Abstract
The FLAGELLIN-SENSING2 (FLS2) receptor kinase recognizes bacterial flagellin and initiates a battery of downstream defense responses to reduce bacterial invasion through stomata in the epidermis and bacterial multiplication in the apoplast of infected plants. Recent studies have shown that during Pseudomonas syringae pv tomato (Pst) DC3000 infection of Arabidopsis (Arabidopsis thaliana), FLS2-mediated immunity is actively suppressed by effector proteins (such as AvrPto and AvrPtoB) secreted through the bacterial type III secretion system (T3SS). We provide evidence here that T3SS effector-based suppression does not appear to be sufficient to overcome FLS2-based immunity during Pst DC3000 infection, but that the phytotoxin coronatine (COR) produced by Pst DC3000 also plays a critical role. COR-deficient mutants of Pst DC3000 are severely reduced in virulence when inoculated onto the leaf surface of wild-type Columbia-0 plants, but this defect was rescued almost fully in fls2 mutant plants. Although bacteria are thought to carry multiple microbe-associated molecular patterns, stomata of fls2 plants are completely unresponsive to COR-deficient mutant Pst DC3000 bacteria. The responses of fls2 plants were similar to those of the Arabidopsis G-protein alpha subunit1-3 mutant, which is defective in abscisic acid-regulated stomatal closure, but were distinct from those of the Arabidopsis non-expressor of PR genes1 mutant, which is defective in salicylic acid-dependent stomatal closure and apoplast defense. Epistasis analyses show that salicylic acid signaling acts upstream of abscisic acid signaling in bacterium-triggered stomatal closure. Taken together, these results suggest a particularly important role of FLS2-mediated resistance to COR-deficient mutant Pst DC3000 bacteria, and nonredundant roles of COR and T3SS effector proteins in the suppression of FLS2-mediated resistance in the Arabidopsis-Pst DC3000 interaction.
Stomata are microscopic pores formed by pairs of guard cells in the epidermis of terrestrial plants; they are essential for CO2 and water exchange with the environment. Plants regulate the stomatal aperture in response to changing abiotic environmental conditions (e.g. light, humidity, CO2 concentration) to optimize CO2 uptake and water transpiration. The molecular mechanisms underlying the stomatal regulation in response to abiotic signals are a subject of intense studies. Research in this area has uncovered many signaling components, indicating that stomatal guard cells have one of the most dynamic regulatory networks in plants (Schroeder et al., 2001; Shimazaki et al., 2007; Neill et al., 2008; Wang and Song, 2008).
Stomatal openings are also a major route of pathogen entry into the plant (Melotto et al., 2006). Accordingly, guard cells have developed mechanisms to regulate stomatal aperture in response to pathogens. Melotto and colleagues found that the bacterial pathogen Pseudomonas syringae pv tomato (Pst) strain DC3000 induces stomatal closure in Arabidopsis (Arabidopsis thaliana) within 1 h post inoculation. However, after 3 to 4 h, stomata reopen (Melotto et al., 2006). The ability of Pst DC3000 to reopen stomata is dependent on the polyketide toxin coronatine (COR), a virulence factor that had previously been shown to be important for bacterial multiplication within the mesophyll space, disease symptom development, and induction of systemic susceptibility of infected plants (Mittal and Davis, 1995; Bender et al., 1999; Budde and Ullrich, 2000; Brooks et al., 2004; Cui et al., 2005; Melotto et al., 2008b). Stomatal reopening by Pst DC3000 was also shown to be dependent on the RPM1-INTERACTING PROTEIN4 in Arabidopsis (Liu et al., 2009). Recently, another bacterial pathogen, Xanthomonas campestris pv campestris, was shown to cause stomatal closure and subsequent reopening during infection (Gudesblat et al., 2009). In this case, a virulence factor of smaller than 2 kD was identified, but the molecular identity of this virulence factor is not yet known. In fungal pathogens, examples of virulence factors that inhibit stomatal closure include fusicoccin (Turner and Graniti, 1969; Assmann and Schwartz, 1992; Kinoshita and Shimazaki, 2001) and oxalic acid (Guimaraes and Stotz, 2004), although their role in pathogen invasion has not been established.
Stomatal guard cells also respond to purified microbe-associated molecular patterns (MAMPs), such as chitosan, a polymer of β-1,4-glucosamine residues derived from fungal chitin (Lee et al., 1999; Amborabe et al., 2008), flg22, a 22-amino acid peptide derived from bacterial flagellin (Melotto et al., 2006; Cho et al., 2008; Desikan et al., 2008; Zhang et al., 2008), and bacterial lipopolysaccharides (LPSs; Melotto et al., 2006; Cho et al., 2008). Peptidoglycan, derived from Gram-positive bacteria, is shown to be able to induce plant innate immune responses (Gust et al., 2007; Erbs et al., 2008). However, peptidoglycan has not yet been shown to trigger stomatal responses. MAMPs are recognized by plant pattern-recognition receptors, such as Arabidopsis proteins FLAGELLIN-SENSING2 (FLS2) that recognizes bacterial flagellin (Gómez-Gómez and Boller, 2000), EF-TU RECEPTOR (EFR) that recognizes bacterial elongation factor TU (Zipfel et al., 2006), and CHITIN ELICITOR RECEPTOR KINASE1 (CERK1) that perceives an unknown MAMP from Pst DC3000 (Gimenez-Ibanez et al., 2009a, 2009b). In the case of flg22-induced stomatal closure, FLS2 is required (Melotto et al., 2006). Stomata from fls2 mutant plants, however, still respond to purified LPS (Melotto et al., 2006), illustrating both specificity in MAMP recognition by guard cells and the capacity of guard cells to recognize multiple MAMPs (Melotto et al., 2006). However, it has not been formally proven that the perception of any individual MAMPs actually contributes to stomatal closure induced by live bacteria, as bacteria produce many other factors in the context of an infection.
Studies using purified MAMPs have shown that stomatal closure in response to biotic signals requires the phytohormone abscisic acid (ABA), the guard cell-specific OPEN STOMATA1 (OST1) kinase, the production of reactive oxygen species and nitric oxide, the heterotrimeric G protein, and the regulation of K+ channels—all of which are hallmarks of abiotic signal-induced stomatal closure (Melotto et al., 2006; Neill et al., 2008; Zhang et al., 2008). These findings suggest that the guard cell signal transductions in response to biotic and abiotic signals share common steps. Besides shared signaling components, however, MAMP-triggered stomatal closure also requires the plant defense hormone salicylic acid (SA; Melotto et al., 2006). At present, it is not clear whether SA per se or a downstream signaling component, such as the NON-EXPRESSOR OF PR GENES1 (NPR1), is required for stomatal closure. Nor do we understand the epistatic relationship between SA and ABA signaling in the regulation of bacterium/MAMP-triggered stomatal closure.
In this study, we conducted experiments to further characterize stomatal regulation during Pst DC3000 infection of Arabidopsis plants. In particular, we sought to determine (1) whether the perception of well-documented MAMPs indeed contributes to stomatal closure in response to live bacteria, (2) the roles of the heterotrimeric G protein (involved in ABA signaling) and NPR1 (involved in SA signaling) in stomatal response during bacterial infection, and (3) the relationship between SA signaling and ABA signaling in regulating bacterium-triggered stomatal closure. These experiments revealed a critical role of FLS2 in mediating disease resistance against COR-deficient mutant Pst DC3000 bacteria.
RESULTS
Restoration of Virulence of Pst DC3000 COR-Deficient Mutants in fls2 Mutant Arabidopsis Plants
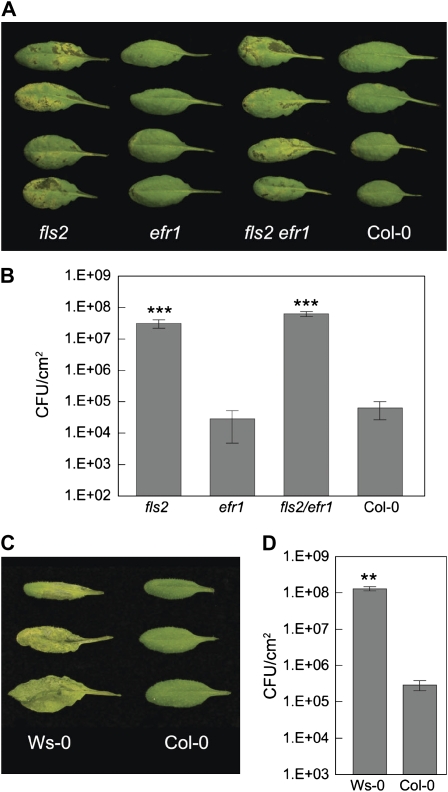
Although purified bacterial MAMPs, such as flg22 and LPS, have been shown to cause stomatal closure (Melotto et al., 2006), it is not known whether they have a relevant biological role in mediating stomatal closure during actual bacterial infection. We hypothesized that if a MAMP plays a substantial role in mediating bacterium-triggered stomatal closure, the corresponding MAMP receptor mutant plants might be able to rescue the virulence defect of COR-deficient mutants, which are unable to overcome stomatal defense. To test this hypothesis, we dip inoculated two well-characterized MAMP receptor kinase mutant plants of Arabidopsis, fls2 and efr-1, with COR-deficient mutants Pst DC3118 (Moore et al., 1989; Ma et al., 1991) and DB29 (Brooks et al., 2004) at the concentration of 1E + 8 colony-forming units (CFU)/mL (Katagiri et al., 2002). We did not observe restoration of the virulence of COR-deficient mutants in efr-1 mutant plants. The disease symptoms and growth of Pst DC3118 (Fig. 1, A and B) and DB29 (Supplemental Fig. S1) were similar in efr-1 mutant plants and in Columbia-0 (Col-0) plants. In contrast, fls2 mutant plants showed severe disease symptoms and allowed the COR-deficient mutants to multiply to levels comparable to that of wild-type Pst DC3000 in wild-type Col-0 plants (Fig. 1, A and B; Supplemental Fig. S1). This observation is consistent with a study published earlier showing that fls2 mutant plants allow spray-inoculated COR-deficient Pst DC3000 to multiply to a higher level (about 10-fold) compared to Col-0 plants (Nekrasov et al., 2009). This restoration of virulence of COR-deficient mutant bacteria in fls2 plants suggests that the lack of functional FLS2 in plants could compensate for the loss of COR production in Pst DC3118 and DB29.
Figure 1.
fls2 and Ws-0 plants exhibit enhanced susceptibility to COR-deficient mutant bacteria. Leaf appearances (A and C) and bacterial populations (B and D) 3 d after surface inoculation with Pst DC3118 at 1E + 8 CFU/mL. Results are displayed as means of four different leaves from four different plants, with ses indicated. Statistical differences are detected with ANOVA (P = 0.0001) followed by Turkey's multiple comparison test (showing comparisons to Col-0; ***, P < 0.001) for B, and with two-tailed t test (**, P < 0.01) for D.
To further confirm our observation, we tested the Wassilewskija (Ws-0) ecotype plants. It was previously shown that Ws-0 does not have a functional FLS2 and therefore is a natural fls2 mutant allele (Bauer et al., 2001; Gómez-Gómez and Boller, 2002; Kunze et al., 2004; Zipfel et al., 2004; Chinchilla et al., 2006). In our experiments, this ecotype behaved similarly to fls2 plants, showing strong disease symptoms and allowing aggressive multiplication of COR-deficient mutant Pst DC3118 after dip inoculation (Fig. 1, C and D).
Next, we examined the susceptibility of the fls2 efr-1 double-mutant plants to Pst DC3118 to determine whether there is any synergistic effect between the two MAMP receptor mutations. The double-mutant plants behaved similarly to the fls2 single-mutant plants (Fig. 1, A and B), suggesting that it is FLS2, not EFR, that plays a decisive role in mediating Arabidopsis resistance to COR-deficient mutants of Pst DC3000.
fls2 Plants Are Defective in Stomatal Closure in Response to Live COR-Deficient Mutant Bacteria
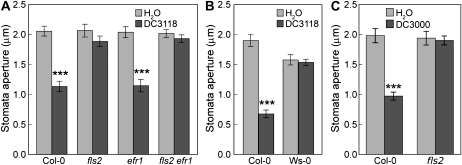
It is known that Arabidopsis stomata respond to several purified bacterial MAMPs, such as flg22, LPS, and elf26 (a bioactive peptide derived from EF-TU; Melotto et al., 2006; Cho et al., 2008; Desikan et al., 2008; Gudesblat et al., 2009), suggesting that Arabidopsis stomatal guard cells can perceive multiple MAMPs. It is surprising, then, that the virulence of Pst DC3000 COR-deficient mutants, which presumably carry multiple MAMPs, is almost fully restored in fls2 mutant plants that are defective in only one MAMP receptor (Fig. 1, A and B). We considered the possibility that flagellin perception by FLS2 may be the primary recognition event during the initial Pst DC3000 invasion through stomata. If so, one would expect that fls2 mutant stomata would not respond to COR-deficient mutant bacteria, even though these bacteria have the potential to release multiple MAMPs. We examined this possibility by conducting stomatal assays with live Pst DC3118 bacteria. The fls2 single mutant, the Ws-0 ecotype, and the fls2 efr-1 double-mutant plants were all impaired in the stomatal closure response to Pst DC3118, whereas efr-1 mutant plants retained the ability to close stomata in a manner similar to that of wild-type Col-0 (Fig. 2). We also tested fls2 plants with the wild-type Pst DC3000 bacteria. Again, stomata of fls2 plants did not show a closure response to the bacteria (Fig. 2C). These results confirm that FLS2 plays an essential role in mediating the stomatal closure response to Pst DC3000 bacteria in Arabidopsis.
Figure 2.
Stomatal closure responses to COR-deficient or wild-type bacteria. Stomatal apertures from leaf peels of Col-0, fls2, efr-1, and fls2 efr-1 plants treated with water or Pst DC3118 at 1E + 8 CFU/mL (A), Col-0 and Ws-0 plants treated with water or Pst DC3118 at 1E + 8 CFU/mL (B), and Col-0 and fls2 plants treated with water or Pst DC3000 at 1E + 8 CFU/mL (C). Results are displayed as means of 30 to 60 stomata, with ses indicated. Statistical differences between water and bacterial treatment are detected with two-tailed t test (***, P < 0.001). In all our experiments, to preserve the stomatal aperture status in plants used for both stomatal and bacterial pathogenesis assays, we did not further treat leaf peels in any stomatal opening buffer.
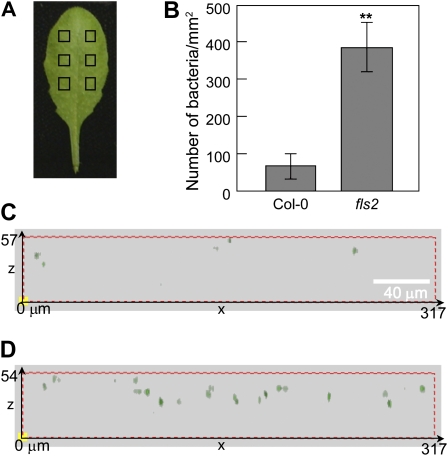
To directly visualize the effect of the fls2 mutation on bacterial entry into Arabidopsis leaves, we quantified bacteria that are inside leaves 1 h after surface inoculation with GFP-labeled Pst DC3118 using confocal microscopy. We found that there were already about 6 times more bacteria inside fls2 leaves compared to wild-type Col-0 leaves at this early time point (Fig. 3; Supplemental Fig. S2).
Figure 3.
Entry of Pst DC3118-GFP into Col-0 and fls2 leaves detected by confocal microscopy. A, A Col-0 leaf with sampling areas indicated by squares. B, Numbers of bacteria observed, showing means from six microscopic views (0.1 mm2 each) with ses. Statistical difference is detected with a two-tailed t test (**, P < 0.01). C and D, Side views of three-dimensional models reconstructed from overlaid z-sections of GFP channel in Col-0 and fls2 leaves, respectively. The dimensions of the leaf section (in μm) of axis (x, z) are labeled. Please note that GFP-labeled bacteria (green dots) are inside the leaves.
FLS2-Mediated Apoplast Defense Does Not Play a Major Role in Restricting Infection of COR-Deficient Mutant Bacteria
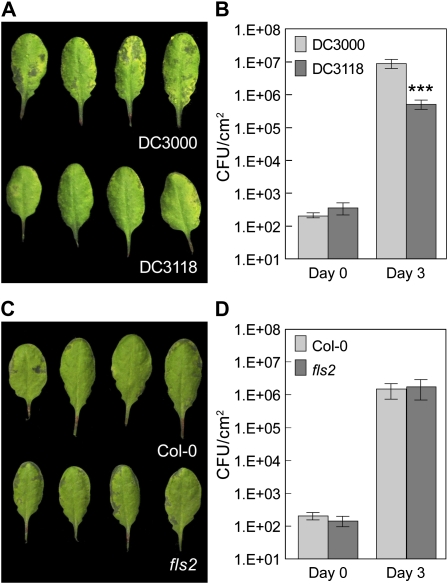
When infiltrated directly into the Arabidopsis leaf apoplast (i.e. bypassing the leaf epidermis) at the inoculum concentration of 1E + 6 CFU/mL, Pst DC3000 COR-deficient mutants multiplied similarly as Pst DC3000 (Mittal and Davis, 1995; Melotto et al., 2006). Furthermore, disease symptom development in infiltration experiments with 1E + 6 CFU/mL Pst DC3000 most closely resembles symptom development in dipping experiments using 1E + 8 CFU/mL Pst DC3000. Interestingly, in infiltration experiments with lower inoculum concentrations, COR-deficient mutants multiplied less than wild-type Pst DC3000 in Arabidopsis (Brooks et al., 2004, 2005; Fig. 4, A and B). These results suggest that COR has roles in not only facilitating bacterial invasion through the epidermis, but also bacterial growth in the apoplast in the Pst DC3000-Arabidopsis interaction. The restoration of virulence of COR-deficient mutants of Pst DC3000 in fls2 plants, as revealed by dip inoculation (Fig. 1), could therefore result from a defect in not only FLS2-mediated defense during bacterial invasion, but also post invasion in the apoplast or a combination of both. To determine the relative contributions of these possible defects, we measured the multiplication of Pst DC3118 in fls2 plants by infiltrating bacteria directly into the Arabidopsis leaf apoplast. At the inoculum of 1E + 5 CFU/mL, fls2 and Col-0 plants showed similarly high susceptibility to Pst DC3118 (Figs. 4, C and D, and 5C). Although variations were observed in some experiments, the difference of susceptibility between fls2 and Col-0 plants was less than 5-fold. This suggests that the FLS2-mediated, post-entry defense in the apoplast does not play a major role in restricting infection by COR-deficient mutant Pst DC3118 bacteria.
Figure 4.
Susceptibility of plants to Pst DC3000 or Pst DC3118 when inoculated by infiltration at 1E + 5 CFU/mL. A, Leaf appearance of Col-0 plants 3 d after bacterial infiltration. B, Bacterial population in Col-0 leaves at day 0 and day 3 after infiltration. C, Leaf appearance (abaxial sides) 3 d after infiltration with Pst DC3118. D, Bacterial populations at day 0 and day 3 after infiltration with Pst DC3118. Results displayed here (B and D) are means of four different leaves from four different plants, with ses indicated. Statistical differences are detected with two-tailed t test (***, P < 0.001).
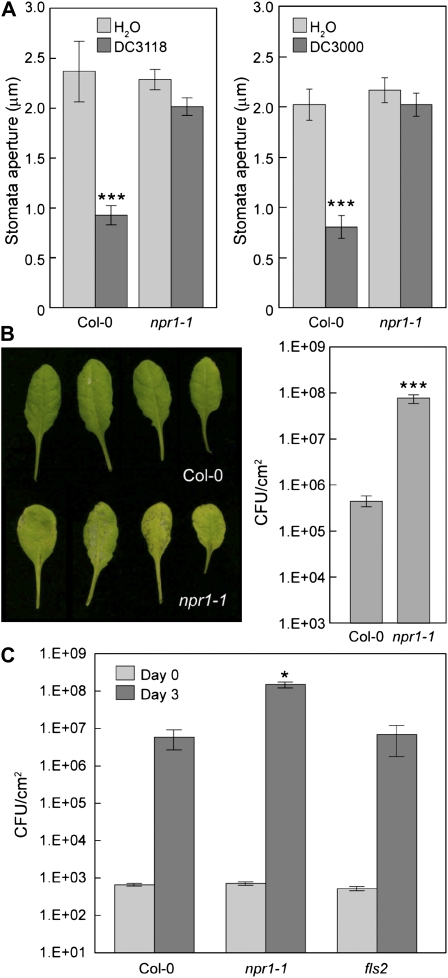
Figure 5.
Role of NPR1 in plant response to COR-deficient or wild-type Pst DC3000 bacteria. A, Stomatal apertures in Col-0 and npr1-1 leaf peels incubated with Pst DC3118 or DC3000 at 1E + 8 CFU/mL. Results are displayed as means of 30 to 60 stomata with ses shown. B, Leaf appearance and bacterial populations 3 d after dip inoculation of Col-0 and npr1-1 plants with Pst DC3118 at 1E + 8 CFU/mL. C, Bacterial populations at day 3 in leaves of Col-0, npr1-1, and fls2 plants infiltrated with Pst DC3118 at 1E + 5 CFU/mL. B and C, Results are displayed as means of four different leaves from four different plants, with ses shown. Statistical differences are detected with two-tailed t test (***, P < 0.001) for A and B, and with ANOVA (P = 0.014) followed by Turkey's multiple comparison test (showing comparisons to Col-0; *, P < 0.05) for C.
Differences in FLS2- and SA-Mediated Resistance against COR-Deficient Mutant Bacteria
COR-deficient mutants of Pst DC3000 can multiply efficiently in SA-deficient nahG and eds16/sid2 plants (Brooks et al., 2005; Melotto et al., 2006). These plants are also defective in the stomatal closure response to Pst DC3000 COR-deficient mutant bacteria (Melotto et al., 2006). However, it is not known whether it is SA per se or SA signaling or both that is required for stomatal defense. Moreover, it has not been determined whether the increased susceptibility of SA-deficient plants to COR-deficient mutant bacteria results from a defect in stomatal defense, SA-mediated apoplast defense, or both. We tested the requirement of NPR1, a key regulator of SA signaling, for stomatal closure response and resistance to COR-deficient mutant bacteria. We found that npr1-1 plants were defective in the stomatal closure response to COR-deficient Pst DC3118 as well as wild-type Pst DC3000 bacteria (Fig. 5A). When dip inoculated, npr1-1 plants showed much enhanced susceptibility to Pst DC3118 (Fig. 5B). Contrary to fls2 plants, however, npr1-1 plants also showed higher susceptibility to DC3118 than wild-type Col-0 plants when bacteria were infiltrated directly into the leaf apoplast (Fig. 5C). These results suggest that NPR1 is involved not only in stomatal defense, but also in post-entry defense in the apoplast in the context of infection by COR-deficient mutant Pst DC3000 bacteria.
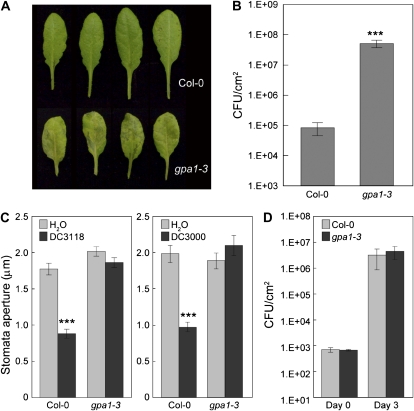
Similarity of FLS2-Mediated Resistance with G-Protein α-Subunit1-Mediated Resistance against COR-Deficient Mutant Bacteria
A recent study has shown that the heterotrimeric G-protein α-subunit1 (GPA1) is required for stomatal closure in response to flg22 (Zhang et al., 2008). However, whether the virulence defect of COR-deficient Pst DC3000 mutant bacteria can also be restored in the gpa1 mutant plants has not been determined. Therefore, we examined the susceptibility of the gpa1-3 mutant plants to Pst DC3118 and found that the responses of gpa1-3 mutant plants were strikingly similar to those of fls2 mutant plants (Fig. 6). First, gpa1-3 mutant plants are highly susceptible to Pst DC3118 when dip inoculated, showing prominent disease symptoms (Fig. 6A) and allowing high levels of bacterial multiplication (Fig. 6B). Second, like fls2 plants, gpa1-3 plants are also defective in stomatal closure response to COR-deficient Pst DC3118 as well as wild-type Pst DC3000 bacteria (Fig. 6C). Finally, unlike npr1-1 plants, gpa1-3 plants did not show significantly increased susceptibility to Pst DC3118 compared with wild-type Col-0 plants, when bacteria were infiltrated directly into the leaf apoplast, bypassing the epidermis (Fig. 6D). These results show that, like FLS2, GPA1 contributes to Arabidopsis resistance to COR-deficient mutant Pst DC3000 bacteria primarily through modulating stomatal defense.
Figure 6.
The involvement of GPA1 in response to COR-deficient or wild-type Pst DC3000 bacteria. Leaf appearance of (A) and bacterial populations in (B) Col-0 and gpa1-3 plants dip inoculated with Pst DC3118 at 1E + 8 CFU/mL. C, Stomatal apertures of Col-0 and gpa1-3 leaf peels treated with water and Pst DC3118 or DC3000 at 1E + 8 CFU/mL. D, Bacterial populations at day 0 and day 3 in leaves of Col-0 and gpa1-3 plants infiltrated with Pst DC3118 at 1E + 5 CFU/mL. Results are displayed as means with ses from four different leaves of four different plants for B and D, and of 30 to 60 stomata for C. Statistical differences are detected with two-tailed t test (***, P < 0.001).
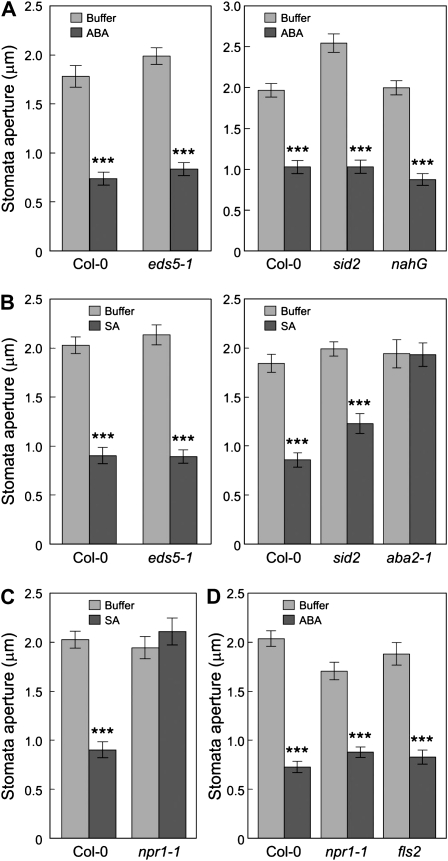
SA Acts Upstream of ABA through NPR1 in the Stomatal Closure Signaling Pathway
Our previous and current studies show an important role for ABA and SA in regulating the stomatal closure response to bacteria and MAMPs. However, the epistatic relationship between SA and ABA in such regulation is not clear. We examined this relationship and found that SA-deficient eds5-1, eds16/sid2, and nahG plants showed normal stomatal closure responses to exogenous ABA (Fig. 7A). On the other hand, exogenously applied SA could induce stomatal closure responses of SA-deficient mutant plants eds5-1 and sid2, but it could not induce stomatal closure in the ABA biosynthetic mutant aba2-1 (Fig. 7B). These results indicate that SA acts upstream of ABA in the signaling pathway, leading to bacterium-triggered stomatal closure.
Figure 7.
SA acts upstream of ABA in stomatal closure response. Data shown here are stomatal apertures of Col-0, eds5-1, sid2, and nahG leaf peels treated with buffer or ABA (10 μm; A); Col-0, eds5-1, sid2, and aba2-1 leaf peels treated with buffer or SA (20 μm; B); Col-0 and npr1-1 leaf peels treated with buffer or SA (20 μm; C); and Col-0, npr1-1, and fls2 leaf peels treated with buffer or ABA (10 μm; D). All results are displayed as means of 30 to 60 stomata, with ses indicated. Statistical differences between chemical treatments and buffer controls are detected with two-tailed t test (***, P < 0.001).
Exogenous SA induced stomatal closure in wild-type Col-0 plants, but it could not induce stomatal closure in npr1-1 plants (Fig. 7C). In contrast, exogenous ABA was still able to induce stomatal closure in npr1-1 as well as in fls2 plants (Fig. 7D). These results indicate that NPR1 acts downstream of SA, but upstream of ABA, in stomatal guard cell signaling.
Requirement of Distinct Signaling Components for FLS2-Mediated Stomatal Closure and Plant Growth Inhibition
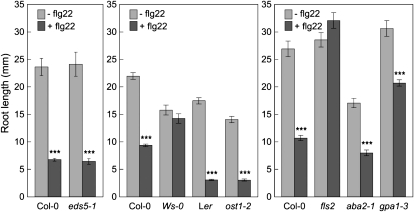
In addition to mediating innate immunity, flagellin and flg22 are also able to inhibit root elongation and seedling growth (Gómez-Gómez et al., 1999). We were interested in knowing whether induction of stomatal closure and inhibition of root elongation and plant growth by FLS2 perception of flg22 share the same components.
SA-deficient, ABA-deficient, fls2, and gpa1-3 mutant plants were examined for their responses to flg22-mediated inhibition of root elongation. As expected, root elongation of wild-type Col-0 plants, but not the fls2 mutant plants or the Ws-0 ecotype plants, was inhibited by the presence of peptide flg22 (Fig. 8). However, inhibition of root elongation was also observed for all the other mutants tested, including eds5-1 (defective in SA biosynthesis), aba2-1 (defective in ABA biosynthesis), ost1-2 (defective in ABA signaling), and gpa1-3 (defective in heterotrimeric G-protein function; Fig. 8). Thus, FLS2 perception of flg22 appears to initiate multiple and distinct signaling pathways. Whereas the stomatal closure pathway involves SA and ABA, the pathway leading to the inhibition of root elongation does not. However, the inhibition of root elongation by flg22 in gpa1-3 plants was not as dramatic as in other mutants. We further tested gpa1-3 and Col-0 plants with lower concentrations of the flg22 peptide. Again, inhibition of root elongation by flg22 in gpa1-3 plants was not as much as in Col-0 (Supplemental Fig. S3). Therefore, GPA1 not only participates in stomatal closure response, but also seems to play some role in flg22-induced root growth inhibition.
Figure 8.
Root elongation response to flg22 treatment. Data shown here are root lengths of wild-type plants Col-0 and Ler; mutant plants eds5-1 (SA synthesis), ost1-2 (ABA signaling, Ler background), aba2-1 (ABA synthesis), and gpa1-3 (α-subunit of the heterotrimeric G protein, ABA signaling) that were grown in liquid Murashige and Skoog medium for 10 d in the presence or absence of flg22 (10 μm). Results are displayed as means of 15 to 24 plants, with ses indicated. Statistical differences between treatments with or without flg22 are detected with two-tailed t test (***, P < 0.001).
DISCUSSION
Previous studies have shown that both wild-type and flagellin-lacking Pst DC3000, when infiltrated into the apoplast, elicit the expression of largely the same set of MAMP-responsive genes in Arabidopsis (Thilmony et al., 2006), and that fls2 mutant plants still respond to infiltration with MAMPs released from Pst DC3000 lysates (Zipfel et al., 2004). Both findings suggest that Pst DC3000 produces MAMPs other than flagellin, and that Arabidopsis leaf cells (at least the mesophyll cells inside the apoplast) are capable of perceiving multiple MAMPs. It has also been shown that stomatal guard cells of Arabidopsis can respond to more than one MAMP (Melotto et al., 2006; Cho et al., 2008; Desikan et al., 2008; Gudesblat et al., 2009). Therefore, it was surprising to observe that (1) stomata in fls2 plants are completely defective in response to Pst DC3000 and its COR-deficient mutants (Fig. 2), as if Pst DC3000 bacteria carry only a single MAMP—flagellin, and (2) Pst DC3000 COR-deficient mutant bacteria are able to multiply aggressively and cause disease symptoms in fls2 mutant plants when surface inoculated (Fig. 1). These observations seem to contradict the idea that multiple MAMP-receptor interactions are at work in the Arabidopsis-Pst DC3000 interaction and suggest that not all potential MAMPs from a given pathogen are presented simultaneously to all plant cell types during the course of an infection. As shown in this study, FLS2-mediated resistance against COR-deficient Pst DC3000 mutant bacteria seems to be manifested mainly at the level of epidermal stomatal defense. It may be that for bacteria to invade through stomata, they must be living and they must possess flagella. At this early stage of infection, other MAMPs (particularly those that are likely released from dead bacteria) may not be present at sufficient concentrations near guard cells to affect stomatal movements. At subsequent stages of infection within the plant apoplast, on the other hand, many different MAMPs, derived from live or dead bacteria, may accumulate to sufficient concentrations to elicit defenses from mesophyll cells, which could mask the unique role of FLS2-mediated resistance in the apoplast.
Alternatively, Pst DC3000 releases multiple MAMPs during infection, but most Pst DC3000-derived MAMPs may not be as potent as flagellin in eliciting defense responses in Arabidopsis. This possibility is supported by earlier observations that, at the same concentration (1 μm), flg22 (derived from the conserved N terminus of eubacterial flagellins; Felix et al., 1999) is more potent than elf18 (derived from Escherichia coli; Kunze et al., 2004) to induce the oxidative burst in Arabidopsis leaves (Zipfel et al., 2006), and elf26 peptides from Agrobacterium tumefaciens and Erwinia amylovora are 50 times more potent to induce medium alkalinization of Arabidopsis cell culture than elf26 from Pst DC3000 (Kunze et al., 2004). Consistent with this observation, our stomata assays showed that the E. coli-derived elf18 is much more potent than the corresponding peptide from Pst in inducing stomata closure in Arabidopsis Col-0 leaf epidermis (Supplemental Fig. S4). This observation might explain why fls2 mutant plants are unable to close stomata in response to Pst DC3118 or DC3000 (Fig. 2), but still responded to E. coli (Melotto et al., 2006). Interestingly, when inoculated by spraying with Pst DC3000 at very high inoculums (1E + 9 CFU/mL), efr-1 mutant plants showed higher susceptibility than wild-type Col-0 plants (Saijo et al., 2009). On the other hand, Nekrasov and colleagues (2009) showed that the efr-1 mutant plants were not more susceptible than wild-type Col-0 plants to wild-type Pst DC3000 or COR-deficient mutant bacteria when spray inoculated at the concentration of 1E + 7 CFU/mL. Similar susceptibilities of efr-1 and Col-0 plants to spray inoculation with 1E + 7 CFU/mL of Pst DC3000 or COR-deficient mutant bacteria were also observed by Li et al. (2009). It seems that only at the high inoculums of 1E + 9 CFU/mL can the concentration of ET-Tu derived from Pst DC3000 bacteria reach a high enough level that the unique contribution of the EFR signaling pathway can be detected in Arabidopsis. Interestingly, flagellin perception has been shown to be critical for Pst DC3000 infection of Nicotiana benthamiana, likely because this plant lacks a functional EFR (Kvitko et al., 2009).
Although we focused on COR-deficient Pst DC3000 mutant bacteria in this study, several studies have shown a role of FLS2-mediated immunity in resistance to wild-type Pst DC3000 (Zipfel et al., 2004; Zhang et al., 2007; Xiang et al., 2008; Clay et al., 2009). However the degrees of enhanced susceptibility of fls2 mutant plants to wild-type Pst DC3000 are generally subtle and variable, ranging from approximately 2- to 10-fold (Zipfel et al., 2004; Sun et al., 2006; Heese et al., 2007; Göhre et al., 2008; Xiang et al., 2008; Clay et al., 2009; Gimenez-Ibanez et al., 2009a; Nekrasov et al., 2009). This variability likely reflects different plant growth and experimental conditions used in these studies. In our own experiments, although significantly enhanced disease symptoms in fls2 mutant plants are observed consistently compared to wild-type Col-0 plants following dip inoculation, the enhanced Pst DC3000 multiplication in fls2 plants was small and variable in different experiments. Results from a representative experiment (about a 3-fold difference at day 2 after inoculation) are shown in Supplemental Figure S5. Under the same conditions, however, the enhanced multiplication of COR-deficient mutant Pst DC3118 was dramatic, measuring 100-fold or higher (Fig. 1). A prevailing explanation for the relatively small contribution of FLS2 to Arabidopsis resistance to Pst DC3000 is that this pathogen produces two type III secretion system (T3SS) effectors, AvrPto and AvrPtoB, that suppress the function of FLS2 and related receptor kinases, such as BAK1 and CERK1 (Shan et al., 2008; Xiang et al., 2008; Gimenez-Ibanez et al., 2009a). The striking FLS2-dependent virulence defect of Pst DC3000 COR-deficient mutants observed in this study, however, suggests that AvrPto and AvrPtoB are not sufficient to suppress FLS2-mediated immunity in the absence of COR. Instead, COR and T3SS effectors are both required for completely disabling the FLS2-mediated resistance mechanism during Pst DC3000 infection of Arabidopsis.
While the requirement of both COR and T3SS effectors for suppressing FLS2-mediated resistance highlights the prominent role of FLS2 in disease resistance, it raises a conceptual issue regarding the functional relationship between various types of virulence factors. As a foliar pathogen, Pst DC3000 encounters different host cell types in a temporal and spatial manner. When inoculated onto the plant surface, Pst DC3000 bacteria first make contact and interact with the epidermal cells (including stomatal guard cells) before they enter the plant apoplast and interact with internal mesophyll cells. Because COR is a small, diffusible molecule, it has the potential to reach many host cells from the site of production. In contrast, T3SS effectors seem to be delivered into only those host cells that are in direct contact with bacteria. With different delivery mechanisms, the two classes of bacterial virulence factors may not reach the same host cells at the same time during every stage of infection. This might explain why Pst DC3000 requires both COR and T3SS effectors AvrPto and AvrPtoB to suppress FLS2-mediated resistance during a presumably multistage infection process that involves distinct host cell types. As already discussed, FLS2-mediated resistance against Pst DC3000 COR-deficient mutant bacteria appears to be manifested mainly at the epidermis. This observation implies that T3SS effectors such as AvrPto and AvrPtoB are not able to substitute for COR in suppressing FLS2-mediated stomatal closure (and possibly other defenses) in the epidermis. Unlike the diffusible COR, AvrPto and AvrPtoB are perhaps not delivered into stomatal guard cells and other epidermal cells in sufficient amounts during early stages of infection. On the other hand, the lack of a major contribution of FLS2-mediated resistance against Pst DC3000 COR-deficient mutant bacteria in the apoplast suggests that the role of COR in suppressing FLS2-mediated resistance in this space is likely redundant to and masked by that of effector proteins such as AvrPto and AvrPtoB.
Extending the previous finding of the important role of SA and ABA in bacterium- and MAMP-triggered stomatal closure, this study demonstrates the involvement of two signaling components, NPR1 and GPA1, and defines the epistatic relationship between SA and ABA signaling in this process (Fig. 7). Although the involvement of NPR1 and GPA1 in bacterium-triggered stomatal closure is not entirely surprising, the demonstrated requirement of NPR1 does expand the role of this key SA signaling regulator to an important plant cell type (the guard cell) and raises a pertinent issue about the function of NPR1. NPR1 is best known for controlling the expression of SA-responsive genes (Wang et al., 2005), and a recent study shows that MAMP signaling later leads to expression of SA response genes in Arabidopsis leaves (Tsuda et al., 2008). Therefore, the requirement of NPR1 for bacterium-triggered stomatal closure could imply an involvement of NPR1-controlled gene expression in stomatal regulation. We examined NPR1-regulated genes from the publicly available microarray data (Blanco et al., 2005; Wang et al., 2005; Tsuda et al., 2008; Blanco et al., 2009), but could not identify any genes that are known to be involved in stomatal regulation. However, these gene expression analyses were performed many hours after chemical or pathogen treatments of whole leaves, which are composed mainly of mesophyll cells. Further experiments will be necessary to determine whether there are NPR1-regulated genes in stomatal guard cells within a shorter duration (e.g. minutes) of treatments with bacteria or MAMPs. Until such genes are identified and confirmed to be required for bacterium-triggered stomatal closure, it remains possible that the npr1 mutant guard cells may be altered in some signaling/metabolic networks that affect stomatal closure independent of gene expression. The epistasis analyses performed in this study place the action of NPR1 between downstream of SA and upstream of ABA (Fig. 7), presenting a clearer picture of the signal transduction events leading to the stomatal closure induced by Pst DC3000.
Recent studies have shown that COR mimics the active form of the plant hormone jasmonate (JA), jasmonoyl Ile, and directly targets the JA receptor complexes (composed of the COI1 F-box protein and JAZ repressor proteins) to activate JA signaling in Arabidopsis (Thines, et al., 2007; Katsir et al., 2008; Melotto et al., 2008a; Browse, 2009; Fonseca et al., 2009). A recent study shows that JA can effectively suppress flg22-induced callose deposition in mesophyll cell walls in Arabidopsis leaves (Clay et al., 2009). Thus, by activating JA signaling, bacteria can suppress not only FLS2-mediated stomatal closure, but also FLS2-mediated mesophyll cell defenses in the leaf. An important direction of future research on the virulence action of COR will be to elucidate how activation of JA signaling inhibits FLS2 signaling in plants in both stomatal guard cells and mesophyll cells.
MATERIALS AND METHODS
Plant Materials
Arabidopsis (Arabidopsis thaliana) wild-type (Col-0, Ws-0, and Landsberg erecta [Ler]) and mutant plants were grown in growth chambers under a day/night cycle of 16 h/8 h at 20°C, with a light intensity of 80 to 100 μE m−2 s−1. Plants were used for experiments when they were about 5 weeks old.
The fls2 mutant was originally identified as Salk_093905 with a T-DNA insertion in At5g46330 (Heese et al., 2007) and is from Dr. Scott Peck (University of Missouri, Columbia, MO). The efr-1 mutant was originally identified as Salk_044334 with a T-DNA insertion in At5g20480 (Zipfel et al., 2006) and is from Dr. Thomas Boller (University of Basel, Basel). The fls2 efr-1 double mutant is also from Dr. Thomas Boller. The npr1-1 mutant is from Dr. Xinnian Dong (Duke University, Durham, NC), and the gpa1-3 mutant is from Dr. Sarah M. Assmann (Pennsylvania State University, University Park, PA). The eds5-1 mutant was purchased from the Arabidopsis Biological Resource Center (CS3735 for gene At4g39030). The sid2 mutant is from Dr. Mary Wildermuth (University of California, Berkeley, CA), and the nahG transgenic plant was purchased from Novartis. The aba2-1 mutant is from Dr. Jan A.D. Zeevaart (Michigan State University, East Lansing, MI), and the ost1-2 mutant is from Dr. Anna-Chiara Mustilli (Centre National de la Recherche Scientifique, Gif-sur-Yvette, France).
Stomatal Assays
Leaf peels were collected from the abaxial side of mature leaves from 5-week-old plants and placed in 250 to 300 μL of distilled, deionized water, bacteria resuspended in distilled, deionized water buffer (25 mm MES, 10 mm KCl, pH 6.15), or buffer containing chemicals that were preplaced on glass slides in square petri dishes with lids on. To preserve the stomatal aperture status in plants used for both stomatal and bacterial pathogenesis assays, we did not further treat leaf peels in any stomatal opening buffer. The petri dishes were left in the growth chamber in which plants were grown for an hour before being observed under a light microscope. Images of leaf peels were randomly taken and there were at least 30 stomata recorded for each sample treatment. Stomatal apertures were measured from these images with Adobe Photoshop. Bacteria were used at 1E + 8 CFU/mL (OD600 = 0.2), flg22 (EZBiolab) at 10 μm, ABA (Sigma) at 10 μm, and SA (Sigma) at 20 μm.
Confocal Microscopy
Whole leaves were detached from 5-week-old plants and incubated with Pst DC3118-GFP suspension at 1E + 8 CFU/mL for 1 h. Leaves were then rinsed in sterile water twice, each in 300 mL water for 2 min. Six leaf sections were excised from each leaf (see Fig. 3A for locations) and observed under an Olympus Fluoview FV1000 confocal microscope. For each microscopic view, 16 to 20 z-sections of 3 μm each were taken and overlaid for bacterial counting.
Pathogen Infection Assays
Bacteria were streaked from a freezer stock onto low-salt Luria-Bertani plates (tryptone 10 g/L, yeast extract 5 g/L, NaCl 5 g/L) with appropriate antibiotics. A Luria-Bertani liquid culture of 10 mL was started from plates that had been stored at −4°C for less than 2 weeks. After this small culture was grown for 12 h at 28°C, a subculture was made using 1:100 dilution and was incubated further at 28°C. When the OD600 reached 0.8 to 1.0, bacterial cells were collected and resuspended in distilled, deionized water to OD600 of 0.2. For dip inoculation, Silwet L-77 was added to a final concentration of 0.03%. Five-week-old plants grown in meshed pots were dipped upside down in the bacterial solution for a few seconds and returned to a growth room in a covered tray. Disease symptoms were recorded by camera, and bacterial populations were monitored by serial-dilution assays (Katagiri et al., 2002).
For infiltration inoculation, bacteria concentration was adjusted with distilled, deionized water according to individual experiments. After hand infiltration with a blunt-end syringe, plants were left in open trays to dry for about 2 h before being covered. The day 0 population was sampled at 2 h after infiltration. Bacteria populations were monitored in the same way as described for dip inoculation.
Root Elongation Assays
Seeds were sterilized in 30% bleach solution with 0.01% Tween 20 for 20 min at room temperature on a shaker, and rinsed with sterile distilled, deionized water five times. Sterilized seeds were resuspended in sterilized 0.1% agarose solution and transferred into the wells of 96-well plates with Murashige and Skoog liquid medium containing various chemicals. Each well contained 150 μL of solution and a single seed. Plates were kept at 4°C for 2 d before being placed in a growth chamber. Root lengths were measured when plants were 10 d old.
Arabidopsis Genome Initiative locus identifiers for the genes mentioned in this article are as follows: ABA2 (At1g52340), EDS5 (At4g39030), EFR (At5g20480), FLS2 (At5g46330), GPA1 (At2g26300), NPR1 (At1g64280), OST1 (At4g33950), and SID2/EDS16 (At1g74710).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Bacterial populations 3 d after dip inoculation with DB29 at 1E + 8 CFU/mL.
Supplemental Figure S2. Entry of Pst DC3118-GFP into Col-0 and fls2 leaves detected by confocal microscopy.
Supplemental Figure S3. Root elongation response of gpa1-3 and Col-0 plants to flg22.
Supplemental Figure S4. Stomata apertures in Col-0 leaf peels treated with flg22 and elf18 from E. coli and Pst DC3000.
Supplemental Figure S5. Responses of Col-0 and fls2 plants after dip inoculation with Pst DC3000 at 1E + 8 CFU/mL.
Supplementary Material
Acknowledgments
We thank Cyril Zipfel for elf18 peptides, Kelly Hannon for her assistance with root measurements, Christy Mecey and Melinda Frame for their help with confocal microscopy, Karen Bird for editing, and Marlene Cameron for figure formatting. We thank William Underwood, Alexandre Brutus, Maeli Melotto, and other members of the S.Y.H. lab for their comments on the manuscript.
References
- Amborabe BE, Bonmort J, Fleurat-Lessard P, Roblin G. (2008) Early events induced by chitosan on plant cells. J Exp Bot 59: 2317–2324 [DOI] [PubMed] [Google Scholar]
- Assmann SM, Schwartz A. (1992) Synergistic effect of light and fusicoccin on stomatal opening: epidermal peel and patch clamp experiments. Plant Physiol 98: 1349–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer Z, Gómez-Gómez L, Boller T, Felix G. (2001) Sensitivity of different ecotypes and mutants of Arabidopsis thaliana toward the bacterial elicitor flagellin correlates with the presence of receptor-binding sites. J Biol Chem 276: 45669–45676 [DOI] [PubMed] [Google Scholar]
- Bender C, Rangaswamy V, Loper J. (1999) Polyketide production by plant associated pseudomonads. Annu Rev Phytopathol 37: 175–196 [DOI] [PubMed] [Google Scholar]
- Blanco F, Garreton V, Frey N, Dominguez C, Perez-Acle T, Van der Straeten D, Jordana X, Holuigue L. (2005) Identification of NPR1-dependent and independent genes early induced by salicylic acid treatment in Arabidopsis. Plant Mol Biol 59: 927–944 [DOI] [PubMed] [Google Scholar]
- Blanco F, Salinas P, Cecchini NM, Jordana X, Van Hummelen P, Alvarez ME, Holuigue L. (2009) Early genomic responses to salicylic acid in Arabidopsis. Plant Mol Biol 70: 79–102 [DOI] [PubMed] [Google Scholar]
- Brooks DM, Bender CL, Kunkel BN. (2005) The Pseudomonas syringae phytotoxin coronatine promotes virulence by overcoming salicylic acid-dependent defenses in Arabidopsis thaliana. Mol Plant Pathol 6: 629–639 [DOI] [PubMed] [Google Scholar]
- Brooks DM, Hernandez-Guzman G, Kloek AP, Alarcon-Chaidez F, Sreedharan A, Rangaswamy V, Penaloza-Vazquez A, Bender CL, Kunkel BN. (2004) Identification and characterization of a well-defined series of coronatine biosynthetic mutants of Pseudomonas syringae pv. tomato DC3000. Mol Plant Microbe Interact 17: 162–174 [DOI] [PubMed] [Google Scholar]
- Browse J. (2009) Jasmonate passes muster: a receptor and targets for the defense hormone. Annu Rev Plant Biol 60: 183–205 [DOI] [PubMed] [Google Scholar]
- Budde IP, Ullrich MS. (2000) Interactions of Pseudomonas syringae pv. glycinea with host and nonhost plants in relation to temperature and phytotoxin synthesis. Mol Plant Microbe Interact 13: 951–961 [DOI] [PubMed] [Google Scholar]
- Chinchilla D, Bauer Z, Regenass M, Boller T, Felix G. (2006) The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell 18: 465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SM, Kang BR, Han SH, Anderson AJ, Park JY, Lee YH, Cho BH, Yang KY, Ryu CM, Kim YC. (2008) 2R,3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol Plant Microbe Interact 21: 1067–1075 [DOI] [PubMed] [Google Scholar]
- Clay NK, Adio AM, Denoux C, Jander G, Ausubel FM. (2009) Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323: 95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Bahrami AK, Pringle EG, Hernandez-Guzman G, Bender CL, Pierce NE, Ausubel FM. (2005) Pseudomonas syringae manipulates systemic plant defenses against pathogens and herbivores. Proc Natl Acad Sci USA 102: 1791–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Horák J, Chaban C, Mira-Rodado V, Witthöft J, Elgass K, Grefen C, Cheung MK, Meixner AJ, Hookey R, et al. (2008) The histidine kinase AHK5 integrates endogenous and environmental signals in Arabidopsis guard cells. PLoS One 3: e2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbs G, Silipo A, Aslam S, De Castro C, Liparoti V, Flagiello A, Pucci P, Lanzetta R, Parrilli M, Molinaro A, et al. (2008) Peptidoglycan and muropeptides from pathogens Agrobacterium and Xanthomonas elicit plant innate immunity: structure and activity. Chem Biol 15: 438–448 [DOI] [PubMed] [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T. (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18: 265–276 [DOI] [PubMed] [Google Scholar]
- Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, Miersch O, Wasternack C, Solano R. (2009) (+)-7-iso-jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol 5: 344–350 [DOI] [PubMed] [Google Scholar]
- Gimenez-Ibanez S, Hann DR, Ntoukakis V, Petutschnig E, Lipka V, Rathjen JP. (2009a) AvrPtoB targets the LysM receptor kinase CERK1 to promote bacterial virulence on plants. Curr Biol 19: 423–429 [DOI] [PubMed] [Google Scholar]
- Gimenez-Ibanez S, Ntoukakis V, Rathjen JP. (2009b) The LysM receptor kinase CERK1 mediates bacterial perception in Arabidopsis. Plant Signal Behav 4: 539–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göhre V, Spallek T, Haweker H, Mersmann S, Mentzel T, Boller T, de Torres M, Mansfield JW, Robatzek S. (2008) Plant pattern-recognition receptor FLS2 is directed for degradation by the bacterial ubiquitin ligase AvrPtoB. Curr Biol 18: 1824–1832 [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L, Boller T. (2000) FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5: 1003–1011 [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L, Boller T. (2002) Flagellin perception: a paradigm for innate immunity. Trends Plant Sci 7: 251–256 [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L, Felix G, Boller T. (1999) A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J 18: 277–284 [DOI] [PubMed] [Google Scholar]
- Gudesblat GE, Torres PS, Vojnov AA. (2009) Xanthomonas campestris overcomes Arabidopsis stomatal innate immunity through a DSF cell-to-cell signal-regulated virulence factor. Plant Physiol 149: 1017–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes RL, Stotz HU. (2004) Oxalate production by Sclerotinia sclerotiorum deregulates guard cells during infection. Plant Physiol 136: 3703–3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust AA, Biswas R, Lenz HD, Rauhut T, Ranf S, Kemmerling B, Götz F, Glawischnig E, Lee J, Felix G, et al. (2007) Bacteria-derived peptidoglycans constitute pathogen-associated molecular patterns triggering innate immunity in Arabidopsis. J Biol Chem 282: 32338–32348 [DOI] [PubMed] [Google Scholar]
- Heese A, Hann DR, Gimenez-Ibanez S, Jones AM, He K, Li J, Schroeder JI, Peck SC, Rathjen JP. (2007) The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA 104: 12217–12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri F, Thilmony R, He SY. (2002) The Arabidopsis thaliana-Pseudomonas syringae interaction. Somerville CR, Meyerowitz EM, , The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, doi/10.1199/tab.0039, http://www.aspb.org/publications/arabidopsis/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA. (2008) COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Natl Acad Sci USA 105: 7100–7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki K. (2001) Analysis of the phosphorylation level in guard-cell plasma membrane H+-ATPase in response to fusicoccin. Plant Cell Physiol 42: 424–432 [DOI] [PubMed] [Google Scholar]
- Kunze G, Zipfel C, Robatzek S, Niehaus K, Boller T, Felix G. (2004) The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 16: 3496–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvitko BH, Park DH, Velásquez AC, Wei CF, Russell AB, Martin GB, Schneider DJ, Collmer A. (2009) Deletions in the repertoire of Pseudomonas syringae pv. tomato DC3000 type III secretion effector genes reveal functional overlap among effectors. PLoS Pathog 5: e1000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Choi H, Huh S, Doo IS, Oh KY, Choi EJ, Taylor ATS, Low PS, Lee Y. (1999) Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol 121: 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chu ZH, Batoux M, Nekrasov V, Roux M, Chinchilla D, Zipfel C, Jones JDG. (2009) Specific ER quality control components required for biogenesis of the plant innate immune receptor EFR. Proc Natl Acad Sci USA 106: 15973–15978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Elmore JM, Fuglsang AT, Palmgren MG, Staskawicz BJ, Coaker G. (2009) RIN4 functions with plasma membrane H+-ATPases to regulate stomatal apertures during pathogen attack. PLoS Biol 7: e1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SW, Morris VL, Cuppels DA. (1991) Characterization of a DNA region required for the production of the phytotoxin coronatine by Pseudomonas syringae pv. tomato. Mol Plant Microbe Interact 4: 69–74 [Google Scholar]
- Melotto M, Mecey C, Niu Y, Chung HS, Katsir L, Yao J, Zeng W, Thines B, Staswick P, Browse J, et al. (2008a) A critical role of two positively charged amino acids in the Jas motif of Arabidopsis JAZ proteins in mediating coronatine- and jasmonoyl isoleucine-dependent interactions with the COI1 F-box protein. Plant J 55: 979–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M, Underwood W, He SY. (2008b) Role of stomata in plant innate immunity and foliar bacterial diseases. Annu Rev Phytopathol 46: 101–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY. (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980 [DOI] [PubMed] [Google Scholar]
- Mittal S, Davis KR. (1995) Role of the phytotoxin coronatine in the infection of Arabidopsis thaliana by Pseudomonas syringae pv. tomato. Mol Plant Microbe Interact 8: 165–171 [DOI] [PubMed] [Google Scholar]
- Moore RA, Starratt N, Ma SW, Morris VL, Cuppels DA. (1989) Identification of a chromosomal region required for biosynthesis of the phytotoxin coronatine by Pseudomonas syringae pv. tomato. Can J Microbiol 35: 910–917 [Google Scholar]
- Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I. (2008) Nitric oxide, stomatal closure, and abiotic stress. J Exp Bot 59: 165–176 [DOI] [PubMed] [Google Scholar]
- Nekrasov V, Li J, Batoux M, Roux M, Chu Z, Lacombe S, Rougon A, Bittel P, Kiss-Papp M, Chinchilla D, et al. (2009) Control of the pattern-recognition receptor EFR by an ER protein complex in plant immunity. EMBO J 28: 3428–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo Y, Tintor N, Lu X, Rauf P, Pajerowska-Mukhtar K, Häweker H, Dong X, Robatzek S, Shulze-Lefert P. (2009) Receptor quality control in the endoplasmic reticulum for plant innate immunity. EMBO J 28: 3439–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D. (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52: 627–658 [DOI] [PubMed] [Google Scholar]
- Shan L, He P, Li J, Heese A, Peck SC, Nurnberger T, Martin GB, Sheen J. (2008) Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe 4: 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K, Doi M, Assmann SM, Kinoshita T. (2007) Light regulation of stomatal movement. Annu Rev Plant Biol 58: 219–247 [DOI] [PubMed] [Google Scholar]
- Sun W, Dunning FM, Pfund C, Weingarten R, Bent AF. (2006) Within-species flagellin polymorphism in Xanthomonas campestris pv campestris and its impact on elicitation of Arabidopsis FLAGELLIN SENSING2-dependent defenses. Plant Cell 18: 764–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilmony R, Underwood W, He SY. (2006) Genome-wide transcriptional analysis of the Arabidopsis thaliana interaction with the plant pathogen Pseudomonas syringae pv. tomato DC3000 and the human pathogen Escherichia coli O157:H7. Plant J 46: 34–53 [DOI] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J. (2007) JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signaling. Nature 448: 661–665 [DOI] [PubMed] [Google Scholar]
- Tsuda K, Sato M, Glazebrook J, Cohen JD, Katagiri F. (2008) Interplay between MAMP-triggered and SA-mediated defense responses. Plant J 53: 763–775 [DOI] [PubMed] [Google Scholar]
- Turner NC, Graniti A. (1969) Fusicoccin: a fungal toxin that opens stomata. Nature 223: 1070–1071 [Google Scholar]
- Wang D, Weaver ND, Kesarwani M, Dong X. (2005) Induction of protein secretory pathway is required for systemic acquired resistance. Science 308: 1036–1040 [DOI] [PubMed] [Google Scholar]
- Wang P, Song CP. (2008) Guard-cell signaling for hydrogen peroxide and abscisic acid. New Phytol 178: 703–718 [DOI] [PubMed] [Google Scholar]
- Xiang T, Zong N, Zou Y, Wu Y, Zhang J, Xing W, Li Y, Tang X, Zhu L, Chai J, et al. (2008) Pseudomonas syringae effector AvrPto blocks innate immunity by targeting receptor kinases. Curr Biol 18: 74–80 [DOI] [PubMed] [Google Scholar]
- Zhang J, Shao F, Li Y, Cui H, Chen L, Li H, Zou Y, Long C, Lan L, Chai J, et al. (2007) A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host Microbe 1: 175–185 [DOI] [PubMed] [Google Scholar]
- Zhang W, He SY, Assmann SM. (2008) The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. Plant J 56: 984–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G. (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125: 749–760 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JD, Felix G, Boller T. (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428: 764–767 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.