Abstract
The lumbar shape in females is thought to be unique, compensating for lumbar hyperlordosis. Yet, the morphological adaptation of various vertebral parameters in the thoracic and lumbar spine to this unique posture in young and adult females has only been partially addressed in the literature. Our aim was to investigate the gender association to vertebral shape in the thoracic and lumbar spine as a possible adaptation to lumbar hyperlordosis in young and adult females. A three-dimensional digitizer was used to measure the vertebral body sagittal wedging, relative spinous process thickness, and relative interfacet width at the T1–L5 level. Two hundred and forty complete, non-pathological skeletons of adults and 32 skeletons of young individuals were assessed. Three major results were found to be independent of age and ethnicity: (a) VB sagittal wedging in females was significantly less kyphotic than males from T9 to L2 (T11 excluded) with a cumulative mean difference of 8.8°; (b) females had a significantly relatively thinner lumbar spinous processes and (c) females had a relatively wider superior interfacet distance (T9–T10 and L1–L4) than males. We conclude that the combination of less kyphotic VB wedging in the lower thoracic and upper lumbar vertebrae, relatively greater interspinous space and larger interfacet width in the lumbar spine in females are key architectural elements in the lumbar hyperlordosis in females and may compensate for the bipedal obstetric load during pregnancy.
Keywords: Lumbar hyperlordosis, Vertebral wedging, Vertebral shape, Lumbar interfacet width, Spinous process thickness
Introduction
The shape of the human vertebrae is gender affected due to genetic, hormonal and environmental factors responsible for growth-spurt timing [13]. Larger vertebral dimensions in males versus females are one of the effects [14]. Another is lumbar hyperlordosis development in females vis-a-vis males [1, 10, 15–17]. This unique lumbar posture in females is thought to compensate for the bipedal obstetric load during pregnancy and is based on a longer series of dorsally wedged vertebrae in the lumbar spine [i.e. an extra dorsally wedged lumbar vertebra (L3) in females] and larger and more frontally oriented lumbar zygoapophyseal facets in females than in males [16]. This distinctive zygoapophyseal facet shape in females, however, is not evident in other studies [5, 6]. The question remains whether the shape of other vertebral parameters along the spine (not only lumbar) contribute to a mechanically stable lumbar hyperlordosis in females.
Following a simple visual biomechanical analysis, we suggest that for this mechanical stability to occur three vertebral morphological parameters are required in females compared to males: (a) morphological compensation in the lower thoracic vertebrae for the hyperlordotic shape existing in the lumbar spine; (b) a wider mechanical basis in the posterior elements of the lumbar region; and (c) a larger intervertebral space between adjacent vertebrae.
Consequently, three major hypotheses are proposed: (1) the lower thoracic and upper lumbar vertebral bodies are less kyphotic in females than in males (providing a larger space for the human fetus to develop); (2) the lumbar interfacet width (i.e. posterior mechanical base) is relatively greater in females compared to males; (3) the lumbar spinous process thickness (i.e. intervertebral space) is relatively smaller in females than in males.
The current study aims to investigate the gender association of the vertebral shape in the thoracic and lumbar spine as a possible adaptation to lumbar hyperlordosis in females.
Subjects and methods
Direct measurements of 4,080 thoracic and lumbar vertebrae were obtained from 240 normal complete spines of adult human skeletons. The skeletons are part of the Hamann–Todd Human (HTH) Osteological Collection housed at the Cleveland Museum of Natural History, Cleveland, OH, USA. This collection, which is professionally reserved for research purposes, comprises about 3,660 human skeletons including 2,252 Caucasians (1,932 males and 320 females) and 1,414 African-Americans (1,038 males and 376 females), all born between 1825 and 1910. In order to reach a statistical power of over 80%, 60 adult skeletons (age range 20–80) were randomly selected (using a random list of numbers) from each population and divided into four groups, according to gender and ethnic affiliation. The skeletons in each group were then subdivided into six cohorts, ranging from 20 to 80 years. A total of 4,080 vertebrae from T1 through L5 were measured. The stature and body weight of all individuals were collected from their medical records. Exclusion criteria were: (a) presence of a systemic disease which could affect the vertebrae/spine (based on the medical reports of all individuals); (b) damaged vertebra/pathological spine—visual inspection of all vertebrae was carried out by three experienced spinal researchers to identify pathological vertebrae, i.e. the presence of spondyloarthropathies, isthmic spondylolysis, idiopathic scoliosis (using Parent et al.’s method of identification [11]), compression fractures, Scheuermann’s kyphosis (vertebrae with anterior extension, as revealed by Scoles et al. [13]), and trauma; (c) missing vertebrae- only individuals with all vertebrae from T1 to L5 were included.
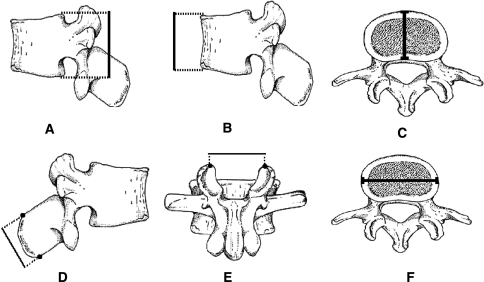
The following three vertebral parameters were measured and calculated using the Microscribe 3D digitizer (Immersion Co. San Jose, CA, USA) (Fig. 1) [5, 6]: (a) vertebral body (VB) sagittal wedging; (b) relative spinous process thickness; and (c) relative superior interfacet width. All vertebral parameters excluded osteophytes. The reliability (both intra- and inter-tester) of the measuring process was found to be high with interclass correlation coefficient (ICC) ranging from 0.96 to 0.98 [5–9].
Fig. 1.
Illustrations of the measured vertebral parameters. The vertebral parameters in the current study were calculated using the following formulas based on vertebral dimensions (a–f). (1) Vertebral body sagittal wedging = 2arctan {[(Centrum dorsal height (a) – Centrum ventral height)(b)/2]/Centrum anterior posterior diameter (c)}); (2) Relative spinous process thickness = spinous process thickness (d)/Centrum dorsal height (a). (3) Relative superior interfacet width: superior interfacet width (e)/superior VB width (f)
Descriptive statistics were calculated for all measurements. The effect of gender, ethnic group and age on the calculated vertebral parameters was examined using analysis of variance (ANOVA). Pearson’s r was used to identify any significant correlation between body physiques parameters (individual’s height and weight) and calculated vertebral parameters. Bonferoni criteria for significant differences was defined as p < 0.003 [e.g. statistical value of 0.05 divided by 17 vertebrae (T1–L5) measured for each individual].
Results
The mean and standard deviations of all calculated vertebral parameters found to be gender affected (T9–L5) in the thoracic and lumbar spine are presented in Table 1. In children, all calculated parameters are not affected by gender (Table 2). Worth noting, however, that although non-significant, L3 in girls is dorsally wedged (i.e. lordotic) (−0.9°) whereas in boys the same vertebra is ventrally wedged (i.e. kyphotic) (+0.6°).
Table 1.
Vertebral shape significant differences between females (N = 120) and males (N = 120) (20 ≤ age ≤ 80)
| Vertebra | T9 | T10 | T11 | T12 | L1 | L2 | L3 | L4 | L5 |
|---|---|---|---|---|---|---|---|---|---|
| Vertebral body (VB) sagittal wedging (degrees)* | |||||||||
| Females | 2.8 ± 0.04 | 2.6 ± 0.04 | 4.6 ± 0.05 | 3.5 ± 0.04 | 2.6 ± 0.05 | 0.7 ± 0.03 | −1.1 ± 0.04 | −3.1 ± 0.04 | −7.8 ± 0.05 |
| Males | 4.8 ± 0.04 | 3.8 ± 0.05 | 5.4 ± 0.05 | 4.9 ± 0.04 | 4.1 ± 0.04 | 2.6 ± 0.04 | 1.3 ± 0.05 | −0.7 ± 0.04 | −4.6 ± 0.05 |
| Ratio: spinous process thickness/VB sagittal height | |||||||||
| Females | 0.47 ± 0.05 | 0.52 ± 0.03 | 0.54 ± 0.02 | 0.70 ± 0.03 | 0.79 ± 0.03 | 0.82 ± 0.04 | 0.79 ± 0.04 | 0.67 ± 0.05 | 0.57 ± 0.06 |
| Males | 0.50 ± 0.04 | 0.54 ± 0.04 | 0.55 ± 0.04 | 0.69 ± 0.05 | 0.87 ± 0.10 | 0.92 ± 0.09 | 0.91 ± 0.09 | 0.80 ± 0.07 | 0.67 ± 0.07 |
| Ratio: interfacet width/VB width | |||||||||
| Females | 0.80 ± 0.02 | 0.80 ± 0.03 | 0.72 ± 0.02 | 0.75 ± 0.04 | 0.73 ± 0.05 | 0.70 ± 0.06 | 0.7 ± 0.05 | 0.73 ± 0.06 | 0.77 ± 0.09 |
| Males | 0.74 ± 0.02 | 0.73 ± 0.01 | 0.71 ± 0.03 | 0.74 ± 0.01 | 0.67 ± 0.04 | 0.63 ± 0.07 | 0.65 ± 0.08 | 0.66 ± 0.02 | 0.74 ± 0.08 |
* Negative values dorsal wedging, positive values ventral wedging, Bold significantly different from males (Bonferoni P < 0.03); ± SD (standard deviation), VB vertebral body
Table 2.
Vertebral shape in children (N = 32) (mean age 11.5 (±4.2) years)
| Vertebra | Vertebral body sagittal wedging (degrees)* (0.2 ≤ SD ≤ 0.9) | Ratio: Spinous process thickness/VB sagittal height (0.01 ≤ SD ≤ 0.09) | Ratio: Interfacet width/VB width (0.01 ≤ SD ≤ 0.09) |
|---|---|---|---|
| T1 | 7.0 | 0.6 | 1.4 |
| T2 | 6.0 | 0.5 | 1.0 |
| T3 | 5.0 | 0.5 | 1.1 |
| T4 | 4.0 | 0.5 | 1.0 |
| T5 | 6.0 | 0.4 | 0.9 |
| T6 | 5.5 | 0.3 | 0.9 |
| T7 | 5.5 | 0.3 | 0.9 |
| T8 | 5.5 | 0.3 | 0.9 |
| T9 | 4.3 | 0.4 | 0.8 |
| T10 | 3.0 | 0.4 | 0.9 |
| T11 | 3.2 | 0.4 | 0.8 |
| T12 | 3.0 | 0.5 | 0.8 |
| L1 | 4.5 | 0.7 | 0.8 |
| L2 | 1.9 | 0.8 | 0.8 |
| L3 | Boys 0.6 and girls −0.9 | 0.7 | 0.8 |
| L4 | −5.0 | 0.6 | 0.8 |
| L5 | −6.0 | 0.5 | 0.9 |
* Negative values dorsal wedging, positive values ventral wedging, ±SD (standard deviation), VB vertebral body
Neither ethnicity nor age was found to have any significant effect on the calculated vertebral parameters. Males were significantly taller and heavier than females (height 171 and 161 cm; weight 137 and 112 lbs., respectively). No correlation was found, however, between the calculated vertebral parameters and the individual’s weight or height [except for VB wedging at L3–L5 where significance, albeit low, correlated with height (Pearson’s r < 0.3, p < 0.05)].
Three major outcomes were found in relation to gender differences:
The vertebral body (VB) sagittal wedging in females was significantly less kyphotic than males from T9 to L2 (T11 excluded) with a cumulative mean difference of 8.8° (ventral wedging of 16.8° in females and 25.6° in males) (Table 1). The total mean wedging (both ventral and dorsal) from T9 to L5 was significantly greater in males than females (a difference of 16.8°; 4.8° in females and 21.6° in males) (Table 1). The sagittal wedging in all other thoracic levels (T1–T8) was gender independent.
The ratio-spinous process (SP) thickness/VB sagittal heights (both anterior and posterior) indicated that the lumbar spinous processes in females were significantly thinner than in males (Table 1). The relative SP thicknesses in all other vertebrae were gender independent.
The ratio—superior interfacet width/VB width—in the lumbar spine (L1–L4) and thoracic vertebrae T9–T10, was significantly higher in females than in males, indicating a relatively wider superior interfacet distance in females (Table 1). The same ratio was gender independent in other thoracic vertebrae and L5.
Discussion
We found that females have less kyphotic lower thoracic and lumbar vertebrae, relatively thinner lumbar spinous processes, and relatively larger superior interfacet width than males.
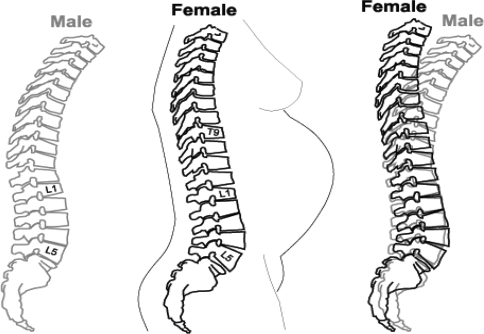
Our finding that females have a less kyphotic posture of the upper lumbar and lower thoracic area than males is a key element in female spine adaptation to lumbar hyperlordosis (Fig. 2). Accordingly, the trunk’s center of mass (COM) is maintained in an approximate sagittal alignment with the hip thus reducing biomechanical load and facilitating spinal extension [7]. An advantage of this ‘deeper lordosis-less kyphotic’ female spine is the providing of a larger superior–inferior space for the human fetus to develop during the last months of growth when the baby is forced into a flexion position due to space limitation (Fig. 2). The disadvantage of this morphological feature is the resultant size reduction of the intervertebral foramen (i.e. due to lumbar extension posture) which in turn may contribute to low back pain commonly experienced in pregnancy. This ‘deeper lordosis’ in females was also demonstrated by Whitcome et al. in a recent study [16] where longer series of dorsally wedged lumbar vertebrae were found in females not males. The authors do not explain how the negligible amount of 1°–2° dorsal wedging at L3 found in their study (with a standard deviation greater than the differences between the sexes) lends a biomechanical advantage to the pregnant female’s posture and “enables them to increase the lordosis with less inter-vertebral rotation”. Furthermore, the authors based their arguments on averages, ignoring the fact that the dorsally wedged L3 does not exist in more than one-third (42.8%) of all females, who have neither non-wedged nor ventrally wedged L3 (Fig. 3). We suggest that a lower amount of kyphotic vertebrae in the lower thoracic region, as found in the current study, provides a broader biomechanical basis for the hyperlolordotic lumbar spine in females.
Fig. 2.
Gender dimorphism in sagittal vertebral body wedging and spinous process thickness. Compared to males, females manifest deeper vertebral lumbar lordosis [due to an extra dorsally wedged lumbar vertebra (L3)] and less vertebral thoracic kyphosis (due to a lower amount of ventral wedging at T9–L1), thus providing a larger superior–inferior space for the human fetus to develop during the last months of growth. Lumbar hyperlordosis in females is probably due to relatively thinner lumbar spinous processes
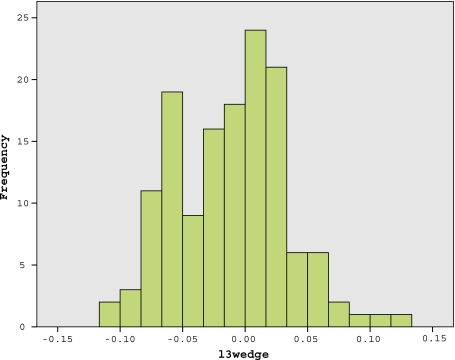
Fig. 3.
The frequency of vertebral body sagittal wedging of lumbar vertebra L3 in females. Almost 43% of the females have either non-wedged or ventrally wedged L3 (lordotic wedging, i.e. negative values up to −1.1°, and kyphotic wedging, i.e. positive values up to 1.2°). It is therefore reasonable to suggest that other anatomical features as well as the dorsally wedged L3 also contribute to the hyperlordotic posture in females
The fact that females possess relatively thinner spinous processes than males provides greater interspinous space in the lumbar region (Fig. 2). This, in turn, enables a greater range for lumbar extension in females associated with increasing lumbar lordosis. This anthropometric feature minimizes the impingement between two adjacent lumbar spinous processes in the hyperlordotic lumbar posture in females. It is reasonable to assume that greater range of lumbar extension requires greater zygoapophyseal facet dimension in the same region. Although Whitcome et al.’s study [16] proved this assumption others found no gender effect on the zygoapophyseal relative dimension [6], which may explain increasing low back pain during pregnancy.
The contradicting information about the uniqueness of facet shape in females may be the result of different measurement tools (caliper vs. digitizer) and different sample size (N = 120 [15] vs. N = 240 [6]). The authors [16] also state that the lumbar zygoapophyseal facet in females are more frontally oriented than in males “conferring greater resistance to forward displacement”. These data are also unconfirmed by others as no gender effect on facet orientation has been found [5]. Furthermore, frontally oriented lumbar facets in females may be of an evolutionary disadvantage as it would increase the shearing stress on the pars interarticularis area [7]. If zygoapophyseal facets in females are more frontally oriented, a lower prevalence of spondylolisthesis would be expected in females than in males. Yet, spondylolisthesis is four times more common in females than in males [4, 12].
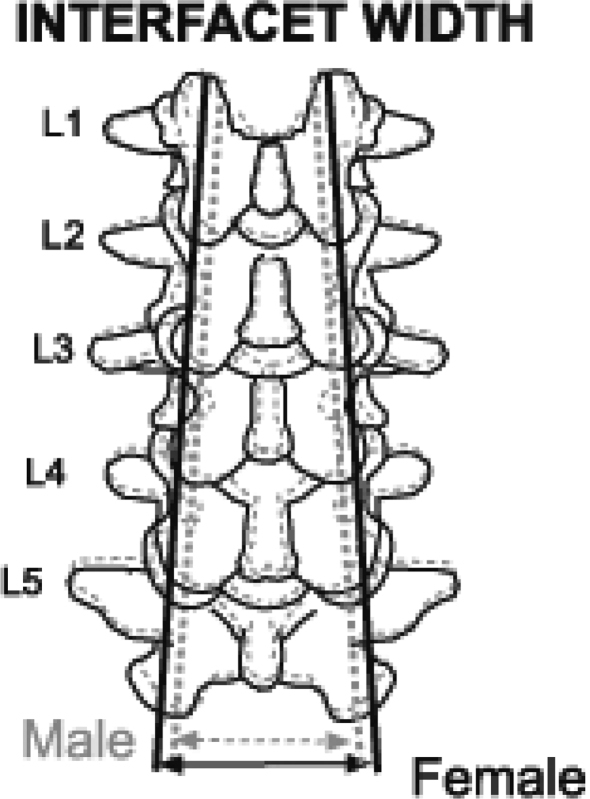
Our third outcome that relatively larger interfacet width is found in females versus males implies a larger area on which the spinal load is transmitted along the dorsal pillar in the hyperlordotic posture in females (Fig. 4). This anatomical feature may provide a better mechanical resistance to the increased shearing forces (up to 40%) developed in the hyperlordotic lumbar spine during pregnancy [2, 3].
Fig. 4.
Gender dimorphism in lumbar interfacet width A relatively larger lumbar interfacet width are found in females than in males implying a larger area on which the spinal load is transmitted along the dorsal pillar in the hyperlordotic posture of females
Although our study was limited only to investigating the dry vertebrae of adults, a recent MRI study on children proved the existence of this anatomical trait in young girls (age range 13–16, i.e. before pregnancy) [8]. This strengthens the validity of our results and highlights their developmental and evolutionary aspects.
Although this study has focused on anthropometric parameters and their uniqueness in females, it also reveals some important clinical aspects. First, when considering surgery in the thoracic or lumbar region, it is necessary to take into account the relative contribution of a specific vertebra to the total amount of kyphosis or lordosis to avoid any mechanical complications after surgery. This is even more applicable in pre-pregnant young females waiting for lumbar surgery. Second, the relatively greater lumbar interspinous space in females may enhance mobility in this region and can be significant as to conservative treatments aimed at restoring lumbar motion. Finally, as the size reduction in the intervertebral foramen (i.e. due to lumbar extension posture) may contribute to low back pain in females, active treatments in the opposite direction (i.e. lumbar flexion) can be suggested. Clearly, further clinical and biomechanical studies are required to validate these clinical implications.
Conclusion
The combination of less kyphotic VB wedging in the lower thoracic and upper lumbar vertebrae, relatively greater interspinous space and larger interfacet width in the lumbar spine in females, are key architectural elements in lumbar hyperlordosis in females. These anatomical features could be the expression of altered vertebral morphology predisposing to pregnancy.
Acknowledgments
The authors wish to thank Phyllis Curchack Kornspan for her editorial and secretarial services.
References
- 1.Bergenudd H, Nilsson B, Uden A, Willner S. Bone mineral content, gender, body posture, and build in relation to back pain in middle age. Spine. 1989;14:577–579. doi: 10.1097/00007632-198906000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Dunlop R, Adams A, Hutton W. Disc space narrowing and the lumbar facet joints. J Bone Joint Surg Br. 1984;66:706–710. doi: 10.1302/0301-620X.66B5.6501365. [DOI] [PubMed] [Google Scholar]
- 3.Lorenz M, Patwardhan A, Vanderby R. Load-bearing characteristics of lumbar facets in normal and surgically altered spinal segments. Spine. 1983;8:122–130. doi: 10.1097/00007632-198303000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Love T, Fagan A, Fraser R. Degenerative spondylolisthesis: developmental or acquired? J Bone Joint Surg Br. 1999;81:670–674. doi: 10.1302/0301-620X.81B4.9682. [DOI] [PubMed] [Google Scholar]
- 5.Masharawi Y, Rothschild B, Dar G, Peleg S, Been E, Robinson D, Hershkovitz I. Facet orientation in the thoraco-lumbar spine: three-dimensional anatomical and biomechanical analysis. Spine. 2004;29:1755–1763. doi: 10.1097/01.BRS.0000134575.04084.EF. [DOI] [PubMed] [Google Scholar]
- 6.Masharawi Y, Rothschild B, Salame K, Dar G, Peleg S, Hershkovitz I. Facet tropism and interfacet shape in the thoraco-lumbar spine: characterization and biomechanical interpretation. Spine. 2005;30:E281–E292. doi: 10.1097/01.brs.0000164098.00201.8d. [DOI] [PubMed] [Google Scholar]
- 7.Masharawi Y, Alperovitch-Najenson D, Dar G, Peleg S, Steinberg N, Rothschild R, Salame K, Hershkovitz I. Lumbar facet orientation in spondylolysis: a skeletal study. Spine. 2007;32:E176–E180. doi: 10.1097/01.brs.0000257565.41856.0f. [DOI] [PubMed] [Google Scholar]
- 8.Masharawi Y, Kjaer P, Bendix T, Manniche C, May H, Mirovski Y, Anekshtein Y, Jensen TS, Hershkovitz I. Lumbar facet and interfacet shape variation during growth in boys and girls from the general population: a three years MRI follow-up study. Spine. 2009;15:408–412. doi: 10.1097/BRS.0b013e3181971b6a. [DOI] [PubMed] [Google Scholar]
- 9.Masharawi Y, Dar G, Peleg S, Steinberg N, Alperovitch-Najenson D, Salame K, Hershkovitz I. Lumbar facet anatomy changes in spondylolysis: a comparative skeletal study. Eur Spine J. 2007;16:993–999. doi: 10.1007/s00586-007-0328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norton B, Sahrmann S, Dillen L. Differences in measurements of lumbar curvature related to gender and low back pain. J Orth Sports Phys Ther. 2004;34:524–534. doi: 10.2519/jospt.2004.34.9.524. [DOI] [PubMed] [Google Scholar]
- 11.Parent S, Labelle H, Skalli W, Latimer B, Guise J. Morphometric analysis of anatomic scoliotic specimens. Spine. 2002;27:2305–2311. doi: 10.1097/00007632-200211010-00002. [DOI] [PubMed] [Google Scholar]
- 12.Sanderson P, Fraser R. The Influence of pregnancy on the development of degenerative spondylolisthesis. J Bone Joint Surg. 1996;78:951–954. doi: 10.1302/0301-620X78B6.1291. [DOI] [PubMed] [Google Scholar]
- 13.Scoles P, Latimer B, DigIovanni B, Vargo E, Bauza S, Jellema L. Vertebral alterations in Scheuermann’s kyphosis. Spine. 1991;16:509–515. doi: 10.1097/00007632-199105000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Tanner JM. Fetus into man: physical growth from conception to maturity. 6. MA: Cambridge; 1990. pp. 165–171. [Google Scholar]
- 15.Taylor J, Twomey L. Sexual dimorphism in human vertebral body shape. J Anat. 1984;138:281–286. [PMC free article] [PubMed] [Google Scholar]
- 16.Whitcome K, Shapiro L, Lieberman D. Fetal load and the evolution of lumbar lordosis in bipedal hominins. Nature. 2007;450:1075–1078. doi: 10.1038/nature06342. [DOI] [PubMed] [Google Scholar]
- 17.Youdas J, Garett T, Harmsen W, Suman V, Carey J. Lumbar lordosis and pelvic inclination of asymptomatic adults. Phy Ther. 1996;76:1066–1081. doi: 10.1093/ptj/76.10.1066. [DOI] [PubMed] [Google Scholar]