Abstract
We evaluated the clinical results of posterior decompression with instrumented fusion (PDF) for thoracic myelopathy due to ossification of the posterior longitudinal ligament (OPLL). A total of 24 patients underwent PDF, and their surgical outcomes were evaluated by the Japanese Orthopaedic Association (JOA) scores (0–11 points) and by recovery rates calculated at 3, 6, 9 and 12 months after surgery and at a mean final follow-up of 4 years and 5 months. The mean JOA score before surgery was 3.7 points. Although transient paralysis occurred immediately after surgery in one patient (3.8%), all patients showed neurological recovery at the final follow-up with a mean JOA score of 8.0 points and a mean recovery rate of 58.1%. The mean recovery rate at 3, 6, 9 and 12 months after surgery was 36.7, 48.8, 54.0 and 56.8%, respectively. The median time point that the JOA score reached its peak value was 9 months after surgery. No patient chose additional anterior decompression surgery via thoracotomy. The present findings demonstrate that despite persistent anterior impingement of the spinal cord by residual OPLL, PDF can result in considerable neurological recovery with a low risk of postoperative paralysis. Since neurological recovery progresses slowly after PDF, we suggest that additional anterior decompression surgery is not desirable during the early stage of recovery.
Keywords: Thoracic myelopathy, Ossification of posterior longitudinal ligament, Kyphosis, Spinal mobility, Instrumented fusion
Introduction
Previous reports have shown that the results of surgery for thoracic myelopathy caused by ossification of the posterior longitudinal ligament (OPLL) unfavorably compare with results for cervical OPLL [12, 19]. Surgeons have employed a variety of surgical procedures to treat thoracic OPLL, including laminectomy [5], OPLL extirpation through thoracotomy [2, 4, 10], OPLL extirpation through a posterior approach [13], and circumspinal decompression [6, 15]. However, postoperative paraplegia remains a major risk [2, 4, 8–10, 13, 15]. At our institute, two patients experienced transient postoperative paraparesis after laminectomy, which resolved after the addition of posterior instrumented fusion without OPLL extirpation [16, 17]. On the basis of these two cases, we hypothesized that stabilizing the spine with instrumentation could yield a certain degree of neurological recovery even without complete OPLL extirpation. Based on this hypothesis, in 1989, we introduced the surgical procedure of posterior decompression with instrumented fusion (PDF) for patients with thoracic OPLL, in whom OPLL extirpation entailed a risk of neurological deterioration [18].
In an earlier series of ours, our patients enjoyed a considerable degree of neurological recovery following PDF despite persistent anterior impingement of the spinal cord by residual OPLL [18]. In addition, PDF was associated with an extremely low risk of postoperative paralysis and late neurological deterioration, compared with complication rates for laminectomy [5] and OPLL extirpation [2, 4]. However, the mechanisms by which PDF produced neurological recovery in thoracic OPLL patients have not yet been fully established. To better elucidate these recovery mechanisms, in the present study, we analyzed the process of neurological recovery after PDF in patients we have treated at our institute. In addition, we analyzed the contribution of thoracic kyphosis correction following PDF to neurological recovery.
Materials and methods
Patient population
From May 1989 through October 2004, a total of 24 patients (7 males and 17 females) with thoracic myelopathy due to OPLL underwent PDF at our institute. In the present study, we analyzed all 24 patients. The mean age at surgery was 54.8 years, ranging from 32 to 74 years. The mean follow-up period was 4 years and 5 months, ranging from 1 year and 2 months to 12 years and 9 months (Table 1).
Table 1.
Key characteristics of the 24 patients who underwent posterior decompression with instrumented fusion
| Case no. | Age (years)/sex | Most stenotic level | Instrumented fusion | |
|---|---|---|---|---|
| Levels | Anchors | |||
| 1 | 41/M | T9/10 | T1–L2 | Hooks |
| 2 | 49/F | T9/10 | T7–L2 | Hooks |
| 3 | 53/F | T6/7 | T1–L1 | Hooks |
| 4 | 64/F | T4/5 | T1–T11 | Hooks |
| 5 | 45/F | T8/9 | T2–L2 | Hooks |
| 6 | 49/F | T9/10 | T2–L2 | Hooks |
| 7 | 51/F | T9/10 | T4–L2 | Hooks, PSs |
| 8 | 44/F | T9/10 | T5–L2 | Hooks, PSs |
| 9 | 57/F | T11/12 | T7–L4 | Hooks, PSs |
| 10 | 43/F | T9/10 | T3–L1 | Hooks, PSs |
| 11 | 74/F | T6/7 | T3–T10 | Hooks |
| 12 | 71/M | T10/11 | T6–L2 | Hooks, PSs |
| 13 | 61/M | T4/5 | T2–T8 | PSs |
| 14 | 52/F | T9/10 | T3–T12 | Hooks, PSs |
| 15 | 52/F | T5/6 | T2–T9 | PSs |
| 16 | 32/M | T10/11 | T6–L1 | PSs |
| 17 | 66/F | T8/9 | T5–T12 | PSs |
| 18 | 41/F | T6/7 | T1–T10 | Hooks, PSs |
| 19 | 55/M | T9/10 | T6–L2 | PSs |
| 20 | 55/F | T4/5 | T1–T10 | PSs |
| 21 | 72/F | T9/10 | T1–L1 | PSs |
| 22 | 65/F | T5/6 | T1–T10 | PSs |
| 23 | 60/M | T4/5 | T2–T10 | PSs |
| 24 | 64/M | T7/8 | T1–T11 | PSs |
PS pedicle screw
Posterior decompression with instrumented fusion
Informed consent
Before performing PDF, we explained the surgical plan to the patients that laminectomy and posterior instrumented fusion would be performed as the first-step surgery and that if neurological recovery was insufficient after PDF, OPLL extirpation via thoracotomy could be performed as a second-step operation. The choice of adding anterior surgery was then left to the patient.
Surgical procedure
We principally performed laminectomy at sites where preoperative radiographic and magnetic resonance (MR) images showed disappearance of the subarachnoid space on the dorsal side of spinal cord. Regarding instrumented fusion anchors, we initially used hooks when we first introduced PDF, but more recently we have been using pedicle screws (PSs) in most cases (Table 1). After laminectomy, we used intraoperative spinal ultrasonography to assess whether the area of posterior decompression was adequate [3, 14]. After confirming the adequacy of the posterior decompression, we connected the rods to the anchors. We usually did not correct kyphosis at the rod setting but performed the fixation in situ. For bone grafting, we used spinous processes that we had extirpated before laminectomy and grafted them onto the facets and between the transverse processes.
Postoperative course
The patients were allowed to sit and walk with a soft orthosis 2 days after surgery. The patients generally wore the orthosis for at least 12 weeks to prevent PSs from pulling out and/or displacement of hooks.
Clinical assessment
Surgical outcomes were evaluated using the Japanese Orthopaedic Association (JOA) score (full score = 11 points) [7]. The JOA scoring system evaluates motor function of the lower extremity (0–4 points), sensory function of the trunk (0–2 points) and lower extremity (0–2 points), and bladder function (0–3 points). Recovery rates were calculated using the formula listed in Table 2 [7]. We assessed the JOA score before surgery, at 3, 6, 9, 12 months after surgery and at the final follow-up. In accordance with previous reports [18, 19], the results were ranked as either good (recovery rate ≥ 50%), fair (10% ≤ recovery rate < 50%), unchanged (0% ≤ recovery rate < 10%), or worsened (recovery rate < 0%).
Table 2.
Surgical outcomes after posterior decompression with instrumented fusion
| Surgical outcomes | Before surgery | After surgery | ||||
|---|---|---|---|---|---|---|
| 3 months | 6 months | 9 months | 1 year | Final FU | ||
| JOA score (points)a | 3.7 ± 1.4 (1.0–6.5) | 6.4 ± 1.8† (3.5–11.0) | 7.3 ± 2.0† (4.0–11.0) | 7.7 ± 2.0†,‡ (4.0–11.0) | 7.9 ± 2.0†,‡ (4.0–11.0) | 8.0 ± 2.0†,‡ (4.0–11.0) |
| Recovery rate (%)a,b | 36.7 ± 23.4 (0–100) | 48.8 ± 26.5 (14.3–100) | 54.0 ± 26.9§ (14.3–100) | 56.8 ± 27.4§ (14.3–100) | 58.1 ± 27.5§ (14.3–100) | |
FU follow-up, JOA Japanese Orthopedic Association (full score = 11 points)
†Statistically different from the JOA score before surgery (p < 0.05)
‡Statistically different from the JOA score at 3 months after surgery (p < 0.05)
§Statistically different from the recovery rate at 3 months after surgery (p < 0.05)
aValues are expressed as the mean ± standard deviation, with the range in parenthesis
b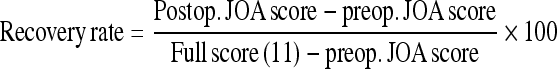
Radiographic assessment
In our last six consecutive patients (Case 19 through Case 24), we measured preoperatively the kyphotic angles at the instrumented fusion levels in the supine, prone, and sitting positions. To calculate the kyphotic angle, we measured the sagittal Cobb angle between the upper endplate of the uppermost vertebra and the lower endplate of the lowest vertebra at the instrumented fusion levels [1]. To accurately identify the landmarks for the angle measurement, we controlled the contrast and brightness of the digital images of the thoracic spine. The kyphotic angle was measured by three spine surgeons independently, and we defined the mean value of these measurements as the value of the kyphotic angle.
Intraoperative spinal ultrasonography
After laminectomy, we assessed the posterior shift of the spinal cord away from the anterior ossified mass by means of intraoperative ultrasonography. We looked for the presence of the subarachnoid space on the ventral side of the spinal cord, on the basis of which we classified the decompression status as either non-contact type or contact type [3, 14]. The non-contact type is characterized by visualization of the subarachnoid space between the OPLL and the spinal cord, indicating that sufficient decompression of the spinal cord from the anterior ossified mass has been achieved. Conversely, in the contact type, the spinal cord always touches the OPLL, and no subarachnoid space is evident between the OPLL and the spinal cord, indicating persistent impingement of the spinal cord from the anterior direction even after the posterior decompression procedure.
Statistical analysis
Statistical analysis was performed using the Mann–Whitney U test. A p value <0.05 was considered statistically significant. Results are presented as the mean ± standard deviation of the mean.
Results
Surgical outcome
The mean JOA score before surgery was 3.7 (range 1.0–6.5). All patients showed neurological recovery at the final follow-up; the mean JOA score at final follow-up was 8.0 (range 4.0–11.0 points), and the mean recovery rate was 58.1% (range 14.3–100%) (Table 2).
The mean JOA score was 6.4 at 3 months after surgery, 7.3 at 6 months after surgery, 7.7 at 9 months after surgery and 7.9 at 12 months after surgery (Table 2). The JOA scores at 3 months after surgery and later were significantly higher than the JOA score before surgery. In addition, the JOA scores at 9 months after surgery and later were significantly higher than the JOA score at 3 months after surgery (Table 2). The mean recovery rate was 36.7% at 3 months after surgery, 48.8% at 6 months after surgery, 54.0% at 9 months after surgery and 56.8% at 12 months after surgery (Table 2). The recovery rates at 9 months after surgery and later were significantly higher than the recovery rate at 3 months after surgery (Table 2).
Surgical outcome at the final follow-up was good in 15 patients (Cases 2, 3, 4, 6, 8, 10, 11, 13, 14, 16, 19, 20, 21, 22, and 24) and fair in 9 patients (Cases 1, 5, 7, 9, 12, 15, 17, 18, and 23). No patient was unchanged or worsened.
The JOA score reached its peak value at 3 months after surgery in 3 patients (Cases 5, 9, 13), at 6 months after surgery in 7 patients (Cases 3, 7, 10, 12, 16, 18, 23), at 9 months after surgery in 4 patients (Cases 1, 4, 8, 21), at 12 months after surgery in 7 patients (Cases 2, 6, 14, 15, 17, 20, 22), at 24 months in 2 patients (Cases 11, 19), and at the final follow-up in 1 patient (Case 24). The median time point that the JOA score reached its peak value was 9 months after surgery.
No patient chose additional anterior decompression surgery via thoracotomy.
Complications
In the present series, no patient developed persistent paralysis after surgery, but one patient (3.8%) had transient paralysis immediately after surgery (Case 23). Cerebro-spinal fluid leakage occurred after laminectomy in one patient (3.8%) (Case 6). No instrumented failures occurred, such as PS loosening, hook displacement, or rod breakage.
Kyphotic angles at instrumented fusion levels
In the last six consecutive patients (Case 19 through Case 24), the preoperative kyphotic angles at the instrumented fusion levels were measured in the supine, prone, and sitting positions (Table 3). The difference between the kyphotic angle in the supine position and the kyphotic angle in the sitting position ranged from 8° (Case 20) to 20° (Case 24), indicating that some mobility remained in the thoracic spine in spite of the presence of OPLL. The mean spinal mobility per disc was 1.3°, ranging from 0.9° to 2.0°.
Table 3.
Kyphotic angles at instrumented fusion levels
| Case no. | Instrumented fusion levels/(no. of discs) | Preop. kyphotic angle (°) | Spinal mobility (°) | Spinal mobility per disc (°) | Postop. kyphotic angle (°) | ||
|---|---|---|---|---|---|---|---|
| Supine position | Prone position | Sitting position | |||||
| 19 | T6–L2/(8) | 15 | 23 | 25 | 10 | 1.3 | 23 |
| 20 | T1–T10/(9) | 27 | 32 | 35 | 8 | 0.9 | 32 |
| 21 | T1–L1/(12) | 40 | 47 | 51 | 11 | 0.9 | 49 |
| 22 | T1–T10/(11) | 23 | 26 | 34 | 11 | 1.2 | 32 |
| 23 | T2–T10/(8) | 30 | 37 | 40 | 10 | 1.3 | 38 |
| 24 | T1–T11/(10) | 25 | 33 | 45 | 20 | 2.0 | 37 |
| Average ± SD | 26.7 ± 1.3 | 33.0 ± 8.5 | 38.3 ± 9.1 | 11.7 ± 4.2 | 1.3 ± 0.4 | 35.2 ± 8.6 | |
Spinal mobility = Preop. kyphotic angle in sitting position − preop. kyphotic angle in supine position
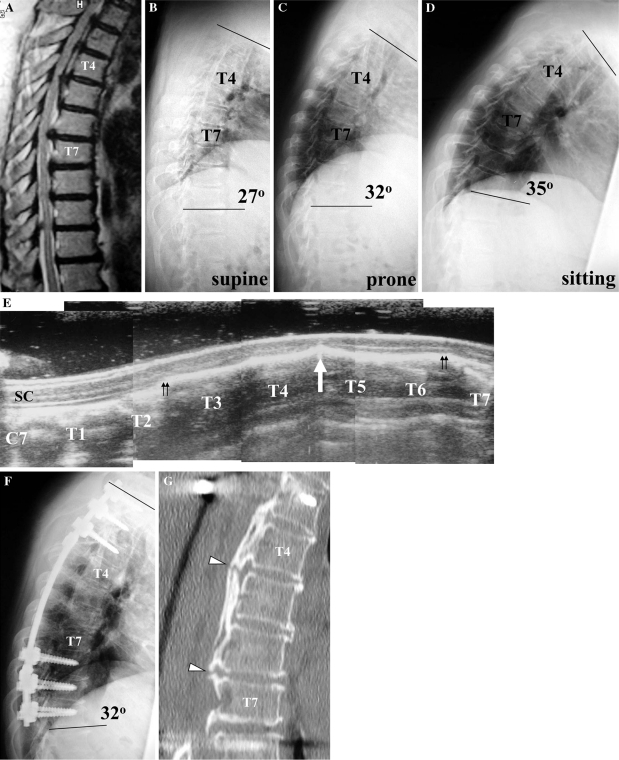
In all six patients, the postoperative kyphotic angle at the instrumented fusion levels was greater than the preoperative kyphotic angle in the supine position, but less than the preoperative kyphotic angle in the sitting position. Evaluating the correction of kyphosis after surgery with respect to the preoperative kyphotic angle in the sitting position demonstrated some correction of the kyphosis, with a mean change in kyphotic angle of 3.2° (Table 4). However, evaluating the correction of kyphosis after surgery with respect to the preoperative kyphotic angle in the supine position demonstrated increase of the kyphosis, with a mean change in kyphotic angle of −7.8° (Table 4). Figure 1 presents an illustrative example (Case 20) of the change in kyphotic angle following PDF, as demonstrated by a comparison of preoperative (b–d) and postoperative (f) radiographs.
Table 4.
Correction of kyphosis at instrumented fusion levels
| Case no. | Preop. kyphotic angle at supine position − postop. kyphotic angle (°) | Preop. kyphotic angle at prone position − postop. kyphotic angle (°) | Preop. kyphotic angle at sitting position − postop. kyphotic angle (°) |
|---|---|---|---|
| 19 | −8 | 0 | 2 |
| 20 | −5 | 0 | 3 |
| 21 | −9 | −2 | 2 |
| 22 | −9 | −6 | 2 |
| 23 | −8 | −1 | 2 |
| 24 | −8 | −4 | 8 |
| Average ± SD | −7.8 ± 1.5 | −2.2 ± 2.4 | 3.2 ± 2.4 |
Fig. 1.
Preoperative T2-weighted MR image at the midsagittal plane (a) of a 55-year-old woman (Case 20), showing severe narrowing of the spinal cord at T4/5 and T6/7. Preoperative radiographic images show that the kyphotic angle at T1–T10 was 27° in the supine position (b), 32° in the prone position (c) and 35° in the sitting position (d). Intraoperative spinal ultrasonography at the midsagittal plane after laminectomy shows anterior impingement of the spinal cord by a beak-type OPLL at T4/5 (e, arrow) and absence of the subarachnoid space on the ventral side of the spinal cord from the T2/3 to T6/7 levels (e, double arrows). SC spinal cord. A postoperative radiographic image (f) shows a kyphotic angle at T1–T10 of 32°. A midsagittal reconstruction CT image (g) shows a non-ossified area at the mid-portion of the ossified mass at T4/5 and T6/7 (g, arrowheads)
Intraoperative ultrasonography
Intraoperative ultrasonography demonstrated that the decompression status for all 24 patients was the contact type, indicating that the posterior shift of the spinal cord was insufficient to prevent persistent impingement of the spinal cord from the anterior direction after laminectomy.
Discussion
Mechanisms for the improvement of myelopathy
The results of this study demonstrated that all patients showed neurological recovery after PDF at the final follow-up, with an average recovery rate of 58.1%. Compared with the results from previous published reports regarding thoracic OPLL, the surgical outcome of PDF was superior to the surgical outcome of laminectomy alone [5, 19]. When laminectomy alone is performed for thoracic OPLL, the backward shift of the spinal cord is often restricted because the thoracic spine is physiologically kyphotic, leading to persistent anterior impingement of the spinal cord by OPLL. In the present study, we evaluated the posterior shift of the spinal cord after laminectomy using intraoperative sonography and observed persistent anterior impingement of the spinal cord by OPLL in all cases. Despite this insufficient decompression of the spinal cord, PDF did result in considerable neurological recovery, indicating that posterior instrumented fusion has some positive effect on myelopathy after laminectomy for thoracic OPLL.
In this study, we also demonstrated that, in patients with thoracic OPLL, the spinal column still showed some mobility at the cord compression level in spite of the presence of massive heterotopic vertebral ossification. Our previous studies on patients with cervical myelopathy due to OPLL have shown that hypermobility of the vertebra at the cord compression level is a risk factor for the development and aggravation of myelopathy [11] and for poor surgical outcome after laminoplasty [7]. Taken together with the present findings, our investigations suggest that the remaining mobility of the spinal column at the cord compression level correlates with the development and aggravation of myelopathy in patients with thoracic OPLL.
Regarding correction of kyphosis by posterior instrumented fusion, we measured kyphotic angles in our last six cases. In all six cases, the postoperative kyphotic angle was smaller than the preoperative kyphotic angle in the supine position but greater than the preoperative kyphotic angle in the sitting position. However, even if we based our calculation of the correction of kyphosis upon the preoperative kyphotic angle in the sitting position, the mean correction still was only 3.2°, indicating that our procedure of posterior instrumented fusion did not sufficiently correct our patients’ kyphosis. In turn, these results suggest that correction of kyphosis is not a major factor contributing to the neurological improvements observed after PDF.
In the present study, the JOA scores reached a peak value from 3 to 24 months after PDF (median 9 months), indicating that improvement of myelopathy in our patients was slowly progressed. Our findings suggest that suppression of spinal column mobility by posterior instrumented fusion is a more powerful factor than correction of kyphosis for producing neurological recovery after PDF. After PDF, anterior impingement of the spinal cord by OPLL persists, but the stabilization of the spine appears to decrease the damage to the spinal cord at the cord compression level, enabling a slow neurological recovery to commence.
Risk of postoperative paralysis
In our earlier study of 17 patients who underwent PDF for thoracic OPLL, no postoperative paralysis occurred after PDF [18]. Based on this indication that PDF entailed a low risk of postoperative neurological deterioration, we have employed PDF for all cases of thoracic OPLL treated surgically at our institute since 2003. However, we did encounter our first case of postoperative paralysis with our 23rd patient. Fortunately, the paralysis spontaneously resolved without adding OPLL extirpation. This incident thus suggests that the decompression procedure itself in patients with a severely compressed spinal cord entails a risk of postoperative paralysis, such that even the selection of PDF as our surgical procedure for thoracic OPLL cannot completely eliminate the risk of postoperative paralysis. However, in light of what appears to be a higher risk of postoperative paralysis following other surgical procedures such as laminectomy alone [5, 19] and OPLL extirpation [2, 4, 10, 13], we would suggest that PDF is the safest surgical procedure among the alternatives for thoracic OPLL.
Indication of additional OPLL extirpation
We have employed the same concepts in planning PDF as we use in planning circumspinal decompression [6, 15]. We explained to patients that PDF was the first operation and that we could add OPLL extirpation via thoracotomy as the second operation if their neurological recovery after PDF was insufficient. At our institute, the informed consent procedure for patients undergoing the first operation included our presenting all the information we had about the advantages and the disadvantages of additional OPLL extirpation surgery, including the high neurological recovery rate in successful cases of OPLL extirpation as well as the attendant risk of postoperative deterioration [6, 13, 18]. The choice of adding a second surgery was then left to the patient. All 24 patients in the present series have been sufficiently satisfied with the surgical outcome obtained by PDF alone such that no patient to date has elected additional anterior decompression surgery over a mean postoperative follow-up of 4 years and 5 months. Although all the patients understand the likelihood of much better neurological recovery after the addition of OPLL extirpation, thus far they appear to prefer not subjecting themselves to a new risk of postoperative paralysis that a second procedure would entail.
When neurological recovery after PDF is insufficient, we should consider adding anterior OPLL extirpation surgery. The findings from our patient group demonstrating gradual neurological recovery after PDF indicate that additional anterior surgery during the early stage of recovery after PDF generally is not desirable. In particular, since the patients’ JOA scores reached their peak value at 9 months after PDF, we should follow postoperative neurological recovery in patients for at least 9 months to a year before arriving at a decision regarding additional anterior surgery.
Acknowledgments
The authors thank Professor Kazuhisa Takahashi (Department of Orthopaedic Surgery, Chiba University Graduate School of Medicine) for his kind support in the present analysis. This work was supported by the Health Labour Science Research Grant of Japan.
References
- 1.Ataka H, Tanno T, Yamazaki M. Posterior instrumented fusion without neural decompression for incomplete neurological deficits following vertebral collapse in the osteoporotic thoracolumbar spine. Eur Spine J. 2009;18:69–76. doi: 10.1007/s00586-008-0821-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujimura Y, Nishi Y, Nakamura M, et al. Long-term follow-up study of anterior decompression and fusion for thoracic myelopathy resulting from ossification of the posterior longitudinal ligament. Spine. 1997;22:305–311. doi: 10.1097/00007632-199702010-00015. [DOI] [PubMed] [Google Scholar]
- 3.Fujiyoshi T, Yamazaki M, Kawabe J, et al. A new concept for making decisions regarding the surgical approach for cervical ossification of the posterior longitudinal ligament: the K-line. Spine. 2008;33:E990–E993. doi: 10.1097/BRS.0b013e318188b300. [DOI] [PubMed] [Google Scholar]
- 4.Hanai K, Ogikubo O, Miyashita T. Anterior decompression for myelopathy resulting from thoracic ossification of the posterior longitudinal ligament. Spine. 2002;27:1070–1076. doi: 10.1097/00007632-200205150-00012. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa K, Kaneda K, Abumi K, et al (1996) Operative indication of posterior decompression for thoracic ossification of the posterior longitudinal ligament. Investigation Committee Report on the Ossification of the Spinal Ligaments. Japanese Ministry of Health, Labour and Welfare, Tokyo, pp 305–309 (in Japanese)
- 6.Kawahara N, Tomita K, Murakami H, et al. Circumspinal decompression with dekyphosis stabilization for thoracic myelopathy due to ossification of the posterior longitudinal ligament. Spine. 2008;33:39–46. doi: 10.1097/BRS.0b013e31815e3911. [DOI] [PubMed] [Google Scholar]
- 7.Masaki Y, Yamazaki M, Okawa A, et al. An analysis of factors causing poor surgical outcome in patients with cervical myelopathy due to ossification of the posterior longitudinal ligament: anterior decompression with spinal fusion versus laminoplasty. J Spinal Disord Tech. 2007;20:7–13. doi: 10.1097/01.bsd.0000211260.28497.35. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto M, Chiba K, Toyama Y, et al. Surgical results and related factors for ossification of posterior longitudinal ligament of the thoracic spine: a multi-institutional retrospective study. Spine. 2008;33:1034–1041. doi: 10.1097/BRS.0b013e31816c913b. [DOI] [PubMed] [Google Scholar]
- 9.Matsuyama Y, Yoshihara H, Tsuji T, et al. Surgical outcome of ossification of the posterior longitudinal ligament (OPLL) of the thoracic spine: implication of the type of ossification and surgical options. J Spinal Disord Tech. 2005;18:492–497. doi: 10.1097/01.bsd.0000155033.63557.9c. [DOI] [PubMed] [Google Scholar]
- 10.Min JH, Jang JS, Lee SH. Clinical results of ossification of the posterior longitudinal ligament (OPLL) of the thoracic spine treated by anterior decompression. J Spinal Disord Tech. 2008;21:116–119. doi: 10.1097/BSD.0b013e318060091a. [DOI] [PubMed] [Google Scholar]
- 11.Mochizuki M, Aiba A, Hashimoto M, et al. Cervical myelopathy in patients with ossification of the posterior longitudinal ligament. J Neurosurg Spine. 2009;10:122–128. doi: 10.3171/2008.10.SPI08480. [DOI] [PubMed] [Google Scholar]
- 12.Ohtani K, Nakai S, Fujimura Y, et al. Anterior surgical decompression for thoracic myelopathy as a result of ossification of the posterior longitudinal ligament. Clin Orthop. 1982;166:82–88. [PubMed] [Google Scholar]
- 13.Takahata M, Ito M, Abumi K, et al. Clinical results and complications of circumferential spinal cord decompression through a single posterior approach for thoracic myelopathy caused by ossification of posterior longitudinal ligament. Spine. 2008;33:1199–1208. doi: 10.1097/BRS.0b013e3181714515. [DOI] [PubMed] [Google Scholar]
- 14.Tokuhashi Y, Matsuzaki H, Oda H, et al. Effectiveness of posterior decompression for patients with ossification of the posterior longitudinal ligament in the thoracic spine: usefulness of the ossification-kyphosis angle on MRI. Spine. 2006;31:E26–E30. doi: 10.1097/01.brs.0000193940.75354.e5. [DOI] [PubMed] [Google Scholar]
- 15.Tomita K, Kawahara N, Baba H, et al. Circumspinal decompression for thoracic myelopathy due to combined ossification of the posterior longitudinal ligament and ligamentum flavum. Spine. 1990;15:1114–1120. doi: 10.1097/00007632-199011010-00006. [DOI] [PubMed] [Google Scholar]
- 16.Yamazaki M, Koda M, Okawa A, et al. Transient paraparesis after laminectomy for thoracic ossification of the posterior longitudinal ligament and ossification of the ligamentum flavum. Spinal Cord. 2006;44:130–134. doi: 10.1038/sj.sc.3101807. [DOI] [PubMed] [Google Scholar]
- 17.Yamazaki M, Okawa A, Koda M, et al. Transient paraparesis after laminectomy for thoracic myelopathy due to ossification of the posterior longitudinal ligament: a case report. Spine. 2005;30:E343–E346. doi: 10.1097/01.brs.0000166504.31627.06. [DOI] [PubMed] [Google Scholar]
- 18.Yamazaki M, Mochizuki M, Ikeda Y, et al. Clinical results of surgery for thoracic myelopathy caused by ossification of the posterior longitudinal ligament: operative indication of posterior decompression with instrumented fusion. Spine. 2006;31:1452–1460. doi: 10.1097/01.brs.0000220834.22131.fb. [DOI] [PubMed] [Google Scholar]
- 19.Yonenobu K, Ebara S, Fujiwara K, et al. Thoracic myelopathy secondary to ossification of the spinal ligament. J Neurosurg. 1987;66:511–518. doi: 10.3171/jns.1987.66.4.0511. [DOI] [PubMed] [Google Scholar]