucose cycling in liver cells. Endocrinology. 2006;147:350–358. doi: 10.1210/en.2005-1014. [DOI] [PubMed] [Google Scholar]
- 21.Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative Real-Time RT-PCR. BioTechniques. 2002;32:1372–1379. [PubMed] [Google Scholar]
- 22.Pagliassotti MJ, Kang J, Thresher JS, Sung CK, Bizeau ME. Elevated basal PI 3-kinase activity and reduced insulin signaling in sucrose-induced hepatic insulin resistance. Am J Physiol Endocrinol Metab. 2002;282:E170–E176. doi: 10.1152/ajpendo.2002.282.1.E170. [DOI] [PubMed] [Google Scholar]
- 23.Pagliassotti MJ, Wei Y, Wang D. Insulin Protects Liver Cells from Saturated Fatty Acid-Induced Apoptosis via Inhibition of c-Jun NH2 Terminal Kinase Activity. Endocrinology. 2007;148:3338–3345. doi: 10.1210/en.2006-1710. [DOI] [PubMed] [Google Scholar]
- 24.Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14:281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Clark JM, Diehl A. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA. 2003;289:3000–3004. doi: 10.1001/jama.289.22.3000. [DOI] [PubMed] [Google Scholar]
- 26.Moffitt JH, Fielding BA, Evershed R, Berstan R, Currie JM, Clark A. Adverse physicochemical properties of tripalmitin in beta cells lead to morphological changes and lipotoxicity in vitro. Diabetologia. 2005;48:1819–1829. doi: 10.1007/s00125-005-1861-9. [DOI] [PubMed] [Google Scholar]
- 27.Lai E, Bikopoulos G, Wheeler MB, Rozakis-Adcock M, Volchuk A. Differential activation of ER stress and apoptosis in response to chronically elevated free fatty acids in pancreatic B-cells. Am J Physiol Endocrinol Metab. 2008;294:E540–E550. doi: 10.1152/ajpendo.00478.2007. [DOI] [PubMed] [Google Scholar]
- 28.Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic B-cell apoptosis. Endocrinology. 2006;147:3398–3407. doi: 10.1210/en.2005-1494. [DOI] [PubMed] [Google Scholar]
- 29.Gwiazda KS, Yang TL, Lin Y, Johnson JD. Effects of palmitate on ER and cytosolic CA2+ homeostasis in β-cells. Am J Physiol Endocrinol Metab. 2009;296:E690–E701. doi: 10.1152/ajpendo.90525.2008. [DOI] [PubMed] [Google Scholar]
- 30.Bakan E, Yildirim A, Kurtul N, Polat MF, Dursun H, Cayir K. Effects of type 2 diabetes mellitus on plasma fatty acid composition and cholesterol content of erythrocyte and leukocyte membranes. Acta Diabetol. 2006;8:109–113. doi: 10.1007/s00592-007-0224-4. [DOI] [PubMed] [Google Scholar]
- 31.Burdakov D, Petersen OH, Verkhratsky A. Intraluminal calcium as a primary regulator of endoplasmic reticulum function. Cell Calcium. 2005;38:303–310. doi: 10.1016/j.ceca.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 33.Listenberger LJ, Han X, Lewis SE, Cases S, Farese RV, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 35.Barreyro FJ, Kobayashi S, Bronk SF, Werneburg NW, Malhi H, Gores GJ. Transcriptional regulation of Bim by FoxO3A mediates hepatocyte lipoapoptosis. J Biol Chem. 2007;282:27141–27154. doi: 10.1074/jbc.M704391200. [DOI] [PubMed] [Google Scholar]
- 36.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, Gores GJ. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 37.Harwood SM, Yaqoob MM, Allen DA. Caspase and calpain function in cell death: bridging the gap between apoptosis and necrosis. Ann Clin Biochem. 2005;42:415–431. doi: 10.1258/000456305774538238. [DOI] [PubMed] [Google Scholar]
- 38.Di Sano F, Ferraro E, Tufi R, Achsel T, Piacentini M, Cecconi F. ER stress induces apoptosis by an apoptosome-dependent but caspase 12-independent mechanism. J Biol Chem. 2006;281:2693–2700. doi: 10.1074/jbc.M509110200. [DOI] [PubMed] [Google Scholar]
- 39.Ostrander DB, Sparagna GC, Amoscato AA, McMillin JB, Dowhan W. Decreased cardiolipin synthesis corresponds with cytochrome c release in palmitate-induced cardiomyocyte apoptosis. J Biol Chem. 2001;276:38061–38067. doi: 10.1074/jbc.M107067200. [DOI] [PubMed] [Google Scholar]
- 40.Unger RH, Orci L. Lipoapoptosis: its mechanism and its diseases. Biochim Biophys Acta. 2002;1585:202–212. doi: 10.1016/s1388-1981(02)00342-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Caspase-3 activity in H4IIE liver cells or primary hepatocytes incubated for 6 or 16 hours in control media (LG), or control media supplemented with thapsigargin (Thap, 450 nM), oleate (O, 250 µM), or palmitate (P, 250 µM). (B) Caspase-3 activity in H4IIE liver cells or primary rat hepatocytes incubated for 16 hours in control media (LG), or control media supplemented with palmitate (P, 250 µM) or palmitate (P, 250 µM) + oleate (O, 125 µM). (C) Caspase-3 activity in H4IIE liver cells or primary rat hepatocytes incubated for 16 hours in control media (LG), or control media supplemented with oleate (O, 250 µM), linoleate (L, 250 µM), palmitate (P, 250 µM) or stearate (S, 250 µM) in the absence (no additions) or presence of BAPTA-AM (20 µM) or EGTA (1 mM). Data are reported as the mean ± SD for triplicate samples from 4–6 independent experiments. *, significantly (p<0.05) different from LG-. +, significantly (p<0.05) different from No Additions of the same treatment group.