Abstract
The use of frameless stereotactic navigation is gaining popularity in spinal surgery. Although initially used in the spine for placement of lumbar pedicle screws, this technology has expanded to facilitate placement of spinal instrumentation at virtually all spinal levels. While previous reports have described the utility of image guidance for placement of spinal instrumentation, its use in assisting with resection of complex spine tumors has not been extensively reported. Here we describe the use of frameless stereotaxy to guide a complex, four-level sagittal vertebral osteotomy for en bloc resection of a giant cell tumor involving the chest wall and thoracic spine.
Keywords: Frameless stereotaxy, Osteotomy, Giant cell tumor
Introduction
Giant cell tumors (GCT) of the chest wall and spine are rare. They comprise 0.3–1.6% of all GCT [1]. They are benign neoplasms that can be locally aggressive and have high local recurrence rates [2]. Because of their tendency to recur following curettage or intralesional resection, en bloc removal is the treatment of choice when feasible [3].
We describe a multi-level sagittal vertebral osteotomy in the thoracic spine for en bloc resection of a GCT arising from the sixth rib and involving the right lateral portion of the T4–T7 vertebrae. This case demonstrates a technique for the safe and effective en bloc resection of tumors involving the chest wall and spine. Furthermore, it illustrates a novel application of frameless stereotaxy to provide real-time three dimensional imaging during the critical osteotomies required to avoid violating the tumor capsule and the adjacent critical vascular structures.
Case presentation
History and presentation
This 35-year-old male was diagnosed with a GCT of his right posterior chest wall. The patient had a history of a motorcycle accident at 15 years old, during which he sustained right rib fractures. Three years later at age 18 he was involved in a motor vehicle accident and afterwards, he had persistent neck and interscapular pain. Magnetic resonance image (MRI) of the cervical spine was obtained. The images at that time demonstrated a congenital cervical fusion of C5–6, with no other abnormalities seen. The patient remained relatively stable until approximately 16 months prior to surgery. At that time, his pain progressed causing right-sided band-like numbness of the thorax. A chest X-ray was performed demonstrating a mediastinal mass. A computed tomography (CT) scan of the chest was performed, revealing a right posterior chest wall mass. The lesion measured approximately 9 × 9 × 7.5 cm and was involving the fifth, sixth, and seventh ribs as well as the right antero-lateral portion of the fourth, fifth, sixth, and seventh vertebral bodies.
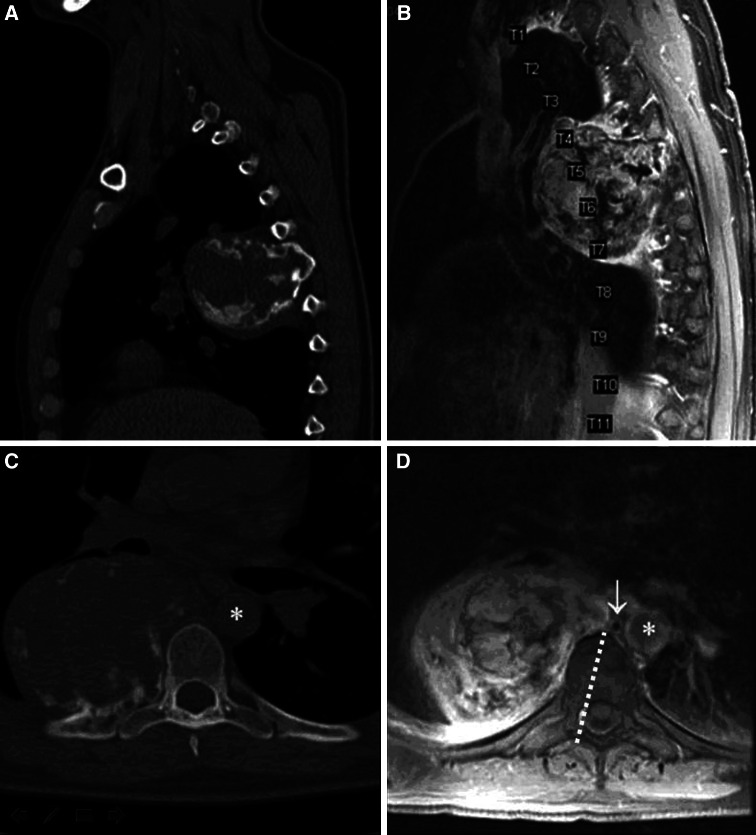
A CT-guided biopsy was performed which revealed histiocytes and giant cells. The pathological diagnosis was GCT of the bone with extensive secondary changes. The patient was referred to the Sarcoma service at our institution and was treated for 1 year with weekly injections of pegylated interferon. He subsequently underwent serial endovascular embolizations of the lesion, including one procedure per month for 4 months. Of note, the artery of Adamkiewicz was not identified at any of the levels to be resected (right T4–T7). The patient was then referred to the neurosurgical service for definitive surgical resection. CT and MRI of the thoracic spine confirmed the intimate association of the tumor with the lateral aspect of the vertebrae (Fig. 1).
Fig. 1.
Sagittal a and axial b bone window CT examination of this patient’s giant cell tumor arising from the sixth rib and involving the right fifth, sixth, and seventh ribs, as well as the right fourth, fifth, sixth, seventh vertebral bodies. Areas of calcification are seen throughout the tumor. c Sagittal MRI demonstrating heterogeneous signal intensity of the tumor. d Axial view through the tumor at T7 showing intimate association of the tumor with the lateral vertebral body. Other cuts (not shown) revealed involvement of the neural foramina and mild inflammatory changes in the bone. Note the proximity of the medial tumor margin to the aorta (asterisk) and azygos vein (white arrow) on the axial image. The proximity of the azygous vein and aorta required that the sagittal osteotomy be precisely directed. The planned osteotomy is indicated by a dashed white line
Operative techniques
An en bloc resection of the mass was planned in two surgical stages, combining the efforts of a multidisciplinary surgical team including neurosurgery, thoracic surgery, and plastic surgery. Intraoperative somatosensory evoked potentials were used during both operations to monitor the patient’s neurologic function.
Stage 1
The first stage consisted of a midline posterior thoracic approach with the patient in the prone position. Laminectomies were performed from T4 to T7. On the right side, medial facetectomies were performed and the decompression exposed the medial aspect of the pedicles. The right-sided T4, T5, T6, and T7 nerve roots were ligated and cut to allow for mobilization of the thecal sac and visualization of the right anterior aspect of the spinal canal where the osteotomies would be initiated (Figs. 1d, 2b, c). Once the laminectomy was completed and the nerve roots ligated, the thecal sac was gently displaced medially with a Penfield #3 dissector. Given the wide laminectomies and medial facetectomies, minimal retraction of the thecal sac was necessary. A high speed pneumatic drill fitted with a 2 mm cutting burr (Midas Rex, Medtronic, Forth Worth, TX, USA) was used to create a sagittal trough approximately 3 mm deep in the right posterior aspect of the vertebral bodies, just medial to the pedicles. This trough extended in the cephalo-caudal direction from the middle of T4 to the T7–8 disc (Fig. 2b). This sagittal trough served as a docking point for the osteotomes during the second stage, marking the initiation point for the sagittal osteotomies.
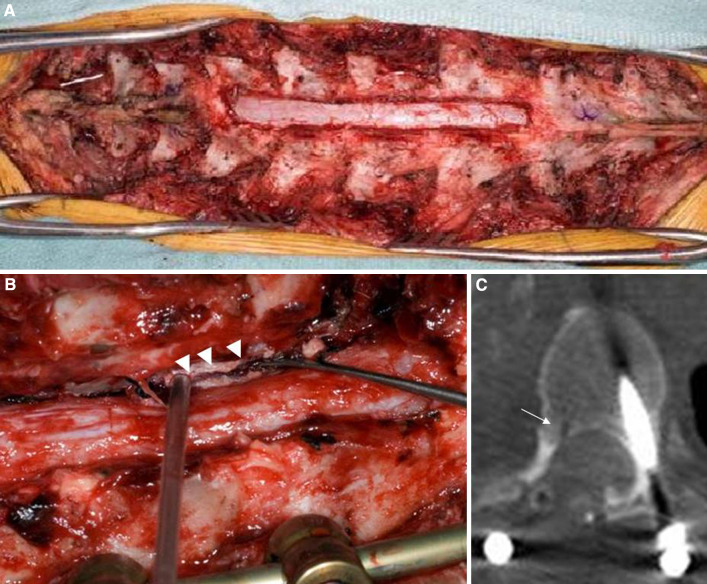
Fig. 2.
a Cephalad (left) and caudal (right) exposure and T4–7 laminectomy with right medial facetectomy. b Sagittal trough (arrowheads) drilled in the posterior T6 vertebral body. This trough was drilled with a 2 mm cutting bur extending from the mid-T4 vertebral body caudally to the T7–8 disc. It was approximately 3 mm deep and allowed the tip of the osteotome to seat safely and securely during the initiation of the sagittal osteotomies. c Axial CT scan of T6 vertebral body demonstrating trough drilled (white arrow)
The importance of this initial bone work cannot be overemphasized. The medial facetectomies are critical to provide unfettered access to the lateral spinal canal for the high speed drill and subsequent osteotomes. The bone removal must be wide enough to allow an appropriate angle of trajectory for the osteotome, allowing its handle to lean out laterally enough to achieve appropriate medialization of the tip. The sagittal trough is also a key step. The bone of the posterior vertebra at the base of the pedicle is quite hard. Initiating the bone cut with the osteotome itself can require significant force and there is clearly a risk of slippage, which can be dangerous given the proximity of the spinal cord. The trough provides a secure slot into which the osteotomes can be inserted down to the cancellous bone and thus directed in a carefully controlled manner. A precise starting point and trajectory for the sagittal osteotomy are critical in order to achieve an en bloc resection and avoid neural and vascular injury. After the trough was created, posterior stabilization using pedicle screw/rod instrumentation was performed from T2–9 on the left and T2, 3, 4, 8, and 9 on the right. Stereotactic navigation was used to facilitate screw placement and the wound was closed in anatomic layers. While these steps could have been performed as part of a single combined anterior/posterior approach, this two stage technique was utilized because it is easier to place the pedicle screws, and the spinal alignment is more neutral, in the prone position.
The patient underwent a CT scan of the thoracic spine between the first and second stages of the operation, following the posterior decompression and instrumentation (see Fig. 2c). The CT images from that study were loaded into the VectorVision (BrainLab Inc., Munich, Germany) frameless stereotaxy system. This data would be utilized during the second stage for trajectory planning and real-time guidance during the vertebral osteotomies.
Stage 2
Two days after the first stage was completed, the patient was taken back to the operating room. The patient was positioned in the left lateral decubitus position and the procedure began with a right posterolateral thoracotomy incision between the fifth and sixth ribs that extended posteriorly to the midline incision from the first stage. The posterior incision was eventually re-opened allowing for a simultaneous anterior and posterior approach to the spine, the advantages of which are well described [4]. The plastic surgery and thoracic surgery services jointly elevated and mobilized the right trapezius, latissimus dorsi, and serratus anterior muscles to provide additional soft tissue coverage at the time of closure.
Next, the thoracotomy was performed by the thoracic surgeon. The fifth, sixth, and seventh ribs were cut distal to the tumor providing a view into the chest. A double lumen endotracheal tube allowed the right lung to be deflated and the tumor to be inspected. The lung was adherent to the tumor capsule and a wedge resection of the right upper and lower lobes was performed, leaving a small portion of each lobe attached to the tumor capsule. This was necessary to avoid violating the tumor capsule and to assure wide negative margins. The medial aspect of the tumor was then palpated and traced to the anterior aspect of the spine using blunt finger dissection. The firm tumor capsule covered by parietal pleura allowed this path to be created without tumor violation. By sweeping the azygos vein, aorta and esophagus medially, an approximately 1 cm space was created along the anterior aspect of the vertebra and this would serve as the end point of the osteotomy in the sagittal plane. A wide margin was achieved as the tumor was covered with a layer of parietal pleura in this area.
With the intrathoracic dissection completed, the posterior incision from the Stage 1 surgery was re-opened. The fourth and eighth ribs were identified and traced back to the spine. These represent the superior and inferior margins of our resection, respectively. The right paraspinal muscle was truncated at the level of the fourth and eighth ribs and left attached to the tumor to ensure a clean dorsal margin (Fig. 3). The frameless stereotaxy unit was brought into the room. The reference array (RA) was clamped to the T9 spinous process, which was below the caudal end of our instrumentation. Using the posterior elements of T9 (lamina and spinous processes) facilitated an accurate registration (less than 1.1 mm mean error) because there was limited hardware artifact from the instrumentation at this level. After successful registration, the pointer probe was placed in the previously drilled sagittal trough in the right posterior aspect of each of the vertebral bodies to assess the accuracy of the registration. Confirming the accuracy of the navigation system by the surgeon using known anatomic landmarks was critical to identify the trajectories needed for the osteotomes (see Fig. 4a). The pointer can then be used alongside the osteotome and guide their trajectories.
Fig. 3.

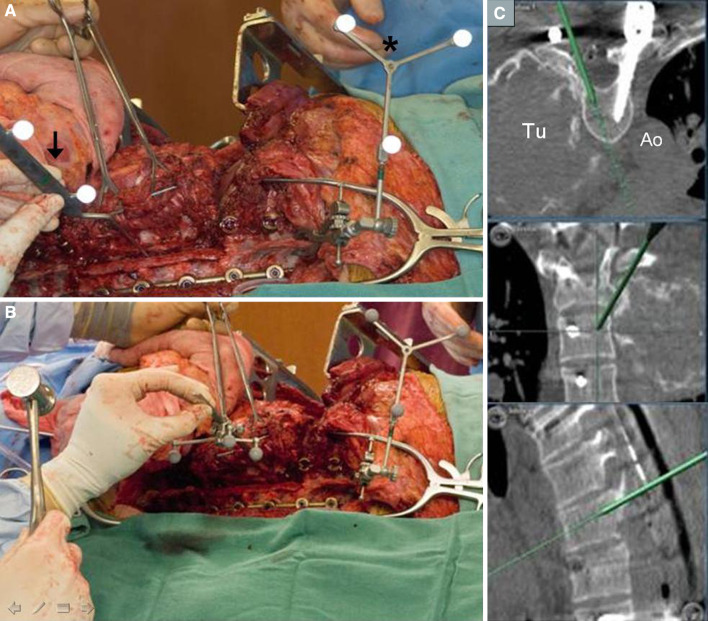
Cephalad (left) and caudal (right) extent of the exposure in Stage 2 of the resection. The tumor has been dissected free of all attachments except for the sagittal osteotomies at T4–7. There is a retractor (asterisk) under the scapula, the fourth and eighth ribs (dashed white outline) have been exposed, and the paraspinous muscle (black arrows) has been cut and will be removed with the specimen
Fig. 4.
a Reference array (asterisk) clamped to the T9 spinous process. The pointer probe (arrow) is placed in the drilled trough in the posterior vertebral bodies to verify system and registration accuracy. b The osteotome has been registered to the system by clamping a tracking probe to the shaft. Using the proposed trajectory seen on the screen (c), the osteotomy is begun. c Axial, coronal and sagittal views are demonstrated. As the sagittal osteotomy is completed, the depth and trajectory of the instrument is seen in real-time. Tu tumor, Ao aorta
Alternatively, the osteotome itself can be registered to the system by clamping a tracking probe to it (see Fig. 4b). This enables real-time visualization of the osteotome trajectory in the sagittal and axial planes during the osteotomy. Moreover, the imaging provides feedback on the depth of the osteotome tip as it is tapped through the vertebra aiming for the anterior vertebral body surface between the tumor and the aorta. (see Fig. 4c).
Following completion of the initial cut, the tracking probe can be removed and fixed to additional subsequent osteotomes, facilitating the series of cuts required to complete the multilevel osteotomy. Throughout the entire course of these cuts, our thoracic surgeon can palpate the anterior aspect of the spine, feeling for the osteotome tip elevating the ALL, while protecting the aorta.
Once the sagittal osteotomies were completed from the middle of T4 to the T7–8 disc, the only remaining attachments were the lateral vertebral body of T4, the T7–8 disc annulus, and the anterior longitudinal ligament (ALL) and parietal pleura medially. A small transverse osteotomy connecting the sagittal osteotomy with the lateral aspect of the vertebra was completed using a narrow osteotome just below the T4 pedicle. The T7–8 disc was cut sharply with a #15 scalpel. This allowed the entire specimen to be mobilized (see Fig. 5). The remaining attachments to the parietal pleura and ALL were then cauterized and divided, freeing the specimen which was sent to pathology (Fig. 6).
Fig. 5.
a Sagittal osteotomies complete with T4–5, T5–6, T6–7 discs visible (asterisk) and cut vertebral body surfaces seen. b Sagittal osteotomy seen on axial CT scan at T7 level. Note the relationship of the ventral aspect of the osteotomy to the aorta (Ao)
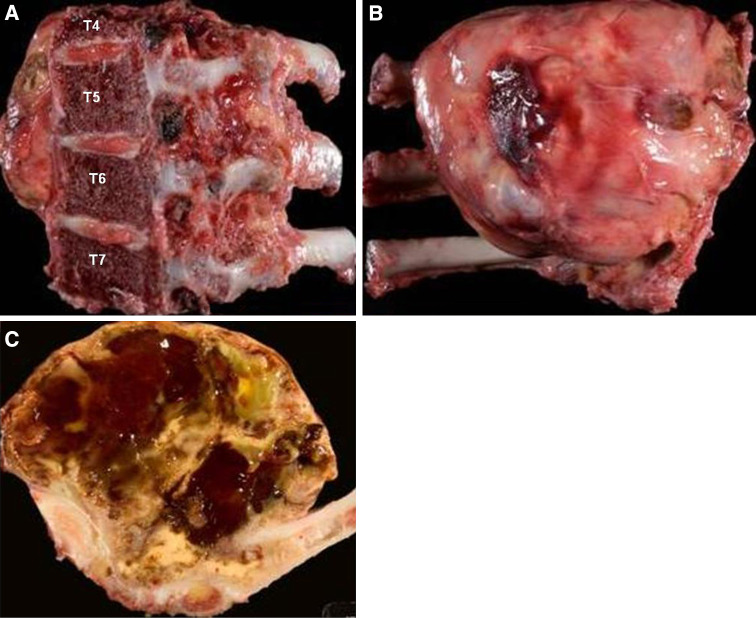
Fig. 6.
The tumor specimen. a En face view of the sagittal osteotomy surface. b Ventral view of the tumor resected in en bloc fashion. c Cross-section of the specimen demonstrating the gross appearance of the heterogeneous contents of the tumor
This resulted in an en bloc resection of the tumor and margins were negative for tumor on final pathological evaluation. While there was no tumor invasion of the vertebra, the capsule was densely adherent to the vertebral periosteum. Dissection along this plane would most certainly have led to an intratumoral margin.
Prior to closure, the right posterior stabilization rod was replaced and the posterior bony surfaces from T2 to T9 were decorticated using a high speed drill. Corticocancellous allograft and demineralized bone matrix were placed over the bleeding bony surfaces to achieve arthrodesis. At closure, the trapezius muscle was used to provide coverage for the posterior instrumentation. This was necessary as the paraspinal musculature at the involved levels had been resected with the specimen in order to ensure a clean margin. The latissimus dorsi muscle was used to reconstruct the posterior chest wall where the ribs had been resected, and to provide a barrier between the exposed thecal sac and the thoracic cavity. The serratus anterior muscle was used to provide further coverage of the posterior chest wall defect.
At 1-year follow-up, the patient has no radiographic evidence of recurrence, and his spinal alignment has been maintained without evidence of hardware failure. There is evidence of successful posterior arthrodesis. The post operative CT scans, when compared with the intraoperative navigational images, confirm that the vertebral osteotomies are at the location predicted by the frameless stereotaxy during the surgery (Figs. 7a–c, 4c). The patient is neurologically intact.
Fig. 7.
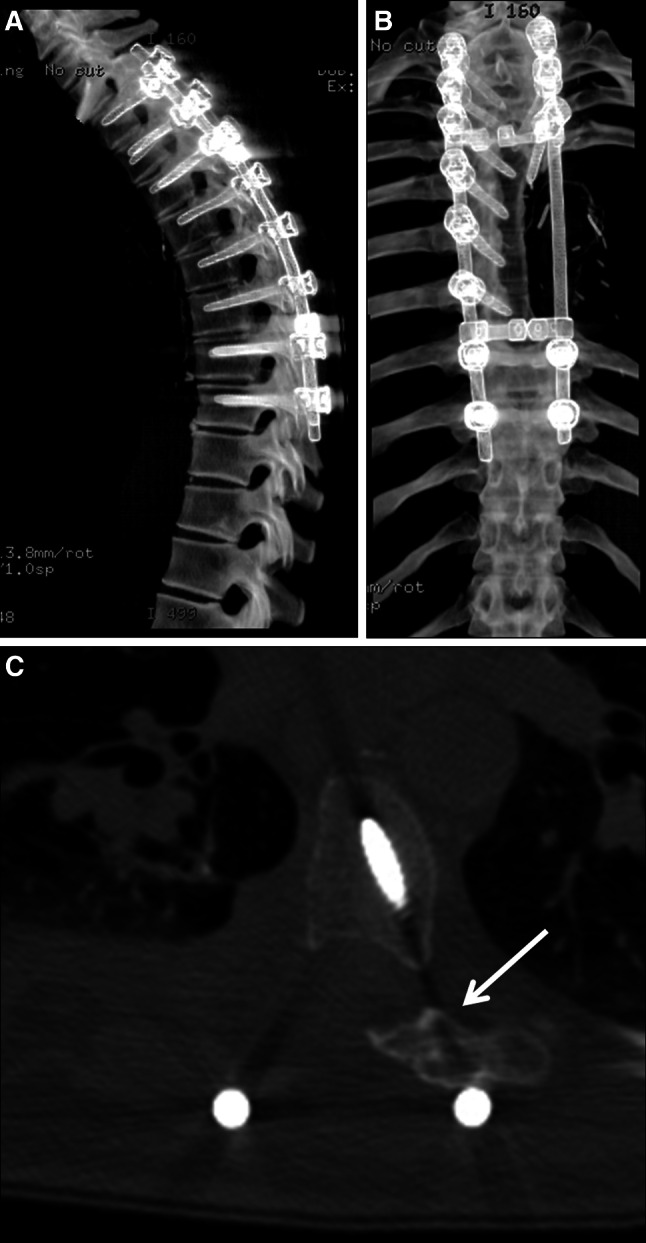
a Sagittal CT reconstruction at 1 year postoperative demonstrating maintenance of spinal alignment without evidence of hardware failure. b Coronal CT reconstruction at 1 year postoperative demonstrating maintained coronal alignment. c Axial CT at 1 year postoperative. The osteotomy location correlates with the position predicted by the intraoperative navigation (Fig. 4c). Note presence of posterior fusion mass (indicated by arrow) suggesting successful arthrodesis
Discussion
In this case report, we describe a novel technique for the multilevel sagittal vertebral osteotomy required for en bloc resection of a GCT involving the chest wall and thoracic spine. GCT are benign tumors that typically occur in the epiphysis or distal metaphysis of long bones. They commonly occur in patients in their third to fourth decades of life, and there is a slight female predominance. They are regarded as benign but can be locally aggressive [5]. The rib is a rare site for GCT, accounting for 0.5–1.6% of cases [1, 4, 6]. Positive margins carry a high risk of local recurrence [1]. Other therapies (such as radiation) maintain inherent risks as well. Radiation is generally reserved as a treatment for tumors that cannot be controlled with surgical resection because malignant transformation can occur in as many as 30% of cases [7].
En bloc resection has been advocated as the ideal way to treat these lesions if the procedure is technically feasible and can be performed with acceptable morbidity [8]. Decreased local recurrence rates have clearly been demonstrated when en bloc resection is applied to other primary bone tumors [9, 10]. Techniques for en bloc resection of spinal tumors have been increasingly described in the literature [11–13]. These procedures are technically demanding and provide an excellent opportunity for the application of new technologies.
This case highlights the challenge of maintaining tumor-free margins, consistent with en bloc resection. The tumor involvement of the neural foramina, the intimate association with the lateral vertebral surfaces, and dense adherence to the periosteum (with secondary inflammatory changes in the bone) caused concern that any attempt to peel this tumor off of the lateral vertebral bodies would drastically increase the risks of an intralesional margin and tumor spillage. By resecting a portion of the vertebral body, we could ensure a wide margin. One of the difficulties faced in this case was establishing the ideal osteotome trajectory, contending with the narrow window between the tumor and aerodigestive and vascular structures in the thorax. Registration of the osteotomes to the frameless stereotactic navigation system, which has not been previously described, allowed for precise bone cuts to be made under real-time image guidance. This prevented violation of the tumor capsule while preserving the integrity of critical adjacent vascular structures.
Frameless stereotaxy has been increasingly utilized for spinal surgery applications. Over the last decade, it has gained popularity as an adjunct to the surgeon’s knowledge of anatomy when placing spinal instrumentation in the thoracic [14, 15], lumbar [16], and cervical spine [17–19]. In situations such as these, intraoperative navigation may decrease the risk of injury to critical neural and/or vascular structures during screw placement [15, 16, 20–23]. Drawing upon our experience using frameless stereotactic navigation to assist in the placement of thoracic pedicle screws, we utilized this technology in a novel manner to help guide our multilevel sagittal osteotomies. Data from a high resolution spinal CT performed between surgical stages was loaded onto the image guidance system in preparation for the second stage of surgery. For the sake of accuracy, we wanted to place the RA as close to the region of the osteotomies as possible. Unfortunately, the laminectomies performed during the first stage eliminated the spinous processes at these levels as a place to attach the RA and also reduced the number of reliable bony surface landmarks that could be used to register the patient to the system. We therefore initially placed the RA at T8, where we encountered a second limitation. The metal artifact from the hardware altered the imaging of the spine creating false surface “landmarks” that made it impossible to achieve accurate registration. This was solved by moving the RA to the T9 spinous process, safely below the caudal end of the metal construct which ended at the T9 pedicles. Here, the CT scan was free of artifact and successful registration could be performed, and importantly, accuracy of the navigation system could be confirmed by touching the probe to visible anatomic landmarks. Given that the patient was fused from T2 to T9, accuracy was still maintained at T4 despite the fact that the RA was five levels away. Whether using the pointer probe held adjacent to the osteotomes or registering the osteotomes themselves with a tracking probe, we found a high level of accuracy with our image guidance and were able to precisely position our osteotomes to achieve a safe en bloc resection.
References
- 1.Hanna RM, Kyriakos M, Quinn SF. Case report 757: giant cell tumor of rib. Skeletal Radiol. 1992;21:482–488. doi: 10.1007/BF00190998. [DOI] [PubMed] [Google Scholar]
- 2.Sakao Y, Sakuragi T, Takeda Y, Natsuaki M, Itoh T. Giant cell tumor of the rib. Jpn J Thorac Cardiovasc Surg. 2003;51:537–540. doi: 10.1007/s11748-003-0119-z. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg RR, Campbell CJ, Bonfiglio M. Giant-cell tumor of bone. An analysis of two hundred and eighteen cases. J Bone Joint Surg Am. 1970;52:619–664. [PubMed] [Google Scholar]
- 4.Fourney DR, Abi-Said D, Rhines LD, Walsh GL, Lang FF, McCutcheon IE, Gokaslan ZL. Simultaneous anterior–posterior approach to the thoracic and lumbar spine for the radical resection of tumors followed by reconstruction and stabilization. J Neurosurg. 2001;94:232–244. doi: 10.3171/spi.2001.94.2.0232. [DOI] [PubMed] [Google Scholar]
- 5.Werner M. Giant cell tumour of bone: morphological, biological and histogenetical aspects. Int Orthop. 2006;30:484–489. doi: 10.1007/s00264-006-0215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta V, Mittal R. Giant cell tumor of rib–rare location on the anterior aspect. Arch Orthop Trauma Surg. 2000;120:231–232. doi: 10.1007/s004020050053. [DOI] [PubMed] [Google Scholar]
- 7.Caudell JJ, Ballo MT, Zagars GK, Lewis VO, Weber KL, Lin PP, Marco RA, El-Naggar AK, Benjamin RS, Yasko AW. Radiotherapy in the management of giant cell tumor of bone. Int J Radiat Oncol Biol Phys. 2003;57:158–165. doi: 10.1016/s0360-3016(03)00416-4. [DOI] [PubMed] [Google Scholar]
- 8.Shimada Y, Hongo M, Miyakoshi N, Kasukawa Y, Ando S, Itoi E, Abe E. Giant cell tumor of fifth lumbar vertebrae: two case reports and review of the literature. Spine J. 2007;7:499–505. doi: 10.1016/j.spinee.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Boriani S, Bandiera S, Biagini R, Bacchini P, Boriani L, Cappuccio M, Chevalley F, Gasbarrini A, Picci P, Weinstein JN. Chordoma of the mobile spine: fifty years of experience. Spine (Phila Pa 1976) 2006;31:493–503. doi: 10.1097/01.brs.0000200038.30869.27. [DOI] [PubMed] [Google Scholar]
- 10.Boriani S, De Iure F, Bandiera S, Campanacci L, Biagini R, Di Fiore M, Bandello L, Picci P, Bacchini P. Chondrosarcoma of the mobile spine: report on 22 cases. Spine (Phila Pa 1976) 2000;25:804–812. doi: 10.1097/00007632-200004010-00008. [DOI] [PubMed] [Google Scholar]
- 11.Marmor E, Rhines LD, Weinberg JS, Gokaslan ZL. Total en bloc lumbar spondylectomy. Case report. J Neurosurg. 2001;95:264–269. doi: 10.3171/spi.2001.95.2.0264. [DOI] [PubMed] [Google Scholar]
- 12.Rhines LD, Fourney DR, Siadati A, Suk I, Gokaslan ZL. En bloc resection of multilevel cervical chordoma with C-2 involvement. Case report and description of operative technique. J Neurosurg Spine. 2005;2:199–205. doi: 10.3171/spi.2005.2.2.0199. [DOI] [PubMed] [Google Scholar]
- 13.Tomita K, Kawahara N, Murakami H, Demura S. Total en bloc spondylectomy for spinal tumors: improvement of the technique and its associated basic background. J Orthop Sci. 2006;11:3–12. doi: 10.1007/s00776-005-0964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolger C, Wigfield C. Image-guided surgery: applications to the cervical and thoracic spine and a review of the first 120 procedures. J Neurosurg. 2000;92:175–180. doi: 10.3171/spi.2000.92.2.0175. [DOI] [PubMed] [Google Scholar]
- 15.Chappell ET, Pare L, Dolich MO, Lekawa ME, Salepour M. Frameless stereotaxy to facilitate anterolateral thoracolumbar surgery: technique. Neurosurgery. 2005;56:110–116. doi: 10.1227/01.NEU.0000144313.91933.44. [DOI] [PubMed] [Google Scholar]
- 16.Kalfas IH, Kormos DW, Murphy MA, McKenzie RL, Barnett GH, Bell GR, Steiner CP, Trimble MB, Weisenberger JP. Application of frameless stereotaxy to pedicle screw fixation of the spine. J Neurosurg. 1995;83:641–647. doi: 10.3171/jns.1995.83.4.0641. [DOI] [PubMed] [Google Scholar]
- 17.Albert TJ, Klein GR, Vaccaro AR. Image-guided anterior cervical corpectomy. A feasibility study. Spine (Phila Pa 1976) 1999;24:826–830. doi: 10.1097/00007632-199904150-00017. [DOI] [PubMed] [Google Scholar]
- 18.Hott JS, Papadopoulos SM, Theodore N, Dickman CA, Sonntag VK. Intraoperative Iso-C C-arm navigation in cervical spinal surgery: review of the first 52 cases. Spine (Phila Pa 1976) 2004;29:2856–2860. doi: 10.1097/01.brs.0000147742.20637.49. [DOI] [PubMed] [Google Scholar]
- 19.Kalfas IH. Image-guided spinal navigation: application to spinal metastases. Neurosurg Focus. 2001;11:e5. doi: 10.3171/foc.2001.11.6.6. [DOI] [PubMed] [Google Scholar]
- 20.Abumi K, Shono Y, Ito M, Taneichi H, Kotani Y, Kaneda K. Complications of pedicle screw fixation in reconstructive surgery of the cervical spine. Spine (Phila Pa 1976) 2000;25:962–969. doi: 10.1097/00007632-200004150-00011. [DOI] [PubMed] [Google Scholar]
- 21.Rajasekaran S, Vidyadhara S, Ramesh P, Shetty AP. Randomized clinical study to compare the accuracy of navigated and non-navigated thoracic pedicle screws in deformity correction surgeries. Spine. 2007;32:E56–E64. doi: 10.1097/01.brs.0000252094.64857.ab. [DOI] [PubMed] [Google Scholar]
- 22.Reinhold M, Bach C, Audige L, Bale R, Attal R, Blauth M, Magerl F. Comparison of two novel fluoroscopy-based stereotactic methods for cervical pedicle screw placement and review of the literature. Eur Spine J. 2008;17:564–575. doi: 10.1007/s00586-008-0584-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy-Camille RM, Saillant G, Benazet J. Rationale and techniques of internal fixation in trauma of the cervical spine. In: Errico T, Bauer RD, Waugh WT, editors. Spinal trauma. Philadelphia: JB Lippincott; 1989. pp. 163–169. [Google Scholar]