Abstract
A prospective, non-randomized multicenter study was initiated to study efficacy and safety of a partly resorbable composite of calcium sulphate and hydroxyapatite (Cerament™ SpineSupport), a novel, injectable bioceramic, in osteoporotic patients with vertebral compression fractures during 18-month follow-up. Fifteen patients with low-energy trauma and 1–2 vertebral compression fractures verified by magnetic resonance imaging were recruited to undergo percutaneous bioceramic vertebral augmentation under fluoroscopy. The patients were treated with a highly flowable bioceramic containing calcium sulphate, hydroxyapatite and the non-ionic radiocontrast agent iohexol, with final setting time within 1 h. After the procedure, the patients were allowed to mobilize after 2 h. Pain (VAS), occurrence of remote and adjacent fractures, and Quality of Life (QoL; SF-36 and EQ-5D) was recorded during 18 months. The injected volume of the composite material ranged from 2.8 to 9 mL (mean 4.2 mL). Pre-operative VAS score was mean 70.3 (CI95% ±8.7) with an immediate post-operative pain relief, which was maintained at the 4-week visit (mean 26.4 with CI95% ±16.1) and 8-week visit (mean 18.0 with CI95% ±13.5 pain relief). Eighty percent of the patients demonstrated a clinical improvement. The pain relief was maintained over 18 months and no adjacent fractures were observed. There was a statistically significant improvement of physical components in the QoL assessment. No extra-vertebral leakage or neurological deficits were reported in this series. This first prospective multicenter study on a partly resorbable bioceramic material indicate that fracture healing can be achieved with sustained pain relief over a follow-up period of 18 months in an osteoporotic patient population with vertebral compression fractures.
Keywords: Bioceramic, Compression fracture, Osteoporosis, Pain, Vertebroplasty
Introduction
Osteoporosis causes more than 8.9 million fractures annually worldwide and about 700,000 of these fractures are vertebral compression fractures [13]. Approximately 40–50% of women above 80 years of age are reported to have suffered at least one vertebral fracture [22]. Percutaneous vertebroplasty has emerged as a treatment option for vertebral compression fractures refractory to conservative treatment. Vertebroplasty involving the injection of a bone cement, polymethylmethacrylate (PMMA), was introduced in 1984 [9].
The incidence of clinically diagnosed vertebral compression fractures (VCF) in women (123/100,000 patient years) is comparable to that for hip fractures [7], with fracture pain usually lasting from 2 weeks to 3 months, associated with intense, deep, and sometimes intractable pain [17]. Each additional new VCF is associated with a further increase in functional limitation, of a magnitude similar to that in patients with diabetes, ischemic heart disease and rheumatism [23]. Both low bone mass and previous fracture independently predict subsequent fracture [30]. Further, women with clinically diagnosed vertebral fractures have a 15% higher mortality than women who do not [6]. A novel, injectable bioceramic bone substitute (Cerament™ SpineSupport) which exerts a dual action by its composition of resorbable calcium sulphate to allow bone in-growth, hydroxyapatite to provide long-term support, and the water-soluble and non-ionic radiocontrast agent iohexol [31] was used. This newly developed material has an immediate compressive strength similar to that of cancellous bone [24], with the purpose of minimizing the risk of implant-induced adjacent fractures. Over weeks and months the calcium sulphate component is replaced by in-growing bone that remodels to form trabeculae [38], while the hydroxyapatite component remains as an armouring of the osteoporotic bone.
The present study was designed to investigate safety aspects, pain relief, Quality of Life (QoL) and the rate of subsequent adjacent fractures following vertebroplasty with this new composite of calcium sulphate and hydroxyapatite that aims for healing of vertebral compression fractures.
Methods
The study was preceded by an initial phase to optimize the treatment procedure and to follow-up on material visibility during fluoroscopy. During this run-in phase one patient suffered from atypical signs (e.g. atrial fibrillation) for 24 h. The CT scan of the lungs and the treated spine region showed no evidence of any suspect material or signs of leakage. Subsequently, a flushing procedure (with saline) of the treated vertebrae was abandoned and the composition of the bioceramic material was improved to give optimum visibility during the injection.
The criteria for inclusion were symptomatic vertebral fractures with a medical history confirming the age of the vertebral compression to be within 8 weeks, low energy trauma causing 1–2 symptomatic vertebral compression(s) at level Th5 to L5 verified by MRI, impaction grade A1.1, A1.2 and A1.3 according to the AO classification, and height compression of the affected vertebra/vertebrae within 0–60% compared to the dorsal vertebral wall.
Exclusion criteria were age below 50, retropulsion of fracture fragment, and concomitant diseases which might be worsened by invasive treatment of the fracture such as local tumor. Patients were also excluded if they had a history of anaphylactic reaction towards iodine-based radio contrast agents, known hyperthyreosis and/or thyroid adenoma, known bleeding disorders, or were treated with anticoagulant medication. Moreover, patients were considered non-eligible for the study if they had an extreme body weight (>100 kg) or had a local infection or otherwise compromised skin of the puncture site.
The screening procedure took place at six investigational sites. Patients with osteoporotic vertebral compression fractures refractory to standard pain medication were consecutively screened for eligibility between 14 days prior to and up to the day of surgery. No study-related procedures were performed before the subjects had signed the informed consent form, which had been approved by the local ethics committee responsible for the research conducted at the respective hospital. If the subjects were found eligible for participation, the fractures were verified by magnetic resonance imaging (MRI) and the patients’ symptoms were recorded. The protocol stipulated a bilateral transpedicular vertebral injection under fluoroscopy. After the procedure, the patient had to stay in prone position for at least 15 min before rolling over in a bed to remain in horizontal position for 2 h while the hardening process was completed.
A total of 16 vertebral levels were treated in 15 patients with primary osteoporotic vertebral compression fractures.
The composite material (Cerament™ SpineSupport, Bonesupport AB, Sweden) is composed of 60 wt% calcium sulphate alpha-hemihydrate and 40% sintered hydroxyapatite. When mixed with the liquid component, iohexol 300 mg/mL in water, it becomes a viscous paste possible to be injected transpedicularly (Fig. 1).
Fig. 1.

Mixed paste of calcium sulphate/hydroxyapatite composite (Cerament™ SpineSupport) in a 1 mL syringe (23G needle)
Follow up
Patient visits were scheduled at the first post-operative day, at 4 and 8 weeks and at 6 and 18 months. X-ray was taken pre-operatively and at 8 weeks to confirm fracture healing and at 18 months to exclude refracture of the treated vertebra or fractures at adjacent vertebral levels. A CT scan was taken within the first 24 h post-operatively to examine possible extravertebral leakage. Pain was assessed using a 100 mm visual analogue ruler (score 0–100) where a score of 0 represents “no pain” and a score of 100 represents “maximum pain”. QoL was followed through SF-36 Health Surveys [39] and EQ-5D questionnaires [5]. Safety was assessed by the registration of adverse events and adverse device effects, and by physical examination and laboratory measurements. The possible occurrence of adjacent or remote vertebral fractures was recorded by two independent examiners using standard radiological images.
Statistical methodology
Data are presented using count of mean and confidence interval as appropriate. Two-sided Student’s paired t test, was used to assess differences between baseline and subsequent VAS measurements, SF-36 and EQ-5D scores. SAS™ statistical software (version 9.1) was used for all analyses. Statistical significance was considered at a probability level <0.05.
Results
Fifteen patients (10 female) with a mean age of 76 (range 68–94) were treated for vertebral compression fractures at spine levels with positive MRI signals that corresponded clinically to the back pain. Sixteen fractures, including seven thoracic and nine lumbar vertebrae, were treated. The injected volume of the composite in each vertebral body ranged from 2.8 to 9 mL (mean 4.2 mL). The radiopacity and intratrabecular material spread are shown in Fig. 2.
Fig. 2.
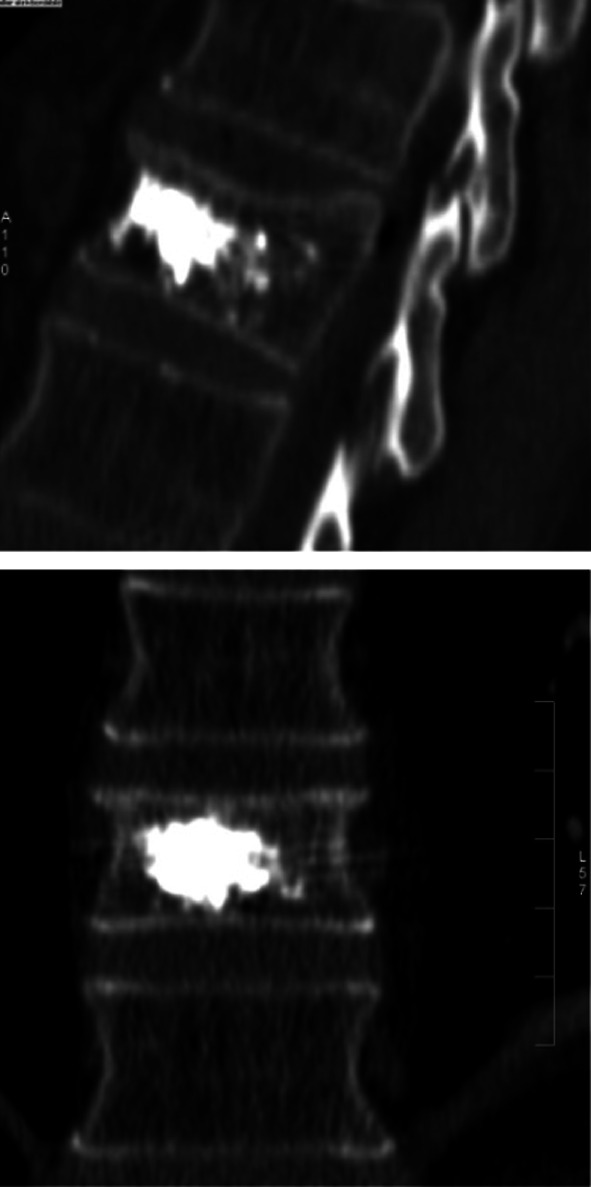
CT control showing good distribution of a composite of calcium sulphate and hydroxyapatite (Cerament™ SpineSupport)
Pain
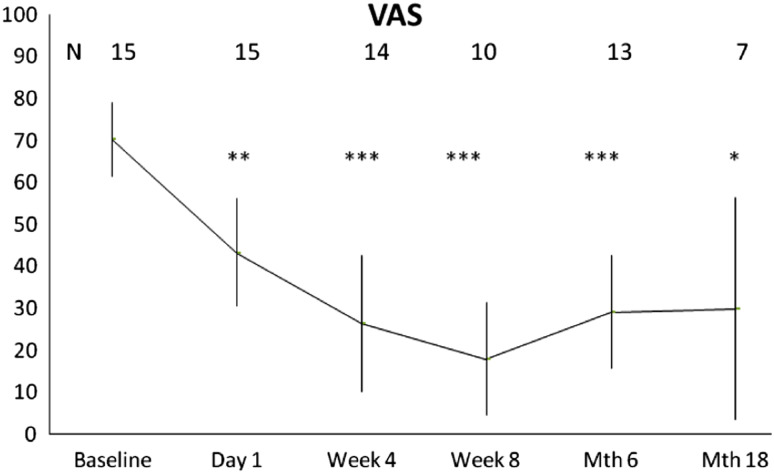
Eighty percent of the patients demonstrated an immediate and statistically significant improvement in pain compared to baseline and assessed by the VAS pain score (Fig. 3). The mean pain relief compared to the assessment immediately before the intervention was 39% on the day after the procedure, and 62% and 74% after 4 and 8 weeks, respectively. A pain relief of approximately 60% was maintained at 6 and 18 months after the treatment.
Fig. 3.
Mean VAS pain score (mm). Error bars represent 95% confidence interval. Numbers in the graph represent number of patients available at follow-up. *p < 0.05; **p < 0.01; ***p < 0.001
Quality of Life
The QoL assessed by the use of SF-36 showed a statistically significant improvement in the mean physical component summary (PCS) of approximately 40% (p < 0.01) and 70% (p < 0.01) at 8 weeks and 18 months, respectively, from a baseline score of 26.3. The mean mental component summary (MCS) remained unchanged.
QoL as evaluated by the EQ-5D Health Index significantly improved from a value of 0.254 at baseline to 0.563 at 8 weeks (p < 0.05) and 0.638 at 18 months.
New vertebral fractures
No adjacent fractures occurred during the 18-month follow-up period. A new remote fracture was diagnosed in one patient.
Safety
No serious events were considered to be related to the calcium sulphate/hydroxyapatite-composite or to the percutaneous procedure. No deaths or neurological complications were reported and there was no evidence for local extravasation or pulmonary embolism. The day after the percutaneous procedure one patient was shown to have an unstable end-plate fracture in the treated vertebra. The patient was (as stipulated in the study protocol) successfully re-treated with PMMA after approximately 1 week.
Discussion
The general purpose of fracture treatment is to stabilize the fracture to allow healing in a correct position and to relieve pain. Vertebroplasty with PMMA only addresses the pain aspect. Further, implantation of a non-resorbable material may entail a risk of future device-related complications. Cerament™ SpineSupport has been designed to stabilize the vertebral fracture and to give both pain relief and allow fracture healing through a gradual bone ingrowth. Although both animal studies [24, 38] and studies on osteoporotic patients undergoing wrist osteotomy [1] have demonstrated a bone remodeling after treatment with Cerament™, the concept still has to be proven in vertebral compression fractures. The present study was designed to test the hypothesis that Cerament™ SpineSupport will give short-term pain relief through fracture stabilization and long-term pain relief through fracture healing in vertebral compression fractures caused by underlying osteoporosis. Further, the fracture healing concept was controlled at 18 months, a time point where the stabilizing calcium sulphate component will be resorbed [26, 28, 32]. As a secondary objective, the possible risk of causing adjacent level fractures was investigated.
The instant and sustained pain relief demonstrated in the present study indicates that Cerament™ SpineSupport has sufficient mechanical strength to stabilize stable compression fractures. It has been speculated that the toxic effects of the monomer methylmetacrylate or the high temperature of PMMA during the setting process [3], may not only cause damage to nerve roots in case of leakage [25] but also contribute to the immediate pain relief seen after vertebroplasty [19]. The present data support the hypothesis that fracture stabilization is the most important factor to relieve pain in vertebral compression fractures. The calcium sulphate/hydroxyapatite composite material investigated in the present study sets at a moderate temperature increase of 4°C.
The sustained pain relief during the first 2–6 months points towards a healing of the vertebral fracture lines, which is supported by bone ingrowth demonstrated in animal studies on the material [24, 38] showing bone remodeling within this time frame. An alternative explanation could be that Cerament™ SpineSupport turnover is slower in osteoporotic patients than in animals and that the implant still has a stabilizing effect after 18 months. This seems, however, less probable considering that Cerament™ was completely remodeled into bone after 12 months, in osteoporotic patients undergoing wrist osteotomy [1]. The final answer can only be obtained by a repeat study including advanced imaging techniques or by bone biopsies in patients treated with the product.
The immediate pain relief obtained was statistically significant compared to baseline and it was maintained over time. These results appear to be in line with the results reported from vertebroplasty studies using PMMA [2, 8, 15, 16, 20, 21, 27, 29, 33, 36, 37, 41].
There was also a statistically significant improvement in QoL as evaluated by the SF-36 PCS. The positive outcome in SF-36 PCS was corroborated by the improvement noted also for the EQ-5D Health Index. Although interpretation of outcomes between studies should be performed with caution, the improvement in QoL after vertebroplasty with Cerament™ SpineSupport appears to be similar to that reported for vertebroplasty with PMMA [2, 8, 21, 29].
It has been reported that the risk of getting a new fracture in adjacent vertebrae following vertebroplasty with PMMA may increase as a result of the high stiffness created in the treated vertebra, with a reported incidence of adjacent fractures in the range between 10 and 25% within the first couple of months [4, 10–12, 14, 18, 34, 35, 40]. An alternative explanation to the occurrence of adjacent fractures is that certain segments of the spine is at higher risk of multiple fractures due to the underlying osteoporosis. Although speculative due to the limited number of patients in the present study and the number of patients lost to follow up at the18-month visit, it is reasonable to assume that a ceramic material, that mimics the stiffness of cancellous bone, is less prone than PMMA to cause adjacent fractures. No adjacent fractures occurred over a follow-up period of 18 months, while one remote fracture was recorded.
Although a partly resorbable ceramic material seems to have an advantage compared to PMMA in that less long-term material-related side effects are to be expected and that the ability to bone remodeling will promote fracture healing, a remodeling implant may still entail certain limitations. Unstable fractures and fractures caused by tumours engaging the cortical walls require a material with certain properties. In the present study, one patient treated with Cerament™ SpineSupport was considered to require re-treatment with PMMA due to an unstable end-plate fracture diagnosed 1 day after the percutaneous administration of the material. From this first prospective study it seems, however, as Cerament™ SpineSupport might become an interesting alternative to PMMA in stable compression fractures classified as A1.1–A1.3 according to the AO classification. Radiological imaging showed no evidence of extra-vertebral leakage and there were no reports of neurological deficits. It is reasonable to believe that leakage occurs not only with PMMA, but also with a bioceramic material. A possible explanation for the lack of extravasation observed might be a propensity for pre-setting dissolution if deposited intravascularly.
Given the limitations of a non-controlled study it may be concluded that the calcium sulphate/hydroxyapatite-composite (Cerament™ SpineSupport) has been shown to be a promising product for bioceramic augmentation in osteoporotic patients with vertebral compression fractures.
Acknowledgments
We are grateful for the support of this study by BoneSupport AB, Lund, Sweden.
Contributor Information
Michael Rauschmann, Phone: +49-69-6705404, Email: m.rauschmann@friedrichsheim.de, Email: m.rauschmann@t-online.de.
Thomas Vogl, Email: T.Vogl@em.uni-frankfurt.de.
Akhil Verheyden, Email: Akhil.verheyden@le.ortenau-klinikum.de.
Robert Pflugmacher, robert.pflugmacher@googlemail.com.
Sven Schmidt, Email: s.schmidt@friedrichsheim.de.
Johannes Hierholzer, Email: jhierholzer@klinikumevb.de.
References
- 1.Abramo A, et al. Osteotomy of distal radius fracture malunion using a fast remodeling bone substitute consisting of calcium sulphate and calcium phosphate. J Biomed Mater Res B Appl Biomater. 2010;92(1):281–286. doi: 10.1002/jbm.b.31524. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez L, et al. Functional improvements in patients with osteoporotic compression fractures. Spine. 2006;31:1113–1118. doi: 10.1097/01.brs.0000216487.97965.38. [DOI] [PubMed] [Google Scholar]
- 3.Belkoff SM, Molloy S. Temperature measurement during polymerization of polymethylmethacrylate cement used for vertebroplasty. Spine. 2003;28(14):1555–1559. doi: 10.1097/00007632-200307150-00015. [DOI] [PubMed] [Google Scholar]
- 4.Berlemann U, et al. Adjacent vertebral failure after vertebroplasty. J Bone Joint Surg. 2002;84-B:748–752. doi: 10.1302/0301-620X.84B5.11841. [DOI] [PubMed] [Google Scholar]
- 5.Brooks R et al (2003) The measurement and valuation of health status using EQ-5D: a European perspective evidence from the EuroQol BIO MED Research Programme
- 6.Cooper C, et al. Population-based study of survival after osteoporotic fractures. Am J Epidemiol. 1993;137:1001–1005. doi: 10.1093/oxfordjournals.aje.a116756. [DOI] [PubMed] [Google Scholar]
- 7.Cooper C, et al. Incidence of clinically diagnosed vertebral fractures: a population based study in Rochester Minnesota, 1985–1989. J Bone Min Res. 1992;7:221–228. doi: 10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]
- 8.Do HM, et al. Prospective analysis of clinical outcomes after percutaneous vertebroplasty for painful osteoporotic vertebral body fractures. Am J Neuroradiol. 2005;26:1623–1628. [PMC free article] [PubMed] [Google Scholar]
- 9.Galibert P, et al. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty [in French] Neurochirurgie. 1987;33:166–168. [PubMed] [Google Scholar]
- 10.Grados F, et al. Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology. 2000;39:1410–1414. doi: 10.1093/rheumatology/39.12.1410. [DOI] [PubMed] [Google Scholar]
- 11.Hardouin P, et al. Should percutaneous vertebroplasty be used to treat osteoporotic fractures? An update. Joint Bone Spine. 2001;68(3):216–221. doi: 10.1016/S1297-319X(01)00265-2. [DOI] [PubMed] [Google Scholar]
- 12.Heini PF. Percutaneous transpedicular vertebroplasty with PMMA: operative technique and early results. A prospective study for the treatment of osteoporotic compression fracture. Eur Spine J. 2000;9:445–450. doi: 10.1007/s005860000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnell O, et al. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, et al. Risk factors of new compression fractures in adjacent vertebrae after percutaneous vertebroplasty. Acta Radiol. 2004;45(4):440–445. doi: 10.1080/02841850410005615. [DOI] [PubMed] [Google Scholar]
- 15.Lane JI, et al. Intravertebral clefts opacified during vertebroplasty: pathogenesis, technical implications, and prognostic significance. Am J Neuroradiol. 2002;23:1642–1646. [PMC free article] [PubMed] [Google Scholar]
- 16.Layton KF, et al. Vertebroplasty, first 1000 levels of a single center: evaluation of the outcomes and complications. Am J Neuroradiol. 2007;28:683–689. [PMC free article] [PubMed] [Google Scholar]
- 17.Leidig, et al. A study of complaints and their relation to vertebral destruction in subjects with osteoporosis. Bone Miner. 1990;8:217–219. doi: 10.1016/0169-6009(90)90107-Q. [DOI] [PubMed] [Google Scholar]
- 18.Lin EP, et al. Vertebroplasty: cement leakage into the disc increases the risk of new fracture of adjacent vertebral body. AJNR Am J Neuroradiol. 2004;25(2):175–180. [PMC free article] [PubMed] [Google Scholar]
- 19.Mathis et al (eds) (2002) Percutaneous vertebroplasty. New York. ISBN 0-387-95306-X
- 20.McGraw JK, et al. Prospective evaluation of pain relief in 100 patients undergoing percutaneous vertebroplasty: results and follow-up. J Vasc Interv Radiol. 2002;13:883–886. doi: 10.1016/S1051-0443(07)61770-9. [DOI] [PubMed] [Google Scholar]
- 21.McKiernan F, et al. Quality of life following vertebroplasty. J Bone Joint Surg. 2004;86-A:2600–2606. doi: 10.2106/00004623-200412000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Melton LJ III (2003) Adverse outcomes of osteoporotic fractures in the general population. J Bone Min Res 18(6):1139–1141 [DOI] [PubMed]
- 23.Nevitt MC, et al. The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Intern Med. 1998;128:793–800. doi: 10.7326/0003-4819-128-10-199805150-00001. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson M, et al. Biogradation and biocompatibility of a calcium sulphate-hydroxyapatite bone substitute. J Bone Joint Surg. 2004;86-B:120–125. [PubMed] [Google Scholar]
- 25.Patel AA, et al. Neurologic deficit following percutaneous vertebral stabilization. Spine. 2007;32:1728–1734. doi: 10.1097/BRS.0b013e3180dc9c36. [DOI] [PubMed] [Google Scholar]
- 26.Peltier LF. The use of plaster of Paris to fill defects in bone. Clin Orthop. 1961;21:1–29. [PubMed] [Google Scholar]
- 27.Perez-Higueras et al (2002) Percutaneous vertebroplasty: long-term clinical and radiological outcome. Neuroradiology 44(11):950–954 [DOI] [PubMed]
- 28.Pietrzak WS, Ronk R. Calcium sulfate bone void filler: a review and a look ahead. J Craniofac Surg. 2000;11:327–333. doi: 10.1097/00001665-200011040-00009. [DOI] [PubMed] [Google Scholar]
- 29.Prather H. Prospective measurement of function and pain in patients with non-neoplastic compression fractures treated with vertebroplasty. J Bone Joint Surg. 2006;88-A(2):334–341. doi: 10.2106/JBJS.D.02670. [DOI] [PubMed] [Google Scholar]
- 30.Ross PD, et al. Preexisting fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med. 1991;114:919–923. doi: 10.7326/0003-4819-114-11-919. [DOI] [PubMed] [Google Scholar]
- 31.Sistrom CL, et al. Extravasation of iopamidol and iohexol during contrast-enhanced CT: report of 28 cases. Radiology. 1991;180:707–710. doi: 10.1148/radiology.180.3.1871281. [DOI] [PubMed] [Google Scholar]
- 32.Tay BKB, et al. Calcium sulfate- and calcium phosphate-based bone substitutes—mimicry of the mineral phase of bone. Orthop Clin North Am. 1999;30:615–623. doi: 10.1016/S0030-5898(05)70114-0. [DOI] [PubMed] [Google Scholar]
- 33.Trout AT, et al. Vertebroplasty in the inpatient population. Am J Neuroradiol. 2005;26:1629–1633. [PMC free article] [PubMed] [Google Scholar]
- 34.Trout AT, et al. New fractures after vertebroplasty. Adjacent fractures occur significantly sooner. Am J Neuroradiol. 2006;27:217–223. [PMC free article] [PubMed] [Google Scholar]
- 35.Uppin AA, et al. Occurrence of new vertebral body fracture after percutaneous vertebroplasty in subjects with osteoporosis. Radiology. 2003;226(1):119–124. doi: 10.1148/radiol.2261011911. [DOI] [PubMed] [Google Scholar]
- 36.Vogl TJ. CT-guided percutaneous vertebroplasty in the therapy of vertebral compression fractures. Eur Radiol. 2006;16:797–803. doi: 10.1007/s00330-005-0021-4. [DOI] [PubMed] [Google Scholar]
- 37.Voormolen MHJ, et al. Pain response in the first trimester after percutaneous vertebroplasty in patients with osteoporotic vertebral compression fractures with or without bone marrow edema. Am J Neuroradiol. 2006;27:1579–1585. [PMC free article] [PubMed] [Google Scholar]
- 38.Wang JS et al (2006) Biomechanics and bone integration on injectable calcium sulphate and hydroxyapatite in large bone defect in rat. Abstract, American Orthopedic Research Society, Chicago, 18–22 March, 2006
- 39.Ware JE, et al. SF-36® Health survey: manual & interpretation guide. Lincoln: QualityMetric Inc.; 1993. [Google Scholar]
- 40.Wilcox RK. The biomechanics of vertebroplasty: a review. Proc Inst Mech Eng [H] 2004;218(1):1–10. doi: 10.1243/095441104322807703. [DOI] [PubMed] [Google Scholar]
- 41.Zoarski GH. Percutaneous vertebroplasty for osteoporotic compression fractures: quantitative prospective evaluation of long-term outcomes. J Vasc Interv Radiol. 2002;13:139–148. doi: 10.1016/S1051-0443(07)61930-7. [DOI] [PubMed] [Google Scholar]