Abstract
Flooding the intercellular air spaces of leaves with water was shown to cause rapid closure of stomata in Tradescantia pallida, Lactuca serriola, Helianthus annuus, and Oenothera caespitosa. The response occurred when water was injected into the intercellular spaces, vacuum infiltrated into the intercellular spaces, or forced into the intercellular spaces by pressurizing the xylem. Injecting 50 mm KCl or silicone oil into the intercellular spaces also caused stomata to close, but the response was slower than with distilled water. Epidermis-mesophyll grafts for T. pallida were created by placing the epidermis of one leaf onto the exposed mesophyll of another leaf. Stomata in these grafts opened under light but closed rapidly when water was allowed to wick between epidermis and the mesophyll. When epidermis-mesophyll grafts were constructed with a thin hydrophobic filter between the mesophyll and epidermis stomata responded normally to light and CO2. These data, when taken together, suggest that the effect of water on stomata is caused partly by dilution of K+ in the guard cell and partly by the existence of a vapor-phase signal that originates in the mesophyll and causes stomata to open in the light.
Stomatal responses to the environment have been studied in leaves for well over 100 years. More recently, the mechanisms for these responses have been investigated using isolated epidermes or isolated guard cell protoplasts. Despite the combination of these two approaches, the mechanisms by which stomata respond to environmental signals are not well understood. Since stomata control CO2 uptake and water loss from leaves, the responses of stomata to environmental factors are important determinants of terrestrial productivity and water use. It is therefore critical that we understand the mechanisms by which stomata respond to the environment if we are to accurately predict the effects of future climates on productivity and water cycles (Randall et al., 1996).
There are two assumptions about stomata that are implicit in much of the recent literature: (1) that stomatal responses result from sensory mechanisms that reside within the guard cells, and (2) that stomata in isolated epidermes respond similarly to those in a leaf. The exception to this generalization is the stomatal response to humidity, which has been suggested to be the result of changes in guard cell water potential (Dewar, 1995, 2002) or of signaling from other cells in the leaf to the guard cells (Buckley et al., 2003). The assumption that guard cells directly sense CO2 and light is largely based on data from isolated epidermes that show effects of light and CO2 on stomatal apertures. As pointed out by Mott (2009), however, stomatal responses to light and CO2 in isolated epidermes are generally much different from those observed in leaves; e.g. responses in isolated epidermes are generally smaller than those in leaves, opening in response to light is slower, and closing in darkness is rarely observed. These observations were used to suggest that the mesophyll is somehow involved in stomatal responses to red light and CO2. This idea is supported by several recent studies that suggest that guard cells do not respond directly to red light. In the first of these studies it was shown that guard cells in an intact leaf do not show hyperpolarization of the plasma membrane in response to red light if the red light is applied to only the guard cell (Roelfsema et al., 2002). In contrast, blue light applied only to the guard cell does cause hyperpolarization, and red light does cause hyperpolarization if applied to the guard cell and the underlying mesophyll. The second study showed that stomata in albino areas of a leaf do not respond to red light, although they contain chloroplasts and do respond to blue light (Roelfsema et al., 2006). Finally, a third study has shown that isolated epidermes are much more sensitive to light and CO2 when placed in close contact with an exposed mesophyll from a leaf from the same or a different species (Mott et al., 2008). These epidermis-mesophyll grafts showed stomatal responses to light and CO2 that were indistinguishable from those in an intact leaf—a sharp contrast to the behavior of stomata in isolated epidermes that are floating on buffer solutions. In that study, illumination of a single stoma in a leaf using a small-diameter fiber optic did not produce stomatal opening, but opening did occur if several stomata and the underlying mesophyll were illuminated. Furthermore, this treatment actually caused opening of adjacent, but unilluminated, stomata (Mott et al., 2008).
In constructing the epidermis-mesophyll grafts in the study described above (Mott et al., 2008), it was noticed that functional grafts could be produced only if both the mesophyll and the epidermis were blotted completely dry of any free water before placing them together. Although the tissues were apparently still fully hydrated, there was very little free water present (i.e. water not contained within the walls of the leaf cells), and both the mesophyll and epidermis felt and looked dry prior to assembly. In addition, even when free water was blotted away initially, stomata did not open in grafts that ended up with visible water on the epidermis or mesophyll that was caused by condensation during the experiment. These observations suggest that the presence of free water somehow prevented the stomata in the grafts from opening. Assuming that the mechanisms operating in the grafts were similar to those in an intact leaf, this result also suggests that free water may have an effect on stomata in leaves as well. In addition, it seems possible that the effect of free water on stomata could be related to the disruption of the signal from the mesophyll that was proposed in an earlier study (Mott et al., 2008). We hypothesize that disruption of this signal could be caused by (1) dilution of some solute that is necessary for opening (such as K+) in the guard cell walls, (2) dilution of an apoplastic, liquid-phase opening signal from the mesophyll to the guard cells, and (3) blockage of a vapor-phase opening signal from the mesophyll to the guard cells. This study was initiated to test these three hypotheses by examining the effect of free water and other liquids on stomatal functioning.
RESULTS
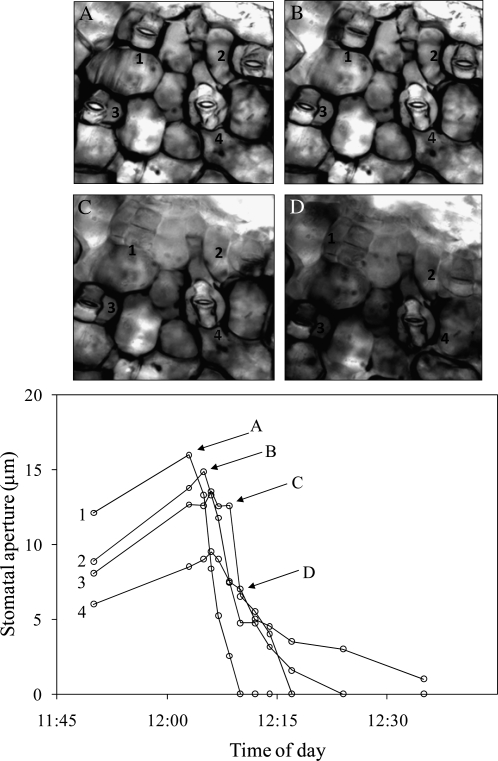
To verify the original observation that stomata do not open in the presence of free water in epidermis-mesophyll grafts from Tradescantia pallida, we constructed grafts and allowed stomata to open until apertures were stable. The effect of free water was then investigated by adding water to the edge of graft. This water then wicked across the space between the epidermis and the mesophyll. The movement of the water along the underside of the epidermis was clearly visible through the microscope by a change in the appearance of the stomata caused by the disappearance of air-water interfaces. As the water moved between the epidermis and mesophyll it caused stomatal apertures to close rapidly, and the closure of each stoma was correlated with the arrival of free water (Fig. 1).
Figure 1.
Stomatal response in an isolated epidermis of T. pallida grafted to an exposed mesophyll of T. pallida after distilled water was added to the edge of the epidermis. Water spread between the mesophyll and epidermis by capillary action over the course of several minutes. Areas with water between the epidermis and mesophyll can be identified by the difference in contrast associated with the loss of air-water interfaces. Water is first visible in the top left corner of image A and spreads from left to right through subsequent images. White circles represent individual stomata on a single grafted epidermis and correspond to numbers in pictures; lettered arrows correspond to images. The experiment was repeated five times with similar results.
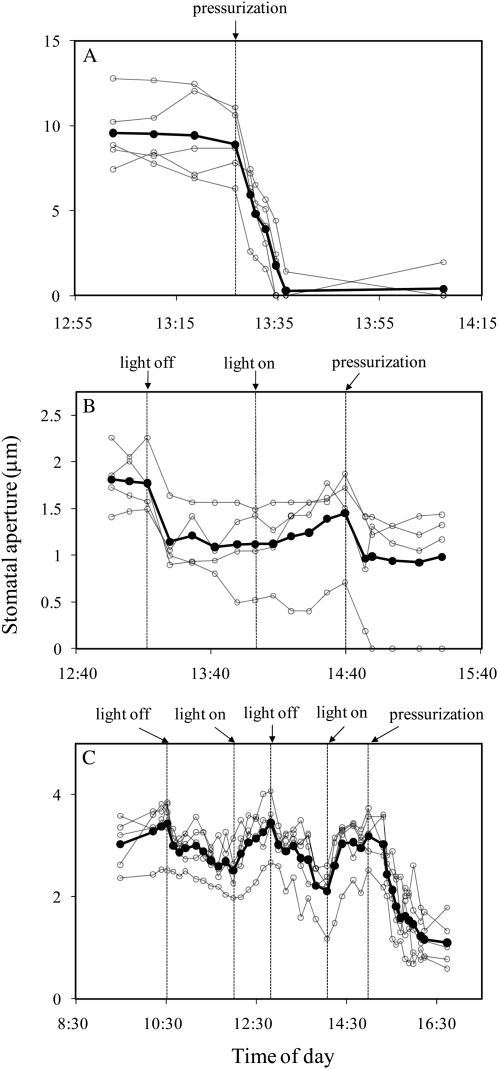
To test the effect of free water on stomata in intact leaves, a pressure chamber was used to pressurize the xylem and flood the mesophyll of detached leaves that were enclosed in a controlled environment chamber. Petioles of detached T. pallida, Helianthus annuus, and Oenothera caespitosa leaves were placed in water and sealed into a pressure chamber as described in “Materials and Methods” section. When stomatal apertures were constant, pressure on the petiole was increased from 0 to 0.5 MPa, causing water to flood the intercellular spaces of the leaf. The flooding of the intercellular spaces took several minutes and was easy to detect visually both macroscopically and microscopically because of changes in the refractive properties of the leaf. In experiments using T. pallida, stomata closed completely in response to infiltration and showed minimal reopening 30 min after pressure was reduced to ambient levels (Fig. 2A). For H. annuus and O. caespitosa, stomata responded to infiltration by closing to 40% to 60% of their original apertures (Fig. 2, B and C). For these species a dark-response experiment was conducted prior to each infiltration to compare the stomatal response to darkness to that of the infiltration response. For each species, the closing response to pressure infiltration was equal to or greater than that observed in response to darkness.
Figure 2.
Stomatal responses to pressure-induced flooding of the intercellular spaces of leaves of T. pallida (A), H. annuus (B), and O. caespitosa (C). White circles represent individual stomata on a single leaf; black symbols represent the average of those observed. In B and C, the effect of darkness on stomatal aperture was tested before pressurization. Each section shows results from a single experiment; each experiment was repeated at least once with similar results.
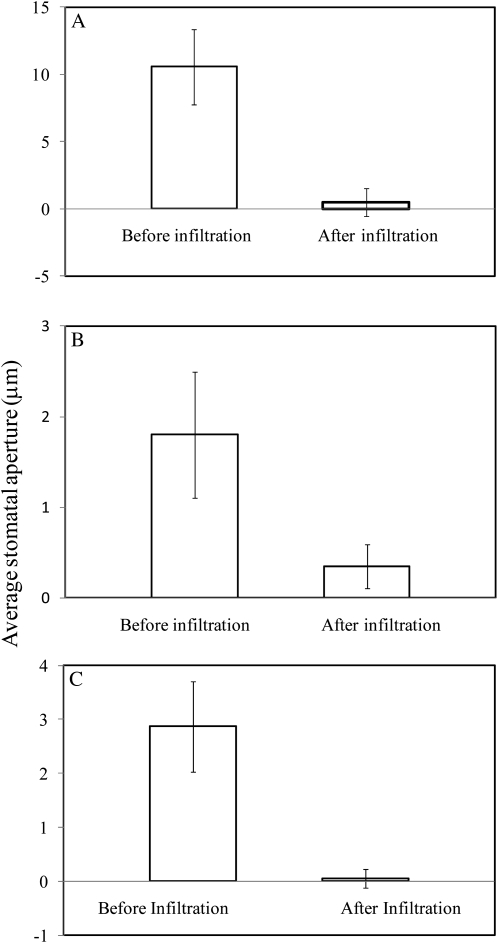
Additional evidence for the effects of flooding the intercellular spaces was gained by vacuum infiltrating water into the intercellular spaces of T. pallida, Lactuca serriola, and O. caespitosa (Fig. 3). Leaves were enclosed in the controlled environment chamber and observed until stomatal apertures were constant. The part of the leaf in the chamber was then excised and vacuum infiltrated as described in “Materials and Methods” sections, and then returned to the chamber. In all experiments and for all species, stomata closed by at least 80% of their original values after water was infiltrated into the intercellular spaces, and for T. palida, stomata closed by 98% of their original values. Stomata had finished closing by the time the leaf material could be returned to the controlled environment chamber (<2 min), so the kinetics of closure could not be determined. Control experiments were also conducted in which the leaf was subjected to the vacuum treatment with no water present in the syringe. In these experiments there was no stomatal response to the vacuum procedure (data not shown).
Figure 3.
Stomatal responses to vacuum-induced flooding of the intercellular spaces with distilled water in leaves of T. pallida (A), L. serriola (B), and O. caespitosa (C). Bars show the average of 25 stomata, and error bars show 1 sd on either side of the mean. Results are for a single experiment; each experiment was repeated at least once with similar results.
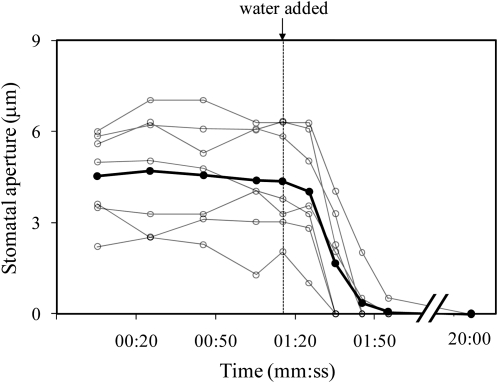
Stomata also closed when the intercellular spaces of the mesophyll were flooded with water by directly injecting water through the epidermis. Using a sharp micropipette, the lower epidermis of an intact T. pallida leaf was pierced at approximately a 45° angle. After approximately 10 min (to be sure that the insertion of the micropipette did not cause closure), enough distilled water was injected into the leaf to fill the intercellular spaces over an area approximately 5 mm in diameter. All stomata in the field of view (which was completely flooded) closed within 2 min of the water injection (Fig. 4). Although these stomata remained closed for at least 5 h after injection, they opened in response to light and appeared fully responsive to changes in light the next day (approximately 16 h later; data not shown).
Figure 4.
Stomatal response when distilled water was microinjected into the intercellular air spaces of an intact T. pallida leaf with a glass micropipette. White circles represent individual stomata on a single leaf; black symbols represent their average. This experiment was repeated five times with similar results.
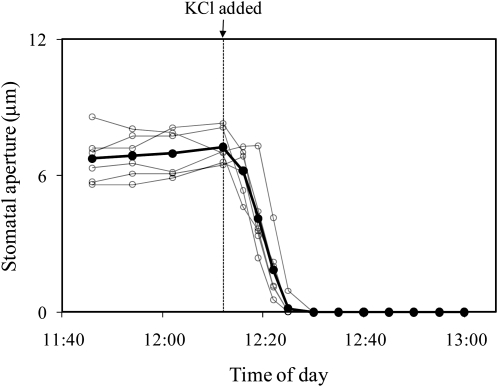
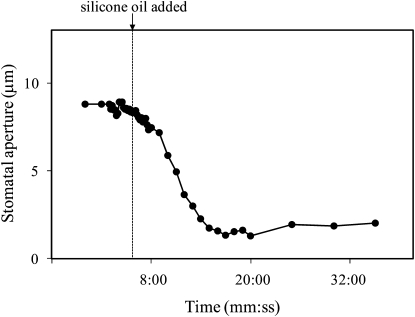
To determine if stomatal closure in response to distilled water was caused by dilution of K+ in the guard cell walls, 50 mm KCl was injected into the intercellular spaces instead of distilled water. Stomata closed in response to injection of 50 mm KCl, but the response was slower than the response to distilled water (Fig. 5). To determine whether the closure of stomata observed with distilled water was caused by wetting of the cell walls or by the blockage of the intercellular spaces, we injected silicon oil into the intercellular spaces using a micropipette. Stomata closed in response to flooding with silicone oil, but as with the 50 mm KCl, the response was slower than the response to distilled water (Fig. 6).
Figure 5.
Stomatal response to a 50 mm KCl solution that was microinjected into the intercellular air spaces of a T. pallida leaf. White circles represent individual stomata on a single leaf; black symbols represent their average. Results are for a single experiment; the experiment was repeated twice with similar results.
Figure 6.
Stomatal response to silicone oil that was microinjected into the intercellular air spaces of a T. pallida leaf. Data show the aperture of a single stoma that was imaged with a confocal microscope. The experiment was repeated three times on different leaves with similar results.
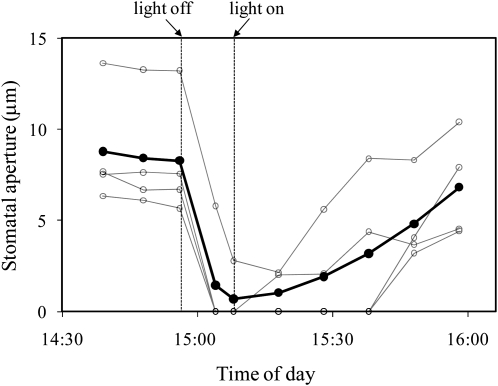
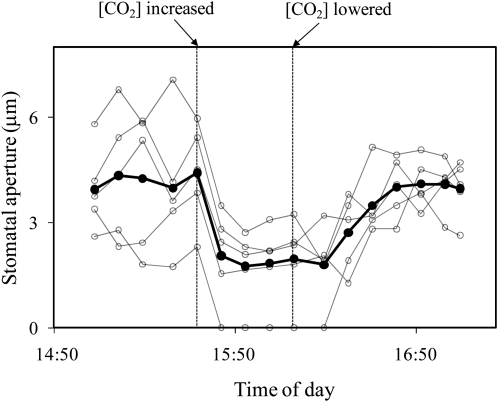
To test the idea of a vapor-phase signal between the epidermis and the mesophyll, experiments were conducted with epidermis-mesophyll grafts of T. pallida in which several types of material were placed between the mesophyll and the epidermis. As a control experiment, grafts were prepared in which the epidermis and mesophyll were separated with low-density polyethylene plastic wrap to block liquid- or vapor-phase signals. Stomata did not open in these experiments (data not shown). To test for a vapor-phase signal and exclude the possibility of a liquid-phase signal, grafts were separated by a thin (approximately 0.25 mm), air permeable, hydrophobic filter (acrylic copolymer membrane cast on a nonwoven nylon support, Versapore-3000 filter, Pall Corporation). Stomata in these grafts opened and reached a stable aperture within about an hour. When photon flux density (PFD) was then changed to 0, stomata closed to 0% to 10% of their original aperture within 10 to 20 min (Fig. 7). In these experiments, grafts were left in darkness only long enough to see a clear response because grafts left in darkness for longer periods of time eventually closed completely and would often not reopen upon illumination (results not shown). However, if the PFD was returned to 350 mmol m−2 s−1 within 30 min, stomata reopened to within 50% to 100% of their original apertures (Fig. 7). Stomata in these experiments were also sensitive to CO2, but increasing the ambient [CO2] from 120 to 540 μmol mol−1 did not close stomata completely (Fig. 8). At the conclusion of the experiments, the filter material between the epidermis and mesophyll appeared dry and was weighed using an analytical balance for fresh, dry, and saturated weights. These were used to calculate relative water content (RWC) of the filter. It was found that RWC of the filters was always below 50%, and usually below 10%, for all five experiments in which stomata opened and were responsive to light. Although the filter material was hydrophobic, it was possible to wet it by prolonged contact with liquid water. To confirm that stomata would not respond to light if the filter was saturated with water, grafts of T. pallida were created with saturated filters between the mesophyll and epidermes. In these experiments the filter remained at 100% RWC throughout the experiment. This experiment was repeated 15 times and in no case did stomata open in response to illumination.
Figure 7.
Stomatal response to light in an isolated epidermis of T. pallida on an exposed mesophyll of T. pallida with Versapor-3000 filter between the mesophyll and epidermis. White circles represent individual stomata on a single grafted epidermis with filter material between the mesophyll and the epidermis; black symbols represent their average. Light intensity was changed from PFD = 340 μmol m−2 s−1 to darkness as indicated. Data are for a single experiment; the experiment was repeated four times with similar results.
Figure 8.
Stomatal response to CO2 in an isolated epidermis of T. pallida on an exposed mesophyll of T. pallida with Versapor-3000 filter between the mesophyll and epidermis. White circles represent individual stomata on a single grafted epidermis with filter between mesophyll and epidermis; black symbols represent their average. [CO2] was changed from 120 to 540 μmol mol−1 as indicated. Data are for a single experiment; the experiment was repeated four times with similar results.
Several other types of filter material were also tested between the epidermis and mesophyll. Stomata did open, and were light responsive, in some grafts constructed with filter paper (Whatman No. 1) or a nylon mesh filter (thickness 70 μm, Spectra/Mesh; data not shown). However, it was difficult to keep these filter materials dry while maintaining hydration of the mesophyll and epidermis. For this reason extensive experiments were not conducted with these materials.
DISCUSSION
The data presented in this study show that infiltration of the intercellular spaces of a leaf with water causes rapid closure of stomata. It seems unlikely that this closure was caused by damage to the mesophyll or guard cells because stomata opened and responded normally to light and CO2 the next day when the water had cleared from the intercellular spaces. Because water flooded the entire mesophyll as well as the substomatal chamber, it was impossible to determine whether stomata closed in response to the flooding of the mesophyll or flooding of the guard cells. It is, however, noteworthy that each individual stoma did not close until it (and the mesophyll directly below it) was flooded. This result suggests that the effect was caused by a local mechanism rather than a general, leaf-wide signal. The effect was demonstrated with a variety of techniques and in four species; it therefore seems probable that it is a common feature of many, and perhaps most, stomata. Yet, to our knowledge, it has apparently never been reported in the literature prior to this study. Flooding of the mesophyll was shown to reduce PSII photon yield, but the effect was attributed to the increase in diffusive resistance to CO2 (Feild et al., 2005). Deleterious effects of leaf wetting have been reported previously, but these have been attributed to blockage of CO2 diffusion by water or reductions in photosynthetic enzymes (Smith and McClean, 1989; Hanba et al., 2004). The exception to this is a study in which wetting leaves with mist was shown to cause rapid stomatal closure (Ishibasha and Terashima, 1995), and it seems possible that the flooding response reported here might be related to that effect.
The rapid closure of stomata in response to flooding of the intercellular spaces with water suggests that in a normally functioning leaf, apoplastic water may be confined to the interstitial spaces of the cell walls rather than existing as a film over the surfaces of the cell walls or filling the intercellular spaces. This conclusion is consistent with the fact that if the osmotic potential of the apoplastic water is near zero, then negative values of water potential for the apoplastic water must involve the retreat of water menisci into the interstitial spaces of the wall (Tyree and Jarvis, 1982). The finding that stomata do not open when the intercellular spaces are flooded with water is surprising since most studies with isolated epidermes are done by floating epidermes on KCl solutions. As noted in a previous study, however, isolated epidermes typically show reduced responses to light and CO2 in comparison with stomata in an intact leaf (Mott, 2009). Based on the data from this study, we suggest that the mechanism for opening and the physiological state of the guard cells in isolated epidermes floating in KCl solutions must be quite different from those of guard cells in intact leaves.
There are many possible reasons for the observed closure of stomata in response to intercellular flooding. Three plausible mechanisms, which are not mutually exclusive, are considered here. They are: (1) dilution of some solute that is necessary for opening (such as K+) in the guard cell walls, (2) dilution of an apoplastic, liquid-phase opening signal from the mesophyll to the guard cells, and (3) blockage of a vapor-phase opening signal from the mesophyll to the guard cells. These hypotheses were tested by flooding the intercellular spaces with liquids other than pure water. A 50 mm KCl solution was used to test for dilution of K+. If the effect of flooding with distilled water was caused by the dilution of K+ in the guard cell walls, then flooding with 50 mm KCl should have caused less stomatal closure than distilled water. The result of this experiment—that stomata closed completely in response to 50 mm KCl, but they closed more slowly than with distilled water—is difficult to interpret. One interpretation is that part, but not all, of the flooding effect was caused by dilution of K+. The remainder of the effect would then have been caused by blocking a signal from the mesophyll. This interpretation is supported by experiments in which the intercellular spaces were flooded with silicone oil, as discussed below.
Because the silicone oil was hydrophobic and had a low viscosity, it spread rapidly inside the leaf and presumably coated the cell walls of the mesophyll and epidermal cells. This assumption is supported by the large change in the visual appearance of the leaf—both microscopically and macroscopically—when the intercellular spaces were flooded with the silicone oil. A similar visual change occurred with water, but the loss of light refraction in the leaf with silicone oil was so severe that it was impossible to accurately measure stomatal apertures using standard light microscopy, and it was necessary to use confocal microscopy to measure apertures after flooding with silicone oil (as described in the “Materials and Methods” section). Because the silicon oil was hydrophobic, it seems reasonable to assume that it coated the cell walls but did not displace the water in the cell walls. It would not, therefore, have diluted the K+ (or any other solutes) in the cell wall. The response of stomata to silicone oil was very similar to that with 50 mm KCl, i.e., silicone oil caused stomata to close completely, but the response was slower than with distilled water. One interpretation of this result is that silicone oil had an effect similar to the KCl solution; it blocked a signal from the mesophyll but it did not dilute the K+ in the guard cell walls. Because flooding with silicone oil is unlikely to have blocked a liquid-phase opening signal traveling in the cell wall water, we conclude that flooding with silicon oil closed stomata because it blocked a vapor-phase signal from the mesophyll.
The existence of a vapor-phase signal from the mesophyll to the guard cells is further supported by the fact that stomata opened and were light- and CO2-sensitive in grafts for which the mesophyll and epidermis were separated by a thin, hydrophobic, filter material. It seems unlikely that a liquid-phase signal could have traveled through these filters using our experimental protocol. The filters were dry when placed between the epidermis and mesophyll, and free water was blotted away from both the epidermis and mesophyll before the filter was inserted. Furthermore, the filters remained dry during the experiments (as determined by weighing), and when filters were deliberately wetted before the experiment, stomata did not open.
CONCLUSION
The data presented in this study conclusively show that flooding of the intercellular spaces causes stomata to close. Part of this response appears to be caused by dilution of K+ in the cell walls of the guard cells because the effect is slower when the intercellular spaces are flooded with a KCl solution or with silicone oil. We suggest that the rest of the response is caused by blockage of an opening signal from the mesophyll to the guard cells, which is generated in the light. None of the experiments in this study, when considered individually, can be considered definitive proof of such a signal, but when taken together, they constitute strong circumstantial evidence. The existence of a signal from the mesophyll that controls stomatal aperture has been suggested several times in the past (Lee and Bowling, 1992, 1993, 1995; Mott et al., 2008; Mott, 2009) but has remained controversial. If such a signal exists, it seems likely that it would be tied to photosynthesis or a component of photosynthesis. This idea has been suggested based on gas-exchange studies (Wong et al., 1979, 1985a, 1985b, 1985c; Farquhar and Wong, 1984; Messinger et al., 2006) and based on antisense mutants for sedoheptulose-1,7-bisphosphatase (Messinger et al., 2006; Lawson et al., 2008). Yet, studies with antisense mutants for Rubisco and cytochrome b6f have shown no correlation between photosynthetic capacity and stomatal conductance (von Caemmerer et al., 2004; Baroli et al., 2008). Although the data in this study suggest the existence of a signal from the mesophyll, they do not address the identity of the signal or the possible involvement of photosynthesis in producing the signal. It has been observed (Farquhar and Wong, 1984) that stomatal responses to light and CO2 are proportional to excess light energy in photosynthesis, and it seems possible that a signal from the mesophyll is related to this observation. These questions await future research.
MATERIALS AND METHODS
Tradescantia pallida was grown in a controlled environment greenhouse with day and night temperatures of 30°C and 20°C, respectively. Day length was extended to 16 h with high-pressure sodium lamps (PFD approximately 1,000 μmol m−2 s−1 at the top of the plant) when necessary. Plants were grown in 1 L pots containing peat:perlite:vermiculite (1:1:1 by volume), and were watered to excess daily with a dilute nutrient solution containing 9.1 mm nitrogen, 1.8 mm phosphorus, 2.7 mm potassium (Peter's 20-10-20; Grace Sierra Horticultural Products Co.), and 11 mm chelated iron. Lactuca serriola, Oenothera caespitosa, and Helianthus annuus leaves were collected from natural environments in Logan, UT. Leaves were removed with a razor blade and petioles were immediately placed in distilled water and cut again to prevent cavitation. Leaves chosen for experiments were fully mature but not senescing.
In all experiments, leaves were mounted in a controlled environment chamber while stomata were observed with a microscope. Two different chambers were used. The first has been described previously (Shope et al., 2008) and consisted of two identical aluminum compartments separated by the leaf (or epidermis-mesophyll graft). The top of the upper compartment and the bottom of the lower compartment were made of optical quality glass, and the temperature of the two compartments was controlled to be 23°C by circulating water from a temperature-controlled bath through water channels in the aluminum body. The second chamber was similar to the first except it had an open top to allow access to the abaxial surface with the glass needles and micromanipulator described below. The chamber temperature was equal to the air temperature (approximately 23°C). In both cases, the leaf was placed upside down (abaxial surface up) in the chamber and was illuminated from below (i.e. on the adaxial surface) with a fiber optic illuminator with a xenon bulb (Schott). The abaxial surface was observed with a standard light microscope. The air in the chamber was provided from compressed-gas tanks of CO2-free air and 1% CO2 in air. These two gases were mixed using mass flow controllers, and the CO2 concentration of the resulting mixture was determined with a CO2 infrared gas analyzer (ADC Mk III; ADC Instruments). The gas mixture was humidified by bubbling through distilled water at 35°C, and then passed through a helical condenser at 22.9°C. The flow rate through the chamber was approximately 1 L min−1. All experiments were performed with the following conditions: [CO2] = 100 μmol mol−1, [O2] = 21 mmol mol−1, PFD = 350 μmol m−2 s−1, leaf temperature = 23°C, water mole fraction difference between leaf and air = 0.016.
To inject the intercellular spaces of T. pallida leaves with different liquids, fine-tipped glass needles (with outside tip diameter of approximately 50 μm) were pulled using a Flaming/Brown micropipette puller (Sutter Instrument Co.). The micropipettes were attached to a syringe and loaded with approximately 1 mL of the appropriate fluid. Intact leaves were secured to the microscope stage and the glass needle was positioned using a micromanipulator. Using the micromanipulator, the tip of the needle was maneuvered through the epidermis of an intact leaf of T. pallida and into the intercellular space of the mesophyll. The leaves were then injected with approximately 1 μL of distilled water, 50 mm KCl, or low-viscosity silicone oil (AK50 silicone fluid; Wacker Solutions), depending on the experiment. After depressing the syringe, complete flooding of the intercellular space with fluid was confirmed optically. In the experiment with silicone oil it was necessary to image stomata using a confocal microscope (MRC 1024; Bio-Rad) due to changes in optical quality after injection. Initially, intact leaves were stained with FM4-64 (20 mm in dimethyl sulfoxide diluted in water to a final concentration of 500 μm) by injecting the stain into the intercellular spaces using the method described above. Once injected with the stain, leaves were left for approximately 15 h to absorb the FM4-64 solution. Details of the procedures for imaging guard cells with confocal microscopy have been reported previously (Shope and Mott, 2006).
Epidermis-mesophyll grafts of T. pallida were prepared as described previously (Mott et al., 2008). Visual examination of the epidermes used for the grafts revealed that (1) all stomata were initially closed, (2) essentially no mesophyll cells remained, and (3) most epidermal cells were alive. In some experiments a small (5 mm × 5 mm) piece of Versapore-3000 micro-pore filter material (acrylic copolymer membrane cast on a nonwoven nylon support; Versapore-3000 filter; Pall Corporation) was placed between the epidermis and mesophyll. RWC of these filter pieces was determined by weight using an analytical balance to measure fresh, dry, and saturated weights.
For pressure- or vacuum-induced infiltration experiments, leaves of T. pallida, L. serriola, O. caespitosa, and H. annuus were removed from the plant using a razor blade, and petioles were immediately cut again under distilled water. In the pressure experiments, the leaf was sealed into the lid of a pressure chamber (PMS Instruments) with the blade outside the chamber and the bottom of the petiole in a beaker of water insider the chamber. The leaf was then mounted into the controlled environment chamber described previously and illuminated. When the stomata were uniformly open, compressed nitrogen gas was used to pressurize the chamber to 0.5 MPa while stomatal apertures were recorded. In the vacuum experiments, leaves were mounted into the controlled environment chamber described earlier with the petioles remaining in a beaker of distilled water. When the stomata were uniformly open, the portion of the leaf in the controlled environment chamber was excised with a razorblade and placed into a translucent syringe filled with distilled water. Vacuum was induced and released in the syringe several times by pulling out and releasing the plunger, until the leaf piece was visibly infiltrated with water. The leaf piece was kept illuminated at a light intensity equivalent to that of the chamber throughout the entire process. The leaf piece was then returned to the controlled environment chamber and stomatal apertures were recorded. For control experiments, the procedure was identical except that the syringe did not contain water.
For experiments involving dark periods, a peltier-cooled CCD camera (CoolSnap HQ; Roper Scientific, Photometrics) was used to acquire images. In these experiments, a low intensity of light above 700 nm was provided to the leaf during periods of darkness using a 700 nm high-pass filter (no. 7-69; Kopp). The peltier-cooled CCD camera was sensitive to wavelengths above 700 nm and could acquire images in the absence of light below 700 nm. In all other experiments, a color digital microscope camera (3.3 MPX; ImagingPlanet) was used to capture images. Stomatal apertures were measured using digital image processing software (ImagePro; Media Cybernetics).
Acknowledgments
We thank Joe Shope for helpful discussions.
References
- Baroli I, Price GD, Badger MR, von Caemmerer S. (2008) The contribution of photosynthesis to the red light response of stomatal conductance. Plant Physiol 146: 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TN, Mott KA, Farquhar GD. (2003) A hydromechanical and biochemical model of stomatal conductance. Plant Cell Environ 26: 1767–1786 [Google Scholar]
- Dewar R. (1995) Interpretation of an empirical model for stomatal conductance in terms of guard cell function. Plant Cell Environ 18: 365–372 [Google Scholar]
- Dewar R. (2002) The Ball-Berry-Leuning and Tardieu-Davies stomatal models: synthesis and extension within a spatially aggregated picture of guard cell function. Plant Cell Environ 25: 1383–1398 [Google Scholar]
- Farquhar GD, Wong SC. (1984) An empirical model of stomatal conductance. Aust J Plant Physiol 11: 191–209 [Google Scholar]
- Feild TS, Sage TL, Czerniak C, Iles WJD. (2005) Hydathodal leaf teeth of Chloranthus japonicus (Chloranthaceae) prevent guttation-induced flooding of the mesophyll. Plant Cell Environ 28: 1179–1190 [Google Scholar]
- Hanba YT, Moriya A, Kimura K. (2004) Effect of leaf surface wetness and wettability on photosynthesis in bean and pea. Plant Cell Environ 27: 413–421 [Google Scholar]
- Ishibasha M, Terashima I. (1995) Effects of continuous leaf wetness on photosynthesis: adverse aspects of rainfall. Plant Cell Environ 18: 431–438 [Google Scholar]
- Lawson T, Lefebvre S, Baker NR, Morison JIL, Raines CA. (2008) Reductions in mesophyll and guard cell photosynthesis impact on the control of stomatal responses to light and CO2. J Exp Bot 59: 3609–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Bowling DJF. (1992) Effect of the mesophyll on stomatal opening in Commelina communis. J Exp Bot 43: 951–957 [Google Scholar]
- Lee JS, Bowling DJF. (1993) The effect of a mesophyll factor on the swelling of guard cell protoplasts of Commelina communis L. J Plant Physiol 142: 203–207 [Google Scholar]
- Lee JS, Bowling DJF. (1995) Influence of the mesophyll on stomatal opening. Aust J Plant Physiol 22: 357–363 [Google Scholar]
- Messinger S, Buckley TN, Mott KA. (2006) Evidence for involvement of photosynthetic processes in the stomatal response to CO2. Plant Physiol 140: 771–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott KA. (2009) Opinion: stomatal responses to light and CO2 depend on the mesophyll. Plant Cell Environ 32: 1479–1486 [DOI] [PubMed] [Google Scholar]
- Mott KA, Sibbernsen ED, Shope JC. (2008) The role of the mesophyll in stomatal responses to light and CO2. Plant Cell Environ 31: 1299–1306 [DOI] [PubMed] [Google Scholar]
- Randall DA, Dazlich DA, Zhang C, Denning AS, Sellers PJ, Tucker CJ, Bounoua L, Berry JA, Collatz GJ, Field CB, et al. (1996) A revised land surface parameterization (SiB2) for GCMs. Part III: the greening of the Colorado State University general circulation model. J Clim 9: 738–763 [Google Scholar]
- Roelfsema MRG, Hanstein S, Felle H, Hedrich R. (2002) CO2 provides an intermediate link in the red light response of guard cells. Plant J 32: 65–75 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Konrad KR, Marten H, Psaras GK, Hartung W, Hedrich R. (2006) Guard cells in albino leaf patches do not respond to photosynthetically active radiation, but are sensitive to blue light, CO2 and abscisic acid. Plant Cell Environ 29: 1595–1605 [DOI] [PubMed] [Google Scholar]
- Shope JC, Mott KA. (2006) Membrane trafficking and osmotically induced volume changes in guard cells. J Exp Bot 57: 4123–4131 [DOI] [PubMed] [Google Scholar]
- Shope JC, Peak D, Mott KA. (2008) Stomatal responses to humidity in isolated epidermes. Plant Cell Environ 31: 1290–1298 [DOI] [PubMed] [Google Scholar]
- Smith WK, McClean TM. (1989) Adaptive relationship between leaf water repellency, stomatal distribution, and gas exchange. Am J Bot 76: 465–469 [Google Scholar]
- Tyree MT, Jarvis PG. (1982) Water in tissues and cells. Lange OL, Nobel PS, Osmond CB, Ziegler H, , Physiological Plant Ecology II. Water Relations and Carbon Assimilation. Springer-Verlag, New York, pp 35–77 [Google Scholar]
- von Caemmerer S, Lawson T, Oxborough K, Baker NR, Andrews TJ, Raines CA. (2004) Stomatal conductance does not correlate with photosynthetic capacity in transgenic tobacco with reduced amounts of Rubisco. J Exp Bot 55: 1157–1166 [DOI] [PubMed] [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD. (1979) Stomatal conductance correlates with photosynthetic capacity. Nature 282: 424–426 [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD. (1985a) Leaf conductance in relation to rate of CO2 assimilation. 1. Influence of nitrogen nutrition, phosphorus-nutrition, photon flux-density, and ambient partial-pressure of CO2 during ontogeny. Plant Physiol 78: 821–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD. (1985b) Leaf conductance in relation to rate of CO2 assimilation. 2. Effects of short-term exposures to different photon flux densities. Plant Physiol 78: 826–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD. (1985c) Leaf conductance in relation to rate of CO2 assimilation. 3. Influences of water-stress and photoinhibition. Plant Physiol 78: 830–834 [DOI] [PMC free article] [PubMed] [Google Scholar]