Abstract
FLOWERING LOCUS C (FLC) is a key repressor of flowering in Arabidopsis (Arabidopsis thaliana) and is regulated, both positively and negatively, by posttranslational histone modifications. For example, vernalization (the promotion of flowering by cold temperatures) epigenetically silences FLC expression through repressive histone modifications such as histone H3 lysine-9 dimethylation (H3K9me2) and H3K27me3. In contrast, an RNA polymerase II-associated complex (Paf1c) activates FLC expression through increased H3K4 and H3K36 methylation. As a result of this regulation, FLC has become a useful model for the study of chromatin structure in Arabidopsis. Here we show that At3g22590 is the Arabidopsis homolog of the yeast (Saccharomyces cerevisiae) Paf1c component CDC73 and is enriched at FLC chromatin. In contrast to other Paf1c component mutants that exhibit pleiotropic developmental phenotypes, the effects of cdc73 mutations are primarily limited to flowering time, suggesting that CDC73 may only be required for Paf1c function at a subset of target genes. In rapid-cycling strains, cdc73 mutants showed reduced FLC mRNA levels and decreased H3K4me3 at the FLC locus. Interestingly, in late-flowering autonomous-pathway mutants, which contain higher levels of FLC, cdc73 mutations only suppressed FLC in a subset of mutants. H3K4me3 was uniformly reduced in all autonomous-pathway cdc73 double mutants tested; however, those showing reduced FLC expression also showed an increase in H3K27me3. Thus, CDC73 is required for high levels of FLC expression in a subset of autonomous-pathway-mutant backgrounds and functions both to promote activating histone modifications (H3K4me3) as well as preventing repressive ones (e.g. H3K27me3).
The timing of the transition from vegetative to reproductive growth is critical for the reproductive success of flowering plants and is highly regulated by both environmental cues and endogenous signals. In Arabidopsis (Arabidopsis thaliana), extensive genetic studies have identified multiple pathways involved in the control of flowering. One of these, the autonomous pathway, contains seven classical genes that promote flowering by repressing the transcription of a potent flowering repressor, FLOWERING LOCUS C (FLC; Michaels and Amasino, 1999; Sheldon et al., 1999; Michaels and Amasino, 2001). Therefore, loss-of-function mutations in any one of the autonomous-pathway genes results in delayed flowering due to an up-regulation in FLC mRNA levels. The genes of the autonomous pathway can be divided into two groups based on predicted protein function.
Four members of the autonomous pathway are associated with RNA-binding (FCA, FPA, and FLOWERING LOCUS K [FLK]) or RNA-processing activities (FY; Macknight et al., 1997; Schomburg et al., 2001; Simpson et al., 2003; Lim et al., 2004; Mockler et al., 2004). FCA and FPA both contain RRM-type RNA-binding domains and have each been shown to regulate poly(A) site selection in their own transcripts (Quesada et al., 2003; Simpson et al., 2003; Hornyik et al., 2010). In the case of FCA, this involves a physical interaction with FY, a homolog of the yeast (Saccharomyces cerevisiae) 3′-end processing factor Pfs2p (Simpson et al., 2003). The remaining three members of the autonomous pathway are predicted to act at DNA/chromatin. LUMINIDEPENDENS (LD) encodes a homeodomain-containing protein with a Gln-rich motif at the C terminus, whereas FLOWERING LOCUS D (FLD) and FVE encode chromatin-remodeling proteins (Lee et al., 1994a; He et al., 2003; Ausin et al., 2004; Kim et al., 2004). FLD is an Arabidopsis homolog of human LYSINE-SPECIFIC DEMETHYLASE1, which demethylates histone H3 Lys-4 (H3K4), and FVE shares homology with yeast Multicopy Suppressor of IRA1 and mammalian Retinoblastoma-Associated Protein, which participate in histone-deacetylase complex recruitment. Thus, the predicted activities of both FLD and FVE are associated with transcriptional repression. It has recently been reported that FCA, FY, and FPA promote the usage of a proximal poly(A) site in FLC antisense transcripts. This has been proposed to trigger the removal of H3K4me2 at the FLC locus, which leads to decreased levels of FLC mRNA (Hornyik et al., 2010; Liu et al., 2010). In agreement with this working model, genetics studies have shown that FLD is required for FPA and FCA to repress FLC expression (Liu et al., 2007, 2010; Baurle and Dean, 2008).
In contrast to rapid-cycling strains, many naturally occurring Arabidopsis accessions are late flowering unless given a long period of cold exposure that acts to promote flowering (vernalization). In these naturally occurring late-flowering accessions, the FRIGIDA (FRI) gene acts epistatically to the autonomous pathway to up-regulate FLC and delay flowering. Vernalization, however, leads to an epigenetic repression of FLC involving an increase in repressive histone modifications at the FLC locus (e.g. H3K9me2 and H3K27me3; Bastow et al., 2004; Sung and Amasino, 2004). Vernalization can repress the high levels of FLC in both FRI-containing and autonomous-pathway-mutant backgrounds. Allelic variation at FRI is a major determinant of naturally occurring variation in flowering time; late-flowering accessions contain active dominant alleles of FRI, whereas most rapid-cycling strains contain loss-of-function alleles of FRI (Johanson et al., 2000; Gazzani et al., 2003; Michaels et al., 2003b).
Much of our understanding of how FLC mRNA is positively regulated has come from the characterization of mutations that suppress the high levels of FLC expression conferred by FRI. One group of genes identified in such screens, VERNALIZATION INDEPENDENCE2 (VIP2)/EARLY FLOWERING7 (ELF7), VIP4, VIP5, and VIP6/ELF8, are homologs of components of the yeast RNA polymerase II-associated factor 1 complex (Paf1c; Paf1, Leo1, Rtf1, and Ctr9, respectively) and VIP3 is homologous to Ski8, which is associated with mammalian Paf1c complexes (Zhang and van Nocker, 2002; Zhang et al., 2003; He et al., 2004; Oh et al., 2004). Evidence suggests that the Paf1c plays roles in transcriptional regulation through both histone modifications and RNA processing. In yeast, Drosophila, and mammals, Paf1c mutations/knockdowns lead to global changes in histone modifications, especially H3K4 and H3K36 methylation (Krogan et al., 2003; Ng et al., 2003a, 2003b; Wood et al., 2003; Zhu et al., 2005; Adelman et al., 2006; Tenney et al., 2006). Despite broad changes in histone modifications throughout the genome, however, altered gene expression was observed for only a subset of genes in yeast Paf1c mutants (Penheiter et al., 2005). It has also been reported that Paf1c is directly or indirectly involved in multiple steps of transcription, including transcription elongation (Squazzo et al., 2002; Rondon et al., 2004; Chen et al., 2009; Zhang et al., 2009b; Kim et al., 2010), poly(A) site selection (Penheiter et al., 2005), mRNA polyadenylation (Mueller et al., 2004; Rozenblatt-Rosen et al., 2009), and snoRNA 3′-end formation (Sheldon et al., 2005).
Similar to yeast Paf1c, the Arabidopsis Paf1c components are required for proper expression of only a subset of genes, including FLC and the FLC-related MADS AFFECTING FLOWERING (MAF) gene family (Zhang and van Nocker, 2002; Zhang et al., 2003; He et al., 2004; Oh et al., 2004). In contrast to yeast, Drosophila, and mammals, global changes in H3 modifications were not observed in Arabidopsis Paf1c mutants (Oh et al., 2004; Xu et al., 2008). The reduction of FLC transcript level in Paf1c mutants, however, is well correlated with the decreased level of H3K4me3 and H3K36me3 at the FLC locus. This suggests that the Paf1c in Arabidopsis may play a similar role to that in other organisms, but its role in mediating histone modifications in Arabidopsis may be restricted to a smaller number of loci (He et al., 2004; Oh et al., 2008; Xu et al., 2008).
In addition to Paf1, Leo1, Rtf1, and Ctr9, the yeast Paf1c contains the protein Cdc73. Here we show that At3g22590 is homologous to Cdc73 and is likely to act in conjunction with the Arabidopsis Paf1c. Like other Paf1c mutants in Arabidopsis, cdc73 mutants flower early, have reduced levels of FLC and MAF family expression, and show reduced levels of H3K4me3 at the FLC locus. Unlike other Paf1c mutants, which show a range of pleiotropic phenotypes, the effects of cdc73 mutations are largely limited to flowering time. Interestingly, cdc73 mutations can suppress FLC expression in backgrounds containing FRI or RNA-associated (fca, flk, and fy) autonomous-pathway mutants, but not in chromatin-associated fld, fve, or ld mutant backgrounds. These data suggest that the role of CDC73 is more flowering specific than the other members of the Arabidopsis Paf1c.
RESULTS AND DISCUSSION
Loss of a CDC73 Homolog Results in Early Flowering
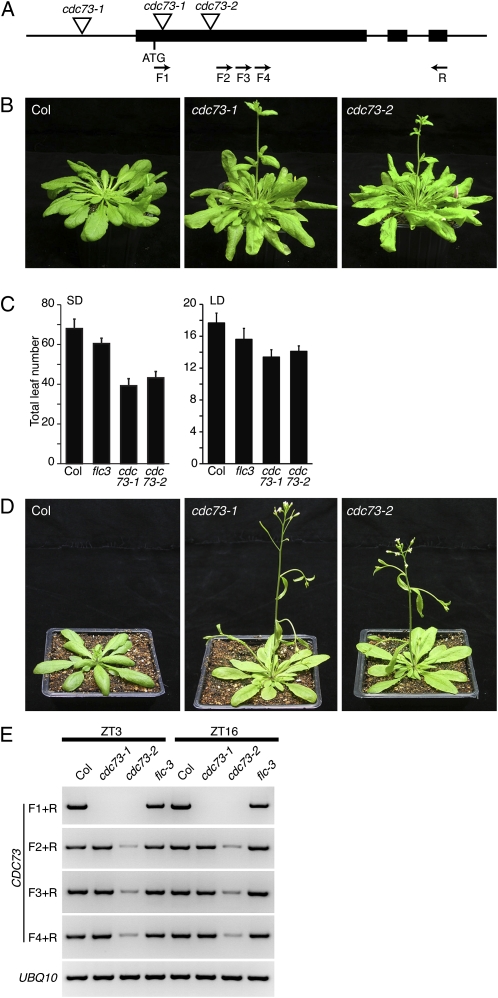
To identify additional components involved in chromatin-mediated flowering-time regulation, lines homozygous for mutations in genes predicted to modify chromatin were screened for altered flowering time under short days. One line that flowered earlier than wild-type Columbia (Col) was SALK_150644C (hereafter cdc73-1), which contains a T-DNA insertion in At3g22590, an Arabidopsis homolog of CDC73 (Fig. 1, A–C). CDC73 in yeast, as well as the human homolog Parafibromin, have been shown to act in Paf1 complexes (Krogan et al., 2002; Rozenblatt-Rosen et al., 2005; Yart et al., 2005). The Arabidopsis homolog of CDC73 shows significant sequence similarity to CDC73 proteins from other species, particularly in the C-terminal half of the protein (Supplemental Fig. S1). Consistent with the behavior of cdc73-1, mutations in other components of the Arabidopsis Paf1c complex also cause early flowering (He et al., 2004; Oh et al., 2004). To confirm that the mutation in cdc73 is responsible for the early flowering phenotype, we obtained a second allele, cdc72-2 (SALK_008357; Fig. 1A), which likewise flowered early under short days (Fig. 1, B and C). Both alleles also flowered earlier than wild type under long days (Fig. 1, C and D). In addition, overexpression of a CDC73 cDNA was able to rescue the flowering-time defects of cdc73 mutants (Supplemental Fig. S2). Thus, CDC73 acts as a repressor of flowering in Arabidopsis. Using primers that amplify the full-length cDNA, no transcript was detected in cdc73-1 or cdc73-2 (Fig. 1E). Primers to the 3′ region of the gene, however, revealed that some truncated transcript is made in both mutant backgrounds. Similar transcripts originating in or downstream of T-DNA insertions have been reported previously (Xu et al., 2007).
Figure 1.
Mutations in CDC73 result in early flowering. A, Schematic of the CDC73 locus. Triangles indicate the positions of T-DNA insertions. For cdc73-1, genomic DNA rescued from the left border of the T-DNA mapped to two different locations, suggesting a complex T-DNA insertion. Arrows indicate primers used for RT-PCR in Figure 1E. B and D, Plants grown under short (B) or long days (D). C, Flowering time of cdc73 mutants under short and long days. Bars indicate the total number of leaves formed prior to flowering. Error bars indicate 1 sd. E, Semiquantitative RT-PCR analysis of CDC73 expression. RNA was extracted from 7-d-old seedlings grown under long days. Samples were taken at 3 h (ZT3) and 16 h (ZT16) after relative dawn. Positions of primers are indicated in A. UBIQUITIN10 was used as a control for loading. [See online article for color version of this figure.]
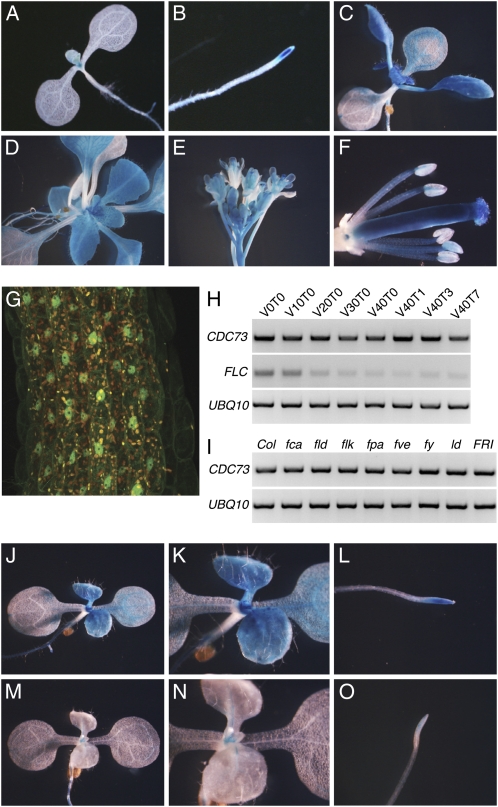
We investigated the spatial expression pattern of CDC73 by creating transgenic plants containing a genomic DNA fragment including the CDC73 promoter and full-length coding sequence fused to the GUS reporter gene (Jefferson et al., 1987). In seedlings, GUS expression was highest in the shoot apex and root tip (Fig. 2, A and B). This result is consistent with microarray data (Genevestigator; Hruz et al., 2008), suggesting that the reporter construct accurately reflects the expression pattern of the endogenous CDC73. In addition to the shoot and root tip, high levels of expression were also observed in young leaves and flowers, especially in stamen filaments and carpels (Fig. 2, C–F). To determine subcellular localization, we created a construct containing the full-length CDC73 cDNA fused to GFP under control of the constitutive 35S promoter. When transformed into FRI cdc73 plants (see below), CDC73::GFP was able to rescue the wild-type phenotype, indicating that construct is functional (Supplemental Fig. S3). Consistent with CDC73's predicted role in transcriptional regulation, CDC73::GFP showed strong nuclear staining (Fig. 2G).
Figure 2.
Analysis of CDC73 expression. A to F, CDC73::GUS expression in seedling (A), root (B), vegetative rosettes (C and D), inflorescence (E), and flower (F; sepals and petals have been removed to expose carpels and stamens). G, Nuclear localization of CDC73::GFP in hypocotyl cells. H and I, Semiquantitative RT-PCR analysis of CDC73 mRNA levels during vernalization (H) and in various late-flowering backgrounds (I). For vernalized samples, V and T indicate the days of cold exposure and subsequent warm temperatures, respectively. FLC was included as a control for vernalization and UBIQUITIN10 was used as a control for loading. J to O, FLC::GUS expression in FRI (J–L) or FRI cdc73 (M–O).
Given the early flowering phenotype of cdc73 mutants, we were curious to determine if CDC73 expression is regulated by factors that affect flowering time. Vernalization promotes flowering through epigenetic repression of FLC; FLC expression is repressed during cold exposure and remains low after return to warm temperatures (Michaels and Amasino, 1999; Sheldon et al., 1999; Fig. 2H). We found that CDC73 transcript levels remained relatively constant both during cold treatment and after plants were returned to warm temperatures (Fig. 2H). Thus, CDC73 does not appear to be regulated by vernalization. Likewise, CDC73 expression was unchanged in late-flowering lines containing dominant late-flowering alleles of FRI or mutations in the autonomous pathway (Fig. 2I). Together, this lack of regulation at the mRNA level suggests that CDC73 plays a constitutive role in floral repression.
The Phenotypes of cdc73 Mutants Are Relatively Flowering Specific Compared to Other Paf1c Component Mutants
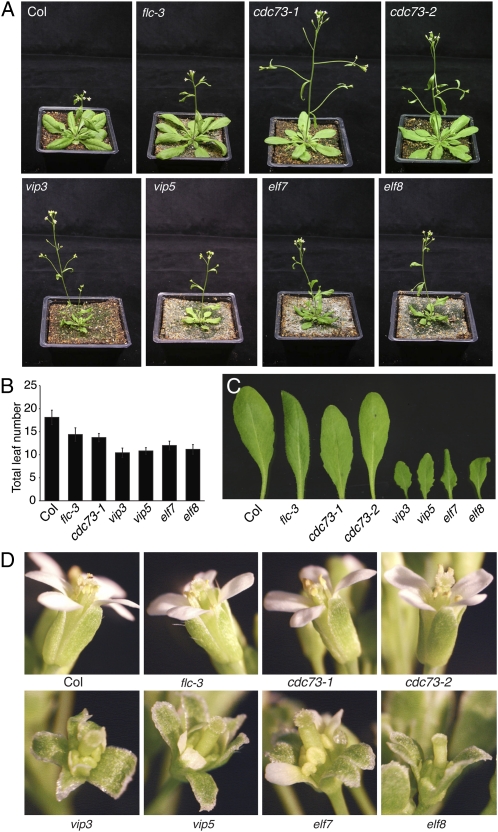
Mutations in Paf1c components such as vip3, vip5, elf7, and elf8 cause a strong early flowering phenotype (Zhang et al., 2003; He et al., 2004; Oh et al., 2004; Fig. 3, A and B). The effects of these mutations, however, are not limited to flowering time. Mutant plants are significantly smaller than wild type with shorter inflorescences and reduced leaf size (Zhang et al., 2003; He et al., 2004; Oh et al., 2004; Fig. 3, A and C). In addition, vip3, vip5, elf7, and elf8 exhibit floral abnormalities including incomplete closure of the sepals over the developing flower bud, diminished petal development, and white petaliod tissue in the margins of sepals (Fig. 3D). In contrast, cdc73 mutations do not affect plant stature, leaf size, or floral development and have a more modest effect on flowering time (Fig. 3). These results are consistent with a model in which VIP3, VIP5, ELF7, and ELF8 act as core components of the Paf1c in Arabidopsis and are essential for the regulation of genes involved in many aspects of development, whereas the requirement for CDC73 is more limited (e.g. flowering time).
Figure 3.
Effect of cdc73 and Paf1c component mutants on growth and development. A, Loss of cdc73 has less severe effects on plant size than other Paf1c-associated mutants (vip3, vip5, elf7, and elf8). B, Flowering time of cdc73 and Paf1c mutants under long days. Bars indicate the total number of leaves formed prior to flowering. Error bars indicate 1 sd. C, Fully expanded fifth leaves are shown for the indicated genotypes. D, Floral abnormalities present in other Paf1c mutants are absent in cdc73. [See online article for color version of this figure.]
cdc73 Mutants Have Reduced Expression of FLC and Several FLC-Related Genes
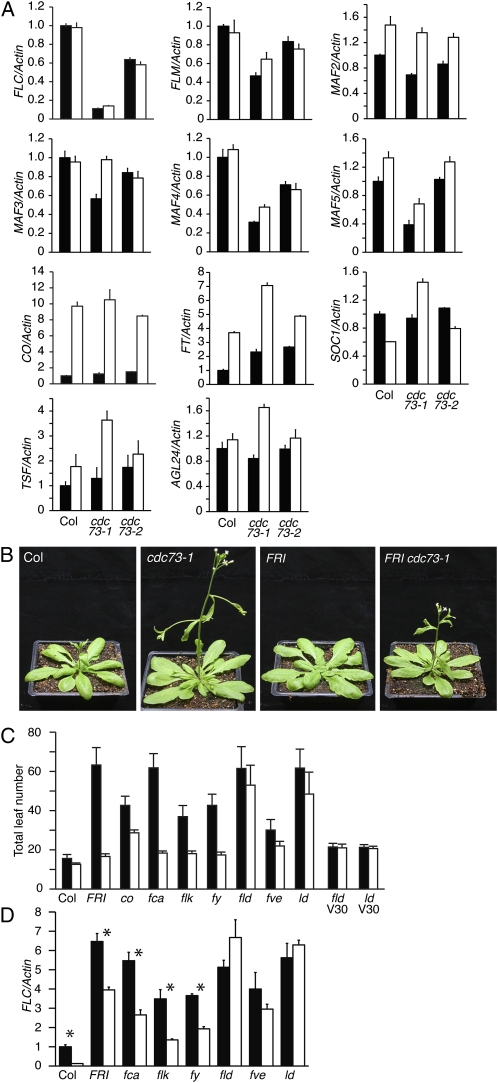
Previous reports have shown that Paf1c components are required for normal expression of the floral repressor FLC (Zhang et al., 2003; He et al., 2004; Oh et al., 2004). FLC levels are reduced in vip3, vip5, elf7, and elf8 mutant backgrounds, which contributes to their early flowering phenotypes. To determine if CDC73 is also required for FLC expression, we examined FLC mRNA levels by quantitative reverse transcription (RT)-PCR and found that they are indeed reduced in cdc73 mutants (Fig. 4A). This reduction in FLC expression is likely to account for part of the early flowering phenotype of cdc73 mutants. It should be noted, however, that cdc73 mutants flowered earlier than an flc-null mutant under both long and short days (Fig. 1C), suggesting the involvement of additional flowering-time genes. FLC is a member of a small clade of MADS-domain transcription factors that also includes FLOWERING LOCUS M (FLM)/MAF1, MAF2, MAF3, MAF4, and MAF5. Although their effect on flowering time is weaker than FLC, several of these FLC-related genes have been shown to play a role in the regulation of flowering time and many are regulated by the Paf1c (Ratcliffe et al., 2001, 2003; Scortecci et al., 2001; Zhang and van Nocker, 2002; Scortecci et al., 2003; Zhang et al., 2003; He et al., 2004; Oh et al., 2004). We therefore examined the effect of CDC73 on the expression of FLC-related genes (Fig. 4A). In particular, the expression of FLM, MAF4, and MAF5 was reduced in cdc73-mutant backgrounds (especially cdc73-1; see below). Thus, the down-regulation of FLC-related genes may also contribute to the early flowering phenotype of cdc73 mutants.
Figure 4.
Effect of cdc73 on the expression of flowering-time genes. A, Quantitative RT-PCR analysis of flowering-time genes. RNA was extracted from 7-d-old seedlings grown under long days. Samples were taken at 3 h (black bars) and 16 h (white bars) after relative dawn. Error bars indicate 1 sd. B, Effect of cdc73 in a FRI-containing background. Plants were grown under long days. C, Flowering time of long-day-grown plants with wild-type CDC73 (black bars) or cdc73-1 (white bars). Error bars indicate 1 sd. D, Quantitative RT-PCR analysis of FLC expression in genotypes containing wild-type CDC73 (black bars) or cdc73-1 (white bars). RNA was extracted from 7-d-old seedlings grown under long days. Error bars indicate 1 sd. Asterisks indicate a statistically significant difference in FLC expression for a given background ± CDC73 (P < 0.05). [See online article for color version of this figure.]
The effect of cdc73 mutations on the expression of FLC and FLC-related genes was consistently stronger for cdc73-1 than for cdc73-2 (Fig. 4A), suggesting that cdc73-1 is a stronger mutant. Therefore, subsequent analyses were carried out using cdc73-1. It is worth noting, however, that the level of transcripts from the 3′ end of CDC73 (downstream of the T-DNA insertions) is higher in cdc73-1 than in cdc73-2 (Fig. 1, A and E). One possible explanation for the stronger effect of cdc73-1 may be that the cdc73-2 transcript (though less abundant) is more likely to be translated into a partially functional protein than the cdc73-1 transcript.
cdc73 Strongly Suppresses the Late-Flowering Phenotype of FRI, But Only Partially Suppresses FLC Expression
The results above show that CDC73 is required for normal FLC expression in a rapid-cycling Col background. We also investigated the requirement for CDC73 in a late-flowering FRI-containing background. Because the late-flowering phenotype of FRI is dependent on FLC (i.e. FRI has no effect on flowering time in a flc-null background; Michaels and Amasino, 2001), a reduction in FLC expression by cdc73 should result in earlier flowering. Indeed cdc73 strongly suppressed the late-flowering phenotype of FRI, with FRI cdc73 plants flowering only slightly later than Col (Fig. 4, B and C). To confirm that the suppression of FRI was due to a reduction of FLC expression, we determined FLC mRNA levels. As expected, FLC levels were lower in FRI cdc73 than those observed in FRI (Figs. 2, J–O, and 4D), however, the suppression of FLC was only partial. Despite the fact that FRI cdc73 flowered almost as early as Col, FLC levels remained much higher in FRI cdc73 (Fig. 4D). This result suggests that the promotion of flowering by cdc73 mutations in the FRI background is only partly due to a reduction in FLC expression and that other flowering pathways may also require CDC73. Consistent with the idea that cdc73 can promote flowering independently of FLC, cdc73 mutants flowered earlier than flc-null mutants (Fig. 1C). Taken together, these results indicate that CDC73 plays a role both in the regulation of FLC, as well as other flowering pathways that are FLC independent.
In an effort to explain the FLC-independent effects of cdc73 mutations on flowering time, we examined the expression of CONSTANS (CO), FLOWERING LOCUS T (FT), TWIN SISTER OF FT (TSF), SUPPRESSOR OF OVEREXPRESSION OF CO (SOC1), and AGAMOUS-LIKE24 (AGL24; Putterill et al., 1995; Kardailsky et al., 1999; Kobayashi et al., 1999; Borner et al., 2000; Lee et al., 2000; Samach et al., 2000; Yu et al., 2002; Michaels et al., 2003a). FT, TSF, SOC1, and AGL24 are strong promoters of flowering that are regulated by multiple pathways that control flowering time and, for this reason, these genes are often referred to as floral integrators. FLC acts to block flowering by repressing the expression of these genes, whereas, a photoperiod-dependent pathway positively regulates the expression of the floral integrators in response to inductive long days (Michaels, 2009). The expression of floral integrators, particularly FT and TSF, is up-regulated in cdc73 mutants (Fig. 4A). Interestingly, however, CO mRNA levels are unchanged, suggesting that the up-regulation of the floral integrators is not a result of the photoperiod pathway. Consistent with this result, the late-flowering phenotype of co mutants (which are photoperiod insensitive) is partially suppressed by cdc73-1 (Fig. 4C). Thus, activity of the photoperiod pathway is not required for the acceleration of flowering by cdc73.
These results suggest that CDC73 plays a role in the regulation of floral integrators that is independent of both FLC and the photoperiod pathway. It is possible that CDC73 has direct effects on the floral integrators. Alternatively, reduced expression of FLC-related genes (FLM, MAF4, and MAF5; described above) in cdc73 mutants may contribute to the observed up-regulation of floral integrators. In theory, one could test the later hypothesis by examining the effect of cdc73 mutations in an flc flm maf4 maf5 quadruple mutant background. Unfortunately, detailed genetic analysis of FLC-related genes is complicated by the fact that MAF2, MAF3, MAF4, and MAF5 exist as a tandem array.
The Up-Regulation of FLC in Chromatin-Associated Autonomous-Pathway Mutants Does Not Require CDC73
We also investigated the effect of cdc73 on flowering time in late-flowering autonomous-pathway-mutant backgrounds. Like FRI, the late-flowering phenotype of autonomous-pathway mutants is also dependent on FLC (Michaels and Amasino, 2001). Interestingly, the autonomous pathway can be divided into two classes based on their interaction with CDC73. Similar to FRI cdc73, the late-flowering phenotype of fca, flk, and fy was almost completely suppressed by cdc73 (Fig. 4C). In contrast, only a slight acceleration of flowering was observed in fld, fve, and ld mutant backgrounds (Fig. 4C). It is worth noting that the groups of autonomous-pathway genes, defined by their interaction with CDC73, correlate with their demonstrated and/or predicted function. FCA, FLK, and FY act in RNA binding/RNA processing, whereas FLD, FVE, and LD are associated with chromatin remodeling/DNA binding. This result suggests that CDC73 plays a larger role in repressing flowering (i.e. promoting FLC expression) in fca, flk, and fy backgrounds than it does in fld, fve, or ld. Interestingly, fld cdc73 and ld cdc73 showed a normal vernalization response, suggesting that CDC73 is not involved in the repression of FLC by cold (Fig. 4C).
To determine the role of CDC73 in the regulation of FLC in autonomous-pathway mutants, we determined FLC mRNA levels by quantitative RT-PCR in various autonomous-pathway-mutant backgrounds ± CDC73. Consistent with the early flowering phenotype observed in the double mutants, fca cdc73, flk cdc73, and fy cdc73 all showed significantly reduced levels of FLC expression relative to the fca, flk, and fy single mutants (Fig. 4D). As with FRI cdc73, however, FLC levels were higher than expected; fca cdc73, flk cdc73, and fy cdc73 all flowered similarly to Col, yet FLC levels remained significantly higher than Col. This result reinforces the hypothesis that CDC73 has FLC-dependent as well as FLC-independent effects on flowering time. Interestingly, fld cdc73, fve cdc73, and ld cdc73 showed no statistically significant difference in FLC expression (Fig. 4D). This indicates that the slight acceleration of flowering observed in these lines may be entirely due to FLC-independent effects of CDC73 and that the up-regulation of FLC in fld, fve, and ld mutants is independent of CDC73.
CDC73 Localizes to FLC Chromatin and Plays a Role in H3K4me3 and H3K27me3
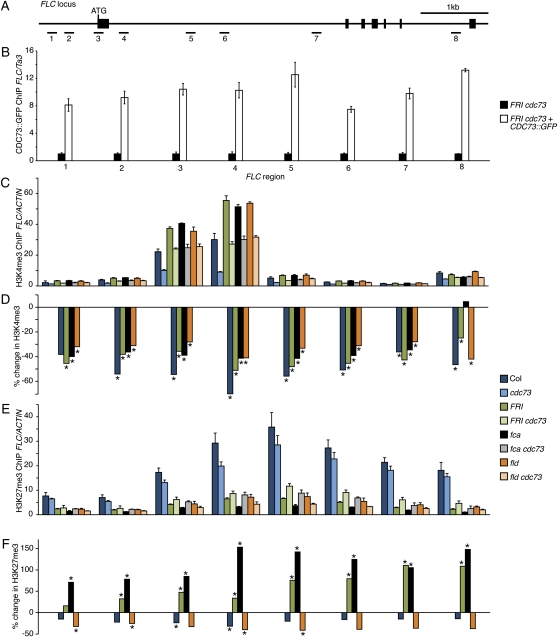
We used chromatin immunoprecipitation (ChIP) to determine if the regulation of FLC by CDC73 might be a direct effect. Anti-GFP antibodies were used to immunoprecipitate chromatin from plants expressing a functional CDC73::GFP fusion protein (Fig. 2G; when transformed into a FRI cdc73 background, CDC73::GFP fully rescued the early flowering phenotype; Supplemental Fig. S3). Enrichment of CDC73::GFP protein was observed across the FLC region (Fig. 5, A and B), indicating that CDC73 directly associates with FLC chromatin.
Figure 5.
ChIP analysis of the FLC locus. A, Schematic drawing of the FLC locus. Exons are depicted as thick black lines. The numbered segments indicate the positions of PCR products generated in ChIP analysis. B, ChIP analysis of CDC73::GFP at the FLC locus. C and E, Analysis of H3K4me3 and H3K27me3 using ChIP followed by quantitative RT-PCR. D and F, Percent change in H3K4me3 and H3K27me3 due to loss of cdc73. Asterisks indicate statistically significant differences (P < 0.05).
The Paf1c has been shown to be required for the activation of FLC through activating histone modifications. In particular, reduced levels of H3K4 and H3K36 are observed at the FLC locus in Paf1c mutants (He et al., 2004; Oh et al., 2008; Xu et al., 2008; Tamada et al., 2009). In addition, a rise in repressive H3K27me3 has also been reported (Oh et al., 2008). If CDC73 acts in conjunction with the Paf1c in Arabidopsis, then one would predict that similar changes in chromatin modifications would be observed in cdc73 mutants and other Paf1c mutants. To test this prediction, we performed ChIP using antibodies to H3K4me3 and H3K27me3 followed by quantitative PCR. In Col, H3K4me3 was enriched near the transcriptional/translational start sites and at the beginning of the first intron, whereas H3K27me3 showed a broader pattern of enrichment along the body of the FLC gene (Fig. 5, C–F). In the cdc73 mutant, there was a significant decrease in H3K4me3 at nearly all locations tested (Fig. 5, C and D; only region 1 failed to show a statistically significant difference). H3K27me3 was not significantly changed in cdc73, with the exception of two regions near the translational start site and beginning of the first intron, where H3K27me3 was decreased slightly (Fig. 5, E and F). Thus, in Col, cdc73 mutations caused a significant reduction in H3K4me3 and a minor decrease in H3K27me3. During the preparation of this manuscript, another group reported on histone modifications at the FLC locus in cdc73 (plant homologous to parafibromin [php]) mutants (Park et al., 2010). Consistent with our results, they observed a decrease in H3K4me3 in the 5′ region of FLC; additionally H3K36me2 was decreased in the 3′ region. Park et al. also observed an increase in H3K27me3 across the FLC region (Park et al., 2010). Although we did not observe similar increase in the Col background, we did find increased H3K27me3 in some late-flowering backgrounds (see below).
In addition to Col, we also examined the effect of cdc73 on histone modifications at the FLC locus in several late-flowering backgrounds. We chose FRI and fca because cdc73 mutations partially suppress FLC expression in these backgrounds and fld, which, in contrast, showed no dependence on CDC73 for high levels of expression (Fig. 4D). Consistent with the high levels of FLC expression in FRI-containing lines and fca and fld mutants, H3K4me3 levels were elevated relative to Col (Fig. 5, C and D). In all three backgrounds, loss of CDC73 reduced H3K4me3 across the FLC locus to a roughly similar extent. Thus, at the level of H3K4me3, no differences were observed between lines that require CDC73 for full levels of FLC expression (i.e. FRI and fca) and fld, which does not. Relative to Col, levels of the repressive H3K27me3 mark were reduced across the FLC locus in FRI, fca, and fld (Fig. 5, E and F). Similar to Col, fld mutants showed a modest decrease in H3K27me3 in the absence of CDC73. In contrast to Col and fld, however, FRI and fca showed a strong increase in H3K27me3 in the absence of CDC73 (Fig. 5, E and F). Overall, these data suggest that the partial suppression of FLC expression by loss of cdc73 in FRI and fca backgrounds may be due to a combination of decreased activating modifications (H3K4me3) and increased repressive modifications (H3K27me3).
CONCLUSION
Here we have shown that the Arabidopsis homolog of yeast CDC73 likely functions as part of a Paf1c. Consistent with this model, the effects of cdc73 mutations on flowering time are generally similar to those reported for mutations in other Paf1c components (e.g. vip3, vip5, elf7, and elf8). In Col, cdc73 and other Paf1c mutants all show reduced expression of FLC and FLC-related genes and reduced H3K4me3 at the FLC locus. It should be noted that, in addition to early flowering, vip3, vip5, elf7, and elf8 mutants show a range of pleiotropic phenotypes that are absent in cdc73 mutants. This suggests that VIP3, VIP5, ELF7, and ELF8 may act as core components of the Paf1c complex, whereas CDC73 function may only be required at a subset of Paf1c targets, such as FLC. Biochemical and transcript profiling experiments published recently support this interpretation. CDC73/PHP physically interacts with other Paf1c proteins and cdc73/php mutants show misexpression of a smaller, but overlapping, set of genes compared to vip3 mutants (Park et al., 2010).
Previous work has shown that ARABIDOPSIS TRITHORAX-LIKE PROTEIN1 (ATX1), ATX2, and ATX7 are required for H3K4 methylation at FLC and may be recruited to the FLC locus by the Paf1c (Pien et al., 2008; Saleh et al., 2008; Tamada et al., 2009). Interestingly, the reduced levels of H3K4me3 observed in atx1, atx7, or atx1 atx7 plants are accompanied by increased H3K27 methylation in FRI-containing backgrounds, suggesting an antagonistic relationship between these two marks (Pien et al., 2008; Tamada et al., 2009). We find that this is also the case for cdc73 mutants in late-flowering FRI-containing or fca-mutant backgrounds; H3K4me3 is decreased, H3K27me3 is increased, and FLC expression is reduced. A decrease in H3K4me3 was also observed in cdc73 fld, however, H3K27me3 and FLC expression were indistinguishable from fld. This suggests that, in late-flowering backgrounds, decreased H3K4me3 is not sufficient to repress FLC expression and that an increase in H3K27me3 is also required for repression.
MATERIALS AND METHODS
Plant Material and Growth Conditions
FRI-Col (Lee et al., 1994b), fca-9 (Bezerra et al., 2004), fld-3 (He et al., 2003), fve-4 (Michaels and Amasino, 2001), ld-1 (Lee et al., 1994a), co (SAIL24H04; Kim and Michaels, 2006), flc-3 (Michaels and Amasino, 1999), flk (SALK_112850; Kim et al., 2008), fy-2 (Simpson et al., 2003), vip5 (Oh et al., 2004), elf7-2, and elf8-1 (He et al., 2004) have been described previously. vip3 (SALK_083364), cdc73-1 (SALK_150644C), and cdc73-2 (SALK_008357) were obtained from the Arabidopsis Biological Resource Center (Columbus, Ohio; Alonso et al., 2003). Plants were grown at 22°C under cool-white fluorescent light with a light intensity of 125 μmol m−2 s−1. Long and short days consisted of 16-h light/8-h dark and 8-h light/16-h dark, respectively.
RNA Expression Analysis
Semiquantitative PCR (Michaels et al., 2004) and quantitative PCR (Mockler et al., 2004) were performed as described previously. Primers are available in the supplemental data. All experiments were replicated at least three times with similar results.
ChIP
ChIP was performed largely as described previously (Jacob et al., 2009). Briefly, seeds were sown on filter paper on top of Murashige and Skoog medium and grown under long-day conditions. Whole 7-d-old seedlings were harvested and fixed with formaldehyde. The cross-linked samples were sheared by sonication and precleared with salmon sperm DNA/protein A agarose slurry. The precleared samples were immunoprecipitated with the antibodies against anti-H3K4me3 (17-614, Millipore) or H3K27me3 (07-449, Millipore) or GFP (A-11122, Invitrogen). The precipitated DNA samples associated with the modified Histone H3 or CDC73-GFP were quantified with real-time PCR. The Histone H3K4me3 and H3K27me3 enrichments at FLC locus were normalized against the Actin 2 locus. The CDC73 enrichments at FLC locus were normalized against the transcriptionally silent Ta3 locus. Because Ta3 lacks H3K4me3 (Zhang et al., 2009a), it should not be bound by the Paf1c. Data presented are an average of three replicates.
Constructs
cDNA and genomic DNA of the Arabidopsis (Arabidopsis thaliana) CDC73 gene were PCR amplified and cloned into pENTR/D-TOPO (Invitrogen). Genomic DNA for the GUS fusion included 1,555 bp upstream of the translational start site and the entire coding region of CDC73. The CDC73 cDNA or genomic DNA in the resulting pENTR clones were transferred to destination vectors (pEG100, pEG103, and pMDC163) by LR clonase reaction according to the manufacturer's instructions (Invitrogen; Curtis and Grossniklaus, 2003; Earley et al., 2006).
Sequence data from this article can be found in EMBL/GenBank under the following accession numbers: CDC73, At3g22590; FCA, At4g16280; FLD, At3g10390; FLK, At3g04610; FPA, At2g43410; FVE, At2g19520; FY, At5g13480; LD, At4g02560; FRI, At4g00650; VIP3, At4g29830; VIP5, At1g61040; ELF7, At1g79730; ELF8, At2g06210; CO, At5g15840; FT, At1g65480; SOC1, At2g45660; TSF, At4g20370; AGL24, At4g24540; FLC, At5g10140; FLM, At1g77080; MAF2, At5g65050; MAF3, At5g65060; MAF4, At5g65070; MAF5, At5g65080; ACTIN2, At3g18780; and UBQ, At4g05320.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Alignment of CDC73 homologs from Arabidopsis, human, and yeast.
Supplemental Figure S2. Rescue of the early flowering phenotype of FRI cdc73 and fca cdc73 mutants by transformation with 35S::CDC73 cDNA.
Supplemental Figure S3. Rescue of the early flowering phenotype of FRI cdc73 mutants by transformation with CDC73::GFP.
Supplemental Data S1. PCR and quantitative PCR primers used in this study.
Supplementary Material
Acknowledgments
We thank C. Walczak for the gift of GFP antibody, J. Lucas and Y. Gu for assistance with microscopy, W. Feng and J. Huang for assistance in genetic analysis, and W. Feng, L. Ding, and G. Parry for useful discussions and assistance in manuscript preparation.
References
- Adelman K, Wei W, Ardehali MB, Werner J, Zhu B, Reinberg D, Lis JT. (2006) Drosophila Paf1 modulates chromatin structure at actively transcribed genes. Mol Cell Biol 26: 250–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Ausin I, Alonso-Blanco C, Jarillo JA, Ruiz-Garcia L, Martinez-Zapater JM. (2004) Regulation of flowering time by FVE, a retinoblastoma-associated protein. Nat Genet 36: 162–166 [DOI] [PubMed] [Google Scholar]
- Bastow R, Mylne JS, Lister C, Lippman Z, Martienssen RA, Dean C. (2004) Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 427: 164–167 [DOI] [PubMed] [Google Scholar]
- Baurle I, Dean C. (2008) Differential interactions of the autonomous pathway RRM proteins and chromatin regulators in the silencing of Arabidopsis targets. PLoS One 3: e2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra IC, Michaels SD, Schomburg FM, Amasino RM. (2004) Lesions in the mRNA cap-binding gene ABA HYPERSENSITIVE 1 suppress FRIGIDA-mediated delayed flowering in Arabidopsis. Plant J 40: 112–119 [DOI] [PubMed] [Google Scholar]
- Borner R, Kampmann G, Chandler J, Gleissner R, Wisman E, Apel K, Melzer S. (2000) A MADS domain gene involved in the transition to flowering in Arabidopsis. Plant J 24: 591–599 [DOI] [PubMed] [Google Scholar]
- Chen Y, Yamaguchi Y, Tsugeno Y, Yamamoto J, Yamada T, Nakamura M, Hisatake K, Handa H. (2009) DSIF, the Paf1 complex, and Tat-SF1 have nonredundant, cooperative roles in RNA polymerase II elongation. Genes Dev 23: 2765–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U. (2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley KW, Haag JR, Pontes O, Opper K, Juehne T, Song K, Pikaard CS. (2006) Gateway-compatible vectors for plant functional genomics and proteomics. Plant J 45: 616–629 [DOI] [PubMed] [Google Scholar]
- Gazzani S, Gendall AR, Lister C, Dean C. (2003) Analysis of the molecular basis of flowering time variation in Arabidopsis accessions. Plant Physiol 132: 1107–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Doyle MR, Amasino RM. (2004) PAF1-complex-mediated histone methylation of FLOWERING LOCUS C chromatin is required for the vernalization-responsive, winter-annual habit in Arabidopsis. Genes Dev 18: 2774–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Michaels SD, Amasino RM. (2003) Regulation of flowering time by histone acetylation in Arabidopsis. Science 302: 1751–1754 [DOI] [PubMed] [Google Scholar]
- Hornyik C, Terzi LC, Simpson GG. (2010) The spen family protein FPA controls alternative cleavage and polyadenylation of RNA. Dev Cell 18: 203–213 [DOI] [PubMed] [Google Scholar]
- Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P. (2008) Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinformatics 2008: 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob Y, Feng S, LeBlanc CA, Bernatavichute YV, Stroud H, Cokus S, Johnson LM, Pellegrini M, Jacobsen SE, Michaels SD. (2009) ATXR5 and ATXR6 are H3K27 monomethyltransferases required for chromatin structure and gene silencing. Nat Struct Mol Biol 16: 763–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson U, West J, Lister C, Michaels S, Amasino R, Dean C. (2000) Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 290: 344–347 [DOI] [PubMed] [Google Scholar]
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D. (1999) Activation tagging of the floral inducer FT. Science 286: 1962–1965 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Hyun Y, Park JY, Park MJ, Park MK, Kim MD, Lee MH, Moon J, Lee I, Kim J. (2004) A genetic link between cold responses and flowering time through FVE in Arabidopsis thaliana. Nat Genet 36: 167–171 [DOI] [PubMed] [Google Scholar]
- Kim J, Guermah M, Roeder RG. (2010) The human PAF1 complex acts in chromatin transcription elongation both independently and cooperatively with SII/TFIIS. Cell 140: 491–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Michaels SD. (2006) SUPPRESSOR OF FRI 4 encodes a nuclear-localized protein that is required for delayed flowering in winter-annual Arabidopsis. Development 133: 4699–4707 [DOI] [PubMed] [Google Scholar]
- Kim SY, Yu X, Michaels SD. (2008) Regulation of CONSTANS and FT expression in response to changing light quality. Plant Physiol 148: 269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. (1999) A pair of related genes with antagonistic roles in mediating flowering signals. Science 286: 1960–1962 [DOI] [PubMed] [Google Scholar]
- Krogan NJ, Dover J, Wood A, Schneider J, Heidt J, Boateng MA, Dean K, Ryan OW, Golshani A, Johnston M, et al. (2003) The Paf1 complex is required for histone H3 methylation by COMPASS and Dot1p: linking transcriptional elongation to histone methylation. Mol Cell 11: 721–729 [DOI] [PubMed] [Google Scholar]
- Krogan NJ, Kim M, Ahn SH, Zhong G, Kobor MS, Cagney G, Emili A, Shilatifard A, Buratowski S, Greenblatt JF. (2002) RNA polymerase II elongation factors of Saccharomyces cerevisiae: a targeted proteomics approach. Mol Cell Biol 22: 6979–6992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Suh SS, Park E, Cho E, Ahn JH, Kim SG, Lee JS, Kwon YM, Lee I. (2000) The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes Dev 14: 2366–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Aukerman MJ, Gore SL, Lohman KN, Michaels SD, Weaver LM, John MC, Feldmann KA, Amasino RM. (1994a) Isolation of LUMINIDEPENDENS: a gene involved in the control of flowering time in Arabidopsis. Plant Cell 6: 75–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Michaels SD, Masshardt AS, Amasino RM. (1994b) The late-flowering phenotype of FRIGIDA and LUMINIDEPENDENS is suppressed in the Landsberg erecta strain of Arabidopsis. Plant J 6: 903–909 [Google Scholar]
- Lim MH, Kim J, Kim YS, Chung KS, Seo YH, Lee I, Hong CB, Kim HJ, Park CM. (2004) A new Arabidopsis gene, FLK, encodes an RNA binding protein with K homology motifs and regulates flowering time via FLOWERING LOCUS C. Plant Cell 16: 731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Marquardt S, Lister C, Swiezewski S, Dean C. (2010) Targeted 3′ processing of antisense transcripts triggers Arabidopsis FLC chromatin silencing. Science 327: 94–97 [DOI] [PubMed] [Google Scholar]
- Liu F, Quesada V, Crevillen P, Baurle I, Swiezewski S, Dean C. (2007) The Arabidopsis RNA-binding protein FCA requires a lysine-specific demethylase 1 homolog to downregulate FLC. Mol Cell 28: 398–407 [DOI] [PubMed] [Google Scholar]
- Macknight R, Bancroft I, Page T, Lister C, Schmidt R, Love K, Westphal L, Murphy G, Sherson S, Cobbett C, et al. (1997) FCA, a gene controlling flowering time in Arabidopsis, encodes a protein containing RNA-binding domains. Cell 89: 737–745 [DOI] [PubMed] [Google Scholar]
- Michaels S, Amasino R. (1999) FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11: 949–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD. (2009) Flowering time regulation produces much fruit. Curr Opin Plant Biol 12: 75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM. (2001) Loss of FLOWERING LOCUS C activity eliminates the late-flowering phenotype of FRIGIDA and autonomous pathway mutations but not responsiveness to vernalization. Plant Cell 13: 935–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, Bezerra IC, Amasino RM. (2004) FRIGIDA-related genes are required for the winter-annual habit in Arabidopsis. Proc Natl Acad Sci USA 101: 3281–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, Ditta G, Gustafson-Brown C, Pelaz S, Yanofsky M, Amasino RM. (2003a) AGL24 acts as a promoter of flowering in Arabidopsis and is positively regulated by vernalization. Plant J 33: 867–874 [DOI] [PubMed] [Google Scholar]
- Michaels SD, He Y, Scortecci KC, Amasino RM. (2003b) Attenuation of FLOWERING LOCUS C activity as a mechanism for the evolution of summer-annual flowering behavior in Arabidopsis. Proc Natl Acad Sci USA 100: 10102–10107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockler TC, Yu X, Shalitin D, Parikh D, Michael TP, Liou J, Huang J, Smith Z, Alonso JM, Ecker JR, et al. (2004) Regulation of flowering time in Arabidopsis by K homology domain proteins. Proc Natl Acad Sci USA 101: 12759–12764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller CL, Porter SE, Hoffman MG, Jaehning JA. (2004) The Paf1 complex has functions independent of actively transcribing RNA polymerase II. Mol Cell 14: 447–456 [DOI] [PubMed] [Google Scholar]
- Ng HH, Dole S, Struhl K. (2003a) The Rtf1 component of the Paf1 transcriptional elongation complex is required for ubiquitination of histone H2B. J Biol Chem 278: 33625–33628 [DOI] [PubMed] [Google Scholar]
- Ng HH, Robert F, Young RA, Struhl K. (2003b) Targeted recruitment of Set1 histone methylase by elongating Pol II provides a localized mark and memory of recent transcriptional activity. Mol Cell 11: 709–719 [DOI] [PubMed] [Google Scholar]
- Oh S, Park S, van Nocker S. (2008) Genic and global functions for Paf1C in chromatin modification and gene expression in Arabidopsis. PLoS Genet 4: e1000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Zhang H, Ludwig P, van Nocker S. (2004) A mechanism related to the yeast transcriptional regulator Paf1c is required for expression of the Arabidopsis FLC/MAF MADS box gene family. Plant Cell 16: 2940–2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Oh S, Ek-Ramos J, van Nocker S. (2010) PLANT HOMOLOGOUS TO PARAFIBROMIN is a component of the Paf1 complex and assists in regulating expression of genes within H3K27me3-enriched chromatin. Plant Physiol 153: 821–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penheiter KL, Washburn TM, Porter SE, Hoffman MG, Jaehning JA. (2005) A posttranscriptional role for the yeast Paf1-RNA polymerase II complex is revealed by identification of primary targets. Mol Cell 20: 213–223 [DOI] [PubMed] [Google Scholar]
- Pien S, Fleury D, Mylne JS, Crevillen P, Inze D, Avramova Z, Dean C, Grossniklaus U. (2008) ARABIDOPSIS TRITHORAX1 dynamically regulates FLOWERING LOCUS C activation via histone 3 lysine 4 trimethylation. Plant Cell 20: 580–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putterill J, Robson F, Lee K, Simon R, Coupland G. (1995) The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80: 847–857 [DOI] [PubMed] [Google Scholar]
- Quesada V, Macknight R, Dean C, Simpson GG. (2003) Autoregulation of FCA pre-mRNA processing controls Arabidopsis flowering time. EMBO J 22: 3142–3152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe OJ, Kumimoto RW, Wong BJ, Riechmann JL. (2003) Analysis of the Arabidopsis MADS AFFECTING FLOWERING gene family: MAF2 prevents vernalization by short periods of cold. Plant Cell 15: 1159–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe OJ, Nadzan GC, Reuber TL, Riechmann JL. (2001) Regulation of flowering in Arabidopsis by an FLC homologue. Plant Physiol 126: 122–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondon AG, Gallardo M, Garcia-Rubio M, Aguilera A. (2004) Molecular evidence indicating that the yeast PAF complex is required for transcription elongation. EMBO Rep 5: 47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt-Rosen O, Hughes CM, Nannepaga SJ, Shanmugam KS, Copeland TD, Guszczynski T, Resau JH, Meyerson M. (2005) The parafibromin tumor suppressor protein is part of a human Paf1 complex. Mol Cell Biol 25: 612–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt-Rosen O, Nagaike T, Francis JM, Kaneko S, Glatt KA, Hughes CM, LaFramboise T, Manley JL, Meyerson M. (2009) The tumor suppressor Cdc73 functionally associates with CPSF and CstF 3′ mRNA processing factors. Proc Natl Acad Sci USA 106: 755–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A, Alvarez-Venegas R, Yilmaz M, Le O, Hou G, Sadder M, Al-Abdallat A, Xia Y, Lu G, Ladunga I, et al. (2008) The highly similar Arabidopsis homologs of trithorax ATX1 and ATX2 encode proteins with divergent biochemical functions. Plant Cell 20: 568–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland G. (2000) Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288: 1613–1616 [DOI] [PubMed] [Google Scholar]
- Schomburg FM, Patton DA, Meinke DW, Amasino RM. (2001) FPA, a gene involved in floral induction in Arabidopsis, encodes a protein containing RNA-recognition motifs. Plant Cell 13: 1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scortecci K, Michaels SD, Amasino RM. (2003) Genetic interactions between FLM and other flowering-time genes in Arabidopsis thaliana. Plant Mol Biol 52: 915–922 [DOI] [PubMed] [Google Scholar]
- Scortecci KC, Michaels SD, Amasino RM. (2001) Identification of a MADS-box gene, FLOWERING LOCUS M, that represses flowering. Plant J 26: 229–236 [DOI] [PubMed] [Google Scholar]
- Sheldon CC, Burn JE, Perez PP, Metzger J, Edwards JA, Peacock WJ, Dennis ES. (1999) The FLF MADS box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 11: 445–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon KE, Mauger DM, Arndt KM. (2005) A requirement for the Saccharomyces cerevisiae Paf1 complex in snoRNA 3′ end formation. Mol Cell 20: 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GG, Dijkwel PP, Quesada V, Henderson I, Dean C. (2003) FY is an RNA 3′ end-processing factor that interacts with FCA to control the Arabidopsis floral transition. Cell 113: 777–787 [DOI] [PubMed] [Google Scholar]
- Squazzo SL, Costa PJ, Lindstrom DL, Kumer KE, Simic R, Jennings JL, Link AJ, Arndt KM, Hartzog GA. (2002) The Paf1 complex physically and functionally associates with transcription elongation factors in vivo. EMBO J 21: 1764–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S, Amasino RM. (2004) Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature 427: 159–164 [DOI] [PubMed] [Google Scholar]
- Tamada Y, Yun JY, Woo SC, Amasino RM. (2009) ARABIDOPSIS TRITHORAX-RELATED7 is required for methylation of lysine 4 of histone H3 and for transcriptional activation of FLOWERING LOCUS C. Plant Cell 21: 3257–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney K, Gerber M, Ilvarsonn A, Schneider J, Gause M, Dorsett D, Eissenberg JC, Shilatifard A. (2006) Drosophila Rtf1 functions in histone methylation, gene expression, and Notch signaling. Proc Natl Acad Sci USA 103: 11970–11974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A, Schneider J, Dover J, Johnston M, Shilatifard A. (2003) The Paf1 complex is essential for histone monoubiquitination by the Rad6-Bre1 complex, which signals for histone methylation by COMPASS and Dot1p. J Biol Chem 278: 34739–34742 [DOI] [PubMed] [Google Scholar]
- Xu L, Zhao Z, Dong A, Soubigou-Taconnat L, Renou JP, Steinmetz A, Shen WH. (2008) Di- and tri- but not monomethylation on histone H3 lysine 36 marks active transcription of genes involved in flowering time regulation and other processes in Arabidopsis thaliana. Mol Cell Biol 28: 1348–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XM, Rose A, Muthuswamy S, Jeong SY, Venkatakrishnan S, Zhao Q, Meier I. (2007) NUCLEAR PORE ANCHOR, the Arabidopsis homolog of Tpr/Mlp1/Mlp2/Megator, is involved in mRNA export and SUMO homeostasis and affects diverse aspects of plant development. Plant Cell 19: 1537–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yart A, Gstaiger M, Wirbelauer C, Pecnik M, Anastasiou D, Hess D, Krek W. (2005) The HRPT2 tumor suppressor gene product parafibromin associates with human PAF1 and RNA polymerase II. Mol Cell Biol 25: 5052–5060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Xu Y, Tan EL, Kumar PP. (2002) AGAMOUS-LIKE 24, a dosage-dependent mediator of the flowering signals. Proc Natl Acad Sci USA 99: 16336–16341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ransom C, Ludwig P, Van Nocker S. (2003) Genetic analysis of early flowering mutants in Arabidopsis defines a class of pleiotropic developmental regulator required for expression of the flowering-time switch flowering locus C. Genetics 164: 347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, van Nocker S. (2002) The VERNALIZATION INDEPENDENCE 4 gene encodes a novel regulator of FLOWERING LOCUS C. Plant J 31: 663–673 [DOI] [PubMed] [Google Scholar]
- Zhang X, Bernatavichute YV, Cokus S, Pellegrini M, Jacobsen SE. (2009a) Genome-wide analysis of mono-, di- and trimethylation of histone H3 lysine 4 in Arabidopsis thaliana. Genome Biol 10: R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sikes ML, Beyer AL, Schneider DA. (2009b) The Paf1 complex is required for efficient transcription elongation by RNA polymerase I. Proc Natl Acad Sci USA 106: 2153–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Mandal SS, Pham AD, Zheng Y, Erdjument-Bromage H, Batra SK, Tempst P, Reinberg D. (2005) The human PAF complex coordinates transcription with events downstream of RNA synthesis. Genes Dev 19: 1668–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.